Abstract
Portal vein occlusion through embolization or ligation (PVE, PVL) offers the possibility of increasing the future liver remnant (FLR) and thus reducing the risk of hepatic failure after extended hepatectomy We reviewed the indications, scope and applicability of PVE/PVL in treatment of primary and secondary liver tumours. A thorough PubMED, Embase, Ovid and Cochrane database search was carried out for all original articles with 30 patients or more undergoing either PVE and any patient series with PVL, irrespective of number with outcome measure in at least one of the following parameters: FLR volume change, complications, length of stay, time to surgery, proportion resectable and survival data. PVE can be performed with a technical success in 98.9 % (95 % confidence interval 97–100) patients, with a mean morbidity of 3.13 % (95 % CI 1.21–5.04) and a median in-hospital stay of 2.1 (range 1–4) days (very few papers had data on length of stay following PVE). The mean increase in volume of the FLR following PVE was 39.75 % (95 % CI 30.8–48.6) facilitating extended liver resection after a mean of 37.13 days (95 % CI 28.51–45.74) with a resectability rate of 76.88 % (95 % CI 70.91–82.84). Morbidity and mortality following such extended liver resections after PVE is 26.58 % (95 % CI 19.20–33.95) and 2.59 % (95 % CI 1.34–3.83) respectively with an in-patient stay of 13.57 days (95 % CI 9.8–17.37). However following post-PVE liver hypertrophy 6.29 % (95 % CI 2.24–10.34) patients still have post-resection liver failure and up to 14.2 % (95 % CI −8.7 to 37) may have positive resection margins. Up to 4.80 % (95 % CI 2.07–7.52) have failure of hypertrophy after PVE and 17.46 % (95 % CI 11.89–23.02) may have disease progression during the interim awaiting hypertrophy and subsequent resection. PVL has a greater morbidity and duration of stay of 5.72 % (95 % CI 0–15.28) and 10.16 days (95 % CI 6.63–13.69) respectively; as compared to PVE. Duration to surgery following PVL was greater at 53.6 days (95 % CI 32.14–75.05). PVL induced FLR hypertrophy by a mean of 64.65 % (95 % CI 0–136.12) giving a resectability rate of 63.68 % (95 % CI 56.82–70.54). PVL failed to produce enough liver hypertrophy in 7.4 % of patients (95 % CI 0–16.12). Progression of disease following PVL was 29.29 (95%CI 15.69–42.88). PVE facilitates an extended hepatectomy in patients with limited or inadequate FLR, with good short and long-term outcomes. Patients need to be adequately counselled and consented for PVE and EH in light of these data. PVL would promote hypertrophy as well, but clearly PVE has advantages as compared to PVL on account of its inherent “minimally invasive” nature, fewer complications, length of stay and its feasibility to have shorter times to surgery.
Keywords: Portal Vein Embolization(PVE), Portal Vein Ligation (PVL), Future Liver Remnant (FLR), Liver hypertrophy
Introduction
Liver resection offers the best survival advantage for primary and secondary liver cancers. Metastases are considered resectable, when all can be resected completely (R0) with negative margins while leaving adequate functioning liver parenchyma behind [1]. Thus the concept of resectability has been freed from the domains of the number and size of metastases and rests exclusively on oncological clearance and Future Liver Remnant (FLR) both in terms of its quality and volume.
Up to 45 % of patients with primary and secondary liver tumours will require an extended hepatectomy to achieve negative margins [2]. The major limitation for extended hepatectomy (EH = excision of five or more than five liver segments) is the lack of adequate and functioning future liver remnant (FLR). PVE (per-cutaneous or at laparotomy) or PVL (at laparotomy or laparoscopy) offers the possibility of increasing the FLR and thus reducing the risk of post-operative hepatic failure after EH. Specifically, morbidity and mortality increases when the FLR in non-cirrhotic patients is less than 20–25 % of the total liver volume and less than 40 % in those with chronic liver disease including cirrhosis [3–6]. With the advent of newer chemotherapeutic agents, such as cetuximab and bevacuzimab, [7, 8] especially for metastatic colo-rectal cancer, and locally ablative therapies such as radiofrequency ablation (RFA), [9] more patients are being increasingly downstaged and rendered suitable for liver resection. In addition, increasing surgical experience and centralisation of hepato-biliary surgery has reduced the risk of peri-operative mortality after major liver resection including extended hepatectomy to less than 5 % [10–12]. Even patients with extra-hepatic metastatic disease are offered hepatic resection, provided all disease can be successfully resected or ablated [13, 14]. Two stage hepatectomy, with intervening PVE or PVL, is now being routinely offered in major liver units to eradicate the liver tumour burden especially for colorectal and neuro-endocrine tumours [15, 16]. Thus, PVE and PVL has become an essential tool in the armamentarium of the multimodality treatment of primary and secondary liver tumours [17, 18]. The liver receives approximately 80 % of its blood from the portal vein and 20 % from the hepatic artery. Occlusion of the portal venous blood flow into the liver segments redirects the entire portal flow into the non-occluded contra lateral lobe/segments and promotes hypertrophy. Rous was the first to describe this atrophy-hypertrophy experimentally in rabbits after portal vein ligation in 1920 [19].
The first human description was in 1956 by intentional occlusion of the portal vein as well as bile ducts [20]. The Japanese applied this clinical observation into clinical practice for extended liver resection in the late 1980s [21–23]. Technically portal vein occlusion can be achieved by two main approaches: portal vein ligation (PVL)-surgical (direct ligation: either open or laparoscopic, PVL) and portal vein embolization – PVE: transileocolic portal embolization, (TIPE) and percutaneous transhepatic portal venous embolization – PTHPE (ipsilateral or contra lateral punctures). TIPE is no longer routinely used. In this article, we discuss the indications and outcomes for PVE and PVL based on systematic review of the major consecutive series with a large cohort of patients and summarise the world experience with this emerging modality in treatment of liver tumours.
Methods
A thorough PubMED, Embase, Ovid and Cochrane database search for all original articles with 30 patients or more undergoing either PVE and patient series (irrespective of number) undergoing PVL was carried out by SV, SM, SP and HMK.
No defined limit in terms of patient numbers was set for PVL, as the procedure itself is not very common and the literature pertaining to the same, when compared to PVE is lesser. Additionally any RCTs or meta-analysis were included. Free text searches were done using phrases such as ‘portal vein embolization’, portal vein embolization’ ‘portal vein ligation’, ‘extended hepatectomy’, ‘clinical trial’, ‘meta-analysis’ and this was further extended with the ‘Related article’ search function of PubMED and hand-searches of bibliography. The articles had to describe at least one of the following parameters as outcome measures: FLR change, complications, length of stay, time to surgery, proportion resectable and/disease progression data. Initial search for PVE revealed 245 articles (of which 27 case series had ten or more patients) and for PVL 23 articles (6 articles eligible). We selected 20 articles containing 30 patients or more who had portal vein embolization (excluding review articles, meta analyses and articles from the same unit over overlapping time periods) [6, 17, 24–40]. We however included one paper with 27 patients coming from a well known unit in Paris, as these patients were a part of the only prospective randomised trial on PVE [26]. Six papers describing Portal Vein Ligation (PVL) were analysed [35, 41–44]. Two papers had a combined cohort of patients who were partly divided between PVE and PVL [33, 35]. Analysis from these papers took into account each group individually. From these article the collated data included 1,532 patients (from published series) who had PVE and 93 patients who had PVL. The data was collated in excel spreadsheets and any controversial interpretations were resolved by DL and TF.
Results
As depicted in Table 1, from the papers studied a total of 1,532 patients underwent PVE to facilitate liver hypertrophy and resection. There was an equitable distribution between Colorectal metastases 656 (42.81 %) and primary hepato-biliary malignancies (CA GB, CC, HCC) 660 (43.08 %). Other metastatic lesions accounted for 195 patients (12.73 %). There were six retrospective studies looking cumulatively at 93 patients who underwent PVL to facilitate liver hypertrophy. In the PVL group (n = 93) also, predictably CRC were the dominant disease pathology with 64 patients (68.81 %) (Table 3). Following PVE the mean resectability rate was 76.88 %. 17.46 % failed resection due to disease progression while failure of hypertrophy in 4.8 % of patients prevented resection (Table 4). Mean post-operative morbidity in the PVE group was 26.58 % (95 % CI 19.2–33.95) with liver failure in 6.29 % (95 % CI 2.24–10.34).%. The mean surgical mortality following liver resection in the PVE group was 2.59 (95 % CI 1.34–3.83)% (Table 4). The mean morbidity from PVL as a surgical procedure was 5.72 %. As would be evident from Tables 1 and 2, the time to discharge following PVL was much higher than PVE (10.16 days v/s 2.1 days). Failure of hypertrophy in the PVL group was 7.4 %. (Table 4). As would be expected due to prolonged stay following PVL, patients after PVL took a longer time to come to definitive respective surgery as opposed to patients who had PVE (53.6 days v/s 37.13 days). From the data given PVL seems to have produced significantly greater FLR hypertrophy when compared to PVE (64.65 % v.s 39.75 %). This data must however be interpreted with caution as one specific paper looking at PVL produced a significantly high FLR hypertrophy when compared to any other publication [43].
Table 1.
Patients with PVE
| Authors | Citation | Nature of study | No | Period of study | Diagnoses | Morbidity of PVE | Time to discharge after PVE | Mean duration to surgery (days) | Resectability rate | Operative mortality | Liver failure | Operative morbidity | Increase of liver volume | Failure of hypertrophy | Post op stay (days) | Disease progression |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azoulay [3] | Ann Surg. 2000 Apr;231(4):480–6. | R | 30 | 1990–1998 | CRC | 3 % | NM | 63+/−45 | 63 % | 3 % | NIL | 16 % | 14–71 %; median =42 % | NIL | NM | 33 % |
| Nimura [6] | Ann Surg. 2006 Mar;243(3):364–72 | P | 240 | 1991–2005 | CC 150 GB Ca 90 | 0.83 % | NM | 14–21 | 80.40 % | 8.80 % | 2.07 % | NM | 7–19 % | NIL | NM | 19.58 % |
| Covey [17] | Ann Surg 2008 Mar;247 (3) :451–5 | P | 100 | 1999–2004 | CRC | 1 % | NM | 41–76 (20–255) | 65.50 % | 2 % | NM | 31 % | 22+/−3 % | 10 % | 8 (4–23) | NM |
| Wakabayashi [24] | Surgery 2002; Jan; 131 (1);26–33 | R | 43 | 1991–2000 | HCC 25, CC 8, OM 10 | NIL | NM | 21–26 | 100 % | NIL | NM | 13.90 % | 32.93 % | NM | NM | NM |
| di Stefano [25] | Radiology 2005;234; 625-30 | R, MC | 188 | 1990–2001 | HCC 32, CC 15, OM 137, BENIGN 4 | 12.80 % | NM | 33–39 | 86 % | NM | NM | NM | 41–62 % | 1.59 % | NM | 6.38 % |
| Farges O, Belghiti [26] | Ann Surg. 2003 Feb;237(2):208–17 | P, RT | 27 | 1998–2000 | CRC10, HCC 14, CC 1, NET 2 | NIL | NM | 49+/−13 | 100 % | 3.57 % | 3.57 % | 25 % | 33+/−10 % | 14 % | 13+/−4 | 6.45 % |
| Cotroneo [27] | Eur J Surg Oncol. 2009 Jan;35(1):71–8 | R | 31 | NM | CRC13, HCC 7, CC 9, GB Ca 2 | 6.45 % | NM | NM | 77.41 % | NIL | NM | NM | 44.2 % non cirrhotic, 32.1 %: cirrhoitic | 6.45 % | NM | 16.12 % |
| Immamura [28] | Hepatology. 1999 Apr;29(4):1099–105. | R | 84 | 1990–1998 | HCC 5, CC 49, GB Ca 22, OM 7, Hemangioma 1 | 2.56 % | NM | 17 | 79 | 3 % | 1.50 % | NM | 30 % −median | NIL | NM | 21.42 % |
| de Baere [29] | Hepatology 1996 Dec;24(6):1386–91. | P | 31 | 1989–1995 | CRC 22, HCC 1, CC 1, OM 7 | 3.22 % | NM | 32 | 77 % | 8.30 % | NIL | 8.30 % | 79+/−50 % | 3.22 % | NM | 12.90 % |
| Jaeck [30] | Surgery. 2008 Apr;143(4):476–82. | R | 146 | 1997–2006 | CRC 106, HCC 10, CC 19, GB Ca 06, NET 5 | 10 % | 4+/−1 days | NM | 79 % | NIL | 4 % | 43 % | 47.7 %+/−31.9 % | 5.51 % | NM | 13.10 % |
| Makuuchi [31] | Hepatogastroenterology. 1991 Aug;38(4):329–36. | P | 54 | NM | CRC 8, HCC 31, CC 12, OM 3 | NIL | NM | 21 | 85 % | 2.17 % | NM | 15.21 % | NM | NM | NM | NM |
| Dong Dae Seo [32] | Ann Surg Oncol. 2007 Dec;14(12):3501–9 | R | 32 | 1999–2001 | HCC | NIL | NM | median = 21 | 58 % | NIL | 12.50 % | 18.75 % | 34 % | 15.60 % | median = 20 | NIL |
| L. Mueller [33] | Ann Surg Oncol. 2008 Jul;15(7):1908–17. | R | 40 | 1995–2004 | CRC | NIL | NM | 50.5 −combined data from PVE/PVL | 55.00 % | 2.30 % | NM | NM | 50.10 % | 10 % | 12 | 35.00 % |
| Vauthey [34] | Br J Surg. 2007 Nov;94(11):1386–94 | R | 112 | 1995–2006 | CRC 50, HCC 24, CC 14, GB Ca 6, OM 18 | 8.90 % | NM | NM | 69.60 % | 3 % | 15.38 % | 44 % | 9.85 % | 4.46 % | 8 days (5–53) | 19.64 % |
| Capussotti [35] | Arch Surg 2008 Oct;143(10):978–82; discussion 982 | R | 31 | 2000–2006 | CRC | NIL | 3 | NM | 77.41 | 3.50 % | NM | 16.66 % | 53.40 % | NM | NM | NM |
| Pamecha [36] | Ann Surg Oncol (2009) 16:1202–1207 | R | 36 | 1999–2005 | CRC | 6 % | NM | 42 | 61 % | 4.50 % | 4.54 % | 36 % | 37 % median | NIL | median = 13 | 33 % |
| Thierry de Baere [37] | Ann Surg Oncol (2010) 17:2081–2089 | R | 107 | 1987–2005 | CRC 79, HCC 10, OM 11, NET 7 | NIL | NM | 34 | 88 % | 3.73 % | 9.34 % | 23.36 % | 69 % | NIL | NM | 12 % |
| D. A. Wicherts [38] | British Journal of Surgery 2010; 97: 240–250 | R | 99 | 1990–2006 | CRC | NM | NM | 56.7 | 68 % | 1.50 % | 19.40 % | 49 % | NM | 3 % | 18 | 27.27 % |
| Ratti [39] | Updates Surg (2010) 62:153–159 | R | 62 | 2006–2009 | CRC 32, HCC 7, CC 10, GB Ca 13 | NIL | NM | 35 +/−10 | 90.32 % | nil | 3.20 % | 32 % | 50.3%mean | 3.22 % | 11+/−4 | 6.45 % |
| Hyunkyung Yoo[40] | Eur Radiol (2009) 19: 1054–1061 | R | 39 | 2006–2007 | HCC 16, CC 19, OM 2, Misc 2 | 4.87 % | NM | NM | 78.04 % | NM | NM | NM | 22.74 % mean | NM | NM | 17.04 % |
R retrospective, P prospective, NM not mentioned, CRC colorectal liver metastasis, HCC hepato-cellular cancer, CC cholangiocellular cancer, GBCa gall bladder cancer, NET nuero-endocrine tumour, OM other metastatic lesions
Table 3.
Distribution of patients
| PVE group | PVL group | |
|---|---|---|
| CRC | 656(42.81 %) | 64 (68.81 %) |
| Primary HPB malignancies | 660 (43.08 %) | 11 (11.82 %) |
| – | - HCC :214 | - HCC :2 |
| - GB Ca:139 | - CC : 9 | |
| - CC :307 s | ||
| OM | 195 (12.73 %) | – |
| NET | 21 (1.37 %) | 18 (19.35 %) |
| Hemangiomas | - NET : 14 | - NET :18 |
| Benign | - Benign/Misc: 06 | |
| Misc Lesions | - Hemangioma: 01 | |
| 1532 | 93 |
CRC colorectal metastases, CCA cholangiocarcinoma, OM other metastases, HCC hepatocellular carcinoma, GB Ca carcinoma gall bladder, NET Neuroendocrine tumours, Misc Miscellaneous lesion
Table 4.
Outcomes
| PVE group | PVL group | |
|---|---|---|
| Morbidity/Complication | 3.13 % (95%CI 1.21–5.04) | 5.72 % (95 % CI 0–15.28 %) |
| Length of stay following procedure -days | 2.1 (95 % CI 1–4) | 10.16 (95%CI 6.63–13.69 %) |
| Increase of FLR (%) | 39.75 % (95%CI 30.8–48.6) | 64.65 % (95 % CI 0–136.12 %) |
| Failure of hyperptrophy precluding surgery (%) | 4.8 % (95 % CI 2.07–7.52) | 7.4 % (95 % CI 0–16.12) |
| Time to surgery - days | 37.13 (95 % CI 28.51–45.74) | 53.6 (95%CI 32.14–75.05) |
| Resectability rate (%) | 76.88 % (95 % CI 70.91–82.84) | 63.68 % (95 % CI 56.82–70.54) |
| Disease progression (%) | 17.46 % (95 % CI 11.89–23.02) | 29.29 % (95 % CI 15.69–42.88) |
| Length of stay following liver resection- days | 13.57 (95 % CI 9.8–17.37) | 13 (95 % CI 10.84–15.16) |
| Morbidity following liver resection | 26.58 (95 % CI 19.2–33.95) | 25.37 %(95 % CI 0–53.39) |
| Post resection liver failure | 6.29 % (2.24–10.34) | 14.28 %a |
| Post resection mortality | 2.59 % (95 % CI 1.34–3.83) | – |
Table 2.
Patients with PVL
| Authors | Citation | Nature of study | Number of patients | Period of study | Diagnoses | Morbidity of PVL | Time to discharge after PVL in days | Mean duration to surgery | Resectability rate | Operative mortality | Liver failure | Operative morbidity | Increase of liver volume | Failure of hypertrophy | Post op stay | Disease progression |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. Mueller [33] | Ann Surg Oncol. 2008 Jul;15(7):1908–17. | R | 13 | 1995–2004 | CRC | 0 | NM | 50.5 | 61.53 % | 0.00 % | NM | NM | 50.10 % | 15 % | 12 | 23.07 % |
| Capussoti [35] | Arch Surg 2008 Oct;143(10):978-82; discussion 982 | R | 17 | 2000–2006 | CRC | 0 | median = 10 | 13–135 days (median = 40 days) | 64.75 % | 0 | NM | 38.46 % | 43.10 % | 0 | 6–30 days (median = 15 days) | 35.30 % |
| Broering [41] | J Gastroint Surg 2002 Nov-Dec;6(6):905–13; discussion 913. | R | 17 | 1995–2000 | CRC 10, HCC 1, CC 6 | 5.80 % | 8.1+/−5.1 | 83+/−30 days | 58.825 | 0 | NM | 27 % | 43.20 % | 17.64 % | 12.2+/−6.72 days | 41.17 % |
| Belghiti [42] | J Gastroint Surg 2008 Feb;12(2):297–303. Epub 2007 Nov 30 | P | 17 | 1998–2005 | CR :7, NET10 | 23.52 % | 13+/−6 | 7–8 weeks | 72 % | 0 | 14.28 % | 36 % | 38+/−26 % | 11.76 % | na | 11.76 % |
| Are [43] | HPB (Oxford). 2008;10(4):229–33 | R | 9 | 2005–2007 | CRC 5, HCC 1, CC:3 | 0 | 6.7+/−1.4 | NM | 55 % | NM | NM | NM | 181.50 % | 0 | na | 44.44 % |
| Kianmanesh [44] | J Am Coll Surg. 2003 Jul;197(1):164–70. | R | 20 | 1996–2001 | CRC 12; NET 8 | 5 % | 6–20 days | 4–8 weeks (median = 6 weeks) | 70 % | 0 | 0 | 0 | 32+/−9 % | 0 | 13 days | 20 % |
R retrospective, P prospective, NM not mentioned, CRC colorectal liver metastasis, HCC hepato-cellular cancer, CC cholangiocellular cancer, GBCa gall bladder cancer, NET neuro-endocrine tumour, OM other metastatic lesions
Inspite of the greater FLR hypertrophy in the PVL group, the resectability rate was higher in the PVE group (76.88 % v/s 63.68 %). This difference we feel must be attributed to the greater time taken for patients to come to resection in the PVL group, which also explains the higher rate of interval disease progression in the PVL group (29.29 % PVL v/s17.46 % PVE). Interestingly failure of hypertrophy was also greater in the PVL group (7.4 % v/s 4.8 %). There was no operative mortality in the PVL group (Tables 2 and 4).
The summary of results is presented in Tables 1, 2, 3, and 4.
Technical Aspects – Percutaneous Portal Vein Embolization
Portal Vein Occlusion (embolization or ligation) is performed to allow hypertrophy of the contralateral FLR Some of the early reports of Portal Vein Embolization described achieving access to the portal vein, through the ileo-colic vein (TIPE) or a jejunal vein at laparotomy [28, 45]. However the laparotomy required for this was a potentially morbid procedure inbuilt with its risks and complications. With improvements in instrumentation and interventional radiology, the “minimally invasive nature” of the percutaneous approach has taken over. As such in a recent meta analysis it was demonstrated that liver hypertrophy following PTHPE was greater than the trans ilio-colic portal route for embolization, making the transhepatic approach a more favoured modality to embolize the portal vein [46]. Peraranau et al., extended their experience with TIPS to achieve Transjugular portal vein embolization (TJPE), and found this to be safe and effective [47]. A peripheral branch of the portal vein to be embolized was accessed through the middle, left or right hepatic vein accessed via the internal jugular vein [47]. The percutaneous transhepatic (PTHPE) approach to portal vein embolization now is the standard uniform method followed world over. Detailed techniques and methods of PVE have been described in more extensive reviews on the subject [48, 49]. As has been suggested by some experts, PVE through an ipsilateral approach avoids instrumentation of the FLR, which could result in damage to the hepatic parenchyma along with increased risk of contralateral portal vein thrombosis [25, 50]. To describe the procedure briefly - intravenous antibiotics (1.2 g co-amoxiclav are administered prior to the procedure and the procedure carried out under conscious sedation). Access is gained via an ultrasound guided fine needle puncture of the ipsilateral or contralateral peripheral portal vein branch. This access is then upsized and an 8 F vascular sheath inserted. A portogram is obtained via a catheter positioned in the main portal vein. This allows assessment of anatomy and patency of the contralateral portal system during the procedure.
The use of various embolic materials has been reported -gelatine sponge polyvinyl alcohol particles, histoacryl glue and/or a combination of the above. A 16 mm nitinol plug. (AMPLATZER® Vascular Plug) may be positioned in the right portal vein (if the right liver is being embolised) approximately 1 cm distal to the left (contralateral) portal vein origin. Once the position is confirmed and is satisfactory, the plug is deployed (Figs. 1 and 2). Since potential intrahepatic collateralisation of the portal veins may occur following occlusion of the main portal vein, therefore a second embolic agent - a mixture of histoacryl glue and iodinised oil in a ratio of 1:6 is used to embolise the more distal branches of the right portal vein until stasis of flow (venous thrombosis does not occur immediately on deployment of the vascular plug and may take up to 15 min) [51]. Once satisfactory embolization has been obtained, the entry tract is embolised with gelatine sponge pledgets (Gelfoam®, Pfizer, New York, USA). This is because histoacryl glue can be very painful if spilled into the abdominal cavity. Likewise in patients where patients qualify for a left extended hepatectomy, with disease sparing right lobe of the liver, hypertrophy of right lobe can be achieved by embolization of the left portal vein, thereby facilitating selective hypertrophy of the posterior segments and hence facilitating a left extended hepatectomy. Among the embolic materials, cyanoacrylate has been found to be a very reliable agent (as compared to gelfoam and thrombin) [28, 29]. Cyanoacrylate produces portal vein occlusion which lasts for at least 4 weeks and also produces a greater volume of liver hypertrophy [28, 29, 46]. However, because of its ability to produce an intense fibrotic reaction – it can cause significant peribiliary fibrosis around the portal vein, making surgery diffucul [28, 29, 46].
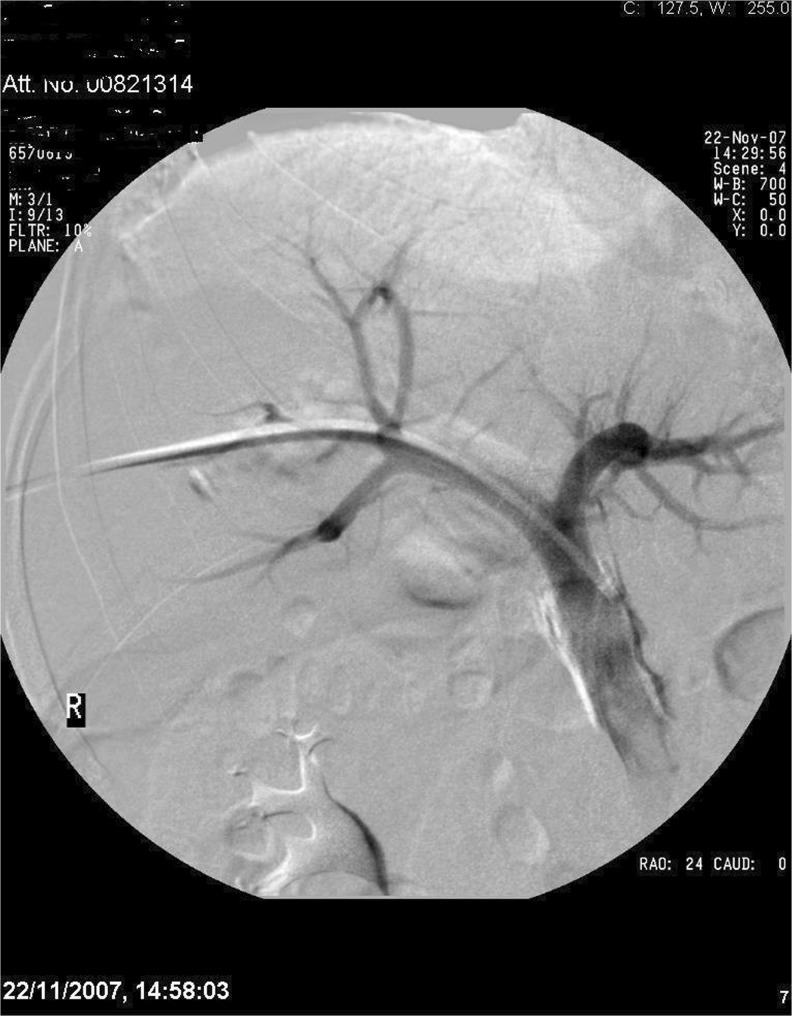
Fig. 1.
PVE in progress
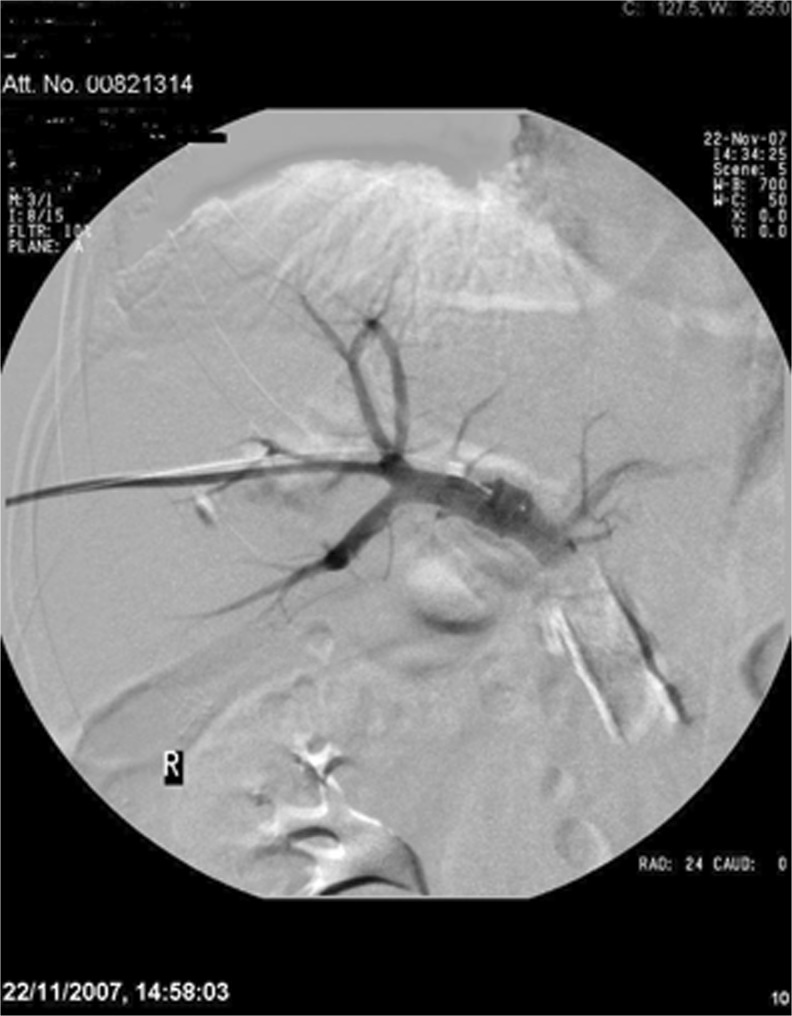
Fig. 2.
Completed PVE
In our experience also, we have found this method to be relatively simple, with a shorter procedural time compared with selection and embolization of subsequent portal vein branches, and there is minimal risk of non-target embolization of glue into the FLR, which may jeopardise hypertrophy. Following the procedure, the patient is admitted overnight for routine post procedural care and analgesia.
Discussion
Indications
PVE is indicated to facilitate a major liver resection to help obtain a complete R0 resection. It may be used prior to a proposed Extended Hepatectomy in patients with a normal liver or even prior to a right/left hepatectomy in patients with cirrhosis or chronic liver disease. This is because the hepatic reserve in these patients is prohibitive to facilitate a standard hemihepatectomy and hence further hypertrophy of the liver may be indicated prior to a hemihepatectomy [3–6]. Individuals with a normal background liver would be able to tolerate removal of up to 70–75 % of the liver without significant risk of post-operative liver failure [12, 27, 29]. In patients with chronic liver disease, including cirrhosis and liver damage from previous chemotherapy, up to 40 % of FLR would be required to permit a safe resection. Beyond these limits, the risk of liver failure and surgery related complications significantly increase [12, 27, 29]. The amount of liver left back after liver resection, has now been defined as the most important single prognostic factor that would determine recovery following a major liver resection.
The planning of an extended resection hence in this context should take into account both the quantity (volume) and the quality of the remnant liver following a proposed resection. Indocyanine Green Clearance and Liver Volumetry provide objective evidence of liver function when planning major liver resections, in borderline situations-1.
ICG clearance – Indocyanine Green Clearance Test – an ICG retention rate of 15 % at 15 min (ICG R15 of 15 %) is considered the upper limit to permit major liver resection [52].
Liver Volumetry – this can be determined either on triple phased contrast enhanced CT scan or MRI Liver. Liver volumetry using technetium −99 m galactosyl human serum albumin (HSA) has also been described [2]. The FLR/TELV ratio is calculated and expressed as a percentage to express the amount of FLR (TELV; Total estimated liver volume = Total liver volume-tumour volume) [1]. As stated earlier a FLR/TELV of a minimum of 25 % in a normal liver would be required to permit a safe resection, while a FLR/TELV of at least 40 % would be a minimum requirement in the background of chronic liver disease, even in livers wherein prior chemotherapy has been given [1].
PVE may therefore be performed in the above situations when the numbers are prohibitive to proceed with a resection straightaway. Some researchers have also proposed a FLR/Body weight ratio as a more accurate indicator of future liver function and defined a value of less than 5 % to be at a significant risk of post-operative liver failure [1, 53, 54].
Contraindications
Presence of disease in the contra lateral lobe and the FLR is a relative contraindication to PVE. This is because as portal venous flow is re directed to the liver lobe following embolization could potentially promote growth of the liver metastases along with that of the liver. In such instances, a two stage hepatectomy could be performed. The first stage hepatectomy is aimed at disease clearance from the “prospective non-embolized liver lobe” followed by a contra-lateral PVE or PVL. The second stage hepatectomy is then performed following PVE to obtain complete disease clearance. Jaeck et al. in their review of 33 patients with a two-stage hepatectomy and PVE for colorectal liver metastasis reported the procedure to be safe and achieve equivalent survival rates as compared to patients undergoing single stage liver resections without PVE [15].
Biliary obstruction is contra-indication for PVE. As PVE can induce changes in the liver function, it is important that liver function be optimised prior to PVE. Hence in patients with obstructed biliary system and jaundice, decompression of the obstructed biliary system to facilitate recovery of liver function pre PVE is an essential prerequisite to facilitate a safe PVE. In a review of 240 patients with biliary cancer who underwent extended resections following PVE, all patients with jaundice had a pre- PVE percutaneous transhepatic biliary drainage (PTBD) [6] Embolization of the portal vein in the presence of an unobstructed biliary tree, predisposes to sepsis and cholangitis. Presence of unresectable local or distant metastatic disease – precluding resection is an absolute contraindication since this patient would never qualify for a resection. Performing Portal Venous Occlusion in such a patient exposes the patient to the potential morbidity of portal occlusion, without any benefit whatsoever. Presence of tumour thrombus in the main portal vein, contra-lateral portal vein is a relative contraindication to PVE for technical reasons. Tumour invasion of the arterial blood supply or thrombosis of the artery to the embolized lobe could induce complete ischemia [3].
Imaging and Assessment of FLR
Contrast enhanced triple phase (pre-contrast, arterial phase and portal venous phase) Multislice Computed Tomography is the standard imaging modality for evaluation of hepatic lesions and volumetry of the proposed FLR. Some liver units have been regularly using Magnetic Resonance Imaging (MRI) to measure liver volumes on a regular basis. Liver regeneration peaks within the first 2 weeks after PVE [48] and reaches a plateau within 1 to 2 months [24, 55]. In cirrhotic patients and in those with chemotherapy induced liver dysfunction, the rate of regeneration and hypertrophy is slower [56]. Imaging of the liver has been reported at varying intervals between 3 and 8 weeks [46]. In a meta-analysis published recently the mean duration to imaging was 29 days [46]. Imaging is done to evaluate.
Degree of hypertrophy and calculate FLR with regard to resectional plan.
Re-stage the tumour burden and exclude disease progression: intra- or extra-hepatic.
Despite various formulas being used to calculate volumes of the FLR [46, 49, 56–58] and inspite of errors in volumetric measurements due to partial volume effect, effects of respiration and inter-observer variations volumetric studies have usually been accurate to within +/− 5 % [56]. Percutaneous PVE has been known to induce an increase of the FLR volume of 34.5 % (95 % CI 23.5–45) [22, 30, 44, 56, 59–61]. Insufficient hypertrophy of the FLR after PVE was seen in 7.3 % (95 % CI 5.1–9.5) of patients [30, 34, 58]. The incidence of interval disease (intra or extra-hepatic) progression is 20 % (95 % CI 15.6–24.7) [30, 34, 51].
Safety, Morbidity and Complications
Percutaneous transhepatic PVE(PTHPE) has a technical success rate of 98.9 % (95 % confidence interval 97–100). PVE is associated with a morbidity of 1.2 % (95 % CI 1.2–4.6) and an in-patient stay of 2.1 days (range 1–4) [17, 30, 46, 59]. PVL has a longer in-patient stay and higher morbidity (Table 4). Changes in liver function are usually minor and transient (50 % of patients have no appreciable change) [6, 48]. The elevated transaminase levels generally return to normal within 7–10 days following the procedure. Transaminase levels when they rise generally peak at 3 times normal in about 3 days following the procedure [46]. The only significant common adverse event is the postembolization syndrome consisting of fever and thrombocytopenia [6, 17]. Rarer events include necrosis, bile leak haemorrhage and abscess formation [17, 62]. As mentioned earlier, cannulation of the ileo-colic vein at laparotomy, to achieve PVE is no longer a favoured method of embolising the liver, due to the attendant morbidity of a laparotomy [28, 45]. The intense fibrotic reaction associated with cyanoacrylate may also be responsible for a greater deterioration of liver functions post-embolization when this agent is used [62].
Resection Rates and Operative Morbidity/Mortality Following Surgery
In a meta analysis with 37 publications and 1,088 patients, 930 (85 %) patients underwent a laparotomy for an intended major hepatectomy, following PVE 772 patients had a resection giving an overall resectability rate of 70.95 % [46]. Resection rates following PVE have varied from 58 to 100 %, with an acceptable post-operative morbidity and mortality [48, 49, 56, 58]. The mean resectability following PVE/PVL was 78 % (95 % CI 73.3–83). Failed resections have resulted due to a combination of factors including interval progression of disease – both systemic and local disease, failure of hypertrophy of the FLR and patient factors including a sub-optimal general condition to permit a safe hepatectomy [3, 6, 30, 46].
Liver Failure, Morbidity and Mortality
The incidence of transient liver failure following 772 post-PVE liver resections has been reported at 2.5 % [46]. The mortality figure for these 772 resections, from 37 publications is. 2.07 %. The morbidity has been reported at 19.2 %, which is comparable to complication rates from liver resections without PVE [46, 56]. Mortality from acute liver failure due to insufficient liver remnant following resection has been reported at 0.8 % [46]. Mortality figures range from 0 to 6.5 % in patients without cirrhosis and about 6–7 % in cirrhotics [56]. Use of neoadjuvant chemotherapy in patients with PVE, is not an adverse prognostic factor. Fong et al. demonstrated that in patients undergoing neoadjuvant chemotherapy, chemotherapy associated steatohepatitis (CASH) did not adversely impact liver regeneration or the patients ability to undergo liver resection [17]. In an interesting study Hemming et al. found no difference in post-operative mortality with or without PVE [59]. However patients with PVE had a lesser post-PVE morbidity in their study [59]. Belghiti et al. in a prospective clinical trail demonstrated that PVE facilitated a safer right hepatectomy in patients with chronic liver disease when compared to patients with normal liver [26]. The entire concept of PVE is therefore to convert a high risk procedure into that of a lower risk (Figs. 3 and 4).
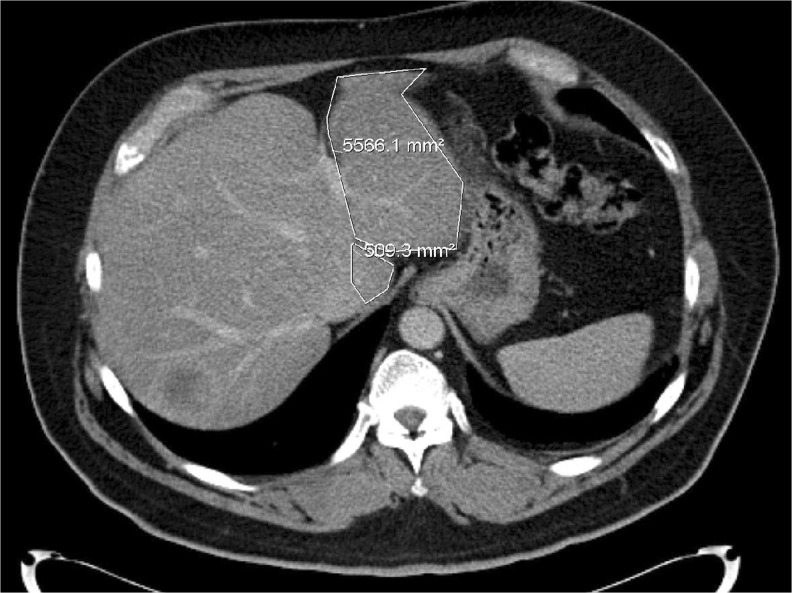
Fig. 3.
Pre-PVE image. The marked area shows the FLR intended for hypertrophy
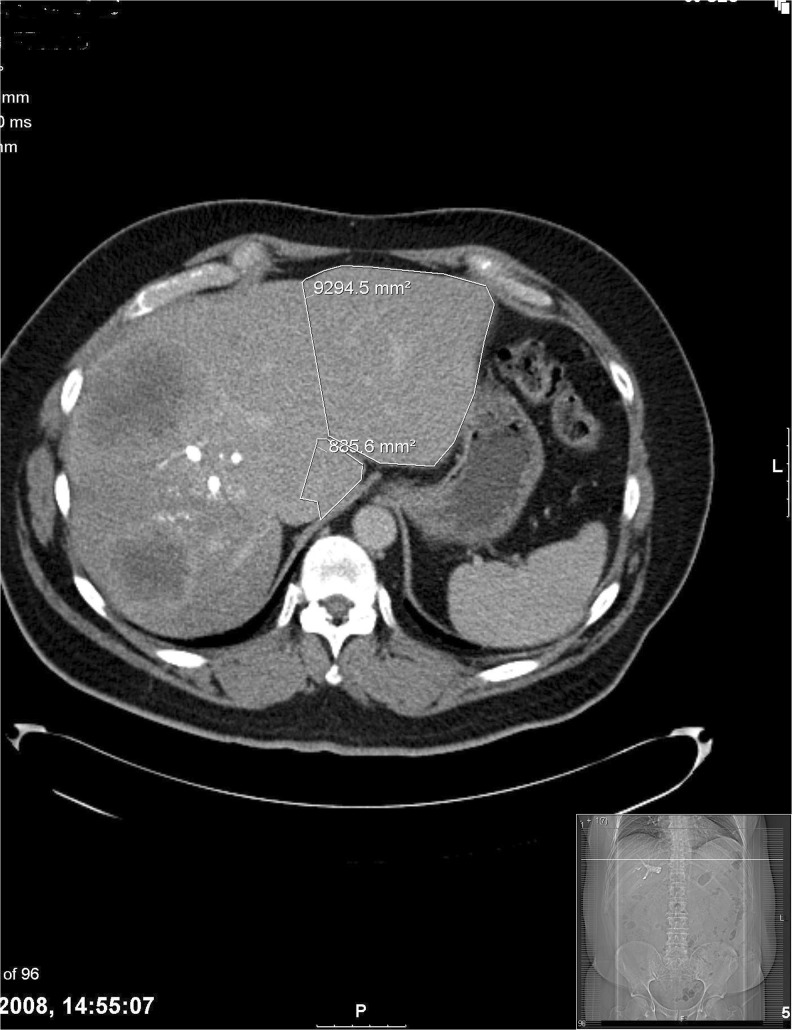
Fig. 4.
Post-PVE image. The marked area shows an increase in the FLR after successful PVE
Long Term Survival
Azoulay et al. reported a 40 % 5 year survival rate for patients with colorectal liver metastases undergoing liver resections [3]. These results are comparable to survival data for patients having liver resections for colorectal metastases without PVE [3, 5]. Similarly Abdalla et al. reported a 65 % 3 years survival rate in patients undergoing liver resection for hepatobiliary malignancies after PVE [5]. These survival data demonstrate an absolute survival advantage for majority of such patients, since in the absence of PVE, they would otherwise have been unsuitable for any treatment with an intended cure in the first instance. Nagino in his study of 240 PVEs for primary hepatobiliary malignancies (cholangiocarcinoma and gallbladder cancers), found an unequivocal benefit in the cholangiocarcinoma group [6]. PVE facilitated extended resections in these patients with cholangiocarcinomas who were otherwise unresectable due to borderline liver volumes, with improved post-operative recovery and acceptable survivals [6]. The benefit of PVE in patients with gall bladder cancers was less obvious presumably due to more aggressive disease biology and more complex surgical procedures that were performed in conjunction with the hepatectomy [6]. In patients with HCC in the background of cirrhosis or fibrosis, PVE increased the possibility of performing a safe hepatectomy in by 47 % [62]. This is significant since in the absence of PVE these patients would not have had surgery, due to lack of adequate FLR. By facilitating complete resectional surgery, in these patients PVE optimised the chance of cure in these patients who were otherwise candidates for non-surgical intervention [62]. In these patients with HCC in the background of cirrhosis/fibrosis, PVE may also potentiate the beneficial effects of TACE (transarterial chemoembolization) apart from preventing metastatic spread via the portal vein [62]. In yet another study on HCC, PVE improved long-term survivals, minimized local recurrence and facilitated curative surgery in a significant proportion of patients with HCC when compared to an equivalent group who underwent TACE [32]. Dong and collegues achieved a 5 years survival rate of 71.9 % in patients who had PVE followed by surgery for HCC [32]. In another interesting study Elie Oussoultzoglou et al. reported a reduced rate of intrahepatic recurrence in patients undergoing right hepatectomy for unilobar CLM following PVE [63]. They suggested that preoperative PVE could contribute to reduce peri-operative tumour shedding and intraoperative intrahepatic dissemination [63].
PVE and Chronic Liver Disease
Belghiti in his study on PVE prior to right hepatectomy reported failure of liver hypertrophy in 14 % of patients with some form of chronic liver disease including cirrhosis [26]. Failure of liver hypertrophy in patients with cirrhosis and chronic liver disease has ranged from 2 to 20 % [56]. Presence of chronic liver disease prevents liver regeneration in these patients and Belghiti suggested that liver regeneration should be considered as a dynamic liver function test [26]. Failure of liver hypertrophy thus contraindicates resection [26]. In addition, diabetes is a poor prognostic factor for liver hypertrophy and patients with diabetes after PVE often have poor liver hypertrophy [4]. It appears that high portal vein pressure and portal hypertension in cirrhosis and chronic liver disease produces significant porta-systemic shunts, thereby diverting blood flow away from the liver thereby restricting liver hypertrophy in portal hypertension [24, 26]. In a meta analysis, Jiao et al. have shown that in patients with borderline liver volumes, PVE is an essential procedure, facilitating liver resection without post-operative liver failure [46].
Debate and Controversy
Although no direct comparisons of ipsilateral v/s contralateral methods of PTHPE have been made, each of these approaches has its advantages and disadvantages.
In certain cases, there is the potential risk of puncture through tumour tissue, which may result in tumour seeding [64]. Technically also embolization of the right portal vein has been reported to be more diffucult through an ipsilateral puncture as compared to the contralateral technique where cannulation of the right portal vein seems to be easier [64]. However an ipsilateral puncture avoids instrumentation of the contralateral healthy liver and portal vein, thereby preventing damage to the liver and portal vein thrombosis which may at times prevent resection [56, 64]. Since an extended right hepatectomy involves removal of segment 4 as well, embolization of the segment 4 branches of the left portal vein in addition to right portal vein could theoretically enhance selective hypertrophy of the caudate lobe and the left lateral segments. Present data and evidence do not show any benefit or increased hypertrophy rates of segment 2 and 3 irrespective of whether segment 4 branches were embolized or not [30, 54]. However embolization of segment 4 vein has been found to be both and effective in some studies [65]. However an attempt to embolize the segment 4 portal radicals along with the right portal vein, may risk occlusion of the left portal vein [24, 30, 54, 65]. Segment IV embolization is advised for oncological reasons when there is disease in segment 4 which may potentially progress after right portal vein embolization [66, 67]. Given the impact PVE has had on resectability it would be unethical to test its efficacy through a randomized trial, since a proportion of patients would then be denied potentially curative surgery, based on inadequate liver size and function [1, 56].
Does PVE Induce Tumour Growth?
It had been reported that PVE may induce tumour growth and hence patients may be at an increased risk of recurrence following PVE [59, 68]. This was analogous to the fact that some studies reported an increased risk of disease recurrence in the liver after a major liver resection [63]. In both instances it is speculated that there may be an increased release of growth factors and cytokines in the circulation leading to a growth stimulus and hence an increased risk of disease recurrence [59]. In patients undergoing major liver resection, intra-operative shedding of tumour cells may also contribute to the increased incidence of disease recurrence [63, 69–71]. However no substantial and convincing evidence has been ever produced to prohibit use of PVE, as it would increase the risk of intra-hepatic disease recurrence.
Evidence in this respect seems to be weak and presently PVE is considered to be a safe oncological procedure with no propensity to accelerate tumour growth in any form. However as PVE redirects the entire portal blood flow to the contralateral liver (FLR), complete absence of disease and disease clearance from this liver segment is an absolute mandatory requirement prior to PVE. This is because portal blood supply along with all its hepatotropic factors while will stimulate liver hypertrophy will also promote increase in the size and number of liver metastasis in the FLR. Hence presence of disease in the FLR is an absolute contraindication to PVE. With the feasibility of safe PVE and its proven success, surgeons extended its role to treat patients with initially unresectable bilobar liver metastases using the 2 stage hepatectomy and PVE in combination [15]. In this process, patients underwent either resection or RFA of the liver metastases located in the FLR (contralateral liver). Subsequently they underwent PVE and had curative resection of the ipsilateral liver [15]. Using the 2 stage hepatectomy combined with PVE, Jaeck et al., could successfully achieve complete disease clearance in 75.75 % of their patients with colorectal liver metastases [15]. Similarly Adam et al. combined PVE with a 2 stage hepatectomy to improve resectability [72]. PVE is recommended to improve resection rates in patients with borderline liver volumes and this translates into better overall survival.
Portal Vein Ligation
Kianmanesh et al. and others ligated the right portal vein in 20 patients during the first step of the 2-stage hepatectomy and found this to be a safe and effective method of inducing contralateral liver hypertrophy and increase tumour resection rates [44]. Although the morbidity of the procedure in this series was 5 %, all patients in this series underwent resection of their primary along with their left sided liver metastasis and hence the morbidity cannot be solely attributed to PVL. PVL, is intended to achieve contralateral liver hypertrophy in a similar manner to that achieved by PVE. Honjo et al. in 1975, first described this approach of PVL to achieve contralateral liver ypertrophy, in their patients with Hepato Cellular Carcinoma (HCC) [41]. A pubmed search for PVL did not generate a comparable volume of citations as it did for PVE. This is obvious because of the ease of PVE when compared to PVL, which is a formal operative procedure attendant with more risks and greater morbidity when compared to PVE. In all the five papers mentioned in Table 2, patients underwent right portal vein ligation. Portal vein ligation was achieved at initial laparoscopy for staging liver disease [43] or laparotomy during the first stage of an intended 2 stage hepatectomy/resection of the primary tumour [35, 41, 42, 44]. In their experience with portal vein ligation in nine patients, Are at al found the technique to be safe and effective resulting in resections in more than half the patients in the cohort, and advocated its selective use where feasible [43]. When performed during a laparoscopic staging as a part of disease evaluation, it avoids a second procedure for PVE, and hence may be more cost and time effective. In their comparative analysis, Belghiti et al., found right PVL as efficient as right PVE to achieve liver hypertrophy and facilitate resection [42]. PVL in particular, was not associated with a more diffucult operation and did not specifically have greater post-operative morbidity when compared to PVE. Belghiti et al. recommended PVL to be performed during the first stage of a 2- stage hepatectomy or during excision of the primary tumour when a subsequent hepatectomy is planned [42]. However in contrast to the above two reports, Broering et al. reported PVE to be more effective than PVL for induction of liver hypertrophy [39]. This may be due to the fact that PVL achieves a more central occlusion of the portal blood flow against the more peripheral occlusion achieved by PVE. This creates more portal collaterals in the region of the right lobe, which apart from making surgery technically diffucult also may limit liver hypertrophy as it may not obtain complete portal flow diversion to the contralateral FLR [30, 41]. This was also seen in our present analysis wherein although PVL produced greater liver hypertrophy as compared to PVE, it also had a greater number of patients with insufficient hypertrophy as compared to PVE, presumably secondary to increased collateralization, limiting hypertrophy. This was inspite of longer waiting times to surgery in the PVL group. However PVL was found to be safe procedure in this study and there were no significant differences in the operative outcomes after either PVE or PVL [41]. PVL has been its increased length of hospital stay, when performed as an independent isolated procedure. Secondly PVL involves hilar manipulation and dissection, which by the virtue of adhesions created make a future hepatectomy more difficult [17, 30, 35, 59]. Based on the reviews on the subject, one can summarize that PVL is safe and may be carried out during a laparotomy for an intended liver resection or during the first stage of a 2 stage hepatectomy. Hence when the patient has had a laparotomy for a reason, the opportunity may be utilized to ligate the portal vein to achieve contralateral liver hypertrophy. This would prevent a second intervention. However in other circumstances PTHPE by the virtue of it’s inherently “minimally invasive” nature when compared to a laparotomy may be a more sutaible procedure to achieve liver hypertrophy. It is safe, involves a short hospital stay and is unquestionably an effective means of achieving liver hypertrophy, facilitating extended resections.
Conclusions
Review of any literature on PVE/PVL will confirm beyond doubt unequivocal evidence that the volume of the residual liver post-resection, is the single most important prognostic factor that determines recovery following liver resection rather than the volume of the liver removed. This therefore indirectly is expected to improve curative resections which would translate into improved disease free and overall survivals. PVE and PVL, both are useful, safe and perhaps an essential measure to induce liver hypertrophy in selected patients. Increase in the FLR in these patients boosts the chances of oncologically curative (R0) extended hepatectomy and hence improves the overall survival. Where used as an independent modality, PVE is “minimally invasive”, quick, associated with a smaller length of stay and facilitates an easier future hepatectomy due to lack of adhesions and collaterals as compared to PVL. As will be evident from the manuscript, it will never be possible to carry out a trial comparing the two modalities. PVE should be used in the multimodality setting alongside surgery, ablation and chemotherapy. PVL promotes hypertrophy as well and may be used in selected cases during a laparotomy for resection of the primary tumour or during a first stage hepatectomy.
References
- 1.Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13(10):1271–1280. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Zhu S. Present status and future perspectives of preoperative portal vein embolization. Am J Surg. 2009;197(5):686–690. doi: 10.1016/j.amjsurg.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent A, Lemoine A, Bismuth H. Resection of nonresectable liver metastases from colorectal cancerafter percutaneous portal vein embolization. Ann Surg. 2000;231(4):480–486. doi: 10.1097/00000658-200004000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, Sugimachi K. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188(3):304–309. doi: 10.1016/S1072-7515(98)00301-9. [DOI] [PubMed] [Google Scholar]
- 5.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extendedhepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137(6):675–680. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- 6.Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243(3):364–372. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 8.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Camci C, Jabbour N. Management of hepatic metastasis from colorectal cancers: an update. J Hepato-Biliary-Pancreat Surg. 2008;15(6):570–580. doi: 10.1007/s00534-008-1350-x. [DOI] [PubMed] [Google Scholar]
- 10.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236(4):397–406. doi: 10.1097/00000658-200210000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choti MA, Bowman HM, Pitt HA, Sosa JA, Sitzmann JV, Cameron JL, Gordon TA. Should hepatic resections be performed at high-volume referral centers? J Gastrointest Surg. 1998;2(1):11–20. doi: 10.1016/S1091-255X(98)80098-X. [DOI] [PubMed] [Google Scholar]
- 12.Melendez J, Ferri E, Zwillman M, Fischer M, DeMatteo R, Leung D, Jarnagin W, Fong Y, Blumgart LH. Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg. 2001;192(1):47–53. doi: 10.1016/S1072-7515(00)00745-6. [DOI] [PubMed] [Google Scholar]
- 13.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elias D, Ouellet JF, Bellon N, Pignon JP, Pocard M, Lasser P. Extrahepatic disease does not contraindicate hepatectomy for colorectal liver metastases. Br J Surg. 2003;90(5):567–574. doi: 10.1002/bjs.4071. [DOI] [PubMed] [Google Scholar]
- 15.Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240(6):1037–1049. doi: 10.1097/01.sla.0000145965.86383.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rees M, Plant G, Bygrave S. Late results justify resection for multiple hepatic metastases from colorectal cancer. Br J Surg. 1997;84(8):1136–1140. doi: 10.1002/bjs.1800840828. [DOI] [PubMed] [Google Scholar]
- 17.Covey AM, Brown KT, Jarnagin WR, Brody LA, Schwartz L, Tuorto S, Sofocleous CT, D’Angelica M, Getrajdman GI, DeMatteo R, Kemeny NE, Fong Y. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg. 2008;247(3):451–455. doi: 10.1097/SLA.0b013e31815ed693. [DOI] [PubMed] [Google Scholar]
- 18.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghémard O, Levi F, Bismuth H. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–657. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rous P, Larimore LD. Relation of the portal blood flow to liver maintenance: a demonstration of liver atrophy conditional on compression. J Exp Med. 1920;31:609–632. doi: 10.1084/jem.31.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bax HR, Mansens BJ, Schalm L. Atrophy of the liver after occlusion of the bile ducts or portal vein and compensatory hypertrophy of the unoccluded portion and its clinical importance. Gastroenterology. 1956;31(2):131–155. [PubMed] [Google Scholar]
- 21.Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107(5):521–527. [PubMed] [Google Scholar]
- 22.Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10(5):803–808. doi: 10.1007/BF01655244. [DOI] [PubMed] [Google Scholar]
- 23.Lee KC, Kinoshita H, Hirohashi K, Kubo S, Iwasa R. Extension of surgical indications for hepatocellular carcinoma by portal vein embolization. World J Surg. 1993;17(1):109–115. doi: 10.1007/BF01655721. [DOI] [PubMed] [Google Scholar]
- 24.Wakabayashi H, Ishimura K, Okano K, Karasawa Y, Goda F, Maeba T, Maeta H. Application of preoperative portal vein embolization before major hepatic resection in patients with normal or abnormal liver parenchyma. Surgery. 2002;131(1):26–33. doi: 10.1067/msy.2002.118259. [DOI] [PubMed] [Google Scholar]
- 25.Di Stefano DR, de Baere T, Denys A, et al. Preoperative percutaneous portal vein embolization: evaluation of adverse events in 188 patients. Radiology. 2005;234:625–630. doi: 10.1148/radiol.2342031996. [DOI] [PubMed] [Google Scholar]
- 26.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237(2):208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotroneo AR, Innocenti P, Marano G, Legnini M, Iezzi R. Pre-hepatectomy portal vein embolization: single center experience. Eur J Surg Oncol. 2009;35(1):71–78. doi: 10.1016/j.ejso.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Imamura H, Shimada R, Kubota M, Matsuyama Y, Nakayama A, Miyagawa S, Makuuchi M, Kawasaki S. Preoperative portal vein embolization: an audit of 84 patients. Hepatology. 1999;29(4):1099–1105. doi: 10.1002/hep.510290415. [DOI] [PubMed] [Google Scholar]
- 29.de Baere T, Roche A, Elias D, Lasser P, Lagrange C, Bousson V. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology. 1996;24(6):1386–1391. doi: 10.1002/hep.510240612. [DOI] [PubMed] [Google Scholar]
- 30.Giraudo G, Greget M, Oussoultzoglou E, Rosso E, Bachellier P, Jaeck D. Preoperative contralateral portal vein embolization before major hepatic resection is a safe and efficient procedure: a large single institution experience. Surgery. 2008;143(4):476–482. doi: 10.1016/j.surg.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Makuuchi M, Kosuge T, Lygidakis NJ. New possibilities for major liver surgery in patients with Klatskin tumors or primary hepatocellular carcinoma—an old problem revisited. Hepatogastroenterology. 1991;38(4):329–336. [PubMed] [Google Scholar]
- 32.Seo DD, Lee HC, Jang MK, Min HJ, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ, Ko GY, Lee YJ, Lee SG. Preoperative portal vein embolization and surgical resection in patients with hepatocellular carcinoma and small future liver remnant volume: comparison with transarterial chemoembolization. Ann Surg Oncol. 2007;14(12):3501–3509. doi: 10.1245/s10434-007-9553-y. [DOI] [PubMed] [Google Scholar]
- 33.Mueller L, Hillert C, Möller L, Krupski-Berdien G, Rogiers X, Broering DC. Major hepatectomy for colorectal metastases: is preoperative portal occlusion an oncological risk factor? Ann Surg Oncol. 2008;15(7):1908–1917. doi: 10.1245/s10434-008-9925-y. [DOI] [PubMed] [Google Scholar]
- 34.Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects On regeneration, resectability and outcome. Br J Surg. 2007;94(11):1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 35.Capussotti L, Muratore A, Baracchi F, Lelong B, Ferrero A, Regge D, Delpero JR. Portal vein ligation as an efficient method of increasing the future liver remnant volume in the surgical treatment of colorectal metastases. Arch Surg. 2008;143(10):978–982. doi: 10.1001/archsurg.143.10.978. [DOI] [PubMed] [Google Scholar]
- 36.Pamecha V, Glantzounis G, Davies N, Fusai G, Sharma D, Davidson B. Long-term survival and disease recurrence following portal vein embolisation prior to major hepatectomy for colorectal metastases. Ann Surg Oncol. 2009;16(5):1202–1207. doi: 10.1245/s10434-008-0269-4. [DOI] [PubMed] [Google Scholar]
- 37.de Baere T, Teriitehau C, Deschamps F, Catherine L, Rao P, Hakime A, Auperin A, Goere D, Elias D, Hechelhammer L. Predictive factors for hypertrophy of the future remnant liver after selective portal vein embolization. Ann Surg Oncol. 2010;17(8):2081–2089. doi: 10.1245/s10434-010-0979-2. [DOI] [PubMed] [Google Scholar]
- 38.Wicherts DA, de Haas RJ, Andreani P, Sotirov D, Salloum C, Castaing D, Adam R, Azoulay D. Impact of portal vein embolization on long-term survival of patients with primarily unresectable colorectal liver metastases. Br J Surg. 2010;97(2):240–250. doi: 10.1002/bjs.6756. [DOI] [PubMed] [Google Scholar]
- 39.Ratti F, Soldati C, Catena M, Paganelli M, Ferla G, Aldrighetti L. Role of portal vein embolization in liver surgery: single centre experience in sixty-two patients. Updates Surg. 2010;62(3-4):153–159. doi: 10.1007/s13304-010-0033-8. [DOI] [PubMed] [Google Scholar]
- 40.Yoo H, Ko GY, Gwon DI, Kim JH, Yoon HK, Sung KB, Kim N, Lee J. Preoperative portal vein embolization using an amplatzer vascular plug. Eur Radiol. 2009;19(5):1054–1061. doi: 10.1007/s00330-008-1240-2. [DOI] [PubMed] [Google Scholar]
- 41.Broering DC, Hillert C, Krupski G, Fischer L, Mueller L, Achilles EG, Schulte am Esch J, Rogiers X. Portal vein embolization vs. portal vein ligation for induction of hypertrophy of the future liver remnant. J Gastrointest Surg. 2002;6(6):905–913. doi: 10.1016/S1091-255X(02)00122-1. [DOI] [PubMed] [Google Scholar]
- 42.Aussilhou B, Lesurtel M, Sauvanet A, Farges O, Dokmak S, Goasguen N, Sibert A, Vilgrain V, Belghiti J. Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg. 2008;12(2):297–303. doi: 10.1007/s11605-007-0410-x. [DOI] [PubMed] [Google Scholar]
- 43.Are C, Iacovitti S, Prete F, Crafa FM. Feasibility of laparoscopic portal vein ligation prior to major hepatectomy. HPB (Oxford) 2008;10(4):229–233. doi: 10.1080/13651820802175261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kianmanesh R, Farges O, Abdalla EK, Sauvanet A, Ruszniewski P, Belghiti J. Right portal vein ligation: a new planned two-step all-surgical approach for complete resection of primary gastrointestinal tumors with multiple bilateral liver metastases. J Am Coll Surg. 2003;197(1):164–170. doi: 10.1016/S1072-7515(03)00334-X. [DOI] [PubMed] [Google Scholar]
- 45.Tsuge H, Mimura H, Kawata N, Orita K. Right portal embolization Before extended right hepatectomy using laparoscopic catheterization of the ileocolic vein: a prospective study. Surg Laparosc Endosc. 1994;4(4):258–263. [PubMed] [Google Scholar]
- 46.Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta analysis. Ann Surg. 2008;247(1):49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 47.Perarnau JM, Daradkeh S, Johann M, Deneuville M, Weinling P, Coniel C. Transjugular preoperative portal embolization (TJPE) a pilot study. Hepatogastroenterology. 2003;50(51):610–613. [PubMed] [Google Scholar]
- 48.Madoff DC, Hicks ME, Vauthey JN, Charnsangavej C, Morello FA, Jr, Ahrar K, Wallace MJ, Gupta S. Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics. 2002;22(5):1063–1076. doi: 10.1148/radiographics.22.5.g02se161063. [DOI] [PubMed] [Google Scholar]
- 49.Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF, Caridi J. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127(5):512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 50.Madoff DC, Abdalla EK, Gupta S, Wu TT, Morris JS, Denys A, Wallace MJ, Morello FA, Jr, Ahrar K, Murthy R, Lunagomez S, Hicks ME, Vauthey JN. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16:215–225. doi: 10.1097/01.RVI.0000147067.79223.85. [DOI] [PubMed] [Google Scholar]
- 51.Capussotti L, Muratore A, Ferrero A, Anselmetti GC, Corgnier A, Regge D. Extension of right portal vein embolization to segment IV portal branches. Arch Surg. 2005;140(11):1100–1103. doi: 10.1001/archsurg.140.11.1100. [DOI] [PubMed] [Google Scholar]
- 52.Yokoyama Y, Nagino M, Nimura Y. Mechanisms of hepatic regeneration Following portal vein embolization and partial hepatectomy: a review. World J Surg. 2007;31(2):367–374. doi: 10.1007/s00268-006-0526-2. [DOI] [PubMed] [Google Scholar]
- 53.Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L, Ernst O, Pruvot FR. Remnant liver volume to body weight ratio > or =0.5%: A new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg. 2007;204(1):22–33. doi: 10.1016/j.jamcollsurg.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Chun YS, Ribero D, Abdalla EK, Madoff DC, Mortenson MM, Wei SH, Vauthey JN. Comparison of two methods of future liver remnant volume measurement. J Gastrointest Surg. 2008;12(1):123–128. doi: 10.1007/s11605-007-0323-8. [DOI] [PubMed] [Google Scholar]
- 55.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191(1):38–46. doi: 10.1016/S1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 56.Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88(2):165–175. doi: 10.1046/j.1365-2168.2001.01658.x. [DOI] [PubMed] [Google Scholar]
- 57.Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26(5):1176–1181. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 58.Kokudo N, Makuuchi M. Current role of portal vein embolization/hepatic artery chemoembolization. Surg Clin N Am. 2004;84(2):643–657. doi: 10.1016/j.suc.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Hemming AW, Reed AI, Howard RJ, Fujita S, Hochwald SN, Caridi JG, Hawkins IF, Vauthey JN. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237(5):686–691. doi: 10.1097/01.SLA.0000065265.16728.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagino M, Nimura Y, Kamiya J, Kondo S, Uesaka K, Kin Y, Hayakawa N, Yamamoto H. Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology. 1995;21(2):434–439. [PubMed] [Google Scholar]
- 61.Elias D, Debaere T, Roche A, Bonvallot S, Lasser P. Preoperative selective portal vein embolizations are an effective means of extending the indications of major hepatectomy in the normal and injured liver. Hepatogastroenterology. 1998;45(19):170–177. [PubMed] [Google Scholar]
- 62.Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine A, Emile JF, Bismuth H. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232(5):665–672. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oussoultzoglou E, Bachellier P, Rosso E, Scurtu R, Lucescu I, Greget M, Jaeck D. Right portal vein embolization before right hepatectomy for unilobar colorectal liver metastases reduces the intrahepatic recurrence rate. Ann Surg. 2006;244(1):71–79. doi: 10.1097/01.sla.0000217609.26178.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Baere T, Denys A, Madoff DC. Preoperative portal vein embolization: indications and technical considerations. Tech Vasc Interv Radiol. 2007;10(1):67–78. doi: 10.1053/j.tvir.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 65.Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94(11):1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 66.Elias D, De Baere T, Roche A, Mducreux, Leclere J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86(6):784–788. doi: 10.1046/j.1365-2168.1999.01154.x. [DOI] [PubMed] [Google Scholar]
- 67.Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, Ohta K, Yamaguchi T, Matsubara T, Takahashi T, Nakajima T, Muto T, Ikari T, Yanagisawa A, Kato Y. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34(2):267–272. doi: 10.1053/jhep.2001.26513. [DOI] [PubMed] [Google Scholar]
- 68.Boerma EJ. Research into the results of resection of hilar bile duct cancer. Surgery. 1990;108(3):572–580. [PubMed] [Google Scholar]
- 69.Weitz J, Koch M, Kienle P, Schrödel A, Willeke F, Benner A, Lehnert T, Herfarth C, von Knebel Doeberitz M. Detection of hematogenic tumor cell dissemination in patients undergoing resection of liver metastases of colorectal cancer. Ann Surg. 2000;232(1):66–72. doi: 10.1097/00000658-200007000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koch M, Kienle P, Hinz U, Antolovic D, Schmidt J, Herfarth C, von Knebel Doeberitz M, Weitz J. Detection of hematogenous tumor cell dissemination Predicts tumor relapse in patients undergoing surgical resection of colorectal liver metastases. Ann Surg. 2005;241(2):199–205. doi: 10.1097/01.sla.0000151795.15068.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kienle P, Koch M, Autschbach F, Benner A, Treiber M, Wannenmacher M, von Knebel Doeberitz M, Büchler M, Herfarth C, Weitz J. Decreased detection rate of disseminated tumor cells of rectal cancer patients after preoperative chemoradiation: a first step towards a molecular surrogate marker for neoadjuvant treatment in colorectal cancer. Ann Surg. 2003;238(3):324–330. doi: 10.1097/01.sla.0000086547.27615.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232(6):777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]