Abstract
Background/Aims
DA-9701, a standardized extract of Pharbitis Semen and Corydalis Tuber, is a new prokinetic agent that exhibits an analgesic effect on the abdomen. We investigated whether DA-9701 affects visceral pain induced by colorectal distension (CRD) in rats.
Methods
A total of 21 rats were divided into three groups: group A (no CRD+no drug), group B (CRD+no drug), and group C (CRD+DA-9701). Expression of pain-related factors, substance P (SP), c-fos, and phosphorylated extracellular signal-regulated kinase (p-ERK) in the dorsal root ganglion (DRG) and spinal cord was determined by immunohistochemical staining and Western blotting.
Results
The proportions of neurons in the DRG and spinal cord expressing SP, c-fos, and p-ERK were higher in group B than in group A. In the group C, the proportion of neurons in the DRG and spinal cord expressing p-ERK was lower than that in group B. Western blot results for p-ERK in the spinal cord indicated a higher level of expression in group B than in group A and a lower level of expression in group C than in group B.
Conclusions
DA-9701 may decrease visceral pain via the downregulation of p-ERK in the DRG and spinal cord.
Keywords: DA-9701, Gastrointestinal diseases, Visceral hypersensitivity, Colorectal distension, Phosphorylated extracellular signal-regulated kinase
INTRODUCTION
Functional gastrointestinal disorders (FGIDs) are disorders of the digestive system in which no structural or biochemical abnormalities can be found to explain their symptoms.1 FGIDs such as functional dyspepsia and irritable bowel syndrome are not life-threatening, but affect quality of life and increase healthcare costs.1 Of multiple pathophysiologic factors involved in FGID, visceral pain and visceral hypersensitivity are known to play a role.2,3 Regarding management of FGID, no single available therapy could sufficiently relieve the FGID symptoms.1-3 Therefore, several herbal products with conventional prokinetics have been used for relieving FGID symptoms.4,5
DA-9701, a new herbal agent obtained from extracts of Pharbitidis Semen and Corydalis Tuber,6 was reported to be effective in relieving gastrointestinal (GI) symptoms.7-10 It was reported that DA-9701 accelerates gastric emptying in normal rats, and improves gastric accommodation in conscious dogs by increasing postprandial gastric volume.6,10 DA-9701 has agonistic activity on serotonergic receptors (5-HT1A, 5-HT1B, and 5-HT4) and antagonistic activity on the dopaminergic D2 receptor,7-9,11 thus it is thought to increase GI motility and reduce visceral hypersensitivity.12
Taken together, we assumed that DA-9701 play a role in modulating visceral pain. However, this role has not been validated by experiments. In this study, visceral pain was produced by colorectal distension (CRD) in rats with and without DA-9701 administration. Then, pain-related factors such as substance P (SP), c-fos, and phosphorylated extracellular signal-regulated kinase (p-ERK) in the dorsal root ganglion (DRG) and spinal cord were assessed by immunohistochemistry and Western blot assay to investigate whether DA-9701 relieves FGID symptoms by reducing visceral pain.
MATERIALS AND METHODS
1. DA-9701
DA-9701 is a new botanical drug formulated with Pharbitidis Semen and Corydalis Tuber. Pharbitidis Semen is the seed of Pharbitis nil Choisy and is used as a folk medicine to relieve abdominal pain. Corydalis Tuber is the root of Corydalis yanhusuo W.T. Wang and is also used as a folk medicine for its analgesic and antiulcer effects. Since early 2012, DA-9701 (Motilitone®; Dong-A Pharm. Co., Seoul, Korea) has been commercially produced in South Korea and has also been demonstrated to have prokinetic effects and good safety profiles.6,10
2. Animals
A total of 21 adult male Sprague-Dawley rats (Chiba, Japan) weighing between 240 and 260 g was used after approval by the Institutional Animal Care and Use Committee of Hanyang University. All rats were kept at a constant temperature (24℃±1℃) and 40% to 60% relative humidity. They were maintained under a 12/12 hours light/dark cycle with free access to normal rat chow and water, and were acclimatized for 7 days (Fig. 1). Before experiments, all rats were fasted for 24 hours except for free access to water. The rats were divided into three groups: group A, in which no CRD and no drug; group B, in which CRD was generated but no drug administered; group C, in which CRD was generated and DA-9701 was administered.
Fig. 1.
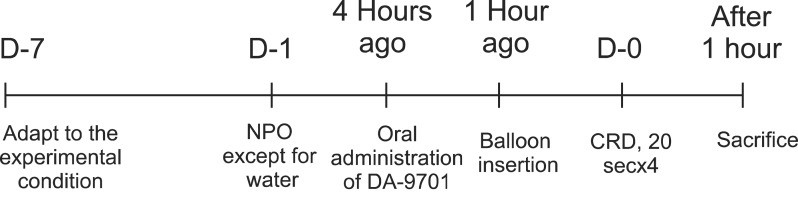
Schematic diagram of the experimental protocol. Rats were adapted to the experimental conditions for 7 days to minimize stress. All of the rats were fasted for 24 hours prior to experimental use but were allowed access to water ad libitum. DA-9701 was administered orally in group C 4 hours before colorectal distension (CRD). A plastic tube with a balloon at the end was inserted through the anus 1 hour before CRD in all groups. CRD was generated in groups B and C. Rats were killed 1 hour after CRD, and the spinal cords and dorsal root ganglion at the level of L6-S1 were removed.
NPO, nil per os.
3. CRD and DA-9701 administration
DA-9701, 3 mg/kg, was administered with oral zoned needle (intragastric feeding catheter) to the rats 4 hours before generating CRD. The dose of DA-9701 was determined according to previous studies showing a potent effect on GI function.6,13 Rats were anesthetized with an intraperitoneal injection of pentobarbital 45 mg/kg and a 6-cm plastic tube with a balloon was inserted intra-anally 1 hour before balloon inflation in all groups. The tube was secured to the tail with a tape and connected to a barostat. CRD was performed in groups B and C by inflating the balloon to 60 mm Hg four times for 20 seconds, at 5 minutes intervals. Rats were under anesthesia during CRD.
4. Measurement of SP, p-ERK, and c-fos
One hour after CRD, rats under anesthesia were sacrificed by intracardiac administration of potassium chloride. Macroscopic and microscopic colonic tissue damages were evaluated and those with damaged colonic mucosa were excluded. Expressions of SP, c-fos, and p-ERK in the DRG and spinal cord at the level of L6-S1 were determined by immunohistochemical staining and Western blotting.
5. Immunohistochemistry
Sacrificed rats were perfused transcardially with warm saline followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer, followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer. After the perfusion, the DRG and spinal cord at the level of L6-S1 were dissected out from four rats in each group (a total of 12 rats). Tissue was fixed in 4% paraformaldehyde overnight at 4℃, and placed in 30% sucrose solution for 24 hour at 4℃. Frozen sections of DRG and spinal cord were cut in a cryostat and placed onto saline coated slides for immunochemistry. Sections were incubated with primary antibody to SP (1:50; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), p-ERK (1:400; Cell Signaling, Danvers, MA, USA), and c-fos (1:50; Santa Cruz Biotechnology Inc.) at 4℃ overnight. The UltraVision LP Detection System HRP Polymer & DAP (diamino benzidine) Plus Chromogen (Thermo Fisher Scientific, Fremont, CA, USA) was used as secondary antibody according to manufacturer's protocol. All samples were handled under the same experimental conditions.
For quantitative assessment of immunostaining, numbers of total and immunoreactive neurons per section were counted in two randomly selected sections for each rat (×400). The number of nerve cells with clearly visible cell bodies was manually counted and among them, the number of immunoreactive cells was counted. To count the number of immunoreactive cells, it was examined whether the nucleus or cytoplasm of nerve cell (DRG neuronal cell or nerve cell of spinal cord) was stained. SP was stained in a cytoplasm, c-fos in a nucleus, and p-ERK in both a cytoplasm and nucleus.14-19 Two investigators quantified the staining and they were blinded to the sections they were counting. Finally, the average of two values was calculated. The intensity of staining was expressed as the percentage of total cells that were immunoreactive.
6. Western blot assay
Samples taken from the spinal cord at the level of L6-S1 of three rats in each group (a total of nine rats) were homogenized in RIPA lysis buffer containing phosphatase inhibitor (Thermo Fisher Scientific, Waltham, MA, USA). Protein samples (30 µg) were separated on sodium dodecylsufate polyacrylamide gel and transferred to polyvinylidene fluoride membrane. The blots were blocked with 4% skim milk and incubated overnight at 4℃ with antibodies to SP (1:200; Santa Cruz Biotechnology Inc.), p-ERK (1:1000; Cell Signaling Technology), and c-fos (1:200; Santa Cruz Biotechnology Inc.). The blots were further incubated with secondary antibody (antigoat and antirabbit antibody). Antigen-antibody complexes were detected with a chemiluminescence reagent kit (Amersham Hybond ECL; GE Healthcare Bio-Sciences, Piscataway, NJ, USA). Chemidoc-Quantity-One software (Biorad Laboratories, Hercules, CA, USA) was used to perform quantitative analyses. Specific bands were evaluated by the apparent molecular size. The intensity of the selected bands was captured and analyzed by Image J 1.43u 2010 software (National Institutes of Health, Bethesda, MD, USA). There was insufficient DRG tissue for Western blot analysis.
7. Statistical analysis
All results were analyzed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Because the values did not follow a normal distribution, nonparameteric tests were used. Data from immunohistochemical stains were analyzed using the Mann-Whitney U test. Data from Western blots were analyzed using the Kruskal-Wallis test with the post hoc test of Tukey test using ranks. A p<0.05 was considered to indicate statistical significance.
RESULTS
1. Colonic tissue damage
Tissue damages in the rectum were macro and microscopically evaluated and no colonic mucosal damages were found in the experimental animals.
2. Immunohistochemistry
1) Spinal cord
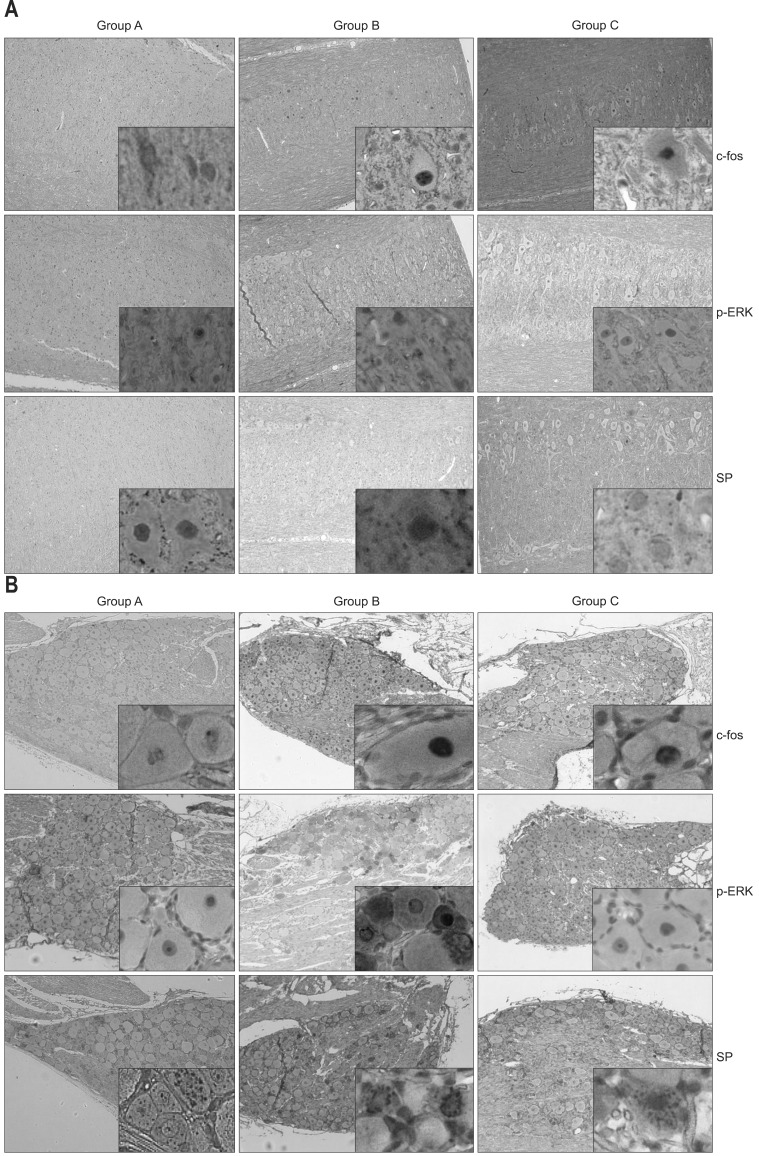
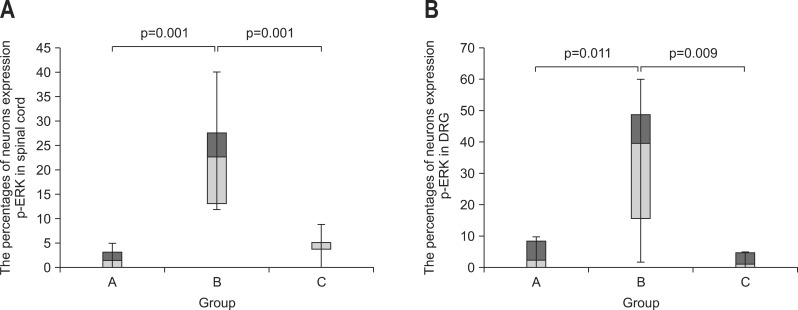
Proportions of spinal cord neurons expressing SP, c-fos, and p-ERK were higher in the group B than in the group A (c-fos, p=0.006; p-ERK, p=0.001; SP p=0.002) (Table 1). In the group C, proportion of neurons expressing p-ERK was lower than in the group B (p=0.001) (Figs 2A and 3A), whereas expressions of SP and c-fos were not different between the groups C and B (c-fos, p=0.673; SP, p=0.206).
Table 1.
Immunohistochemical Staining for c-fos, Phosphorylated Extracellular Signal-Regulated Kinase, and Substance P in the Dorsal Root Ganglion and Spinal Cord
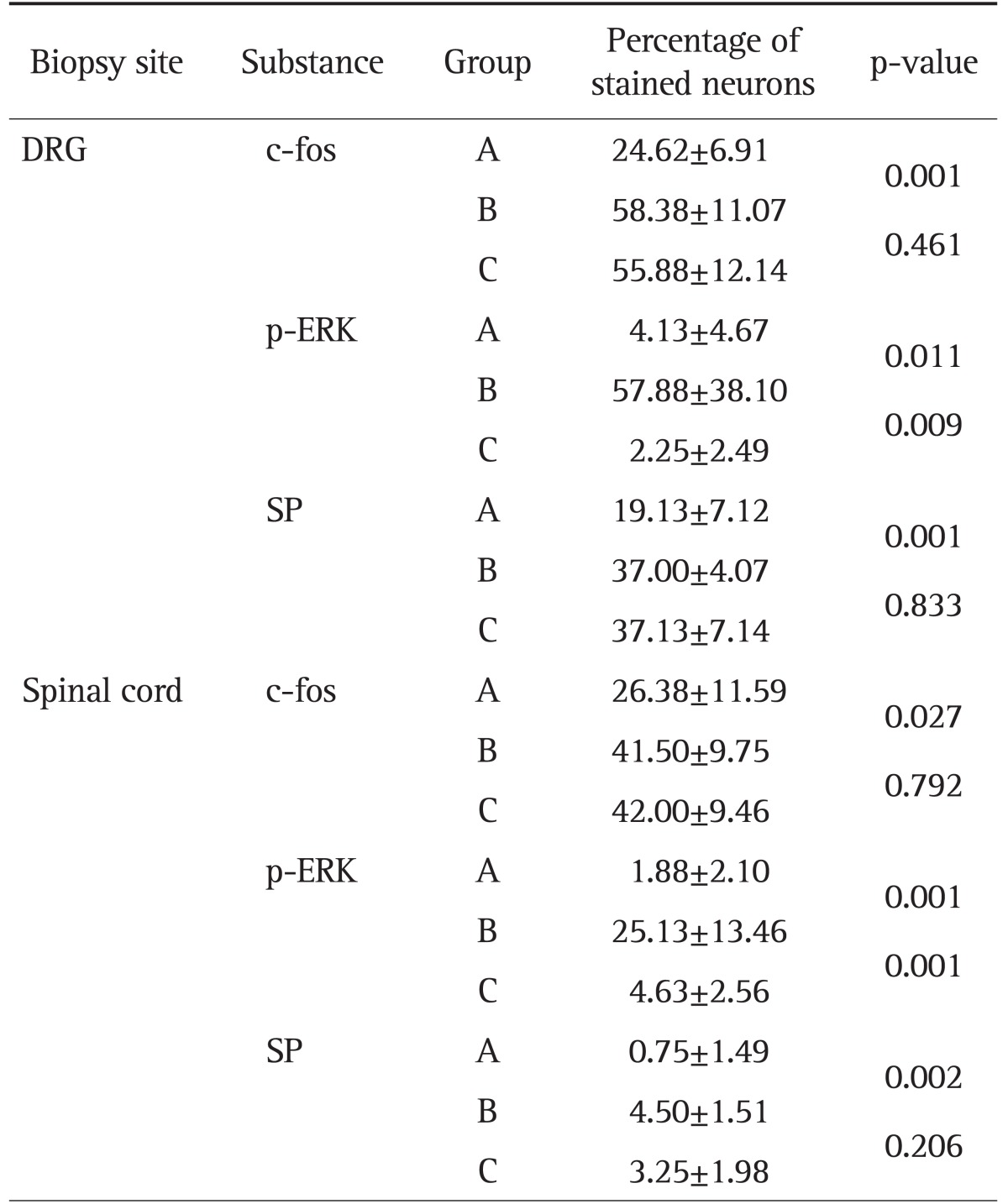
Data are presented as mean±SD.
DRG, dorsal root ganglion; p-ERK, phosphorylated extracellular signal-regulated kinase; SP, substance P.
Fig. 2.
(A) Immunohistochemical staining for c-fos, phosphorylated extracellular signal-regulated kinase (p-ERK), and substance P (SP) in spinal cords. (B) Immunohistochemical staining for c-fos, p-ERK, and SP in the dorsal root ganglion (DRG). In both the spinal cord and DRG, the numbers of positively stained neurons for all substances were higher in group B than in group A, and the numbers of neurons stained for p-ERK were lower in group C than in group B. The groups are marked as A, B, and C from left to right, and the pain-related substances are shown as c-fos, p-ERK, and SP from the top to the bottom of the figures. Each photograph is an image magnified by a factor of 100 (inset, ×400) (A, no colorectal distension [CRD] and no medication; B, CRD+no medication; C, CRD+medication).
Fig. 3.
The percentages of neurons expressing phosphorylated extracellular signal-regulated kinase (p-ERK) as indicated by immunohistochemical staining. The box plot graph demonstrates that the proportion of neurons expressing p-ERK is significantly higher in group B than in group A and lower in group C than in group B. (A) Staining for p-ERK in the spinal cord, p=0.001, n=8 (per group). The percentages of neurons expressing p-ERK in the spinal cord, as indicated by immunostaining, are plotted on the Y-axis, and the groups are plotted on the X-axis. (B) Staining for p-ERK in the dorsal root ganglion (DRG), p=0.009, n=8 (per group). The percentages of neurons expressing p-ERK in the DRG, as indicated by immunostaining, are plotted on the Y-axis, and the groups are plotted on the X-axis.
2) DRG
Proportions of DRG neurons expressing SP, c-fos, and p-ERK were higher in the group B than in the group A (c-fos, p=0.001; p-ERK, p=0.011; SP, p=0.001) (Table 1). Expression of p-ERK in the DRG neurons was greater in the group B than in the group C (p=0.009) (Figs 2B and 3B). Between the groups B and C, expressions of SP and c-fos in the DRG were not different (c-fos, p=0.461; SP, p=0.833).
3. Western blot assays
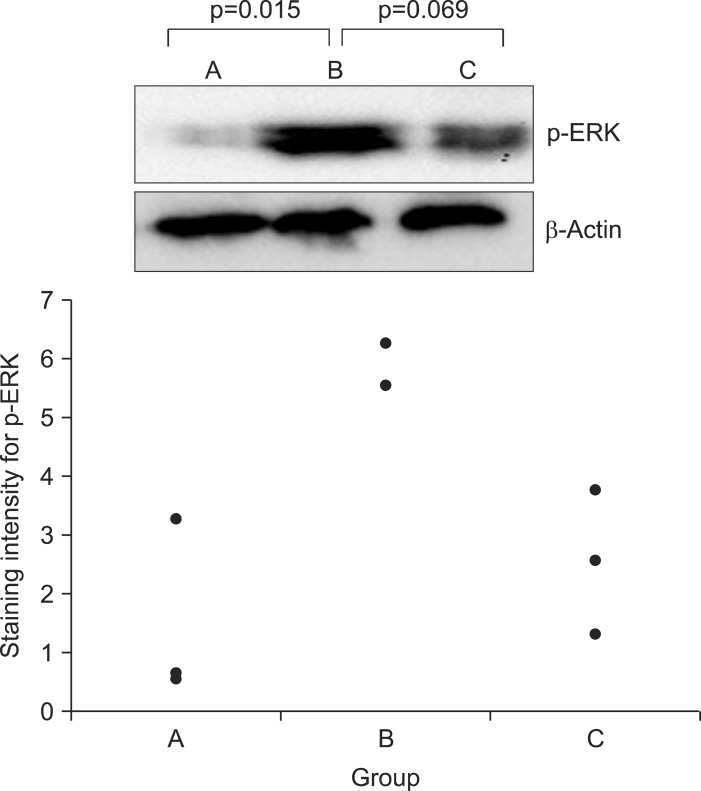
The Western blotting for p-ERK in the spinal cord revealed significant differences among three groups (p=0.025), and posthoc tests revealed significant difference between the group A and B (p=0.015 between the group A and B; p=0.069 between B and C; p=0.457 between A and C). Although post hoc tests showed that there was no significant difference between the group B and C, the staining was less intense in group C than in group B (Fig. 4). There were no differences among groups in staining intensity for SP and c-fos.
Fig. 4.
Western blot results for phosphorylated extracellular signal-regulated kinase (p-ERK) in the spinal cord, p=0.025, n=3 (per group). Staining intensity is higher in group B than in group A and lower in group C than in group B. Staining intensity is plotted along the Y-axis, and the groups are plotted along the X-axis. Black dots represent the staining intensity of individual rats within the indicated groups.
DISCUSSION
In this study, we showed that pain-related factors found in the pain-transmitting nervous pathways, SP, c-fos, and p-ERK were up-regulated in response to CRD and that DA-9701 significantly decreased the CRD-induced upregulation of p-ERK. This means that the expression of SP, c-fos, and p-ERK is associated with CRD-induced visceral pain and DA-9701 decrease visceral pain in rats stimulated by CRD. Consequently, DA-9701 may relieve visceral hypersensitivity related with FGID by downregulating pain-related substance. Visceral hypersensitivity is a condition in which visceral pain is felt after a stimulus that generally does not cause pain. It has been observed in patients with a variety of functional disorders and seems to be important in the pathogenesis of FGID.2,20,21 Visceral hypersensitivity is usually measured by measuring pressure within the rectum, using a balloon inserted into the rectum and slowly filled with air.22-24 Individuals are characterized as having visceral hypersensitivity when they report pain at lower levels of pressure than most tested individuals. Laboratory animals cannot complain of pain accurately and analysis of behavioral response to pain can truly be subjective. Therefore, we need the method to measure visceral pain in animal experiments. In our study, visceral pain was determined objectively by measuring pain-related factors, SP, c-fos, and p-ERK in the DRG and spinal cord of anesthesized rats. Despite there were some ways to measure visceral pain of animals including the abdominal withdrawal reflex,25-27 we used pain-related substances for measuring intensity of pain. This method has an advantage over the other methods in that anesthesized rats are free of experimental stressors other than CRD.28
p-ERK, c-fos, and SP are pain-related factors that are used as objective markers of pain perception.15,19,29 The p-ERK, a kind of mitogen-activated protein kinase, is activated by painful stimuli in DRG neurons,16 and is known to play a role in functional pain-associated diseases by transmitting various noxious stimuli. Previous studies have shown that upregulation of p-ERK plays an essential part in the nociceptive system in DRG neurons and dorsal horn neurons, and that ERK activation in the dorsal horn is associated with c-fos expression.14 The c-fos protein, a product of c-fos proto-oncogene, was also observed to be expressed by both noxious and nonnoxious stimuli in the dorsal horn neurons.15 SP and its neurokinin receptors are ubiquitously expressed in the GI tract and mediate noxious sitmuli through activating visceral pain-transmitting C-fibers.30,31
Changes in the properties of primary afferent neurons and abnormal processing of sensory information within the central nervous system are likely to contribute to the development of visceral pain.3,32,33 Visceral pain is clinically and pathophysiologically different from somatic pain, and is often associated with the characteristic symptoms of allodynia and hyperalgesia.34,35 Sensory signals from the colon reach the central nervous system via primary afferent fibers in the splanchnic and pelvic pathways.29,36,37 These fibers have peripheral endings in the colon wall and cell bodies in the thoracolumbar (T10-L1) and lumbosacral (L6-S1) dorsal root ganglia, respectively.14 We examined the pelvic pathway in rats by generating CRD, killing the rats and conducting autopsies in the spinal cords and DRG at the level of L6-S1, as in many previous studies.38,39
There were some limitations to our study. First, although we found an effect of DA-9701 on p-ERK, the effect of DA-9701 on SP and c-fos was not demonstrated. A possible explanation is that DA-9701 may be selectively related to p-ERK pathway but not SP and c-fos. Alternatively, the two factors may express with a different timescale than p-ERK. Therefore, additional experiments with multiple autopsies at different times might yield significant results. Second, there may be experimenter bias due to errors in the quantitative analysis of immunohistochemistry and Western blot because a standardized method of quantitative analysis does not exist. Third, we observed too many DRG neurons expressed p-ERK in some instances. As only a small proportion of the total number of neurons in the DRG and spinal cord innervates the colon,40 high-level expression of SP, c-fos, and p-ERK might be attributable to other visceral organs rather than the colon, ex, the bladder. It can be partly explained by the pressure effects secondary to local compression of adjacent organs. Fourth, the sample size was small. There were significant differences between group A and B in staining intensity for SP and c-fos on immunohistochemistry. However Western blot showed no difference between group A and B in staining intensity for SP and c-fos. Further experiments need to be conducted with a larger number of rats to maximize the likelihood of obtaining statistically significant results, especially of Western blots. Last, the experimental dose of DA-9701 must be verified. In a previous study, DA-9701 had most potent effects on gastric emptying and GI transit at doses of 0.3 to 3 mg/kg and did not have toxic effects even at a dose of 10 mg/kg.6,10,11 Therefore we set the dose as 3 mg/kg in our study. However further studies to assess multiple doses of DA-9701 are required, because visceral analgesic effect of the drug has not been validated yet.
In summary, our study shows an effect of DA-9701 in modulating visceral pain in a rat model. To our knowledge, this study is the first to provide experimental evidence for this effect. We suggest that DA-9701, by downregulating visceral pain mediators, may be effective in the management of FGID.
ACKNOWLEDGEMENTS
This study was approved by Institutional Animal Care and Use Committee.
Footnotes
This study was supported by Dong-A Pharmaceutical Company, Seoul, Korea, the manufacturer of DA-9701. The funding source had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
References
- 1.Fukudo S, Kuwano H, Miwa H. Management and pathophysiology of functional gastrointestinal disorders. Digestion. 2012;85:85–89. doi: 10.1159/000334652. [DOI] [PubMed] [Google Scholar]
- 2.Feng B, La JH, Schwartz ES, Gebhart GF. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1085–G1098. doi: 10.1152/ajpgi.00542.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sikandar S, Dickenson AH. Visceral pain: the ins and outs, the ups and downs. Curr Opin Support Palliat Care. 2012;6:17–26. doi: 10.1097/SPC.0b013e32834f6ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allescher HD. Functional dyspepsia: a multicausal disease and its therapy. Phytomedicine. 2006;13(Suppl 5):2–11. doi: 10.1016/j.phymed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Thompson Coon J, Ernst E. Systematic review: herbal medicinal products for non-ulcer dyspepsia. Aliment Pharmacol Ther. 2002;16:1689–1699. doi: 10.1046/j.1365-2036.2002.01339.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee TH, Choi JJ, Kim DH, et al. Gastroprokinetic effects of DA-9701, a new prokinetic agent formulated with Pharbitis Semen and Corydalis Tuber. Phytomedicine. 2008;15:836–843. doi: 10.1016/j.phymed.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Choi S, Choi JJ, Jun JY, et al. Induction of pacemaker currents by DA-9701, a prokinetic agent, in interstitial cells of Cajal from murine small intestine. Mol Cells. 2009;27:307–312. doi: 10.1007/s10059-009-0039-6. [DOI] [PubMed] [Google Scholar]
- 8.Lee TH, Kim KH, Lee SO, Lee KR, Son M, Jin M. Tetrahydroberberine, an isoquinoline alkaloid isolated from corydalis tuber, enhances gastrointestinal motor function. J Pharmacol Exp Ther. 2011;338:917–924. doi: 10.1124/jpet.111.182048. [DOI] [PubMed] [Google Scholar]
- 9.Lee TH, Son M, Kim SY. Effects of corydaline from Corydalis tuber on gastric motor function in an animal model. Biol Pharm Bull. 2010;33:958–962. doi: 10.1248/bpb.33.958. [DOI] [PubMed] [Google Scholar]
- 10.Kim ER, Min BH, Lee SO, Lee TH, Son M, Rhee PL. Effects of DA-9701, a novel prokinetic agent, on gastric accommodation in conscious dogs. J Gastroenterol Hepatol. 2012;27:766–772. doi: 10.1111/j.1440-1746.2011.06924.x. [DOI] [PubMed] [Google Scholar]
- 11.Ji HY, Liu KH, Jeong JH, et al. Effect of a new prokinetic agent DA-9701 formulated with corydalis tuber and pharbitidis semen on cytochrome P450 and UDP-glucuronosyltransferase enzyme activities in human liver microsomes. Evid Based Complement Alternat Med. 2012;2012:650718. doi: 10.1155/2012/650718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman JM, Tyler K, MacEachern SJ, et al. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–854. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung YS, Kim MY, Lee HS, Park SL, Lee KJ. Effect of DA-9701, a novel prokinetic agent, on stress-induced delayed gastric emptying and hormonal changes in rats. Neurogastroenterol Motil. 2013;25:254–259. doi: 10.1111/nmo.12053. [DOI] [PubMed] [Google Scholar]
- 14.Harrington AM, Brierley SM, Isaacs N, Hughes PA, Castro J, Blackshaw LA. Sprouting of colonic afferent central terminals and increased spinal mitogen-activated protein kinase expression in a mouse model of chronic visceral hypersensitivity. J Comp Neurol. 2012;520:2241–2255. doi: 10.1002/cne.23042. [DOI] [PubMed] [Google Scholar]
- 15.Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45:1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Micevych P, McDonald J, Rapkin A, Chaban V. Inflammation in the uterus induces phosphorylated extracellular signal-regulated kinase and substance P immunoreactivity in dorsal root ganglia neurons innervating both uterus and colon in rats. J Neurosci Res. 2008;86:2746–2752. doi: 10.1002/jnr.21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roza C, Reeh PW. Substance P, calcitonin gene related peptide and PGE2 co-released from the mouse colon: a new model to study nociceptive and inflammatory responses in viscera, in vitro. Pain. 2001;93:213–219. doi: 10.1016/S0304-3959(01)00318-9. [DOI] [PubMed] [Google Scholar]
- 18.Sakurai J, Obata K, Ozaki N, et al. Activation of extracellular signal-regulated protein kinase in sensory neurons after noxious gastric distention and its involvement in acute visceral pain in rats. Gastroenterology. 2008;134:1094–1103. doi: 10.1053/j.gastro.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 20.Bielefeldt K, Christianson JA, Davis BM. Basic and clinical aspects of visceral sensation: transmission in the CNS. Neurogastroenterol Motil. 2005;17:488–499. doi: 10.1111/j.1365-2982.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- 21.Camilleri M, Coulie B, Tack JF. Visceral hypersensitivity: facts, speculations, and challenges. Gut. 2001;48:125–131. doi: 10.1136/gut.48.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hultin L, Nissen TD, Kakol-Palm D, Lindström E. Colorectal distension-evoked potentials in awake rats: a novel method for studies of visceral sensitivity. Neurogastroenterol Motil. 2012;24:964–e466. doi: 10.1111/nmo.12005. [DOI] [PubMed] [Google Scholar]
- 23.Keszthelyi D, Troost FJ, Masclee AA. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G141–G154. doi: 10.1152/ajpgi.00060.2012. [DOI] [PubMed] [Google Scholar]
- 24.Ludidi S, Conchillo JM, Keszthelyi D, et al. Rectal hypersensitivity as hallmark for irritable bowel syndrome: defining the optimal cutoff. Neurogastroenterol Motil. 2012;24:729–733. doi: 10.1111/j.1365-2982.2012.01926.x. [DOI] [PubMed] [Google Scholar]
- 25.Mertz H, Morgan V, Tanner G, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842–848. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 26.O'Mahony SM, Tramullas M, Fitzgerald P, Cryan JF. Rodent models of colorectal distension. Curr Protoc Neurosci. 2012;Chapter 9:Unit 9.40. doi: 10.1002/0471142301.ns0940s61. [DOI] [PubMed] [Google Scholar]
- 27.Vermeulen W, De Man JG, De Schepper HU, et al. Role of TRPV1 and TRPA1 in visceral hypersensitivity to colorectal distension during experimental colitis in rats. Eur J Pharmacol. 2013;698:404–412. doi: 10.1016/j.ejphar.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Pantellev SS, Martseva AA, Liubashina OA. Responses of neurons of the solitary tract nucleus to noxious colorectal distension in rats. Ross Fiziol Zh Im I M Sechenova. 2011;97:1336–1345. [PubMed] [Google Scholar]
- 29.Bueno L, Fioramonti J, Delvaux M, Frexinos J. Mediators and pharmacology of visceral sensitivity: from basic to clinical investigations. Gastroenterology. 1997;112:1714–1743. doi: 10.1016/s0016-5085(97)70056-8. [DOI] [PubMed] [Google Scholar]
- 30.Bueno L, Fioramonti J. Effects of inflammatory mediators on gut sensitivity. Can J Gastroenterol. 1999;13(Suppl A):42A–46A. doi: 10.1155/1999/846809. [DOI] [PubMed] [Google Scholar]
- 31.Lee WT, Sohn MK, Park SH, Ahn SK, Lee JE, Park KA. Studies on the changes of c-fos protein in spinal cord and neurotransmitter in dorsal root ganglion of the rat with an experimental peripheral neuropathy. Yonsei Med J. 2001;42:30–40. doi: 10.3349/ymj.2001.42.1.30. [DOI] [PubMed] [Google Scholar]
- 32.Gebhart GF. Visceral pain-peripheral sensitisation. Gut. 2000;47(Suppl 4):iv54–iv55. doi: 10.1136/gut.47.suppl_4.iv54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anand P, Aziz Q, Willert R, van Oudenhove L. Peripheral and central mechanisms of visceral sensitization in man. Neurogastroenterol Motil. 2007;19(1 Suppl):29–46. doi: 10.1111/j.1365-2982.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- 34.Cervero F. Visceral pain-central sensitisation. Gut. 2000;47(Suppl 4):iv56–iv57. doi: 10.1136/gut.47.suppl_4.iv56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grundy D, Al-Chaer ED, Aziz Q, et al. Fundamentals of neurogastroenterology: basic science. Gastroenterology. 2006;130:1391–1411. doi: 10.1053/j.gastro.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 36.Brierley SM. Molecular basis of mechanosensitivity. Auton Neurosci. 2010;153:58–68. doi: 10.1016/j.autneu.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Cervero F, Laird JM. Visceral pain. Lancet. 1999;353:2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- 38.Million M, Wang L, Wang Y, et al. CRF2 receptor activation prevents colorectal distension induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut. 2006;55:172–181. doi: 10.1136/gut.2004.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SH, Han JE, Hwang S, Oh DH. The expression of corticotropin-releasing factor in the central nucleus of the amygdala, induced by colorectal distension, is attenuated by general anesthesia. J Korean Med Sci. 2010;25:1646–1651. doi: 10.3346/jkms.2010.25.11.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]