Abstract
COX-2 regulates tumour growth, invasion and metastasis in breast cancer. This study investigated the association between COX-2 expression in human breast cancer versus the expression of ER, PR, HER-2/neu, as well as its association with other established prognostic indicators like age, menopausal status, tumour size, lymph nodal status, stage, grade, NPI and histological subtype, and aims to validate the role of overexpression of COX-2 as a prognostic marker in patients with breast cancer in Indian subcontinent. In this hospital based study of 123 breast cancer patients (Group-A) and 76 female patients with benign breast disease (Group-B) attending a Comprehensive Breast Clinic at a reputed institute in Eastern India, COX-2 protein expression was measured from breast tissue using the Western Blot Technique. COX-2 mRNA expression was measured by RT-PCR Technique. ER, PR and HER-2/neu status was measured by immunohistochemistry methods. COX-2 was not expressed in the control group. The proportion of COX-2 positive tumours was significantly higher in patients of age >50 years [52(91.2 %), p < 0.01], postmenopausal status [64(90.1 %), p < 0.01], advanced stage of disease (p < 0.01), higher grade (p < 0.01), larger tumors (p < 0.01), metastatic lymph nodes (p < 0.01) and NPI ≥ 5.4 (p < 0.01). COX-2 expression was seen in ER-negative [66(95.7 %), p < 0.01], PR-negative [76(92.7 %), p < 0.01], and HER-2/neu positive tumours [29(100.0 %), p < 0.01]. Risk of COX-2 positivity was found to be 2.74 times more for postmenopausal status, 6.90 times more for large size tumours (≥ 2.5), 34.37 times more for node positive tumours, 9.26 times more with ER negative patients and 5.88 times more for PR negative patients. COX-2 expression is associated with established indicators of poor prognosis such as postmenopausal status, age >50 year, advanced stage of disease, large tumour size, higher grade, lymph node metastasis, NPI ≥ 5.4, ER negativity, PR negativity and HER-2/neu positivity. Thus, COX-2 expression implies aggressive tumour biology, and may play an important role as a prognostic marker.
Keywords: Breast cancer, COX-2, Prognostic marker, Nottingham Prognostic Index (NPI), Immunohistochemistry (IHC)
Introduction
The worldwide incidence of breast cancer has increased rapidly in recent years. The scenario in Eastern India is also showing a similar trend [1, 2]. The prognosis of breast cancer depends on various biological and molecular factors [3, 4]. Cyclooxygenase (COX) group of enzymes are important for the conversion of arachidonic acid to prostaglandins. Cyclooxygenase-1 (COX-1) is constitutively expressed at a constant level throughout the cell cycle in most of the tissues. The inducible isoform, Cyclooxygenase-2 (COX-2), is often overexpressed in breast cancer [5]. Various research articles suggest that COX-2-derived metabolites may contribute to maintenance of tumour viability, premalignant hyper proliferation, tumour growth, transformation, invasion and metastatic spread [2, 6, 7] and COX-2 has been shown to be overexpressed in many human malignant tumours[8]. Prostaglandins increase the expression and activation of aromatase [9], an enzyme that coverts androgen to estrogen. Estrogen can stimulate the growth of cancer cells via activation of the estrogen receptor (ER) and its target genes. This is one mechanism by which COX-2 could stimulate breast tumour growth and angiogenesis [7, 10]. COX-2 expression is itself up-regulated by ER via modulation of Activated Protein – 1 (AP-1) activity [11]. In breast cancer the human epidermal growth factor receptor type 2 (HER-2) is overexpressed in 20–30 % of tumours due to amplification of the HER-2/neu gene [12]. HER-2/neu acts via Mitogen-activated protein kinase (MAPK) to stimulate COX-2 expression in colorectal cancer cells [13] and can stimulate COX-2 expression in the same way when transfected into breast cancer cells [11, 14]. Stimulation of COX-2 expression in ER-negative breast cells may occur via Protein kinase C (PKC) and/or RAS/MAPK pathways [7]. COX-2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer [15]. Estrogen plays an important role in breast cancer initiation and progression. Breast cancer over time acquires different mutations and the proportion of estrogen receptor negative cells in tumour increases. This transformation confers aggressive biological characteristics to breast cancer such as rapid growth, poor differentiation, and poor response to hormone therapy. Nottingham Prognostic Index (NPI) is a good prognostic marker and correlates well with survival in clinical practice.
This study investigated the association between COX-2 expression in human breast cancer versus the expression of ER, PR, HER-2/neu, as well as its association with other established prognostic indicators like age, menopausal status, tumour size, lymph nodal status, stage, grade, NPI and histological subtype, and also aims to validate the role of overexpression of COX-2 as a prognostic marker in patients with breast cancer in Indian subcontinent.
Materials and Methods
Patient Selection
This was a prospective study. The patients were divided into two groups. The first group (Group-A) comprised of 123 female patients with primary breast carcinoma previous untreated by chemotherapy, radiotherapy, hormone therapy or a combination of any of the modalities who presented to the Comprehensive Breast Clinic & Breast Cancer Research Unit, IPGME&R/SSKM Hospital, Kolkata, West Bengal, India between 2010 and 2012. The control group (Group-B) comprised of 76 female patients with fibro adenoma or benign breast disease (clinically diagnosed and thereafter, proven histologically).
Tissue Processing
The specimens were washed with phosphate buffered saline (PBS), cut into small pieces and immersed in collagenase at 37 °C for 4–6 h. Collagenase incubated tissue was minced and treated with 0.125 % trypsin-EDTA for 10 min. Total protein was extracted by homogenizing cells in ripa : lysis buffer mixture (1:3) at 4 °C and measured spectrophotometrically by Lowry’s method.
Immunoblotting
Cells were lysed in buffer (10 mM Hepes, pH 7.9, 1.5 mM MgCl2, 10 mM KCl and 0.5 mM dithiothreitol) and spun at 3,300 g to get cytosolic fraction. Pellet was resuspended in buffer (20 mM Hepes, pH 7.9, 0.4 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA and 0.5 mM dithiothreitol) and spun down at 12 000 g for 30 min to get nuclear fraction. For whole cell lysates, cells were resuspended and homogenized in buffer (100 mM Tris-Cl, pH 7.4, 300 mM NaCl, 1 % NP-40 and 0.25 % sodium-deoxycholate). All the buffers were supplemented with protease and phosphatase inhibitor mixtures. For direct western blot analysis, the cell lysates or the particular fractions were separated by SDS polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane and probed with specific antibodies, for example, -COX-2 produced from Santa Cruz (Santa Cruz, CA, USA), thereafter the immunoblots were visualized by chemiluminescence. Equal protein loading was confirmed with α-actin antibody (Santa Cruz).
RT–PCR Assay
Two mg of the total RNA, extracted from cells with TRIzol reagent (Invitrogen, Carlsbad, Carlsbard, CA, USA) was reverse transcribed and then subjected to PCR with enzymes and reagents of the RTplusPCR system (Eppendorf, Hamburg,Germany) using GeneAmpPCR 2720 (Applied Biosystems; Foster City, CA, USA). The cDNAs were amplified with primers specific for COX-2 (5′-TGA TCGAAGACTACGTGCAACA-3′/5′-GCG GATGCCAGTGATAGAGTG-3′), and GAPDH (internal control): (5′-CA-GAACATCATCCCTGCCTCT-3′/5′-GCTTGACAAAGTGGTCGTTGAG-3′).
Histology and Immunohistochemistry
Breast carcinoma tumours were fixed in 10 % neutral-buffered formalin for 24 h, and the tumour size was measured. The tumour was then embedded in paraffin, sectioned, following which the lymph nodal status and grade was determined. For immunohistochemistry, paraffin sections of tumours were deparaffinized and hydrated by successive washes with xylene, 100 % ethanol, and a phosphate buffer [10 mM],(pH 7.4) and 0.138 M saline containing 2.7 mM KCl. Antigen retrieval was accomplished with diluted antigen retrieval buffer (DAKO Corp.) Endogenous peroxidase was blocked with 3 % hydrogen peroxide. Subsequently, slides were washed in PBS/KCl, incubated with 10 % normal horse serum followed by the primary antibody (rabbit anti-ER antibody or rabbit anti-PR antibody rabbit anti-c-erbB2; HER-2/neu) and incubated overnight at 4 °C. The slides were then incubated with biotinylated secondary antibody for 45 min, followed by ABC reagent and diaminobenzidine. Counterstaining was done with hematoxylin. Sections were dehydrated by washing sequentially with 95 % ethanol, 100 % ethanol, and xylene. Coverslips were mounted on slides using Paramount. Digital images of stained and unstained cells were obtained using an Olympus microscope equipped with a SPOT digital camera [1].
Statistical Analysis
Statistical Analysis was performed with help of Epi Info (TM) 3.5.3. EPI INFO is a trademark of the Centers for Disease Control and Prevention (CDC). Using this software, basic cross-tabulation, inferences and associations were performed. Chi-square test was used to test the association of different study variables with the expression of COX-2. Test of proportions was used to test the significant difference between two proportions and corresponding standard normal deviate (Z-values) were calculated with corresponding p-values. Odds ratio (OR) with 95 % Confidence Interval (CI) was calculated to measure the different risk factors under univariate analysis. Under multivariate analysis Logistic Regression was used to find the risk factors. p < 0.05 was considered statistically significant.
Results and Analysis
COX-2 was not expressed in the control group by either western blot or RT-PCR technique. The mean age (mean ± s.e) of the patients was 50.78 ± 8.81 years with range 37–75 years and the median age was 50 years.
As per Table 1, test of proportion showed that there was no significant difference in COX-2 expression for Stage-I tumours, small size (0 – 1.99 cm) and a NPI <5.4. Similarly, histology of lobular carcinoma and ductal carcinoma in situ also did not show any significant difference in COX-2 expression. For all other variables proportions of COX-2 positive cases were significantly higher than COX-2 negative cases. The proportion of COX-2 positive tumours in postmenopausal patients [64(90.1 %)] was significantly higher than in the pre-menopausal group [40 (76.9 %)], (p < 0.01). Likewise, the proportion of COX-2 positive tumours in patients of age >50 years [52(91.2 %)] was significantly higher than those of age ≤50 years [52 (78.8 %)], (p < 0.01). 8 (66.7 %) patients with stage I, 13 (81.3 %) patients with stage II, 77 (87.5 %) patients with stage III and 6(85.7 %) patients with stage IV disease had COX-2 positive tumours. The histological grades were measured by the Modified Bloom-Richardson Grading Scheme. In grade I, 2 out of 9 patients (22.2 %), in grade II 17 out of 23 patients (73.9 %), in grade III 85 out of 91 patients (93.4 %) had COX-2 positive tumours and this was statistically significantly higher than the COX-2 negative tumours. 96.3 % (52 out of 54) of large (≥4 cm) tumours, 75.8 % (47 out of 62) of middle size (2 – 3.99 cm) tumours, and 71.4 % (5 out of 7) small (<2 cm) tumours were COX-2 positive. 4 patients with no lymph node metastasis(26.7 %), 35 patients with 1–3 metastatic lymph nodes (81.4 %), 47 patients with 4–9 metastatic lymph nodes (100.0 %) and 18 patients with metastasis in >9 lymph nodes (100.0 %) were found to have COX-2 positive tumours and this was statistically significant. COX-2 expression was seen in 86 patients with a NPI ≥ 5.4 (100.0 %) as compared to 18 patients with a NPI <5.4 (48.6 %). For tumours with a NPI ≥ 5.4, the difference between COX-2 positive and negative tumours was statistically significant (p < 0.01).
 |
Table 1.
Clinicopathological details according to COX-2 status
| Table-1 | COX-2 positive | COX-2 negative | Z-value | p-value | |||
|---|---|---|---|---|---|---|---|
| No of patients | Percentage (%) | No of patients | Percentage (%) | ||||
| Menopausal Status | Premenopausal | 40 | 76.9 | 12 | 23.1 | 5.49 | <0.01* |
| Postmenopausal | 64 | 90.1 | 7 | 9.9 | 9.56 | <0.01* | |
| Age (years) | ≤50 | 52 | 78.8 | 14 | 21.1 | 6.61 | <0.01* |
| >50 | 52 | 91.2 | 5 | 8.8 | 8.80 | <0.01* | |
| Stage | I | 8 | 66.7 | 4 | 33.3 | 1.63 | >0.05 |
| II | 13 | 81.3 | 3 | 18.8 | 3.53 | <0.01* | |
| III | 77 | 87.5 | 11 | 12.5 | 9.94 | <0.01* | |
| IV | 6 | 85.7 | 1 | 14.3 | 2.67 | <0.01* | |
| Tumor size (cm) | 0 – 1.99 | 5 | 71.4 | 2 | 28.6 | 1.60 | >0.05 |
| 2 – 3.99 | 47 | 75.8 | 15 | 24.2 | 5.74 | <0.01* | |
| ≥ 4 | 52 | 96.3 | 2 | 3.7 | 9.62 | <0.01* | |
| Grade | I | 2 | 22.2 | 7 | 77.8 | 2.35 | <0.05* |
| II | 17 | 73.9 | 6 | 26.1 | 3.24 | <0.01* | |
| III | 85 | 93.4 | 6 | 6.6 | 11.71 | <0.01* | |
| Lymph node metastasis | No Node | 4 | 26.7 | 11 | 73.3 | 2.55 | <0.05* |
| 1 – 3 Node | 35 | 81.4 | 8 | 18.6 | 5.82 | <0.01* | |
| 4 – 9 Node | 47 | 100.0 | 0 | 0.0 | 9.69 | <0.01* | |
| 10 – More Node | 18 | 100.0 | 0 | 0.0 | 6.00 | <0.01* | |
| NPI | < 5.4 | 18 | 48.6 | 19 | 51.4 | 0.23 | >0.05 |
| ≥ 5.4 | 86 | 100.0 | 0 | 0.0 | 13.11 | <0.01* | |
| ER | Positive | 38 | 70.4 | 16 | 29.6 | 4.23 | <0.01* |
| Negative | 66 | 95.7 | 3 | 4.3 | 10.72 | <0.01* | |
| PR | Positive | 28 | 68.3 | 13 | 31. 7 | 3.31 | <0.01* |
| Negative | 76 | 92.7 | 6 | 7.3 | 10.93 | <0.01* | |
| HER-2/neu | Positive | 29 | 100.0 | 0 | 0.0 | 7.61 | <0.01* |
| Negative | 75 | 79.8 | 19 | 20.2 | 8.16 | <0.01* | |
| Histology type | IDC | 83 | 92.2 | 7 | 7.8 | 11.32 | <0.01* |
| LC | 12 | 63.2 | 7 | 36.8 | 1.62 | >0.05 | |
| DCIS | 9 | 64.3 | 5 | 35.7 | 1.51 | >0.05 | |
DCIS ductal carcinoma in situ, IDC invasive ductal carcinoma, LC lobular carcinoma
* Statistically significant
COX-2 expression was more common in ER-negative tumours 66(95.7 %) than ER-positive tumours 38(70.4 %) which was statistically significant (p < 0.01). COX-2 expression was more common in PR-negative tumours 76(92.7 %) than PR-positive tumours 28(68.3 %) and this difference was statistically significant (p < 0.01). COX-2 expression was seen more frequently in HER-2/neu positive tumours 29(100.0 %) compared with HER-2/neu negative tumours 75(79.8 %) and this was statistically significant (p < 0.01). According to histological type, 83(92.2 %) patients with invasive ductal carcinoma, 12(63.2 %) patients with lobular carcinoma and 9(64.3 %) patients with ductal carcinoma in situ were COX-2 positive.
As per Table 2, the risk of COX-2 positivity was 2.74[OR-2.74(1.00, 7.54); p = 0.04] times more for postmenopausal patients, 6.90[OR-6.90(2.33,20–37); p < 0.001] times more for large tumours size(>2.5 cm), 34.37[OR-34.37(8.89,132.88);p < 0.001] times more for node positive tumours, 9.26 [OR-9.26(2.53, 33.85); p < 0.001] for ER negative tumours and 5.88[OR-5.88(2.03, 16.97); p < 0.001] times more for PR negative tumours and the risks were statistically significant. The risk of COX-2 positivity was 2.80 [OR-2.80(0.94, 8.33); p = 0.06] times more for the patients having higher age (>50 years) compared to the patients having lower age (≤50 years) and 2.30[OR-2.30(0.81, 6.57); p = 0.11] times more for advanced stage but the risks were not significant.
Table 2.
Different risk factors under univariate analysis according to COX-2 status
| Table-2 | COX-2 positive | COX-2 negative | Odds ratio (OR) with 95 % CI and p-values | |||
|---|---|---|---|---|---|---|
| No of patients | Percentage (%) | No of patients | Percentage (%) | |||
| Menopausal status | Premenopausal | 40 | 76.9 | 12 | 23.1 | 2.74(1.00,7.54); p = 0.04* |
| Postmenopausal | 64 | 90.1 | 7 | 9.9 | ||
| Age (years) | ≤50 | 52 | 78.8 | 14 | 21.1 | 2.80(0.94, 8.33); p = 0.06 |
| >50 | 52 | 91.2 | 5 | 8.8 | ||
| Stage | Early (I, II) | 21 | 75.0 | 7 | 25 | 2.30(0.81, 6.57); p = 0.11 |
| Advance (III, IV) | 83 | 87.4 | 12 | 12.6 | ||
| Tumor size (cm) | ≤ 2.5 | 12 | 57.1 | 9 | 42.9 | 6.90(2.33, 20.37); p < 0.001* |
| >2.5 | 92 | 90.2 | 10 | 9.8 | ||
| Lymph node metastasis | No Node | 4 | 26.7 | 11 | 73.3 | 34.37(8.89, 132.88); p < 0.001* |
| Node Positive | 100 | 92.6 | 8 | 7.4 | ||
| ER | Positive | 38 | 70.4 | 16 | 29.6 | 9.26(2.53, 33.85); p < 0.001* |
| Negative | 66 | 95. 7 | 3 | 4.3 | ||
| PR | Positive | 28 | 68.3 | 13 | 31. 7 | 5.88(2.03, 16.97); p < 0.001* |
| Negative | 76 | 92.7 | 6 | 7.3 | ||
* Statistically significant
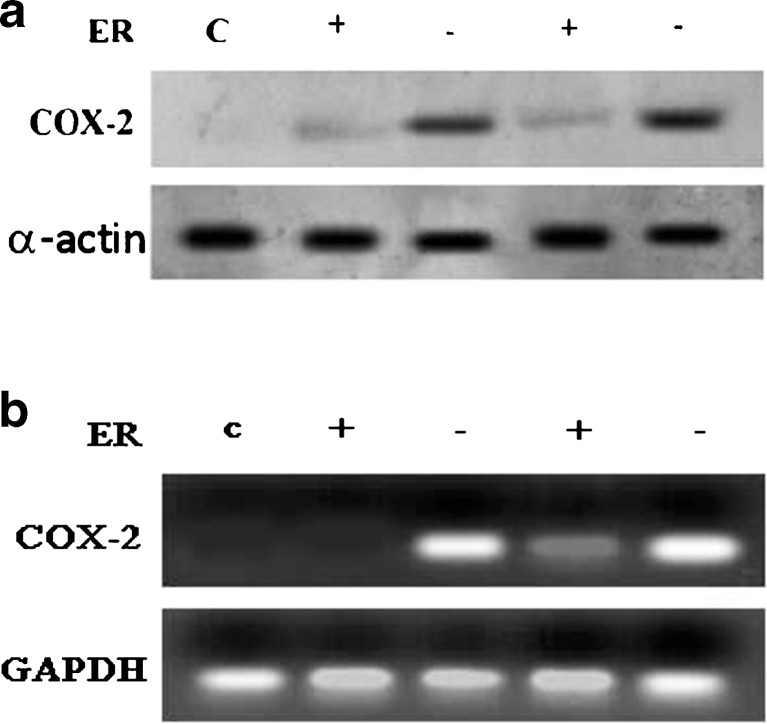
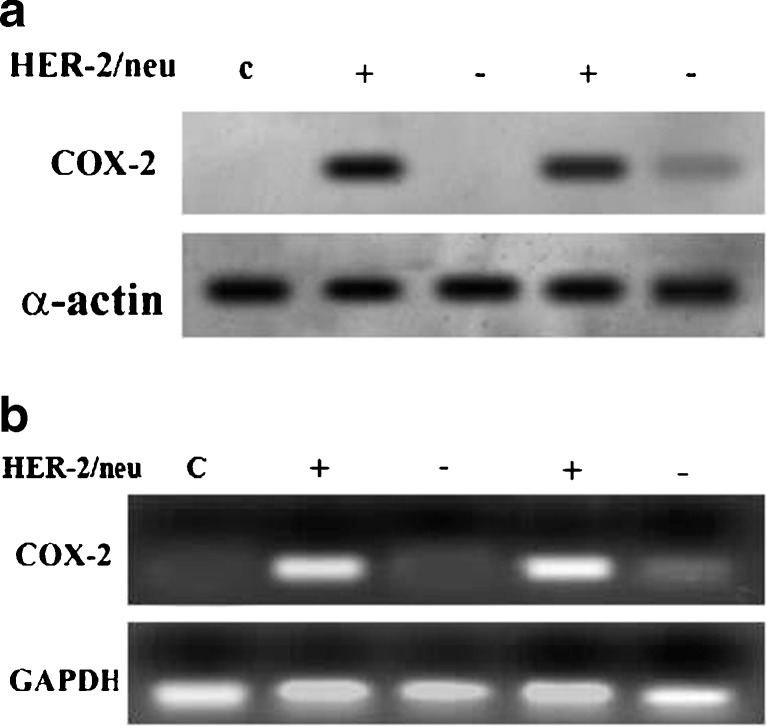
Figure 1a, represent that COX-2 was expressed more in ER negative tumours as compared to ER positive tumours as determined by western blot. Figure 1b, represents that COX-2 was expressed more in ER negative tumours as compared to ER positive tumours as determined by RT-PCR technique. 2A, represents that COX-2 was expressed more in HER-2/neu positive tumours as compared to HER-2/neu negative tumour as determined by western blot method. 2B, represents that COX-2 was expressed more in HER-2/neu positive tumours as compared to HER-2/neu negative tumour as determined by RT-PCR technique.
Fig. 1.
a. represents that COX-2 was expressed more in ER negative tumours as compared to ER positive tumours as determined by western blot method. b. represents that COX-2 was expressed more in ER negative tumours as compared to ER positive tumours as determined by RT-PCR technique
Discussion
In our study, COX-2 was activated in 84.55 % cases of human breast carcinoma as analyzed by western blotting and RT-PCR, where as COX-2 was undetectable in the control group.
COX-2 has been shown to be expressed in both ductal carcinoma in situ and invasive ductal carcinoma, but not in normal breast tissue in several research articles [16–18]. We also saw COX-2 expression in invasive ductal carcinoma, lobular carcinoma and ductal carcinoma in situ but not in fibroadenoma and benign breast disease (Figs. 1 and 2). In this study, COX-2 expression was seen more frequently in postmenopausal patients.
Fig. 2.
a. represents that COX-2 was expressed more in HER-2/neu positive tumours as compared to HER-2/neu negative tumour as determined by western blot method. b. represents that COX-2 was expressed more in HER-2/neu positive tumours as compared to HER-2/neu negative tumour as determined by RT-PCR technique
Many studies have demonstrated that COX-2 expression was significantly correlated with large tumour size and advanced stage of disease [19–22]. It has been reported that elevated COX-2 expression was more common in tumours with axillary lymph node metastasis and a larger size [15, 17, 23]. We showed that COX-2 was expressed in advanced stage compare to early breast carcinoma. In this study, activation of COX-2 significantly correlated with large size and high grade tumours. Like the western data, this study shows COX-2 expression to be more frequent in patients with lymph node involvement.
Various studies reported that COX-2 expression was correlated with ER negative [23], PR negative and HER-2/neu positive status [24]. HER-2/neu is over expressed in approximately 20–30 % of invasive breast cancers and is an independent marker of poor prognosis [25]. We found that high levels of COX-2 expression correlated with HER-2⁄neu overexpression and also correlated with absence of ER and PR expression. COX-2 expression in ER negative cell lines is also associated with mutated RAS. Increased expression of this protein has been associated with reduced estrogen dependence in breast cells [26]. Both PKC [27] and mutated RAS [28] have been associated with an increased metastatic potential in cell lines. ER positive tumours were associated with a good prognosis compared to ER negative tumours. We observed that COX-2 was expressed in HER-2/neu positive tumours.
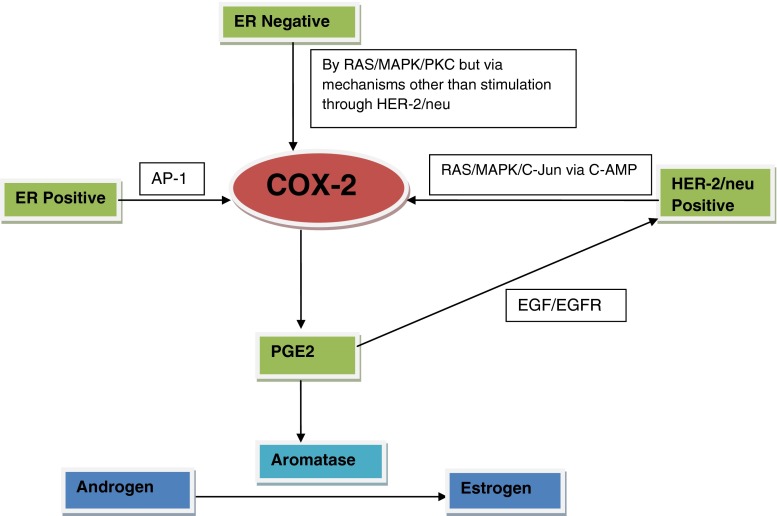
NPI is already an accepted prognostic marker in breast cancer in the western population. Andre Albergaria et al. found that NPI is a good predictor of survival in breast cancer [29]. NPI is a reliable index to predict overall survival of breast cancer patients over 5 years [30]. NPI < 5.4 is associated with good prognosis (about 70 % survival over 10 years) while NPI ≥ 5.4 has a less than 50 % 10-year survival rate. We observed that COX-2 was expressed patients with a high NPI, suggestive of a poor prognosis. The induction of COX-2 by HER-2/neu was mediated by the RAS pathway. RAS can regulate gene expression by stimulating MAPK activities [31]. HER-2/neu and COX-2 expression could be stimulated by PKC [32] and MAPK/C-Jun via the c-AMP response element [33]. We may conclude that the high level of COX-2 in breast cancer is due to overexpression of HER-2/neu through RAS/MAPK/C-Jun via C-AMP pathways (Fig. 3). COX-2 can stimulate HER-2/neu expression via EGFR through PGE2. So COX-2 mediates variety of cellular processes including tumour growth, apoptosis, differentiation, cell cycle, lymph node metastasis and angiogenesis. COX-2 overexpression correlates with aggressive phenotypic features, such as high grade, large tumour size, lymph node metastasis, high NPI, HER-2/neu overexpression and ER negative status in breast cancer patients. It can be hypothesized that COX-2 expression may play an important role as a biomarker for estimating tumour aggressiveness in clinical practice. If COX-2 expression is an early initiating event in the development of breast cancer, novel therapeutic strategies that inhibit its aberrant expression or function may well have a major role in the prevention of human breast cancer.
Fig. 3.
A model for ER, COX-2 and HER-2/neu interactions. ER regulates gene expression through protein-protein interactions with other transcription factors, e.g. activator protein1 (AP-1) and then COX-2 expressed through Ras, Raf, MAPK pathway. Our study demonstrates that COX-2 can stimulate HER-2/neu expression via EGFR through the major role of PGE-2
Conclusion
COX-2 expression was seen to be associated with parameters that indicate poor prognosis in breast cancer such as postmenopausal status, age >50 year, advanced stage of disease, large tumour size, higher grade, lymph node metastasis and a NPI ≥ 5.4. Similarly, COX-2 was expressed in ER-, PR- and HER-2/neu + tumours. This indicates that COX-2 expression is associated with aggressive tumour biology, and can act as a predictor of tumours with a poor prognosis. As a result, inhibition of COX-2 expression may decrease tumour progression and block breast carcinogenesis, which may have implications in prevention of breast cancer in patients at high risk.
References
- 1.Jana D, Das S, Sarkar DK, Mandal S, Maji A, Mukhopadhyay M. Role of nuclear factor-κB in female breast cancer: a study in Indian patients. Asian Pac J Cancer Prev. 2012;13:5511–5515. doi: 10.7314/APJCP.2012.13.11.5511. [DOI] [PubMed] [Google Scholar]
- 2.Jana D, Sarkar DK, Maji A, Chikkala BR, Hassanujjaman S, Mukhopadhyay M, et al. Can cyclo-oxygenase-2 be a useful prognostic and risk stratification marker in breast cancer? J Indian Med Assoc. 2012;110:429–433. [PubMed] [Google Scholar]
- 3.Jana D, Mandal S, Mukhopadhyay M, Mitra D, Mukhopadhyay SK, Sarkar DK. Prognostic signifcance of HER-2/neu and survival of breast cancer patients attending a specialized breast clinic in Kolkata, Eastern India. Asian Pac J Cancer Prev. 2012;13:3851–3855. doi: 10.7314/APJCP.2012.13.8.3851. [DOI] [PubMed] [Google Scholar]
- 4.Sarkar DK, Jana D, Patil PS, Chaudhari KS, Chattopadhyay BK, Chikkala BR, et al. Role of NF-κB as a prognostic marker in breast cancer : a pilot study in Indian patients. Indian J Surg Oncol. 2013;13:0234–0238. doi: 10.1007/s13193-013-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowa LW-C, Yip AY-S, Loo WT-Y, Lam C-K, Toi M. Celecoxib anti-aromatase neoadjuvant (CAAN) trial for locally advanced breast cancer. J Steroid Biochem Mol Biol. 2008;111:13–17. doi: 10.1016/j.jsbmb.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Witton CJ, Hawe SJK, Cooke TG, Bartlett JMS. Cyclooxygenase 2 (COX2) expression is associated with poor outcome in ER-negative, but not ER-positive, breast cancer. Histopathology. 2004;45:47–54. doi: 10.1111/j.1365-2559.2004.01898.x. [DOI] [PubMed] [Google Scholar]
- 7.Williams CS, Mann M, Dubois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 8.Boland GP, Butt IS, Prasad R, Knox WF, Bundred NJ. COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer. 2004;90:423–429. doi: 10.1038/sj.bjc.6601534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Estrogen biosynthesis proximal to a breast tumour is stimulated by PGE(2) via cyclic AMP, reading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996;137:5739–5742. doi: 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- 10.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, Dubois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/S0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 11.Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ. Cyclooxygenase- 2 is overexpressed in HER-2 ⁄ neu-positive breast cancer—evidence for involvement of AP-1 and PEA3. J Biol Chem. 2002;277:18649–18657. doi: 10.1074/jbc.M111415200. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett JMS, Mallon EA, Cooke TG. The clinical evaluation of HER2 status, which test to use? J Pathol. 2003;99:411–417. doi: 10.1002/path.1354. [DOI] [PubMed] [Google Scholar]
- 13.Vadlamudi R, Mandal M, Adam L, Steibach G, Mendelsohn J, Kumar R. Regulation of cyclooxygenase-2 pathway by HER2 receptor. Oncogene. 1999;18:305–314. doi: 10.1038/sj.onc.1202307. [DOI] [PubMed] [Google Scholar]
- 14.Half E, Tang XM, Gwyn K, Sahin A, Wathen K, Sinicrope FA. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002;62:1676–1681. [PubMed] [Google Scholar]
- 15.Costa C, Soares R, Reis-Filho JS, Leitão D, Amendoeira I, Schmitt FC. Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol. 2002;55:429–434. doi: 10.1136/jcp.55.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciris IM, Bozkurt KK, Baspinar S, Kapucuoglu FN. Immunohistochemical COX-2 overexpression correlates with HER-2/neu overexpression in invasive breast carcinomas: a pilot study. Pathol Res Pract. 2011;207:182–187. doi: 10.1016/j.prp.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Ristimaki A, Sivula A, Lundin J, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- 18.Perrone G, Santini D, Vincenzi B, Zagami M, La Cesa A, Bianchi A, Altomare V, Primavera A, Battista C, Vetrani A, Tonini G, Rabitti C. COX-2 expression in DCIS: correlation with VEGF, HER-2⁄neu, prognostic molecular markers and clinicopathological features. Histopathology. 2005;46:561–568. doi: 10.1111/j.1365-2559.2005.02132.x. [DOI] [PubMed] [Google Scholar]
- 19.Shim JY, An HJ, Lee YH, Kim SK. Overexpression of cyclooxygenase-2 is associated with breast carcinoma and its poor prognostic factors. Mod Pathol. 2003;16:1199–1204. doi: 10.1097/01.MP.0000097372.73582.CB. [DOI] [PubMed] [Google Scholar]
- 20.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NFkappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumours. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::AID-CNCR17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe O, Shimizu T, Kinoshita J, Utada Y, Okabe T, Kimura K, et al. Expression of cyclooxygenase-2 in malignant and benign breast tumours. Anticancer Res. 2003;23:3215–3221. [PubMed] [Google Scholar]
- 23.Denkert C, Winzer K-J, Muller B-M, Weichert W, Pest S, Kobel M, et al. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease survival and overall survival in patients with breast cancer. Cancer. 2003;97:2978–2987. doi: 10.1002/cncr.11437. [DOI] [PubMed] [Google Scholar]
- 24.Zeeneldin AA, Mohamed AM, Abdel HA, Taha FM, Goda IA, AboDeef WT. Survival effects of cyclooxygenase-2 and 12-lypooxygenase in Egyptian women with operable breast cancer. Indian J Cancer. 2009;46:54–60. doi: 10.4103/0019-509X.48597. [DOI] [PubMed] [Google Scholar]
- 25.Tsutsui S, Ohno S, Murakami S, Hachitanda Y, Oda S. Prognostic value of c-erbB2 expression in breast cancer. J Surg Oncol. 2002;79:216–223. doi: 10.1002/jso.10079. [DOI] [PubMed] [Google Scholar]
- 26.Welch DR, Wei LL. Genetic and epigenetic regulation of human breast cancer progression and metastasis. Endocr Relat Cancer. 1998;5:155–197. doi: 10.1677/erc.0.0050155. [DOI] [Google Scholar]
- 27.Kiley SC, Clark KJ, Goodnough M, Welch DR, Jaken S. Protein kinase C delta involvement in mammary tumour cell metastasis. Cancer Res. 1999;59:3230–3238. [PubMed] [Google Scholar]
- 28.Gilhooly EM, Rose DP. The association between a mutated ras gene and cyclooxygenase-2 expression in human breast cancer cell lines. Int J Oncol. 1999;15:267–270. [PubMed] [Google Scholar]
- 29.Albergaria A, Ricardo S, Milanezi F, Carneiro V, Amendoeira I, Vieira D, et al. Nottingham prognostic index in triple-negative breast cancer: a reliable prognostic tool? BMC Cancer. 2011;11:299. doi: 10.1186/1471-2407-11-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das S, Paul R, De U, Mukhopadhyay M. The lady with raised prostate specific antigen: Do we need to worry? Asian Pac J Cancer Prev. 2011;12:2051–2053. [PubMed] [Google Scholar]
- 31.Vojtek AB, Der CJ. Increasing complexity of the Ras signaling pathway. J Biol Chem. 1998;273:19925–19928. doi: 10.1074/jbc.273.32.19925. [DOI] [PubMed] [Google Scholar]
- 32.Richards JA, Petrel TA, Brueggemeier RW. Signaling pathways regulating aromatase and cyclooxygenases in normal and malignant breast cells. J Steroid Biochem Mol Biol. 2002;80:203–212. doi: 10.1016/S0960-0760(01)00187-X. [DOI] [PubMed] [Google Scholar]
- 33.Subbaramaiah K, Chung WJ, Dannenberg AJ. Ceramide regulates the transcription of cyclooxygenase-2—evidence forinvolvement of extracellular signal-regulated kinase c-Jun N-terminal kinase and p38 mitogen-activated protein kinase pathways. J Biol Chem. 1998;273:32943–32949. doi: 10.1074/jbc.273.49.32943. [DOI] [PubMed] [Google Scholar]