Abstract
As a result of treatment innovations, the survival rates of young people with cancer have increased substantially. The cancers most frequently diagnosed in adults aged 25–49 years include breast, colorectal and cervical cancer and malignant melanoma (Cancer Research UK, 2009). The 5-year survival rates of over 90 % for many malignancies are now reported in young people. But the diagnosis and treatment of cancer often poses a threat to fertility. Methods of fertility preservation are evolving quickly and awareness needs to grow in the medical community regarding these methods. Studies suggest that the ability to have biological children is of great importance to many people. The possible future effects of chemotherapy or radiotherapy on fertility should be discussed with all cancer patients who have reproductive potential. Moreover, fertility preservation should be considered for all young people undergoing potentially gonadotoxic treatment. This article covers the various methods of fertility preserving options in young men and women with respect to the various treatment modalities that they may be subjected to. Sperm banking is a simple and low cost intervention. Embryo cryopreservation is the only established method of female fertility preservation. Oocyte cryopreservation offers a useful option for women without a male partner. Emergency ovarian stimulation and cryopreservation of ovarian tissue (followed by tissue transplantation or in-vitro maturation of oocytes) are experimental techniques for women who require urgent cancer treatment. Large, well-controlled studies are also required to identify any unexpected long-term sequelae of cryopreservation of oocytes and ovarian tissue.
Keywords: Fertility preservation, Cryopreservation of oocytes and ovarian tissue
Review
Advances in cancer therapy have given a chance for a good percentage of patients suffering from malignancies to escape or at least extend their span of enjoyable and productive life. During the course of treatment, the primary objective in the minds of most oncologists is to cure the system of killer illness. Everything else appears as secondary, to the extent of ignoring all other sequele of cancer and its treatment. It has been well established that young men and women lose their reproductive potential either because of chemotherapy or radiotherapy. Several young patients who are victims of leukemia, blood dyscrasia may often be unaware of the seriousness of their problems and much less the possible adverse consequences of the treatment given to them. With 65 % overall survival rates of young cancer patients treated with radiation or chemotherapeutic agents, the prevalence of long-time survivors in young adult population has been estimated to be 1 in 1,000 [1]. Gonadal function has been evaluated in 101 postpubertal children treated with multiple chemotherapies for childhood Hodgkin’s disease [2]. 89.1 % of the males had elevated serum follicular stimulating hormone (FSH) levels indicative of severe germinal epithelial damage and 24.4 % of them had raised serum luteinizing hormone (LH) suggesting subtle Leydig cell dysfunction. Among women, 17 (53 %) of them had elevated serum gonadotrophins, and ten of them had symptoms of ovarian failure. In view of this, maximum attempts should be made to preserve gonadal function of young men and women treated with cancer drug therapy.
Anatomy and Physiology
Female
Female children are born with a store of oocytes in their ovaries from their embryonic stage; the maximum number being approximately 7 million oocytes noticeable at 5 months of gestation. After this point, there is no further increase in formation of germ cells; instead progressive atresia occurs. At birth this number decreases to 1–2 million, and at puberty, it reaches about 0.3 million. Reproductive era in a women’s life is about approximately 30 years from the age of 15 to the age of 45 years. During this period, nearly all the follicles become atretic averaging at the rate of 1,000 follicles per month. The reproductive capacity develops at about the age of 14–15 years. In the first phase, the primordial germ cells and somatic cells (cumulus and stromal cells) become an integrated ovarian mass. The first phase of oocyte maturation starts in utero and is gonadotrophin independent. The next phase of follicular growth is known as recruitment of primordial follicles for maturity also known as folliculogenesis in which oogenesis, granulogenesis and thecogenesis occur (Fig. 1). This phase begins at puberty and is entirely gonadotrophin dependent. The third phase is that of ovulation when the egg becomes liberated from the follicle, the process being triggered off by change of gonadotrophin milieu. The final phase of follicular growth ends up in formation of Corpus Luteum (CL) which develops further during the implantation period. If implantation of embryo fails, the CL undergoes lysis and disappears (Fig. 1).
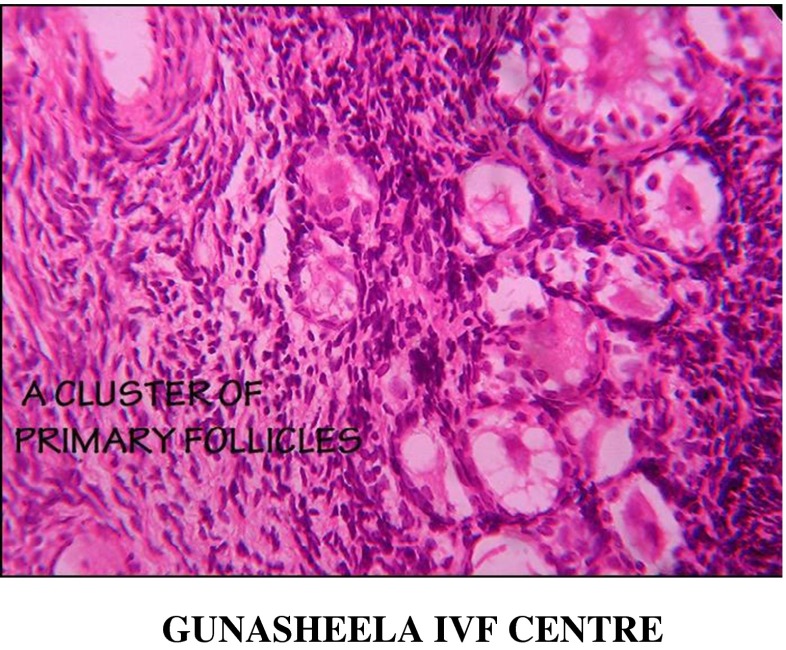
Fig. 1.
Microscopic picture of primary follicle seen in ovarian cortical strips
Male
The human male reproductive system includes the hypothalamic-pituitary-gonadals axis, the epididymis, vas deferens, seminal vesicles, prostate and the urethra. Production of spermatozoa requires approximately 3 months from the initial mitotic division. The testis is composed primarily of seminiferous tubules packed closely together (95 % of testicular volume), and interstitial cells. Each tubule is 30–70 cms long and 200–300 μm in diameter. There are approximately 500 tubules per testis. The cells within the seminiferous tubules are germ cells that mature into spermatozoa, and Sertoli cells that serve as supporting cells for developing germ cells.
The major cell in the interstitial space outside the seminiferous tubule is the Leydig cell, which produces testosterone, a necessary component for germ cell maturation. The mature spermatozoon is released into the tubule lumen. All the seminiferous tubule joins to form the vas deferens which is an approximately 35 cms long tubular structure. Vas deferens is an androgen-dependent organ and transports sperm into the pelvis, where it joins the seminal vesicles to form the ejaculatory ducts, which enter the prostatic urethra.
Endocrinology of Male Reproduction
Pulsatile hypothalamic release of GnRH stimulates the secretion of FSH and LH by anterior pituitary. These hormones then act at the level of the testis, LH stimulating testosterone production by the Leydig cells, and FSH acting on the Sertoli cell to support spermatogenesis.
The Impact of Oncology Therapy on Fertility
Radiotherapy
The damage caused by radiotherapy depends on the type and dose of radiation and dose per fraction. The principle of radiotherapy is based on the ionisation of cellular atoms and molecules leading to the destruction of double and single DNA structures within the cell structure. A chain of events is set up, disrupting the cell-cycle leading to apoptosis of the cells. Radiotherapy has its use in oncology because unlike malignant cells, most normal cells have the inert ability to recover from the effects of radiotherapy. Clearly radiotherapy can be administered as external beam therapy (teletherapy), or as intracavity (brachytherapy) treatments. It is important to consider the effect of scattered radiation as well [3]
Male
In male patients, immature sperm cells are very sensitive even to low radiation dose. The application of greater than 6 Gray to the testes will result in irreversible azoospermia. At levels of 3.5 Gray, sterility does occur, but this is reversible although commonly such recovery will take 18 to 24 months. Interestingly the Leydig cells (responsible for producing testosterone) [4] are more resistant to chemotherapeutic agents. That is why testosterone production and the development of secondary sexual characteristics are preserved even when the spermatogenesis has failed completely [5]. Leydig cell damage is directly related to the dose of cytotoxic agent and inversely to the age of the child [1]. In addition, libido and erection will usually remain normal in the male and it is sterility that is the main concern. However it is not unusual for patients who have had pelvic irradiation to suffer from erectile dysfunction as a long-term complication. This may in part be explained by radiation induced vascular disease leading to reduced blood flow in the pelvic and penile vessels [6].
Female
In the female gonads, the mature cells take the onslaught of the effect of radiotherapy while the immature resting cells escape. The results of a number of studies have shown that the sensitivity of an oocyte/follicle to radiation induced apoptosis/atresia may widely differ between species or between follicular stages. However, in females, the follicular function and sex steroid functions are interdependent where damage of either results in failure of both functions [1].
Recent results suggest that the dose necessary to destroy 50 % of primordial follicles (LD 50) would be, 2 Gy [7], instead of 6–18 Gy [8] or 4 Gy [9] as previously reported. The degree of ovarian impairment is related to the volume treated, total irradiation dose, fractionation schedule and age at time of treatment, with older women being at greater risk of damage [10–13]. Thus, the effective sterilizing dose (ESD: dose of fractionated radiotherapy at which premature ovarian failure occurs immediately after treatment in 97.5 % of patients) decreases with increasing age at treatment, as the remaining population of primordial follicles falls. ESD at birth is 20.3 Gy; at 10 years 18.4 Gy; at 20 years 16.5 Gy; and at 30 years 14.3 Gy [13]. Based on the determination of the LD 50 for human oocytes as being, 2 Gy, calculations could be made to determine the surviving fraction of the follicle pool for any given dose of radiotherapy [7].
It has been demonstrated that there is an increased incidence of fetal loss and intrauterine growth restriction in young girls who have been irradiated in prepubertal age. This may be due to reduced elasticity of the myometrial tissue, and decreased endometrial receptivity due to uterine vascular damage [4, 14].
Apart from the effect of ovary, radiation effect is seen on the uterus and pregnancy outcomes. Pelvic irradiation is associated with infertility, spontaneous miscarriage and intrauterine growth retardation [15]. There are direct effects on the uterus by irradiation, irreversible changes in the uterine musculature and blood flow, as well as hormone resistant endometrial insufficiency. These effects may be to some extent countered by sex steroid replacement therapy. There are other complications also related to irradiation of uterus. This includes spontaneous abortions (38 % vs.12 %), preterm labor (62 % vs. 9 %) and low-birth weight infants (62 % vs. 6 %) [16].
The vagina is relatively radio-resistant however, irradiation of this organ carries with it the risk of loss of lubrication and stenosis which may result in physical impairments to fertility as well as major psychosexual issues [17].
The following Tables are estimated risks of premature ovarian failure (listed from highest to lowest) and, consequently, loss of future fertility potential in a patient of reproductive age [18] (Tables 1, 2 and 3):
Table 1.
Risk to fertility with different modalities of radiotherapy & chemotherapy
| Risk | Treatment [18] |
|---|---|
| High >80 % | 1. High dose external beam radiation to the ovaries: • Adult women ≥6 Gy • Post-Pubertal girls ≥10 Gy • Pre-Pubertal girls ≥15 Gy (Gy-Gray: Absorbed Dose of Radiation) 2. Bone marrow transplant or stem cell transplant • Alkylating agent chemotherapy (Cyclophosphamide, Busulfan or Melaphan) in preparation for transplant 3. Breast cancer combination chemotherapy in women over 40 year: • Alkylating agent chemotherapy (Cyclophosphamide, Busulfan or Melaphan) plus Methotrexate, Fluororacil, Doxorubicin plus Epirubicin |
Table 2.
Risk to fertility with different modalities of radiotherapy & chemotherapy
| Risk | Treatment [18] |
|---|---|
| Intermediate ~30–70 % | 1. Breast cancer or severe autoimmune disease chemotherapy in women 30–39 years: • Alkylating chemotherapy (Cyclophosphamide, Busulfan Melphalan or Chlorambucil) 2. Breast cancer combination chemotherapy in women over 40 years: • Alkylating agent chemotherapy (Cyclophosphamide, Busulfan or Melaphan) plus Doxorubicin |
Table 3.
Risk to fertility with different modalities of radiotherapy & chemotherapy
| Risk | Treatment [18] |
|---|---|
| Low <20 % | 1. Breast cancer or severe autoimmune disease chemotherapy in women less than 30 years: • Alkylating agent chemotherapy (Cyclophosphamide, Busulfan, Melphalan or Chlorambucil) 2. Breast cancer combination chemotherapy in women less than 40 years: • Alkylating agent chemotherapy (Cyclophosphamide, Busulfan or Melaphan) plus Doxorubicin 3. Cancer chemotherapy for: • Leukemia (ALL, AML) • Non Hodgkin Lymphoma |
Chemotherapy Effects on Fertility
It has been found that almost every chemotherapeutic agent used for cancer has a degree of gonadotoxicity. Chemotherapy can produce significant effects upon patient fertility. These effects are dependent on a number of factors [19]:
-
(i)
radicalversusadjuvant chemotherapy—Radical chemotherapy generally has more profound effects on fertility than adjuvant chemotherapy,
-
(ii)
single agentversuscombination chemotherapy—Increasing complexities of regimes are more likely to have impacts upon fertility than single agent [20–22]
-
(iii)
dose-dependent effects—Increasing doses are likely to have more profound effects on fertility than lower doses.
-
(iv)
drug-dependent effects—Different agents have a markedly different impact upon fertility with some chemo-therapeutic agents sparing fertility whilst others are extremely toxic in this regard.
-
(v)
age-dependent effects. In the female in particular, age has a profound effect on chemotherapy toxicity [23].
-
(vi)
maleversusfemale physiology—The testis in the male is exquisitely sensitive to chemotherapy while in the female it is variable in terms of the tolerance to chemotherapy agents.
Chemotherapy & the Testis
In humans, the testis is more sensitive to chemotherapy than the ovary [4, 24, 25]. Gonadal toxicity of the testis affects spermatogenesis more than it does testosterone production as the germinal epithelium is extremely sensitive when compared to the Leydig cells. The germinal cell division is extremely high through increased meiotic and mitotic activity thus allowing for increase sensitivity to cytotoxic agents [26–28]. Sexual maturation of the testis also influences the degree of gonadal damage experienced when exposed to cytotoxic drugs, the prepubertal testis being less susceptible than post-pubertal testis [27].
Chemotherapy and the Ovary
As mentioned before, every woman is born with a fixed number of primordial follicles which constitute her ovarian reserve. Postpuberty these primordial follicles contain single oocytes arrested in the prophase of the first meiotic division and are highly sensitive to cytotoxic drugs leading to cellular death [29]. Follicular depletion has been shown to be physiologically age dependent, the maximum rate of depletion occurring around the age of 38 years when the reserve is just about 10 % the number present at menarche [30]. Hence the cytotoxic affects are dependent on the types of cytotoxic drugs, the dosage of the drugs and also the age of the women.
The following table illustrates the risk of ovarian failure with various chemotherapeutic agents (Table 4):
Table 4.
Risk levels for infertility promoted by various chemotherapeutic agents
| Risk level | Risk due to nature or risks caused by nature of treatment of malignancy |
|---|---|
| High | Alkylating agents: cyclophosphamide, ifosfamide, chlormethine, busulfan, melphalan, chlorambucil, carmustine, lomustine, mechlorethamine; procarbazide |
| Medium | Cisplatin, carboplatin, doxorubicin |
| Low <20 % | Vincristine, methotrexate, dactinomycin, bleomycin, mercaptopurine, vinca alkaloids (vinblastine), fluorouracil |
| Risk exists unknown level | Nitrosources & antimetabolites: cytosine arabinoside |
| Unknown risk | Taxanes, oxaliplatin, monoclonal antibodies (trastuzumab, bevacizumab, cetuximab), tyrosine kinase inhibitors (eriotinib, imatinib) |
Taken with kind permission from Ronit Abir
Cancer in Female Adolescents [31]. Elad Feigin, Ronit Abir, Fertility Preservation in Female Adolescents with Malignancies
© 2008 Nova Science Publishers, Inc
Strategies for Fertility Preservation
In Males
Fertility preservation in males depends on the sexual maturity of the patient. Sperm banking remains the choice for males capable of producing a semen sample. However young males will only start producing sperm cells suitable for cryopreservation around the age of 12–13 years [32]. There is now a good evidence base to suggest that if the testicular volume is less than 10mls, it is very unlikely that the patient will demonstrate any significant spermatogenesis. When young males are unable to ejaculate, then electro ejaculation [33] or epididymal or testicular sperm extraction [34] may be done. The spermatozoa then obtained can be used for Intracytoplasmic Sperm Injection (ICSI) whenever necessary [34].
Gonadotrophin suppression either by GnRH analogue, be it agonist or antagonist, has failed to suppress the testis [35, 36].
Cryopreservation of spermatogonia may be performed by testicular biopsy in prepubertal boys where spermatogenesis has not yet commenced. Testicular biopsy can produce psychological trauma to young boys and also cause anxiety in parents [37].
Obstacles to Cryopreservation
Patient may not feel up to collecting sample(s)
Added stress of having the semen frozen may not be worth the possible benefits
Patient may not be able to fit the process into a busy treatment schedule
Use of Frozen Semen Sample
- Artificial Insemination
- Requires at least 10 million motile sperm per insemination (about 20 million per sample)
- Almost always requires multiple samples
- IVF
- Best for limited number of samples
- Good success rates
- Expensive
- Intracytoplasmic sperm insemination (ICSI)
- Required for low sperm numbers
- Good success rates even with low numbers
- Slightly more expensive than IVF
Storage Period of Semen Samples
Indefinitely in liquid nitrogen
Cryopreservation fee is about $350 with additional cost of approximately $100/specimen
$250.00 annual storage fee (at University of Colorado)
FROZEN FOR FREE IN GUNASHEELA IVF CENTRE for a period of 10 years (Tables 5 and 6).
Table 5.
Gunasheela Institute of Research in Cancer & Fertility (Charitable Trust) Males (86 pts)
| Age | No. of pts., |
|---|---|
| 15–20 years | 11 pts |
| 21–25 years | 28 |
| 26–30 years | 30 |
| 31–35 years | 13 |
| 36–40 years | 04 |
Table 6.
Distribution of types of cancer Gunasheela Institute of Research in Cancer & Fertility (Charitable Trust) males (86 pts)
| Type of cancer | No. of pts. |
|---|---|
| Testicular tumours | 38 |
| Hodgkins & Non-Hodgkins Lymphoma | 17 |
| Mixed others | 31 |
Strategies for Fertility Preservation
In Females
Radiotherapy
Laparoscopic Ovarian Suspension Before Irradiation [38]
Radiation therapy is one of the treatment modalities used in the management of patients with cancer. It depends upon the site and the extent of the disease and the dose of radiation administered locally or to a larger area. In some women with genitourinary or low intestinal tumors, pelvic irradiation may be indicated. It is highly effective in patients with early stage cancers, but it results in the loss of ovarian function.
In an attempt to protect the ovaries from radiation, ovarian transposition to an extrapelvic site out of the field of radiation has been advocated. This is done by laparotomy either as a part of surgical staging or as a separate procedure [39–46] (Fig. 2).
Fig. 2.
Laparoscopic lateral ovarian transposition [38]
Discussion
Lateral ovarian transposition is associated with preservation of ovarian function in 83 % of patients after pelvic irradiation [42]. This technique is more effective than medial transposition of the ovaries behind the uterus. The upper and lower poles of the ovaries can be marked with hemoclips (Fig. 2). At the end of the procedure, good blood supply to both ovaries should be confirmed. The metal clips around the ovaries help to verify that they are out of the radiation portals on radiation verification films [47]
Another method is exteriorization of the ovaries under the skin through an opening in the flank. This approach, however, is not used widely and has been associated with ovarian cyst formation [45]. Possible complications of ovarian transposition are injury and torsion of the ovarian blood vessels [42].
Success with ovarian salvage by oophoropexy has also been varied in the hands of different workers because the ovaries frequently migrate back into the pelvic irradiation fields, especially when there is a long delay between oophoropexy and onset of irradiation therapy [48]. In view of this, oophoropexy must be done immediately before starting pelvic irradiation.
Chemotherapy
Ovarian Suppressor Agents
A meta-analysis published in 2009 by Clowse et al. [49], which included 366 women and three studies involved included women with autoimmune disease receiving cyclophosphamide (CYC) and the remaining six with hematologic malignancy receiving combination chemotherapy. Out of 178 women who were treated with GnRHa during chemotherapy, 93 % maintained ovarian function. Of the 188 women not treated with GnRHa, 48 % maintained ovarian function. The use of a GnRHa during chemotherapy was associated with a 68 % increase in the rate of preserved ovarian function compared with women not receiving a GnRHa.
Several randomized trials are underway to define the role of GnRHa in the preservation of ovarian function.
GnRHa cannot provide protection from ovarian damage caused by irradiation. It must also be understood that some alkylating agents can destroy the resting primordial follicles on which GnRH agonist has no effect at all.
Elgindy et al. published a study [50] where 100 women with breast cancer were divided into four groups. Women who needed chemotherapy immediately were assigned either to GnRH agonist combined with GnRH antagonist (GnRH agonist + GnRH antagonist) to produce quick ovarian suppression (and then continued with GnRH agonist) or to no adjuvant therapy. Women who could delay chemotherapy were assigned either to GnRH agonist or to no adjuvant therapy. All women received similar doses of doxorubicin, 5-fluorouracil, and cyclophosphamide during chemotherapy. They concluded that GnRH treatment has minimal benefit at best when used during chemotherapy for breast cancer. In addition to the side effects of chemotherapy, prolonged GnRH agonist use is accompanied by undesired adverse effects (hot flashes, vaginal dryness, mood swings, osteoporosis). The patient’s quality of life must be considered as well, and adjuvant therapy that is associated with minimal or no benefit should not be offered.
The PROMISE-GIM6 (Prevention of Menopause Induced by Chemotherapy: A Study in Early Breast Cancer Patients–Gruppo Italiano Mammella 6) study [51], a parallel, randomized, open-label, phase 3 superiority trial, was conducted at 16 sites in Italy and enrolled 281 patients between October 2003 and January 2008. The patients were premenopausal women with stage I through III breast cancer who were candidates for adjuvant or neoadjuvant chemotherapy.
The results of the study showed that the administration of the GnRH analogue triptorelin, before and during chemotherapy, led to a 17 % absolute reduction in the occurrence of early menopause in premenopausal patients with breast cancer undergoing adjuvant or neoadjuvant chemotherapy. Early menopause (defined as no resumption of menses and postmenopausal or unknown levels of FSH and E2, 12 months after the end of chemotherapy) occurred in 25.9 % of the patients treated with chemotherapy alone and 8.9 % of those treated with chemotherapy plus triptorelin.
The mechanisms of action by means of which of GnRH analogues preserve ovarian function are not fully understood but may include the interruption of FSH secretion, a decrease in utero-ovarian perfusion, the activation of GnRH receptors, the up-regulation of intragonadal antiapoptotic molecules such as sphingosine-I-phosphate, or the protection of undifferentiated germline stem cells [52]. But the same effect was unfortunately not seen in patients with Lymphoma.
But in a study done by Demeestere et al. [53] on the use of Gonadotropin-Releasing Hormone Agonist for the Prevention of Chemotherapy-Induced Ovarian Failure in Patients With Lymphoma, after a year 20 % and 19 % of patients in the GnRHa and control groups, respectively, exhibited POF (P1.00). More than half of patients in each group completely restored their ovarian function (FSH 10 IU/L), but the anti–Mullerian hormone values were higher in the GnRHa group than in the control group.
But the debate is still on and use of GnRH analogues may be justified in specific cancers alone rather than its generalized application.
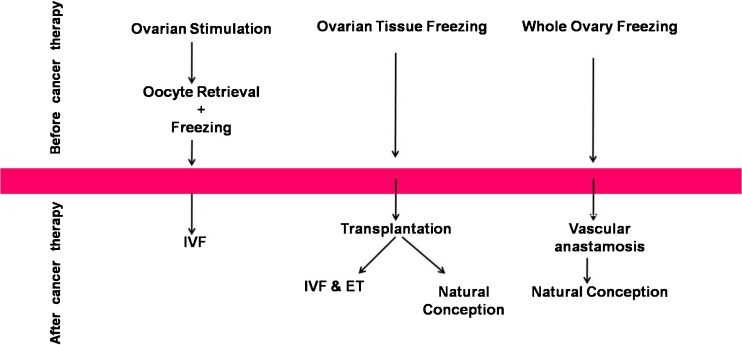
Cryopreservation of Embryos, Oocytes and Ovarian Tissue (Fig. 3)
Fig. 3.
Strategies to preserve fertility in female cancer patients through freezing [85]
Embryo Storage
Embryo storage is the best option for an adult woman who is married or in a stable relationship. It is a technique that has been available since the mid 1980s. It involves stimulating the ovaries using gonadotrophins to produce mature eggs which are then aspirated and injected by the partner’s sperm. This technique is called as ICSI and it yields a success rate of approximately 40 % per cycle (age dependent). The limitations would include :
-
i.
The paucity of time that there is between the detection of the cancer and the start of the treatment.
-
ii.
The fact that only a limited number of embryos would be available for transfer at a later date.
-
iii.
Using gonadotrophins results in high estrogen levels which is of concern in some tumours such as breast cancer with an estrogen receptor positive status. It is still unclear what the risks of such techniques in terms of tumour progression or relapse in hormone dependent cancers are [3].
In a study conducted by Oktay et al. [54], he reported that 600 women with breast cancer underwent ovarian stimulation with either Tamoxifen or Letrozole in combination with a low dose of follicle stimulating hormone (FSH). It was seen that a combination of low dose Letrozole with FSH resulted in a higher embryo yield and lower peak estradiol levels [54].
The non-predictability of the number of good oocytes that one gets in IVF in any one cycle makes it a dubious method of preserving reproductive potential. Secondly, IVF demands that there should be a stable male partner for the individual concerned. If this criterion is absent, one has no alternative but to arrange for cryopreservation of oocytes either by slow-cooling or by vitrification.
Oocyte Storage
This technique is suitable for adults and for older teenagers who do not have a current partner. The technique involves stimulation of the ovaries, harvesting of eggs and then freezing them, which is technically slightly more difficult than embryo cryopreservation. Stored eggs can later be thawed and ICSI techniques can be used.
With the advent of Vitrification (Rapid Freezing method), freezing of oocytes has become highly successful. Pregnancy rates after warming of these oocytes and fertilizing them are in the range of 32–65 % per embryo transfer and live birth rates of over 50 % have been reported [55] in certain centers world over.
For younger patients, oocyte storage is an option and the oocytes can be harvested if required by laparoscopy.
Ovarian Tissue Storage
This is a technique which can be used for young adults and adolescent children. Laparoscopy is required to undertake a biopsy of an ovary or to remove the whole ovary for preservation. It is therefore an invasive procedure under general anaesthetic and carries a mortality rate of 1 in 12,000. The cortical tissue of the removed ovary contains the maximal number of ovarian follicles which can be pared off the medullary stromal tissue. The cortical tissue can then be made into several strips of 8–10 mm2 and cryopreserved for future use. The frozen cortical tissue can then be thawed when the patient is declared disease free and can be transplanted onto the patient’s ovary or other sites as appropriate. Oocytes from isolated antral follicles of the size of 2–3 mm can also be aspirated, maturated in-vitro and then cryopreserved (Tables 7 and 8).
Table 7.
Gunasheela Institute of Research in Cancer & Fertility (Charitable Trust) total female patients (86 pts)
| Breast Cancer Pts. | Stimulation | OPU | Embryos | Oocytes | Ov tissue | Follow up |
|---|---|---|---|---|---|---|
| 23 M ER,PR neg | Straight stim 8 days, poor resp. | Had aspiration of immature oocytes during laparoscopy. 18 M1 | – | 6 M2 oocytes vitrified | Yes 9 vials | On Rx |
| 26,M ER,PR neg | Straight stim 8 days | 18-oocytes-12 m 2 + 6 m1 4 + 1(ICSI) (poor sperm morph) | 2 + 1-embryos fragmented, Discarded | 8 M2 oocytes vitrified | Nil | On CT |
| 26,M ER,PR neg | Straight stim 10 days | 2 M2 | 2 embryos,4C | Yes 9 vials | Nov for ET | |
| 31,M ER,PR pos | Letroz + FSH 10 days | 10 oocytes | 4 + 1 | Yes 15 vials | March | |
| 6 M2 + 3 M1 | D3 + D2 | |||||
| 6 + 1 ICSI | ||||||
| 26,UM ER,PR pos | Letroz + FSH 4 days | 4-oocytes | 3 M2 oocytes vitrified | Yes 14 vials | On RT | |
| 1 M2 + 3 M1 | ||||||
| 30 M ER,PR pos | Straight stim 8 days | 13-oocytes | 6D3 | nil | On Rx | |
| 10 M2 +3 M1 | ||||||
| 10 ICSI |
Table 8.
Gunasheela Institute of Research in Cancer & Fertility (Charitable Trust) other cancer details
| 16 years, UM Hodgkins Lymphoma | Flare up, protocol-12 oocytes, 7 M2 + 5 M1. 7 + 3(IVM) vitrified | Ovarian tissue 16 Vials frozen | Patient expired |
| 23 Y, UM Hodgkins lymphoma | Straight stim 10 days | 9 oocytes, 5 + 4 (IVM) vitrified | On treatment |
| 34 Y, Married Ca Left Ovary | Ovarian tissue Cryopreservation done during staging Laprotomy- 9 Vials frozen, | Patient underwent left salpingo-oophorectomy, right ovary free of tumour | Conceived spontaneously, delivered by LSCS, Nov 2012 |
| 36, married rectal ca | Ovarian tissue cryopreservation done, 7 Vials frozen, | On chemotherapy | |
| 33 married colon Ca | Ovarian tissue Cryopreservation done, 12 Vials frozen, | Underwent surgery | Still on chemotherapy |
Sherman Silber et al. [56] reported that oocytes obtained from ultra-fast frozen ovarian tissue produced the same success rates as fresh tissue claiming that vitrification of thin cortical ovarian tissue containing follicles of various stages of growth, had the same kind of viability as that in fresh oocytes. They also found that 91.9 % of fresh oocytes and 88.9 % of vitrified and thawed oocytes were viable, whereas among the cohort of oocytes tested after slow freezing and thawing only 56 % were viable.
Ovarian Tissue Transplantation and Outcomes
Autologous ovarian cortical tissue transplantation has been successfully applied with demonstration of restoration of ovarian function, including achievement of pregnancy and endogenous hormone production.
Autotransplantation reimplants viable cortical ovarian tissue into a pelvic (orthotopic) site or into an extrapelvic (heterotopic) site such as the forearm or the abdominal wall [57–67]
Autologous Orthotopic Transplantation of Cortical Tissue
Autologous orthotopic transplantation involves re-implanting the thawed ovarian tissue back into its anatomic pelvic location, allowing for the possibility of natural fertilization.
Regrafting of ovarian cortical strips can be considered once the patient is certified as disease free. The concept of ovarian cortical grafting is based on the fact that all follicles containing the eggs are located in the outer 1 mm of the surface of the ovary, which can be sutured to the ovarian medulla of the recipient ovary like a split full-thickness skin graft. Resumption of normal ovulatory menstrual cycles has been reported to occur within 4–9 months post transplantation, which is consistent with the time necessary to initiate follicular growth and final maturation [57–59, 68]. The limitation of this technique is that the graft generally loses two thirds of its follicles from ischemia during revascularization reducing the life span of the graft [69].
Studies have shown variability in graft survival and ovarian function from several months to years with the longest graft survival noted to be seven years [59, 66, 70–73]
The first pregnancy was reported in 2004 by Donnez et al. in the Lancet in a patient who developed ovarian failure after chemotherapy and radiation for clinical stage IV Hodgkin’s lymphoma [58]. Subsequent laparoscopic reimplantation of frozen/thawed ovarian cortical tissue resulted in resumption of menses and then spontaneous pregnancy. The efficiency of pregnancy in patients with orthotopic transplantation is currently unknown.
The disadvantages include a second surgery to replace the tissue, and more importantly the possibility of re-implanting microscopic deposits of cancer.
In a recent review of case reports of pregnancies after orthotopic transplantation [60], most of the initial surgical procedures did not remove both ovaries. As a consequence, the success of the procedure is confounded by the possibility that any pregnancy that was conceived could have been the result of ovulation of oocytes from the native ovary and not the transplanted tissue.
So far there have been only 17 live births worldwide from frozen ovarian cortical tissue transplantation in humans (see table below) [74] (Table 9).
Table 9.
Worldwide pregnancies from ovarian tissue cryopreservation & reimplantation [74]
| Case | Diagnosis | No. of babies | Main researcher |
|---|---|---|---|
| 1 | Hodgkin’s Lymphoma | 1 | Donnez |
| 2 | Neurotumor | 1 | Donnez |
| 3 | Non-Hodgkin’s Lymphoma | 1 | Meirow |
| 4 | Hodgkin’s Lymphoma | 1 | Demeestere |
| 5 | Ewing’s sarcoma | 3 | Andersen |
| 6 | Hodgkin’s Lymphoma | 1 | Andersen |
| 7 | Premature ovarian failure | 1 | Silber |
| 8 | Hodgkin’s Lymphoma | 2 | Silber |
| 9 | Polyangiitis | 1 | Piver |
| 10 | Breast cancer | 2 | Pellicer |
| 11 | Sickle cell anemia | 1 | Piver |
| 12 | Hodgkin’s Lymphoma | 2 | Revel |
| A total of 12 patients | A total of 17 babies | A total of 8 centers | |
| Fresh + Frozen | 28 babies | Silber—14 babies | |
The risk of re-implanting tissue with occult cancer while small remains significant. Only patients with cancer cases associated with low risk of ovarian metastasis such as squamous cell carcinoma of the cervix, Wilm’s tumour, Hodgkin and non-Hodgkin’s lymphoma should be considered for future autotransplantation. Patients with moderate and in particular high risk of ovarian involvement should not be considered for future autotransplantation [75].
Autologous Heterotopic Transplantation
Autologous heterotopic transplantation is when the ovarian cortical strips are transplanted into the sites other than the anatomical location of the ovary. For e.g., forearm abdominal wall, etc. With this option pregnancy can only be achieved by oocyte retrieval and in-vitro fertilization (IVF) utilizing assisted reproductive technologies. While successful oocyte retrieval and fertilization have been possible, there have been no live births reported with this technique
Whole Ovary Transplantation
Whole ovarian tissue transplant with its vascular pedicle has been performed and is a technique that allows for immediate revascularization of the transplant [76–82]. In humans, fresh whole ovary transplantation between living related donor and recipient has been performed successfully [81, 82].
Silber [74] has reported a series of nine monozygotic twin pairs discordant for premature ovarian failure who underwent ovary transplantation at one center. All of these fresh ovary transplants were successful, resulting in 11 healthy babies in 7 of the 9 recipients.
Although technically more challenging than ovarian cortical grafting, whole-ovary microvascular transplantation may result in a longer duration of graft function. The delay of 101 days between the operation and the first menstruation is consistent with the projected time of growth of eggs from small preantral follicles to reach sufficient maturity to enter the menstrual and ovulatory cycles [83].
Limitations to the success of whole ovary transplantation include the small size of the ovarian artery, the inadequacy of the length of the vascular pedicle and the risk that if the microvascular anastomosis fails then the survival of the entire ovary is compromised with no option for another attempt at transplantation [84] (Fig. 3).
Ovarian Transplantation in Monozygotic Twins
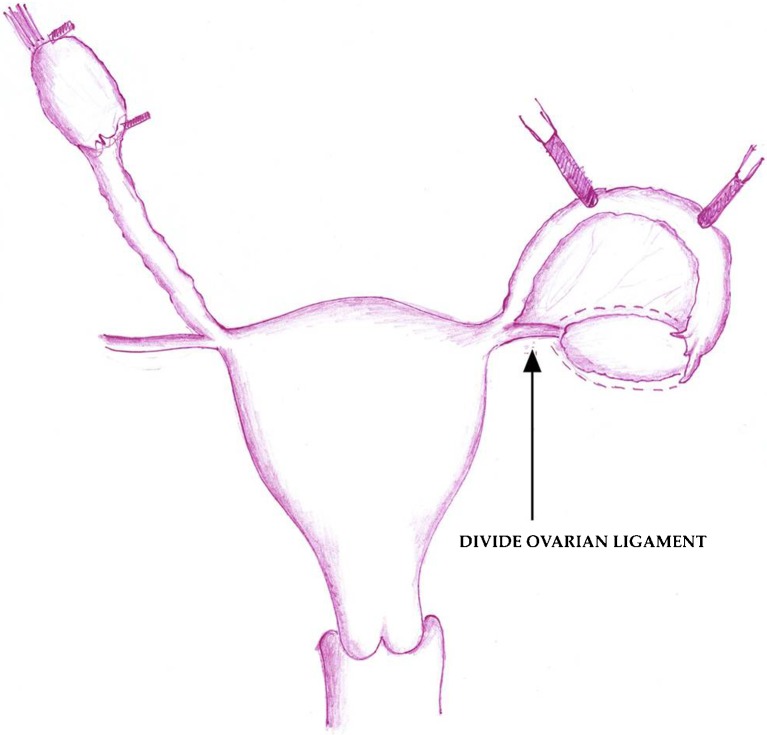
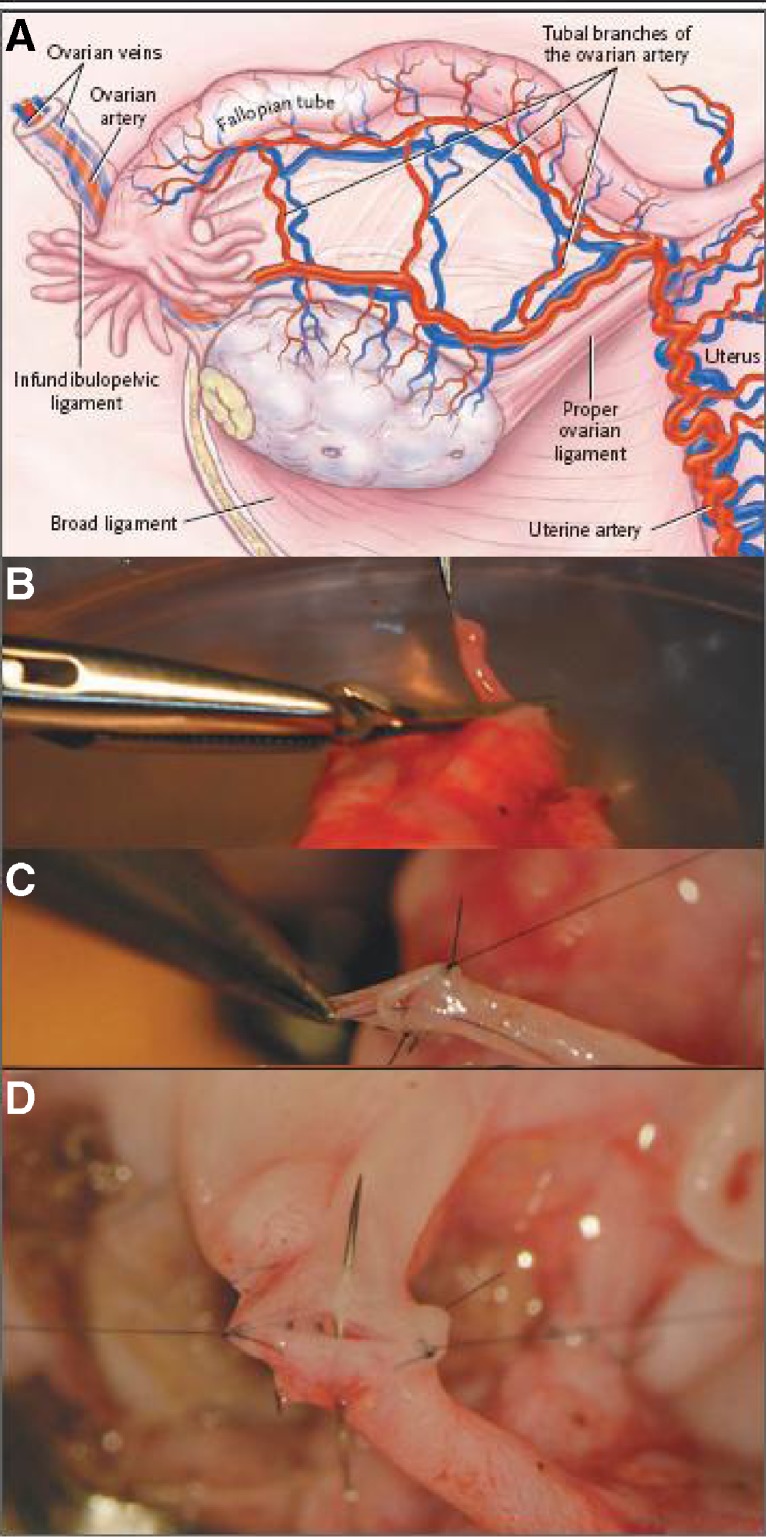
Sherman Silber [82] reported on a case of twins with discordant ovarian failure with amenorrhoea for 23 years and FSH hormone at the level of 81mIU per milliliter. The healthy, fertile twin offered to have one of her ovaries removed laparoscopically. The ovary was released by dividing the infundibulopelvic ligament at its base. The donated ovary was then prepared for microvascular transplantation on the recipient. With the use of mini laparotomy, the donor’s ovarian veins were anastomosed to the recipient’s blood vessels with 9-0–10-0 nylon sutures (Fig. 4 below). Normal blood flow was immediately observed after a short period of ischemia of 100 min.
Fig. 4.
Whole-ovary transplantation between monozygotic twins [82]. Panel a shows various structures in the ovarian vasculature that are key during transplantation of an ovary. In Panel b, the donor’s ovarian artery (0.5 mm in diameter) is prepared for transplantation. The donor’s ovarian artery and ovarian vein are then anastomosed to the recipient’s ovarian artery and vein, as show in Panels c and d, respectively. (Taken with kind permission from Dr. Sherman Silber [82]
The recipient’s first menstruation in 22 years occurred on day 101 after transplantation. At the end of 158 days after transplantation, the FSH level had dropped from 81mIU per milliliter to 7.4mIU per milliliter. The patient had 11 regular menstrual cycles. The patient conceived spontaneously and produced a healthy baby girl of 8 lb.
Safety Concerns and Alternative Treatments
The magnitude of risk for reintroducing a malignancy is currently unknown for most types of cancer. It appears that blood born cancers, such as leukemia, carry the highest risk of reintroduction with autotransplantation.
Given the uncertainties with regards to transmission of disease, ovarian tissue transplantation is not recommended for patients with blood born malignancies, malignancies that metastasize to the ovary or for those with an inherent predisposition to ovarian cancer. However, ovarian tissue transplantation in women with cancers that have a negligible risk of ovarian involvement may be considered for future autotransplantation [3].
Other risks or concerns associated with ovarian tissue transplantation include surgical and anesthetic risks involved in obtaining the tissue and the requirement to provide clear directives regarding disposition of the tissue in the event of the death of the donor.
Ethical Issues
The options to overcome difficulty in conception for young cancer patients must be laid open to them or at least to their parents if the victims are not old enough to understand the implications. Anti cancer treatment along with the effects of chemo and radiotherapy can be very difficult to endure causing physical and mental distress and even depression. The problem is further complicated in adoloscents during which time they start comparing themselves with their healthy peers and start developing psychological instability. The realization that their chances of having their own offsprings are remote can be devastating. Many observers have seen positive psychological effect in these patients in whom the option of parenthood is preserved. It gives them a source of hope and reassurance and a strong morale for seeking a partner in life without the sense of deficiency.
Oocytes do not have cancer deposits on them and they are usually collected before chemo or radiotherapy is given. Various epidemiological studies showed no increase in congenital malformations in cancer patients who underwent chemotherapy [86].
Fertility preservation raises several ethical issues which include candid discussion of danger or safety for fertility preservation in the setting of available techniques, cultural issues, the consent process [87, 88], and the dilemma of counselling a young candidate who has not yet reached adulthood to make decisions, regarding his or her reproductive health.
Most men who completed a survey given by Schover et al. [89] felt that having experienced cancer increased the value of family closeness and that would make them better parents. Many men lamented that timely information was not given to them. The cancer patient or their guardians must interact not only with the oncologists but also the fertility specialists and the andrology/embryology laboratories where the biological material of cancer sufferers is going to be preserved. Treatment for cancer—be it surgery, chemo or radiotherapy, must be started expeditiously and may not allow the luxury of time for necessary consultations and decision-making. Their decision making is not very complicated in sperm banking as it is in ovarian tissue banking.
The patient must also know that technique does not guarantee either the birth of a child or its well-being. The technology of cryopreservation of gametes and embryos is not a part of the care for cancer; but it runs parallel to the main treatment which may be accepted or rejected depending on patient’s philosophy of life. She may opt adoption or a gamete donation and in which case one must agree that it may not reduce the joy of parenting ultimately.
With the exception of a few heritable cancer syndromes, a history of cancer or its treatment does not seem to increase the rate of congenital abnormalities or cancer in the offspring [90]. Some types of cancer pose a greater relative risk of ovarian or testicular metastasis, including leukemia and lymphoma. The potential risk of development of a metastatic tumor in the reproductive tract must be disclosed to the patient and the family before proceeding [91].
Embryo cryopreservation is an entirely different situation especially:
If the candidate is either engaged to be married or not yet married.
If her future partner is different and the one who has donated the sperm for embryo formation is different, would she like to abandon her embryo?
Patients’ questions to be answered
Is it morally acceptable to subject a minor adolescent to oocyte retrieval?
In case the candidate dies of cancer before marriage, the consent should have been given by the patient’s guardians as to how the embryos to be disposed off.
There are no laws as yet defining the responsibility of the guardian, of the laboratory, of the adolescent patient or the healthcare providers.
In India there are ICMR guidelines that have been formulated on cryopreservation of oocytes and sperm being used for commercial purposes. But there are no guidelines regarding freezing of the gametes or ovarian tissue of cancer afflicted patients for their own use at a later date when they are deemed to be cured of the disease.
After an extensive search on the internet we did not come across any data/clinics from India pursuing an active programme of oocyte/sperm/ovarian tissue/embryo cryopreservation in patients afflicted with cancer. Hence we do not know the costs of these various procedures. But in our hospital, these procedures are done on a completely charitable basis under the aegis of Gunasheela Institute of Research in Cancer and Fertility.
American Society of Clinical Oncology (ASCO) Recommendations [92]
ASCO convened an expert panel to conduct a systematic review of 233 studies published between 1987 and 2005 to develop recommendation. The fact that ASCO convened an expert panel to publish guidelines underscores the importance of fertility preservation and related issues in people undergoing cancer treatment. The panel concludes that oncologists should address the possibility of infertility and options for fertility preservation with all patients who are to be treated in their reproductive years, and that these discussion and resultant referrals to reproductive endocrinologists should occur as soon as possible during treatment planning.
After an exhaustive review of admittedly limited data, the panel noted that there did not appear to be an increased risk of disease recurrence associated with most fertility preservation options and with pregnancy, and that a history of cancer, cancer therapy, and fertility interventions did not appear to have negative effects on future offspring.
Conclusion
Fertility preservation in cancer patients can only be approached with a multi disciplinary setting. Increasing long term survival rates along with advances in fertility treatment means that it is now perpetual that fertility preservation should be offered to these patients.
Till 2010, fertility preservation with cryopreservation of ovarian tissue was thought to be only experimental. But the papers read in the European Society of Human Reproduction and Embryology (ESHRE) Meeting at Istanbul in July 2012 [93], showed that it is no more an experimental procedure. It has moved into the main stream of Reproductive Medicine and in particular, for nulliparous women anxious to conceive. As per the reports claimed by the workers in this field, there are 28 babies born out of this technique (freezing, thawing, grafting of ovarian tissue and whole ovary transplantation).
However, the patients must be willing to take the gamble and the surgeon must be anxious to offer the treatment available. Surely this kind of trepidation will be felt while undertaking any treatment programmes on human beings.
There are many obvious challenges and ethical issues that still need to be resolved especially in the area of fertility preservation in prepubertal patients.
Footnotes
Sulochana Gunasheela deceased.
References
- 1.Waring A, Wallace W. Subfertility following treatment for childhood cancer. Hosp Med. 2000;61:550–557. doi: 10.12968/hosp.2000.61.8.1398. [DOI] [PubMed] [Google Scholar]
- 2.Mackie EJ, Radford M, Shalet SM. Gonadal function following chemotherapy fur childhood Hodgkin’s disease. Med Pediatr Oncol. 1996;27:74–78. doi: 10.1002/(SICI)1096-911X(199608)27:2<74::AID-MPO2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 3.Ajala T, Rafi J, Larsen-Disney P, Howell R (2010) Fertility preservation for cancer patients: a review. Hindawi Publishing Corporation. Obstet Gynecol Int Article ID 160386:1–9 [DOI] [PMC free article] [PubMed]
- 4.Janson PO. Possibilities of fertility preservation in children and young adults undergoing treatment for malignancy. Acta Obstet Gynecol Scand. 2000;79:240–243. doi: 10.1080/j.1600-0412.2000.079004240.x. [DOI] [PubMed] [Google Scholar]
- 5.Petersen PM, Daugaard G, Rørth M, et al. Endocrine function in patients treated for carcinoma in situ in the testis with irradiation. Acta Pathol Microbiol Immunol Scand. 2003;111(1):93–99. doi: 10.1034/j.1600-0463.2003.11101131.x. [DOI] [PubMed] [Google Scholar]
- 6.Basavaraju SR, Easterly CE. Pathophysiological effects of radiation on atherosclerosis development and progression, and the incidence of cardiovascular complications. Med Phys. 2002;29(10):2391–2403. doi: 10.1118/1.1509442. [DOI] [PubMed] [Google Scholar]
- 7.Wallace W, Thomson A, Kelsey T. The radiosensitivity of the human oocyte. Hum Reprod. 2003;18:117–121. doi: 10.1093/humrep/deg016. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi M. Cytotoxic insult to germinal tissue, part 2; the ovary. In: Potten CS, Hendry JH, editors. Cytotoxic insult to tissue: effects on cell lineages. Edinburgh: Churchill-Livingstone; 1983. pp. 309–328. [Google Scholar]
- 9.Wallace WHB, Shalet SM, Hendry JH, Jones PHM, Gattamaneni HR. Ovarian failure following abdominal irradiation in childhood: the radiosensitivity of the human oocyte. Br J Radiol. 1989;62:995–998. doi: 10.1259/0007-1285-62-743-995. [DOI] [PubMed] [Google Scholar]
- 10.Lushbaugh CC, Casarett GW. The effects of gonadal irradiation in clinical radiation therapy: a review. Cancer. 1976;37:1111–1125. doi: 10.1002/1097-0142(197602)37:2+<1111::AID-CNCR2820370821>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 11.Green DM. Fertility and pregnancy outcome after treatment for cancer in childhood or adolescence. Oncologist. 1997;2:171–179. [PubMed] [Google Scholar]
- 12.Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7:535–543. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 13.Wallace W, Thomson A, Saran F, Kelsey T. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738–744. doi: 10.1016/j.ijrobp.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 14.Green DM, Hall B, Zevon MA. Pregnancy outcome after treatment for acute lymphoplastic leukemia during childhood or adolescence. Cancer. 1989;64:2335–2339. doi: 10.1002/1097-0142(19891201)64:11<2335::AID-CNCR2820641124>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Wallace WH, Thomson AB. Preservation of fertility in children treated for cancer. Arch Dis Child. 2003;88:493–496. doi: 10.1136/adc.88.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkins MM, Smith RA. Pregnancy outcomes in childhood cancer survivors: probable effects of abdominal irradiation. Int J Cancer. 1989;43:399–402. doi: 10.1002/ijc.2910430309. [DOI] [PubMed] [Google Scholar]
- 17.Liao L-M, Doyle J, Crouch NS, Creighton SM. Dilation as treatment for vaginal agenesis and hypoplasia: a pilot exploration of benefits and barriers as perceived by patients. J Obstet Gynaecol. 2006;26(2):144–148. doi: 10.1080/01443610500443527. [DOI] [PubMed] [Google Scholar]
- 18.Scoccia B, Hirshfeld-Cytron J (2013) Fertility preservation program. University of Illinois Medical Centre
- 19.Howell S, Shalet S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol Metab Clin N Am. 1998;27(4):927–943. doi: 10.1016/S0889-8529(05)70048-7. [DOI] [PubMed] [Google Scholar]
- 20.Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000;169:123–131. doi: 10.1016/S0303-7207(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 21.Viviani S, Santoro A, Ragni G, Bonfante V, Bestetti O, Bonadonna G. Gonadal toxicity after combination chemotherapy for Hodgkin’s disease. Comparative results of MOPP vs ABVD. Eur J Cancer Clin Oncol. 1985;21:601–605. doi: 10.1016/0277-5379(85)90088-4. [DOI] [PubMed] [Google Scholar]
- 22.Papdakis V, Valchopapadopoulou E, Van Syckle K, Ganshaw L, Kalmanti M, Tan C, et al. Gonadal function following therapy for childhood Hodgkin’s disease. Med Pediatr Oncol. 1999;32:366–372. doi: 10.1002/(SICI)1096-911X(199905)32:5<366::AID-MPO10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Byrne J, Fears TR, Gail MH. Spontaneous recovery of chemotherapy-induced primary ovarian failure: implication for management. Clin Endocrinol. 1997;46:217–219. doi: 10.1046/j.1365-2265.1997.771575.x. [DOI] [PubMed] [Google Scholar]
- 24.Thomson A, Critchley H, Kelnar C, Wallace WH. Late reproductive sequelae following treatment of childhood cancer and options for fertility preservation. Best Pract Res Clin Endocrinol Metab. 2002;16:311–334. doi: 10.1053/beem.2002.0200. [DOI] [PubMed] [Google Scholar]
- 25.Bahadur G, Ralph D. Gonadal tissue cryopreservation in boys with paediatric cancers. Hum Reprod. 1999;14:11–17. doi: 10.1093/humrep/14.1.11. [DOI] [PubMed] [Google Scholar]
- 26.Schilsky RL, Lewis BJ, Sherins RJ, Young RC. Gonadal dysfunction in patients receiving chemotherapy for cancer. Ann Intern Med. 1980;93(1):109–114. doi: 10.7326/0003-4819-93-1-109. [DOI] [PubMed] [Google Scholar]
- 27.Rivkees SA, Crawford JD. The relationship of gonadal activity and chemotherapy-induced gonadal damage. J Am Med Assoc. 1988;259(14):2123–2125. doi: 10.1001/jama.1988.03720140043031. [DOI] [PubMed] [Google Scholar]
- 28.Roeser HP, Stocks AE, Smith AJ. Testicular damage due to cytotoxic drugs and recovery after cessation of therapy. Aust NZ J Med. 1978;8(3):250–254. doi: 10.1111/j.1445-5994.1978.tb04518.x. [DOI] [PubMed] [Google Scholar]
- 29.Warne GL, Fairley KF, Hobbs JB, Martin FIR. Cyclophosphamide induced ovarian failure. N Engl J Med. 1973;289(22):1159–1162. doi: 10.1056/NEJM197311292892202. [DOI] [PubMed] [Google Scholar]
- 30.Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65(6):1231–1237. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- 31.Feigin E, Freud E, Fisch B, Orvieto R, Kravarusic D, Avrahami G, Ben-Haroush A, Abir R Fertility preservation in female adolescents with malignancies. In: Cancer in female adolescents. Chapter II. 2008 Nova Science Publishers, Inc
- 32.Aslam I, Fishel S, Moore H, Dowell K, Thornton S. Fertility preservation of boys undergoing anti-cancer therapy: a review of the existing situation and prospects for the future. Hum Reprod. 2000;15(10):2154–2159. doi: 10.1093/humrep/15.10.2154. [DOI] [PubMed] [Google Scholar]
- 33.Rosenlund B, Sjoblom P, Tornblom M, Hultling C, Hillensjo T. In-vitro fertilization and intracytoplasmic sperm injection in the treatment of infertility after testicular cancer. Hum Reprod. 1998;13(2):414–418. doi: 10.1093/humrep/13.2.414. [DOI] [PubMed] [Google Scholar]
- 34.Schwarzer JU, Fiedler K, Hertwig IV, et al. Sperm retrieval procedures and intracytoplasmatic spermatozoa injection with epididymal and testicular sperms. Urol Int. 2003;70(2):119–123. doi: 10.1159/000068185. [DOI] [PubMed] [Google Scholar]
- 35.Johnson DH, Linde R, Hainsworth JD, Vale W, Rivier J, Stein R, et al. Effect of a luteinizing hormone releasing hormone agonist given during combination chemotherapy on post therapy fertility in male patients with lymphoma: preliminary observations. Blood. 1985;65:832–836. [PubMed] [Google Scholar]
- 36.Waxman JH, Ahmed R, Smith D, Wrigley PF, Gregory W, Shalet S, et al. Failure to preserve fertility in patients with Hodgkin’s disease. Cancer Chemother Pharmacol. 1987;19:159–162. doi: 10.1007/BF00254570. [DOI] [PubMed] [Google Scholar]
- 37.Hovatta O. Cryopreservation of testicular tissue. Mol Cell Endocrinol. 2000;169:113–115. doi: 10.1016/S0303-7207(00)00363-4. [DOI] [PubMed] [Google Scholar]
- 38.Togas Tulandi MD, Sundus Al-Took MD. Laparoscopic ovarian suspension before irradiation. Fertil Steril. 1998;70:381–383. doi: 10.1016/S0015-0282(98)00155-1. [DOI] [PubMed] [Google Scholar]
- 39.Ray GR, Trueblood HW, Enright LP, Kaplan HS, Nelsen TS. Oophoropexy (a means of preserving ovarian function following pelvic megavoltage radiotherapy for Hodgkin’s disease) Radiology. 1970;96:175–180. doi: 10.1148/96.1.175. [DOI] [PubMed] [Google Scholar]
- 40.Nahhas WA, Nisce LZ, D’Angio GL, Lewis JL. Lateral ovarian transposition. Obstet Gynecol. 1971;38:785–788. doi: 10.1097/00006250-197110000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Belinson JL, Doherty M. A new technique for ovarian transposition. Surg Gynaecol Obstet. 1984;159:157–160. [PubMed] [Google Scholar]
- 42.Husseinzadeh N, Nahhas WA, Velkley DE, Whitney CW, Mortel R. The preservation of ovarian function in young women undergoing pelvic radiation therapy. Gynecol Oncol. 1984;18:373–379. doi: 10.1016/0090-8258(84)90049-0. [DOI] [PubMed] [Google Scholar]
- 43.Gabriel DA, Bernard SA, Lambert J, Croom RD. Oophoropexy and the management of Hodgkin’s disease. Arch Surg. 1986;121:1083–1085. doi: 10.1001/archsurg.1986.01400090115021. [DOI] [PubMed] [Google Scholar]
- 44.Thomas PRM, Winstanly D, Peckham MJ, Austin DE, Murray MAF, Jacobs HS. Reproductive and endocrine function in patients with Hodgkin’s disease (effects of oophoropexy and irradiation) Br J Cancer. 1976;33:226–231. doi: 10.1038/bjc.1976.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovacev M. Exteriorization of ovaries under the skin of young women operated upon for cancer of the cervix. Am J Obstet Gynecol. 1968;101:756–759. doi: 10.1016/0002-9378(68)90026-4. [DOI] [PubMed] [Google Scholar]
- 46.Leporrier M, von Theobald P, Roffe JL, Mueller G. A new technique to protect ovarian function before pelvic irradiation. Cancer. 1987;60:2201–2204. doi: 10.1002/1097-0142(19871101)60:9<2201::AID-CNCR2820600915>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 47.Farber LA, Ames JW, Rush S, Gal D. Laparoscopic ovarian transposition to preserve ovarian function before pelvic radiation and chemotherapy in a young patient with rectal cancer. Med Gen Med. 2005;7(1):66. [PMC free article] [PubMed] [Google Scholar]
- 48.Stan Williams R, Ramey D. Little, Nancy P. Mendenhall. Laparoscopic oophoropexy and ovarian function in the treatment of Hodgkin disease. Cancer. 1999;86(10):2138–2142. doi: 10.1002/(SICI)1097-0142(19991115)86:10<2138::AID-CNCR36>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 49.Clowse MEB, Behera MA, Anders CK, Copland S, Coffman CJ, Leppert PC, Bastian LA. Ovarian preservation by GnRH agonists during chemotherapy: a meta-analysis. Journal of Women’s Health. 2009;18:311–319. doi: 10.1089/jwh.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elgindy EA, El-Haieg DO, Khorshid OM, et al. Gonadotrophin suppression to prevent Chemotherapy induced ovarian damage : A randomized controlled trial. Obstet Gynaecol. 2013;121:178–186. doi: 10.1097/aog.0b013e31827374e2. [DOI] [PubMed] [Google Scholar]
- 51.Del Mastro L, Boni L, Michelotti A, Gamucci T, Olmeo N, et al. Effect of the gonadotropin-releasing hormone analogue Triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer-a randomized trial. JAMA. 2011;306:269–276. doi: 10.1001/jama.2011.991. [DOI] [PubMed] [Google Scholar]
- 52.Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12:1044–1054. doi: 10.1634/theoncologist.12-9-1044. [DOI] [PubMed] [Google Scholar]
- 53.Demeestere I, Brice P, Peccatori FA, Kentos A, Gaillard I, et al. Gonadotropin-releasing hormone agonist for the prevention of chemotherapy-induced ovarian failure in patients with lymphoma: 1-year follow-up of a prospective randomized trial. JCO. 2013;31(7):903–909. doi: 10.1200/JCO.2012.42.8185. [DOI] [PubMed] [Google Scholar]
- 54.Oktay K, Buyuk E, Linertella N, Akar M, Rosenwaks Z. Fertility preservation in Breast Cancer patients: A prospective controlled comparison of ovarian stimulation with Tamoxifen and Letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 55.Homburg R, van der Veen F, Silber SJ. Oocyte vitrification—Women’s emancipation set in stone. Fertil Steril. 2009;91:1319–1320. doi: 10.1016/j.fertnstert.2008.02.127. [DOI] [PubMed] [Google Scholar]
- 56.Silber S (2009) Ovarian transplantation: new technique gives greatly improved results in this delicate operation. Abstract no: O-037. 25th annual conference of the European Society of Human Reproduction and Embryology. Amsterdam, The Netherlands
- 57.Oktay K. Karlikaya G Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000;342:1919. doi: 10.1056/NEJM200006223422516. [DOI] [PubMed] [Google Scholar]
- 58.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Aquifflet J, et al. Live birth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 59.Radford JA, Lieberman BA, Brison D, Smith AR, Critchlow JD, Russell SA, et al. Orthotopic reimplantation of cryopreserved ovarian cortical strips after high-dose chemotherapy for Hodgkin’s lymphma. Lancet. 2001;357:1172–1175. doi: 10.1016/S0140-6736(00)04335-X. [DOI] [PubMed] [Google Scholar]
- 60.Grynberg M, Poulain M, Sebag-Peyrelevade S, le Parco S, Fanchin R, Frydman N. Ovarian tissue and follicle transplantation as an option for fertility preservation. Fertil Steril. 2012;97:1260–1268. doi: 10.1016/j.fertnstert.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 61.Meirow D, Levron J, Eldar-Geva T, Hardan I, Firdman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt KL, Andersen CY, Loft A, Byskov AG, Ernst E, Andersen A. Follow-up of ovarian function post chemotherapy following ovarian cryopreservation and transplantation. Hum Reprod. 2005;20:3539–3546. doi: 10.1093/humrep/dei250. [DOI] [PubMed] [Google Scholar]
- 63.DemeestereI I, Simon P, Buxant F, Robin V, Fernandez-Aguilar S, Centner J, et al. Ovarian function and spontaneous pregnancy after combined heterotopic and orthotopic cryopreserved ovarian tissue transplantation in a patient previously treated with bone marrow transplantation: case report. Hum Reprod. 2006;21:2010–2014. doi: 10.1093/humrep/del092. [DOI] [PubMed] [Google Scholar]
- 64.Callejo J, Salvador C, Miralles A, Vilaseca S, Lailla JM, Balasch J. Long-term ovarian function evaluation after autografting by implantation with fresh and frozen-thawed human ovarian tissue. J Clin Endocrinol Metab. 2001;86:4489–4494. doi: 10.1210/jcem.86.9.7871. [DOI] [PubMed] [Google Scholar]
- 65.Donnez J, Squifflet J, Dolmans MM, Martinez-Madrid B, Jadoul P, Van Langendonckt A. Orthotopic transplantation of fresh ovarian cortex: a report of two cases. Fertil Steril. 2005;84:1018. doi: 10.1016/j.fertnstert.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Kim SS, Lee WS, Chung MK, Lee HC, Lee HH, Hill D. Long-term ovarian function and fertility after heterotopic autotransplantation of cryobanked human ovarian tissue: 8-year experience in cancer patients. Fertil Steril. 2009;91:2349. doi: 10.1016/j.fertnstert.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 67.Oktay K, Economos K, Kan M, Rucinski J, Veek L, Rosenewaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA. 2001;286:1490–1493. doi: 10.1001/jama.286.12.1490. [DOI] [PubMed] [Google Scholar]
- 68.Donnez J, Jadoul P, Squifflet J, Van Langendonckt A, Donnez O, Van Eyck AS, et al. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24:87–100. doi: 10.1016/j.bpobgyn.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 69.Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519–535. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- 70.Bedaiwy MA, El-Nashar SA, El Saman AM, Evers JL, Sandadi S, Desai N, et al. Reproductive outcome after transplantation of ovarian tissue: a systemic review. Hum Reprod. 2008;23:2709–2717. doi: 10.1093/humrep/den301. [DOI] [PubMed] [Google Scholar]
- 71.Janse F, Donnez J, Anckaert E, de Jong FH, Fauser BC, Dolmans MM. Limited value of ovarian function markers following orthotopic transplantation of ovarian tissue after gonadotoxic treatment. J Clin Endocrinol Metab. 2011;96:1136–1144. doi: 10.1210/jc.2010-2188. [DOI] [PubMed] [Google Scholar]
- 72.Kim SS. Assessment of long term endocrine function after ovarian transplantation of frozen-thawed human ovarian tissue to the heterotopic site: 10 year longitudinal follow-up study. J Assist Reprod Genet. 2012;29:489–493. doi: 10.1007/s10815-012-9757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McLaren JF, Bates GW. Fertility preservation in women of reproductive age with cancer. Am J Obstet Gynecol. 2012;207:455–462. doi: 10.1016/j.ajog.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 74.Sonmezer M, Shamonki MI, Oktay K. Ovarian tissue cryopreservation: benefits and risks. Cell Tissue Res. 2005;322(1):125–132. doi: 10.1007/s00441-005-1098-4. [DOI] [PubMed] [Google Scholar]
- 75.Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18:59–67. doi: 10.1093/molehr/gar082. [DOI] [PubMed] [Google Scholar]
- 76.Wang Z, Chen H, Yin H, Kim SS, Lin tan S, Gosden RG. Fertlity after intact ovary transplantation. Nature. 2002;415:385. doi: 10.1038/415385a. [DOI] [PubMed] [Google Scholar]
- 77.Imhof M, Bergmeister H, Lipovac M, Rudas M, Hofstetter G, Huber J. Orthotopic microvascular reanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth. Fertil Steril. 2006;85(1 Suppl):1208–1215. doi: 10.1016/j.fertnstert.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 78.Hilders CG, Baranski AG, Peters L, Ramkhelawan A, Trimbos JB. Successful human ovarian autotransplantation to the upper arm. Cancer. 2004;101:2771–2778. doi: 10.1002/cncr.20715. [DOI] [PubMed] [Google Scholar]
- 79.Leporrier M, von Theobald P, Roffe JL, Muller G. A new technique to protect ovarian function before pelvic irradiation. Heterotopic ovarian autotransplantation. Cancer. 1987;60:2201–2204. doi: 10.1002/1097-0142(19871101)60:9<2201::AID-CNCR2820600915>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 80.Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrere S, Donnez J. Freeze thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril. 2004;82:1390–1394. doi: 10.1016/j.fertnstert.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 81.Mhatre P, Mhatre J, Magotra R. Ovarian transplant: a new frontier. Transplant Proc. 2005;37:1396–1398. doi: 10.1016/j.transproceed.2004.11.083. [DOI] [PubMed] [Google Scholar]
- 82.Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med. 2008;359(24):2617–2618. doi: 10.1056/NEJMc0804321. [DOI] [PubMed] [Google Scholar]
- 83.Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1:81–87. doi: 10.1093/oxfordjournals.humrep.a136365. [DOI] [PubMed] [Google Scholar]
- 84.Courbiere B, Caquant L, Mazoyer C, Franck M, Lornage J, Salle B. Difficulties improving ovarian functional recovery by microvascular transplantation and whole ovary vitrification. Fertil Steril. 2009;91:2697–2706. doi: 10.1016/j.fertnstert.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 85.Samuel Kim S. Fertility preservation in female cancer patients: current developments and future directions. Fertil Steril. 2006;85:1–11. doi: 10.1016/j.fertnstert.2005.04.071. [DOI] [PubMed] [Google Scholar]
- 86.Arnon J, Meirow D, Lewis-Roness H, Ornoy A. Genetic and teratogenic effects of cancer treatments on gametes and embryos. Hum Reprod Update. 2001;7:394–403. doi: 10.1093/humupd/7.4.394. [DOI] [PubMed] [Google Scholar]
- 87.Grundy R, Gosden RG, Hewitt M, et al. Personal practice: fertility preservation for children treated for cancer (1): scientific advances and research dilemmas. Arch Dis Child. 2001;84(4):355–359. doi: 10.1136/adc.84.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grundy R, Larcher V, Gosden RG, et al. Fertility preservation for children treated for cancer (2): ethics of consent for gamete storage and experimentation. Arch Dis Child. 2001;84(4):360–362. doi: 10.1136/adc.84.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. 2002;20(7):1880–1889. doi: 10.1200/JCO.2002.07.175. [DOI] [PubMed] [Google Scholar]
- 90.Hawkins MM, Draper GJ, Smith RA. Cancer among 1,348 offspring of survivors of childhood cancer. Int J Cancer. 1989;43(6):975–978. doi: 10.1002/ijc.2910430604. [DOI] [PubMed] [Google Scholar]
- 91.Kim SS, Radford J, Harris M, et al. Ovarian tissue harvested from lymphoma patients to preserve fertility may be safe for autotransplantation. Hum Reprod. 2001;16(10):2056–2060. doi: 10.1093/humrep/16.10.2056. [DOI] [PubMed] [Google Scholar]
- 92.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 93.Gennarelli G (2012) Fertility preservation with the cryopreservation of ovarian tissue moves from the experimental to the mainstream. Italy’s first successful case shows that time in freeze-storage is no barrier to success. Abstract. Press information from the 28th Annual Meeting of the European Society of Human Reproduction and Embryology. Istanbul, 4 July