Abstract
INTRODUCTION
This study aims to make an indirect comparison between enzalutamide and abiraterone acetate for mCRPC post-docetaxel.
METHODS
A search for published phase 3 trials was performed with PubMed. Indirect comparisons of enzalutamide (AFFIRM) to abiraterone acetate (COU-AA-301) on outcomes overall survival (OS), time to prostate-specific-antigen (PSA) progression, radiographic progression-free survival (PFS), and PSA response were constructed in the context of log-linear regression models.
RESULTS
There was no statistically significant difference in OS (hazard ratio (HR) 0.85, 95% CI 0.68–1.07). However, there was some evidence that enzalutamide may outperform abiraterone acetate with respect to secondary outcomes: time to PSA progression (HR 0.40, 95% CI 0.30–0.53), radiographic PFS (HR 0.61, 95% CI 0.50–0.74), and PSA response rates (RRs) (OR 10.69, 95% CI 3.92–29.20).
CONCLUSION
While there was no statistically significant difference in OS, enzalutamide may be advantageous for secondary endpoints. Findings of this indirect comparison serve to be hypothesis-generating for future head-to-head trials.
Keywords: enzalutamide, abiraterone acetate, metastatic castration-resistant prostate cancer, mCRPC, post-docetaxel
Introduction
Prostate cancer is the second leading incident cancer after lung cancer and the sixth leading cause of cancer mortality in men globally.1 Metastatic castration-resistant prostate cancer (mCRPC) is defined as persistent tumor proliferation despite castrate testosterone levels, not responding to standard surgical or medical androgen-deprivation therapy, with bone being the most common organ site of metastatic disease.2,3
A combination of mitoxantrone and prednisone had previously been the standard of care for treatment of mCRPC. Following landmark trials that demonstrated the superiority of docetaxel over mitoxantrone, docetaxel plus prednisone emerged as the standard of care for first-line therapy for mCRPC.4–6 Nevertheless, patients treated with docetaxel commonly develop progression or relapse and hence, the availability of second-line therapeutic options remains pivotal. More recently, the TROPIC trial has reported superiority in overall survival (OS) of cabazitaxel (a second-generation taxane) plus prednisone over mitoxantrone plus prednisone in post-docetaxel mCRPC patients.7
Tumor biology indicates that despite castrate levels of testosterone, signaling in hormone-related pathways remains a critical driver in prostate cancer progression including (a) upregulation of androgen-producing enzymes, (b) genetic modifications stimulating upregulation of androgen-receptors, and (c) androgen-receptor mutations enabling cross-talk activation by mediators.8–10
Recent basic research has provided insights into the molecular mechanisms of mCRPC. A two-compartment model describes the prostate tumor epithelial compartment interaction with its microenvironment stromal compartment. The cross-talk between these two compartments contributes to progression of mCRPC through signaling mechanisms of the androgenic, tyrosine kinase, angiogenic, apoptotic, and immune pathways.11–14
As a consequence of a more thorough understanding of prostate cancer biology and molecular mechanisms, novel hormonal therapeutic targets for post-docetaxel mCRPC have recently been tested in phase III trials: enzalutamide15 and abiraterone acetate.16,17
Enzalutamide (MDV3100) is an oral androgen-receptor blocker that binds more tightly and has a novel mechanism of action compared to older anti-androgens. It is thought to work by disrupting androgen-receptor nuclear translocation, binding of hormone response elements, and subsequent mobilization of regulatory proteins for downstream transcription and translation.11,18 The phase 3 AFFIRM trial compared enzalutamide to placebo, potentially with prednisone or other glucocorticoids, and demonstrated superiority in all outcomes, including OS, time to prostate-specific-antigen (PSA) progression, radiographic progression-free survival (PFS), and PSA RR.15
Abiraterone acetate is a pro-drug developed for oral administration. Its metabolite, abiraterone, is a CYP17 inhibitor in the steroidogenesis pathway, which is believed to work primarily through inhibition of 17α-hydroxylase and c17,20-lyase. This in turn inhibits production of dehydroepiandrostenedione (DHEA) and androstenedione, precursors of testosterone.19 The phase 3 COU-AA-301 trial compared abiraterone acetate plus prednisone versus placebo plus prednisone and demonstrated superiority in all outcomes, including OS, time to PSA progression, radiographic PFS, and PSA response rate (RR).16,17
A recent meta-analysis by Iacovelli et al has demonstrated significant improvement in OS for second-line treatment of mCRPC using pooled data on cabazitaxel, abiraterone acetate, and enzalutamide with their respective comparators.20 Unfortunately, there is no currently available phase 3 data directly comparing the effectiveness of hormonal therapeutics enzalutamide to abiraterone acetate in patients with post-docetaxel mCRPC. Hence, we aim to perform a literature-based systematic review and make an indirect comparison of hormonal therapeutics between enzalutamide and abiraterone acetate in terms of OS, time to PSA progression, radiographic PFS, PSA RRs, and adverse events.
Methods
Study selection
Randomized phase III clinical trials having hormonal therapeutics enzalutamide or abiraterone acetate as a comparator in mCRPC patients post-docetaxel were included. Endpoints of interest were OS, time to PSA progression, radiographic PFS, PSA RRs, and adverse events. Literature search was performed with PubMed to identify published randomized phase 3 trials using the search phrase “enzalutamide” OR “abiraterone”. The search results were further limited to clinical trials. Abstracts were screened, and non-relevant studies were excluded.
As an assessment of risk of publication bias, clinical-trials.gov was searched for any registered trials using either enzalutamide or abiraterone and having accessible results. As an assessment of risk of bias within the included studies, each study’s design and execution were examined in terms of patient selection, interventions applied to study arms, loss to follow-up, endpoint assessment, and reporting.
Statistical analysis
Indirect comparisons are increasingly used to draw preliminary insights based on the best available evidence for comparative effectiveness of interventions when direct head-to-head comparisons are not available.21–23
Indirect comparisons of enzalutamide to abiraterone acetate on each outcome, OS, time to PSA progression, radiographic PFS, and PSA response, in terms of hazard or odds ratios, as appropriate, were constructed in the context of log-linear regression models with standard errors fixed at those respectively reported in the enzalutamide versus placebo (AFFIRM)15 and abiraterone acetate versus placebo (COU-AA-301)17 studies. Owing to lack of replication of comparisons, it was not possible to estimate study-to-study heterogeneity, and the primary estimates are subject to the assumption of no heterogeneity in treatment effects across studies. However, the potential impact of a broad range of heterogeneity scenarios was examined in a sensitivity analysis.
Where alike adverse events were reported in both studies, indirect comparisons of adverse event rates were generated using methods similar to RR. In the case of drug-specific adverse events, individual odds ratios for comparison with placebo were computed using rates reported in the individual studies.
As there was only one study for each treatment comparison, it was not possible to estimate study-to-study heterogeneity. Hence, we performed a sensitivity analysis to assess the robustness of our results to a range of heterogeneity scenarios in terms of I2, the proportion of variability across studies which is because of actual study-specific differences in treatment effect rather than chance.24,25 In the context of a mixed model for meta-analysis similar to DerSimonian and Laird,26 it can be shown that if the estimates of the included studies have similar standard errors, then study-to-study variance is approximately (I2/(1 − I2))SE2. To assess the sensitivity of results to potential study-to-study heterogeneity, for each outcome, I2 was varied over (0, 25, 50, 75%) whereas SE2 was set at its average value over AFFIRM15 and COU-AA-301.17 Then, 95% confidence intervals (95% CIs) and 95% predictive intervals (95% PIs) were constructed in the context of log-linear mixed models with study-to-study variance set using the above equation.
A further sensitivity analysis was performed by comparing indirect estimates obtained with comparison of AFFIRM15 to COU-AA-30117 in the full analysis to those obtained with comparison of AFFIRM15 to COU-AA-30116 in the interim analysis.
Results
The methods and results of this indirect comparison are reported in accordance with PRISMA guidelines.27 Literature search yielded two published randomized phase 3 trials that fulfilled the search criteria of a trial containing an experimental arm with either enzalutamide or abiraterone as treatment of patients with mCRPC post-docetaxel (Fig. 1). Results from the AFFIRM15 and COU-AA-30117 trials comparing enzalutamide to placebo and abiraterone acetate to placebo, respectively, were used to indirectly compare enzalutamide to abiraterone acetate. Table 1 summarizes each study.
Figure 1.
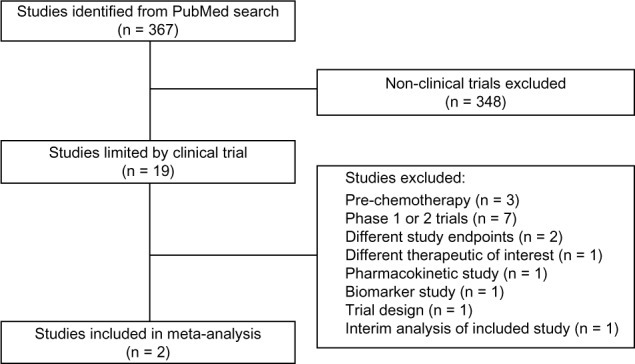
Search strategy and study selection.27
Table 1.
Summary of individual trial characteristics.
| TRIAL | PATIENT GROUPS (N) | MEDIAN FOLLOW-UPa | TREATMENT ARMS (N) | OSa | TIME TO PSA PROGRESSIONa | RADIOGRAPHIC PFSa | PSA RRb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| MEDIAN | HR (95% CI) |
MEDIAN | HR (95% CI) |
MEDIAN | HR (95% CI) |
RR | OR (95% CI) |
||||
| AFFIRM15 | Progressive CRPC post-docetaxel (N = 1199) |
14.4 | Enzalutamidec (n = 800) |
18.4 | 0.63 (0.53–0.75) | 8.3 | 0.25 (0.20–0.30) | 8.3 | 0.40 (0.35–0.47) | 54.0%e | 76.41 (31.22–187.04) |
| Placeboc (n = 399) |
13.6 | 3.0 | 2.9 | 1.5%e | |||||||
|
| |||||||||||
| COU-AA-30117 | Metastatic CRPC post-docetaxel (N = 1195) |
20.2 | Abiraterone acetated (n = 797) |
15.8 | 0.74 (0.64–0.86) | 8.5 | 0.63 (0.52–0.78) | 5.6 | 0.66 (0.58–0.76) | 29.5% | 7.15 (4.53–11.28) |
| Placebod (n = 398) |
11.2 | 6.6 | 3.6 | 5.5% | |||||||
Notes:
Reported in units of months.
RR defined as PSA decline ≥50%.
Concomitant administration with prednisone was allowed but not needed.
Concomitant administration with prednisone.
Denominator – enzalutamide – was 731; denominator – placebo – was 330.
OS
The hazard ratios (HRs) (95% CIs) in the AFFIRM15 and COU-AA-30117 trials for OS for enzalutamide versus placebo and abiraterone acetate versus placebo were 0.63 (0.53–0.75) and 0.74 (0.64–0.86), respectively. The indirect estimate of the HR (95% CI; P-value) for enzalutamide versus abiraterone acetate was 0.85 (0.68–1.07; P = 0.17) as illustrated in Figure 2.
Figure 2.
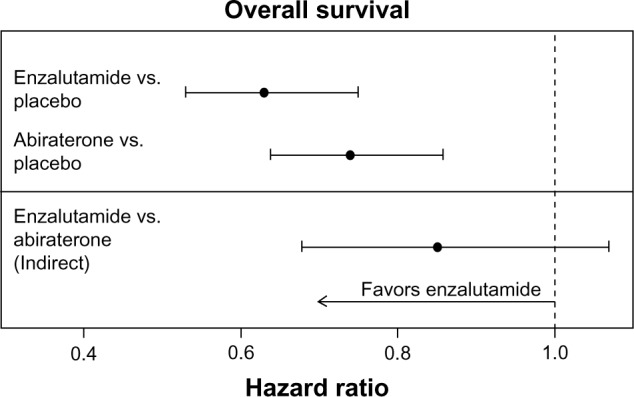
Individual study HR estimates and indirect enzalutamide versus abiraterone acetate estimate for OS.
Time to PSA progression
The HRs (95% CIs) in the AFFIRM15 and COU-AA-30117 trials for time to PSA progression for enzalutamide versus placebo and abiraterone acetate versus placebo were 0.25 (0.20–0.30) and 0.63 (0.52–0.78), respectively. The indirect estimate of the HR (95% CIs; P-value) for enzalutamide versus abiraterone acetate was 0.40 (0.30–0.53; P < 0.001) as illustrated in Figure 3.
Figure 3.
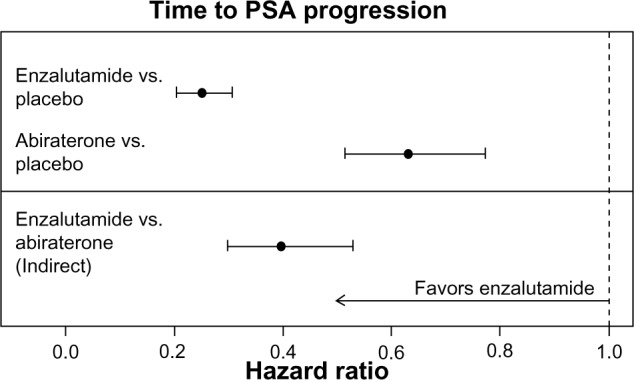
Individual study HR estimates and indirect enzalutamide versus abiraterone acetate estimate for time to PSA progression.
Radiographic PFS
The respective HRs (95% CIs) in the AFFIRM15 and COU-AA-30117 trials for radiographic PFS for enzalutamide versus placebo and abiraterone acetate versus placebo were 0.40 (0.35–0.47) and 0.66 (0.58–0.76), respectively. The indirect estimate of the HR (95% CIs; P-value) for enzalutamide versus abiraterone acetate was 0.61 (0.50–0.74; P < 0.001) as illustrated in Figure 4.
Figure 4.
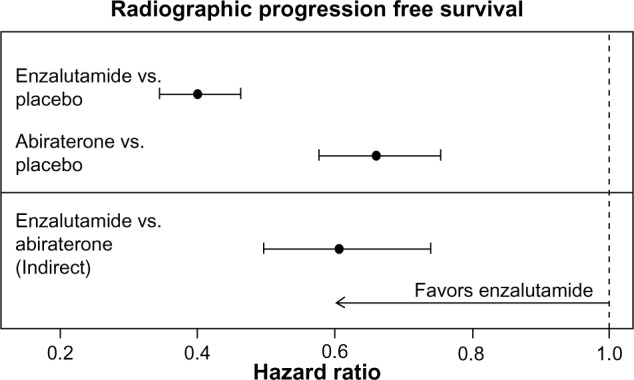
Individual study HR estimates and indirect enzalutamide versus abiraterone acetate estimate for radiographic PFS.
PSA response
The odds ratios (95% CIs) in the AFFIRM15 and COU-AA-30117 trials for PSA response for enzalutamide versus placebo and abiraterone acetate versus placebo were 76.41 (31.22–187.04) and 7.15 (4.53–11.28), respectively. The indirect estimate of the odds ratio (95% CIs; P-value) for enzalutamide versus abiraterone acetate was 10.69 (3.92–29.20; P < 0.001) as illustrated in Figure 5.
Figure 5.
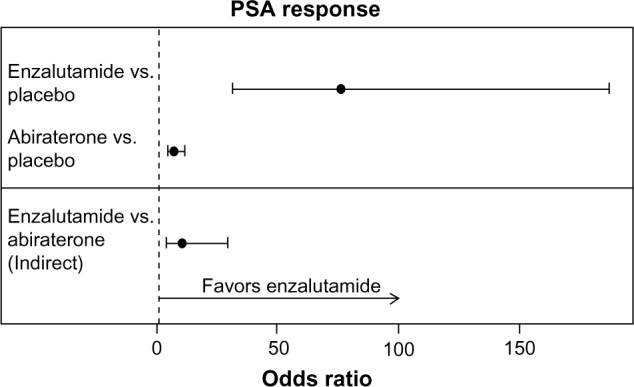
Individual study odds ratio estimates and indirect enzalutamide versus abiraterone acetate estimate for PSA response.
Adverse events
There were no statistically significant differences in fatigue, diarrhea, or liver function abnormalities with enzalutamide versus abiraterone acetate, albeit marginally more cardiac disorders with abiraterone acetate. Specific adverse events from abiraterone acetate include fluid retention and hypokalemia, which were significantly more common in the treatment group compared to placebo. In all, 5 of 800 patients treated with enzalutamide had seizures while no patients in the placebo group did (Table 2).
Table 2.
Indirect comparisons of adverse events.
| ADVERSE EVENTS | AFFIRM15 | COU-AA-30117 | INDIRECT COMPARISON | |||||
|---|---|---|---|---|---|---|---|---|
| N (%) | OR§ (95% CI) | N (%) | OR§ (95% CI) | OR§ (95% CI) | P | |||
| ENZALUTAMIDE (N = 800) | PLACEBO (N = 399) | ABIRATERONE ACETATE (N = 791) | PLACEBO (N = 394) | |||||
| Fatigue | 269 (34%) | 116 (29%) | 1.24 (0.95–1.60) | 372 (47%) | 174 (44%) | 1.12 (0.88–1.43) | 1.10 (0.77–1.57) | 0.60 |
| Diarrhea | 171 (21%) | 70 (18%) | 1.28 (0.94–1.74) | 156 (20%) | 58 (15%) | 1.42 (1.02–1.98) | 0.90 (0.57–1.41) | 0.64 |
| Cardiac disorders† | 49 (6%) | 30 (8%) | 0.80 (0.50–1.29) | 126 (16%) | 46 (12%) | 1.43 (1.00–2.06) | 0.56 (0.31–1.01) | 0.06 |
| Liver function test abnormalities | 8 (1%) | 6 (2%) | 0.66 (0.23–1.92) | 89 (11%) | 35 (9%) | 1.30 (0.86–1.96) | 0.51 (0.16–1.59) | 0.25 |
| Fluid retention | – | – | – | 261 (33%) | 94 (24%) | 1.57 (1.19–2.07) | – | – |
| Hypokalemia | – | – | – | 143 (18%) | 36 (9%) | 2.19 (1.49–3.23) | – | – |
| Hypertension | – | – | – | 88 (11%) | 32 (8%) | 1.42 (0.93–2.16) | – | – |
| Seizure | 5 (<1%) | 0 | Inf. (0.46–Inf.)‡ | – | – | – | – | – |
Notes:
Cardiac disorders were defined in COU-AA-301 to include any of the following: cardiac ischemia, myocardial infarction, supraventricular or ventricular tachyarrhythmias, cardiac failure, or other arrhythmia-related problems. In the AFFIRM study, cardiac disorder was defined more broadly as either any disorder or myocardial infarction.
Abbreviations: Inf., infinity;
OR, odds ratio.
Assessment of risk of publication bias and within included study bias
Search of clinicaltrials.gov for registered clinical trials using either enzalutamide or abiraterone for the same indication did not yield any additional relevant studies.28,29 We conclude that the risk of publication bias is low. In both included studies, well-defined and similar patient populations were recruited. Both trials were randomized, double blind, and placebo controlled. In both, intention-to-treat estimates were reported. We conclude that the risk of bias within the included studies is also low.
Sensitivity analyses
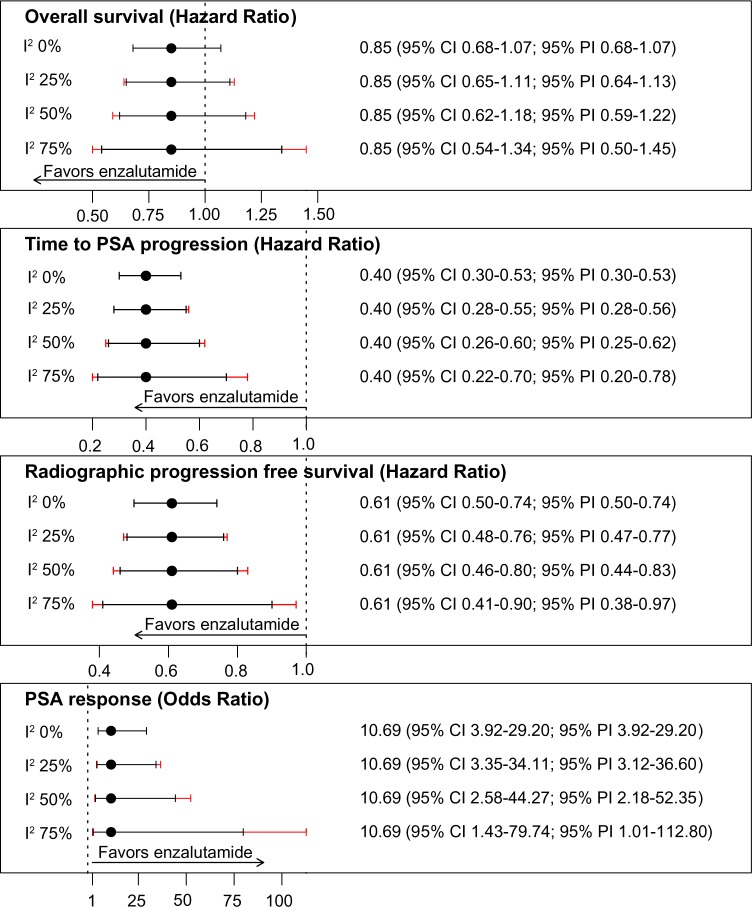
Sensitivity analysis of the robustness of our results to a broad range of heterogeneity scenarios showed that findings were qualitatively unchanged even in the hypothetical presence of high levels of study-to-study heterogeneity (Fig. 6). That is, even if 75% of the variability in estimates were because of study-specific differences in treatment effectiveness not chance, we would still find no statistically significant difference in OS, whereas enzalutamide would outperform abiraterone acetate in terms of time to PSA progression, radiographic PFS, and PSA RR.
Figure 6.
Sensitivity analysis of estimates with potential study-to-study heterogeneity.
In our study, we compared the interim analysis of AFFIRM15 and full analysis of COU-AA-301,17 which had somewhat different follow-up times, median 14.4 and 20.2 months, respectively. Hence, we repeated the indirect comparisons utilizing the interim analysis for both studies, AFFIRM15 and COU-AA-301,16 with comparable interim follow-up times, median 14.4 and 12.8 months, respectively. We found similar results; a non-significant improvement in OS with enzalutamide versus abiraterone acetate, but significant benefits of enzalutamide over abiraterone acetate in secondary outcomes: time to PSA progression, radiographic PFS, and PSA RRs (Table 3).
Table 3.
Sensitivity analysis—comparison of indirect estimates of AFFIRM (interim analysis)15 with COU-AA-301 (full set17 and interim analysis16).
| AFFIRM VERSUS COU-AA-301 (FULL SET) |
P | AFFIRM VERSUS COU-AA-301 (INTERIM) |
P | |
|---|---|---|---|---|
| Median follow-up (month) | 14.4 versus 20.2 | – | 14.4 versus 12.8 | – |
| OS, HR (95% CI) | 0.85 (0.68–1.07) | 0.17 | 0.97 (0.76–1.24) | 0.81 |
| Time to PSA progression, HR (95% CI) | 0.40 (0.30–0.53) | <0.01 | 0.43 (0.32–0.59) | <0.01 |
| Radiographic PFS, HR (95% CI) | 0.61 (0.50–0.74) | <0.01 | 0.60 (0.49–0.73) | <0.01 |
| PSA response,a OR (95% CI) | 10.69 (3.92–29.20) | <0.01 | 10.89 (3.99–29.74) | <0.01 |
Note:
RR defined as PSA decline ≥50%.
Discussion
In this indirect comparison study of enzalutamide and abiraterone acetate, we found no statistically significant difference in OS in patients with mCRPC post-docetaxel. However, enzalutamide showed significant benefits over abiraterone acetate for secondary outcomes: time to PSA progression, radiographic PFS, and PSA RRs. There were marginally more cardiac disorders with abiraterone acetate. In addition, both drugs had some adverse events that were unique to each: mineralocorticoid events for abiraterone acetate and a risk of seizures for enzalutamide.
A possible explanation for the superiority of enzalutamide over abiraterone could be the fact that prednisone administration was compulsory in the abiraterone study, COU-AA-301,17 whereas prednisone was not compulsory in the enzalutamide study, AFFIRM.15 Conventionally, prednisone is thought to have a therapeutic impact, potentially by exerting anti-cancer effects on its own or alleviating toxicities of other anti-tumor agents.30 Recently however, Richards et al. have shown that corticosteroids may activate the mutated androgen-receptor causing disease progression.31 Following that, Scher et al. conducted a post hoc analysis of the enzalutamide study, AFFIRM15, and found that patients given prednisone had poorer OS and reduced benefit of enzalutamide compared to patients without prednisone, with marginal differences observed for OS and more evident differences for PSA progression and radiographic PFS.32,33 Hence, compulsory administration of prednisone in the abiraterone study, COU-AA-301,17 could exert a greater attenuation of drug effect compared to AFFIRM,15 potentially explaining findings in our study of no significant difference in OS but significant benefits of enzalutamide over abiraterone in secondary endpoints.
The process of selecting a particular systemic therapy for patients with an incurable malignancy involves several different considerations, with the toxicity profile being one of the most important considerations. Enzalutamide is known to have off-site actions on GABAA receptors in reducing seizure thresholds and hence should be used with close monitoring in patients with known seizure disorders or brain injury.15,34 Abiraterone acetate inhibits the steroidogenic pathway to cause mineralocorticoid disturbances and hence should be used with caution in patients with metabolite disturbances, renal failure, or congestive heart failure.16,17,19
Ryan et al have recently reported evidence of significant improvement in OS and radiographic PFS for use of abiraterone acetate in mCRPC patients in the pre-chemotherapy setting.35 In the same mode, the PREVAIL trial comparing enzalutamide versus placebo in mCRPC patients in the pre-chemotherapy setting also reported favorable preliminary outcomes with significant improvement in OS and radiographic PFS.36,37 These provided a paradigm shift to the sequencing of therapeutic options and a question for future research to compare therapeutic outcomes of abiraterone acetate used in the pre- versus post-chemotherapy settings. However, a major limitation of these randomized phase III trials was that they were placebo-controlled, whereas for many patients with mCRPC, particularly symptomatic patients or asymptomatic patients with rapidly progressing disease, the standard of care would not be observation but rather treatment with chemotherapy. Even though abiraterone acetate was approved by the FDA for use in the pre-chemotherapy setting based on the results from this study, the optimal sequence of treatments remains poorly defined. To the best of our knowledge, there are no ongoing trials comparing abiraterone acetate to chemotherapy as first-line treatment for mCRPC.
Other therapeutic directions being explored for treatment of mCRPC include combining docetaxel with molecular targets for potential synergistic effects.12,38 However, Araujo et al. have recently reported no favorable outcomes for combination therapy involving docetaxel with tyrosine kinase inhibitor dasatinib.39 An ongoing phase 3 trial, SYNERGY (NCT01188187), is comparing docetaxel plus prednisone to docetaxel plus prednisone with custirsen sodium, a clusterin inhibitor.40 In addition, the combination of enzalutamide and abiraterone is also being investigated in a single arm phase 2 trial (NCT01650194).41
The main limitation of our indirect comparison study is the assumption of no study-specific differences in the effectiveness of treatments. However, sensitivity analysis showed qualitatively unchanged findings across a broad range of potential study-to-study heterogeneity scenarios, as summarized by I2, ranging from 0 to 75%. Another limitation was the comparison of the interim analysis of AFFIRM15 and full analysis of COU-AA-301,17 which had different follow-up times. However, our sensitivity analysis showed that findings remained qualitatively unchanged when the interim analyses of each trial were compared.15,16 Nonetheless, this is the first study to compare (indirectly) the effects of enzalutamide and abiraterone acetate for the treatment of mCRPC post-docetaxel.
In conclusion, our indirect comparison suggests no statistically significant difference in OS between the two drugs. However, there is an indication that enzalutamide could be superior to abiraterone acetate in time to PSA progression, radiographic PFS, PSA RRs, and have a more favorable side effect profile than abiraterone acetate in the treatment of mCRPC post-docetaxel. The findings of this indirect comparison serve to be hypothesis-generating for future head-to-head trials comparing enzalutamide to abiraterone acetate in mCRPC post-docetaxel.
Footnotes
ACADEMIC EDITOR: William C.S. Cho, Editor in Chief
FUNDING: Author(s) disclose no funding sources.
COMPETING INTERESTS: Author(s) disclose no potential conflicts of interest.
Author Contributions
GL, PST, and BH conceived and designed the study. PST and BH analyzed the data. PST wrote the first draft of the manuscript. PST, BH, AJM, CEK, and GL contributed to the writing of the manuscript. PST, BH, AJM, CEK, and GL agreed with manuscript results and conclusions. PST, BH, AJM, CEK, and GL jointly developed the structure and arguments for the paper. PST, BH, AJM, CEK, and GL made critical revisions and approved the final version. All authors reviewed and approved the final manuscript.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
REFERENCES
- 1.Globocan Country Fast Stat. 2008. [Accessed June 22, 2013]. http://globocan.iarc.fr/factsheet.asp.
- 2.Saad F, Hotte SJ. Guidelines for the management of castrate-resistant prostate cancer. Can Urol Assoc J. 2010;4(6):380–4. doi: 10.5489/cuaj.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotte SJ, Saad F. Current management of castrate-resistant prostate cancer. Curr Oncol. 2010;17(suppl 2):S72–9. doi: 10.3747/co.v17i0.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26(2):242–5. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 7.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 8.Sartor AO. Progression of metastatic castrate-resistant prostate cancer: impact of therapeutic intervention in the post-docetaxel space. J Hematol Oncol. 2011;(4):18. doi: 10.1186/1756-8722-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2003;10(1):33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 10.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23(32):8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 11.Dayyani F, Gallick GE, Logothetis CJ, Corn PG. Novel therapies for metastatic castrate-resistant prostate cancer. J Natl Cancer Inst. 2011;103(22):1665–75. doi: 10.1093/jnci/djr362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saad F, Miller K. Treatment options in castration-resistant prostate cancer: current therapies and emerging docetaxel-based regimens. Urol Oncol. 2014;32(2):70–9. doi: 10.1016/j.urolonc.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Chi KN, Bjartell A, Dearnaley D, et al. Castration-resistant prostate cancer: from new pathophysiology to new treatment targets. Eur Urol. 2009;56(4):594–605. doi: 10.1016/j.eururo.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal N, Sonpavde G, Sternberg CN. Novel molecular targets for the therapy of castration-resistant prostate cancer. Eur Urol. 2012;61(5):950–60. doi: 10.1016/j.eururo.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 16.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13(10):983–92. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 18.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Sci Signal. 2009;324(5928):787. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ang JE, Olmos D, de Bono JS. CYP17 blockade by abiraterone: further evidence for frequent continued hormone-dependence in castration-resistant prostate cancer. Br J Cancer. 2009;100(5):671–5. doi: 10.1038/sj.bjc.6604904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iacovelli R, Altavilla A, Procopio G, et al. Are post-docetaxel treatments effective in patients with castration-resistant prostate cancer and performance of 2? A meta-analysis of published trials. Prostate Cancer Prostatic Dis. 2013;16(4):323–7. doi: 10.1038/pcan.2013.20. [DOI] [PubMed] [Google Scholar]
- 21.Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21(16):2313–24. doi: 10.1002/sim.1201. [DOI] [PubMed] [Google Scholar]
- 22.Mills EJ, Thorlund K, Ioannidis JPA. Demystifying trial networks and network meta-analysis. BMJ. 2013;346:f2914. doi: 10.1136/bmj.f2914. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- 24.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DerSimonian R Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ClinicalTrials.gov Enzalutamide. [Accessed June 12, 2013]. http://www.clinicaltrials.gov/ct2/results?term=enzalutamide&Search=Search.
- 29.ClinicalTrials.gov Abiraterone. [Accessed June 12, 2013]. http://www.clinicaltrials.gov/ct2/results?term=abiraterone&Search=Search.
- 30.Prednisone—National Cancer Institute. [Accessed June 12, 2013]. http://www.cancer.gov/cancertopics/druginfo/prednisone.
- 31.Richards J, Lim AC, Hay CW, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 2012;72(9):2176–82. doi: 10.1158/0008-5472.CAN-11-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scher HI, Fizazi K, Saad F, et al. Association of baseline corticosteroid with outcomes in a multivariate analysis of the phase 3 affirm study of enzalutamide (ENZA), an androgen receptor signaling inhibitor (ARSI) ESMO. European Society for Medical Oncology. 2012 Abstract 2887. Vienna. [Google Scholar]
- 33.Scher HI, Fizazi K, Saad F, et al. Impact of on-study corticosteroid use on efficacy and safety in the phase III AFFIRM study of enzalutamide (ENZA), an androgen receptor inhibitor; 2013 Genitourinary Cancers Symposium; Orlando, Florida. [Journal of Clinical Oncology. 2013; 31(suppl 6):abstr 6] [Google Scholar]
- 34.Foster WR, Car BD, Shi H, et al. Drug safety is a barrier to the discovery and development of new androgen receptor antagonists. Prostate. 2011;71(5):480–8. doi: 10.1002/pros.21263. [DOI] [PubMed] [Google Scholar]
- 35.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ClinicalTrials.gov A Safety and Efficacy Study of Oral MDV3100 in Chemotherapy-Naive Patients with Progressive Metastatic Prostate Cancer—Full Text View—ClinicalTrialsgov. [Accessed June 12, 2013]. http://www.clinicaltrials.gov/ct2/show/NCT01212991?term=prevail+trial+enzalutamide&rank=1.
- 37.Machado P, Bowdidge A, Kite J, Beyer M. Medivation and Astellas Announce the Phase 3 PREVAIL Trial of Enzalutamide Meets Both Co-Primary Endpoints of Overall Survival and Radiographic Progression-Free Survival in Chemotherapy-Naïve Patients with Advanced Prostate Cancer. Press Release (Medivation, Astellas) 2013. [Accessed December 19, 2013]. pp. 1–5. Available at: http://www.astellas.com/en/corporate/news/pdf/131022_1_Eg.pdf.
- 38.ClinicalTrials.gov Randomized Study Comparing Docetaxel Plus Dasatinib to Docetaxel Plus Placebo in Castration-Resistant Prostate Cancer—Full Text View—ClinicalTrialsgov. [Accessed June 12, 2013]. http://clinicaltrials.gov/show/NCT00744497.
- 39.Araujo JC, Trudel GC, Saad F, et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol. 2013;14(13):1307–16. doi: 10.1016/S1470-2045(13)70479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ClinicalTrials.gov Comparison of Docetaxel/Prednisone to Docetaxel/Prednisone in Combination with OGX-011 in Men with Prostate Cancer—Full Text View—ClinicalTrialsgov. [Accessed June 12, 2013]. http://clinicaltrials.gov/show/NCT01188187.
- 41.ClinicalTrials.gov A Study to Determine Safety and Tolerability of Enzalutamide (MDV3100) in Combination with Abiraterone Acetate in Bone Metastatic Castration-Resistant Prostate Cancer Patients—Full Text View—ClinicalTrialsgov. [Accessed June 12, 2013]. http://clinicaltrials.gov/show/NCT01650194.