Abstract
Background/Aims
α-Fetoprotein (AFP) is the biomarker most widely used to detect hepatocellular carcinoma (HCC), despite its suboptimal diagnostic accuracy. Glypican-3 (GPC3) and osteopontin (OPN) are secreted glycoproteins that are reportedly associated with tumorigenesis and metastasis. This study was conducted to evaluate the clinical utility of using plasma GPC3 and OPN as diagnostic biomarkers for HCC.
Methods
We measured the plasma levels of GPC3 and OPN in 120 HCC and 40 chronic liver disease (CLD) patients via an enzyme-linked immunosorbent assay. The diagnostic accuracy of each tumor marker was evaluated using receiver operating characteristic (ROC) curve analysis.
Results
The GPC3 levels in the HCC patients (75.8 ng/mL) were significantly higher (p=0.020) than the levels in patients with CLD (66.4 ng/mL). The area under the ROC curve (AUROC) values for GPC3 and OPN were 0.62 and 0.51, respectively. In subgroup analyses, including subgroups of HCC patients with low serum AFP and PIVKA II levels, the AUROC of GPC3 remained relatively high (0.66), and GPC3 showed a high sensitivity (62.1%) for detecting small HCC tumors.
Conclusions
The plasma levels of GPC3 and OPN demonstrated low diagnostic accuracy for HCC. However, GPC3 may have a complementary role in diagnosing HCC in patients with nondiagnostic levels of conventional tumor markers and with small-sized tumors.
Keywords: Hepatocellular carcinoma, Glypican-3, Osteopontin
INTRODUCTION
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death worldwide, and the burden of this disease is expected to increase in the future.1 Major risk factors for HCC are hepatitis B virus (HBV) and hepatitis C virus infections, alcoholic liver disease, and nonalcoholic fatty liver disease. Among these risk factors, HBV infection accounts for >50% of HCC cases worldwide, and especially in areas such as Asia and Africa where the infection is endemic.1,2
Because the poor outcomes of HCC patients are often related to late detection, recent practice guidelines recommend continued surveillance for patients at high risk.3,4 Screening procedures for HCC include serological and radiological tests, and among the serological tests, α-fetoprotein (AFP) and prothrombin induced by vitamin K absence II (PIVKA II) are widely used as biomarkers for HCC.5-8 However, ~30% of HCC patients are negative for AFP and PIVKA II, and screening for these biomarkers may not be satisfactory due to low sensitivity and specificity. In a recent prospective study, both serological markers demonstrated to be inadequate for surveillance purposes, even when combined.9 Thus, the American Association for the Study of Liver Disease (AASLD) Practice Guidelines Committee recommended that ultrasound examination alone (without AFP) should be used for HCC surveillance,4 and consequently, a number of novel biomarkers for HCC have been suggested.
Glypican-3 (GPC3) is a heparan sulfate proteoglycan molecule anchored to the plasma membrane and has been reported to be a useful serologic and histochemical marker for HCC.10,11 Osteopontin (OPN) is a secreted glycophosphoprotein and has a pivotal role in tumorigenesis and metastasis in a variety of human cancers.12-14 In several studies, overexpression of OPN was associated with poor clinical outcome and poor prognosis of HCC;15,16 however, the vast majority of studies exploring the use of GPC3 and OPN for diagnosing HCC were based only on immunohistochemical parameters, and more evidence is needed regarding their use in clinical practice.
In this study, we evaluated and compared the plasma levels of GPC3 and OPN in HCC patients and liver disease (chronic liver disease, CLD) patients. The clinical utility of using GPC3 and OPN, as compared to using AFP and PIVKA II as serological markers for the diagnosis of HCC was also evaluated.
MATERIALS AND METHODS
1. Patients and blood samples
A cohort of 120 patients diagnosed with HCC for the first time at Korea University Guro Hospital between July 2007 and March 2011 was recruited for this study. The diagnosis of HCC was based on typical imaging patterns and/or histologic examinations conducted according to the AASLD guidelines proposed in 2005.17 The criteria for diagnosing the presence HCC via imaging were based on reports of hyperattenuation at the arterial phase and hypoattenuation at the portal phase in dynamic computed tomography or magnetic resonance imaging, and tumor staining on angiography. In this study, 82 patients were diagnosed with HCC by histologic examination, and the remaining 38 patients were diagnosed by having imaging patterns typical of HCC. Demographic and clinical data on the etiology of HCC, the presence of cirrhosis, the status of liver function in terms of Child-Pugh class, and the tumor-node-metastases (TNM) stage of HCC were evaluated as recommended by the American Joint Committee on Cancer/United International Consensus Committee (AJCC/UICC) staging system for HCC (6th edition),18 and the Barcelona Clinic Liver Cancer (BCLC) stage19 was obtained by reviewing medical records and radiological studies. Serum AFP and PIVKA-II levels were routinely evaluated in all patients prior to receiving therapy.
Blood samples from 120 HCC patients were collected at the time of HCC diagnosis and prior to initiating therapy, and isolated plasma samples were stored at -80℃ until measurements of GPC3 and OPN were conducted. Blood samples from 40 patients with CLD but without HCC, and that were obtained during the same time period as the blood samples from HCC patients, were used as control samples.
The study was approved by the Institutional Review Board of Korea University Guro Hospital, and written informed consent was obtained from all patients when they were enrolled.
2. Measurement of GPC3 and OPN levels
Plasma GPC3 and OPN levels were measured in the same plasma samples. Plasma GPC3 levels were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Cusabio Biotech Co., Ltd., Wuhan, China) according to the manufacturer's instructions. Plasma OPN levels were determined by an ELISA kit (Immuno-Biological Laboratories, Gunma, Japan) according to the manufacturer's instructions. All sample assays performed in duplicate.
3. Statistical analysis
All data are expressed as the mean±SD or median values and ranges. Statistical comparisons of the baseline data were analyzed by the chi-square or Fisher exact test. Differences in quantitative values were compared using the Mann-Whitney U test. All p-values were derived from two-sided tests, and p<0.05 was considered statistically significant. Receiver operating characteristics (ROC) analysis was used to evaluate the diagnostic value of each tumor marker. The optimal cutoff values were calculated using the maximum sum of sensitivity and specificity. Statistical analyses were conducted using SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Patient characteristics
The demographic characteristics of patients included in the analysis are summarized in Table 1. The patients were diagnosed as having HBV-related HCC (62.5%) or CLD (100%). The data show that 72.5% of the patients with HCC and 50% of the control subjects also had cirrhosis. Eighty percent of the patients with HCC were Child-Pugh class A and 20% were class B. The clinical characteristics of HCC are summarized in Table 2. The percentage of patients with stage I or stage II HCC was 55%, as based on TNM staging guidelines published by the AJCC/UICC (6th edition).
Table 1.
Clinical Characteristics of the Study Patients (n=160)
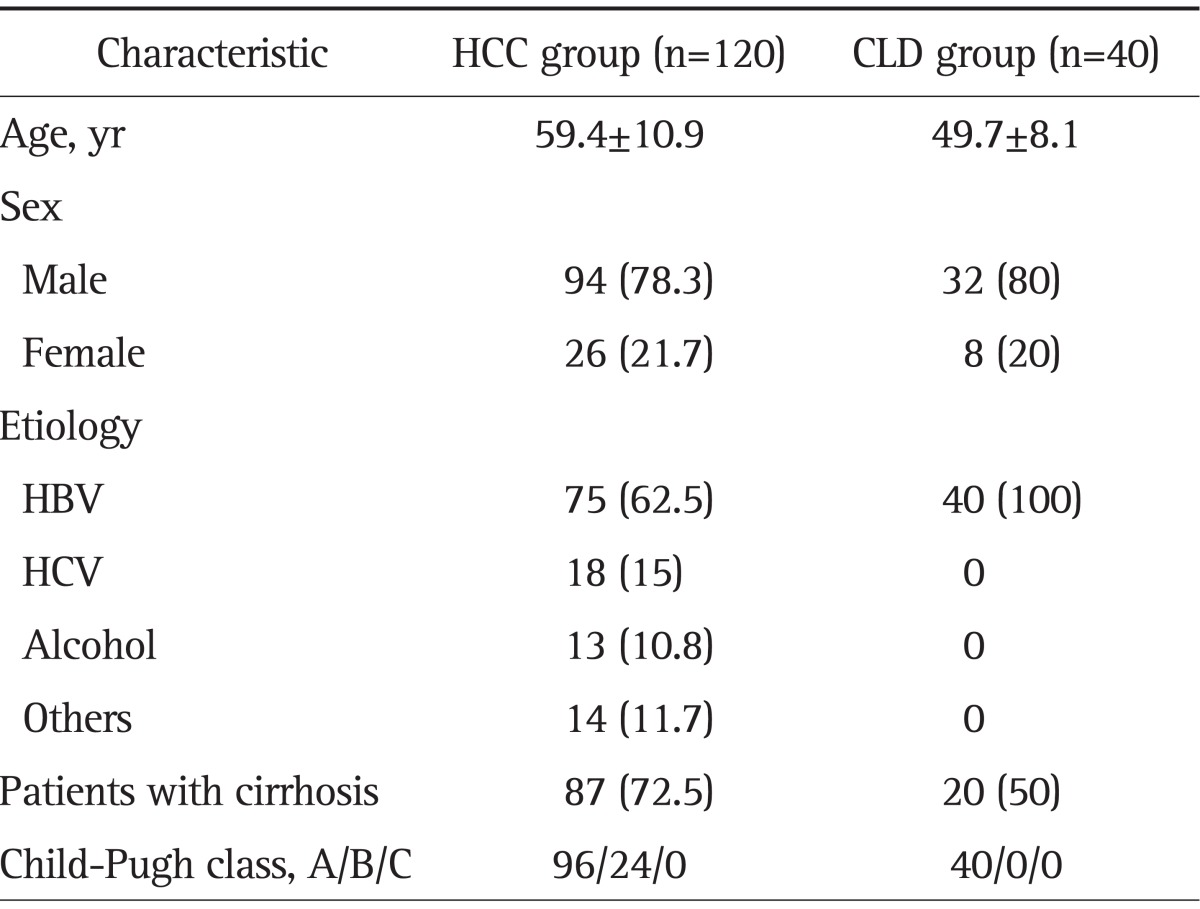
Data are presented as number (%).
HCC, hepatocellular carcinoma; CLD, chronic liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus.
Table 2.
Characteristics of Hepatocelluar Carcinoma (n=120)
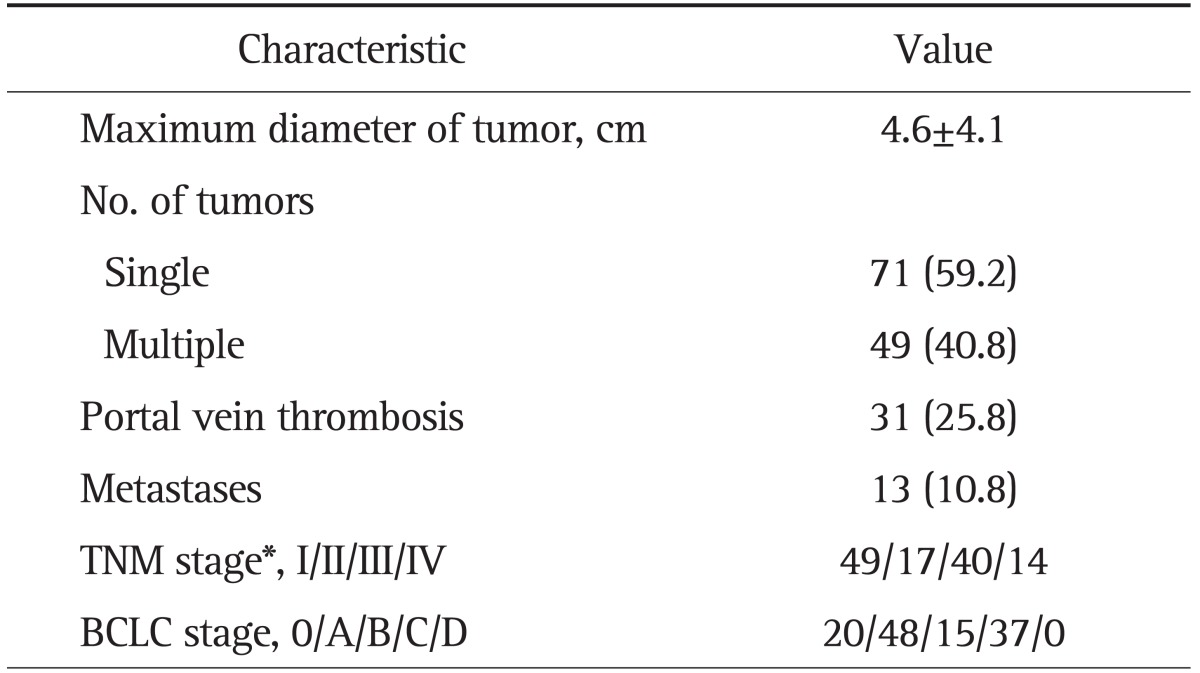
Data are presented as mean±SD or number (%).
TNM, tumor node metastasis; BCLC, the Barcelona Clinic Liver Cancer.
*TNM stage for hepatocelluar carcinoma was determined according to the American Joint Committee on Cancer/United International Consensus Committee, 6th edition.
2. Plasma levels of GPC3 and OPN
Plasma levels of GPC3, OPN, AFP, and PIVKA II are summarized in Table 3. The median GPC3 values in patients with HCC and those with CLD were 75.8 and 66.4 ng/mL, respectively; patients with HCC showed significantly higher GPC3 concentrations than patients with CLD (p=0.020). In contrast, plasma levels of OPN in patients with HCC were not significantly higher than those in patients with CLD (345.2 ng/mL vs 306.8 ng/mL, p=0.821) (Fig. 1).
Table 3.
Plasma or Serum Levels of Tumor Markers for Hepatocelluar Carcinoma (n=160)
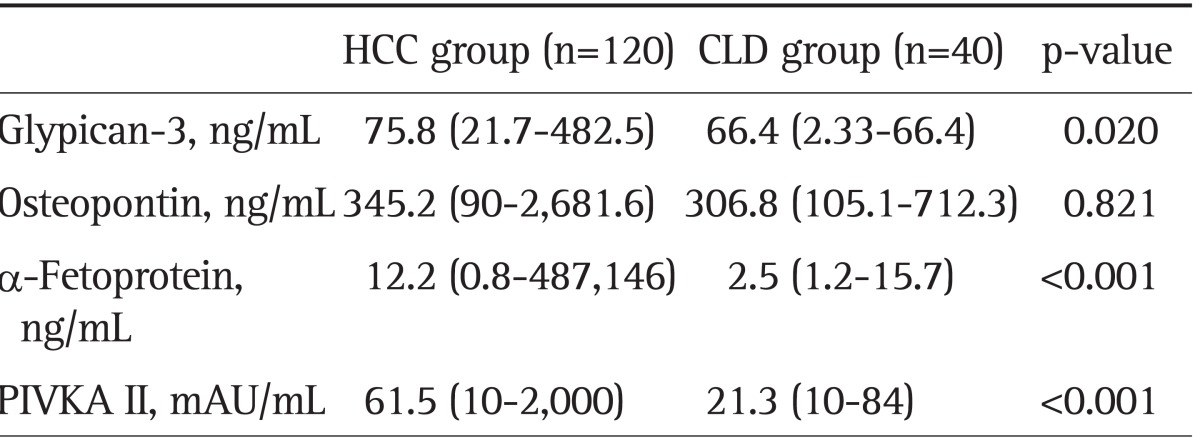
Data are presented as median (range).
HCC, hepatocellular carcinoma; CLD, chronic liver disease; PIVKA II, prothrombin induced vitamin K absence II.
Fig. 1.
(A) Plasma levels of glypican-3 (GPC3) and (B) osteopontin (OPN) in patients with hepatocellular carcinoma (HCC) or chronic liver disease (CLD). The box indicates the 25th and 75th percentile values, and the line indicates the median level, whereas the interquartile range (IQR) extends outside the box. The points outside the IQR are outliers. The plasma GPC3 levels were higher in patients with HCC (75.8 ng/mL) than in patients with CLD (66.4 ng/mL, p=0.020). However, we found no difference in plasma OPN levels based on study group (345.2 ng/mL vs 306.8 ng/mL, p=0.821).
The GPC3 and OPN levels in HCC, as based on various clinical parameters, are summarized in Table 4. Plasma GPC3 levels were not significantly affected by almost all clinical parameters including age, sex, the etiology of HCC, the presence of cirrhosis, or the presence of portal vein thrombosis. The plasma GPC3 levels in the HCC group were also not affected by the tumor stage of HCC; however, plasma OPN levels were higher in males than in females (374.5 ng/mL vs 220.4 ng/mL, p=0.023). The presence of cirrhosis was negatively associated with plasma OPN levels (234.4 ng/mL vs 400.7 ng/mL, p=0.016). Plasma OPN levels were significantly higher in patients with large tumors (>2 cm in diameter), multiple tumors, portal vein thrombosis, or distant metastasis, and there were meaningful differences in the OPN levels according to the tumor stage as described by TNM or BCLC.
Table 4.
Plasma Levels of Glypican-3 and Osteopontin in the Patients with Hepatocelluar Carcinoma
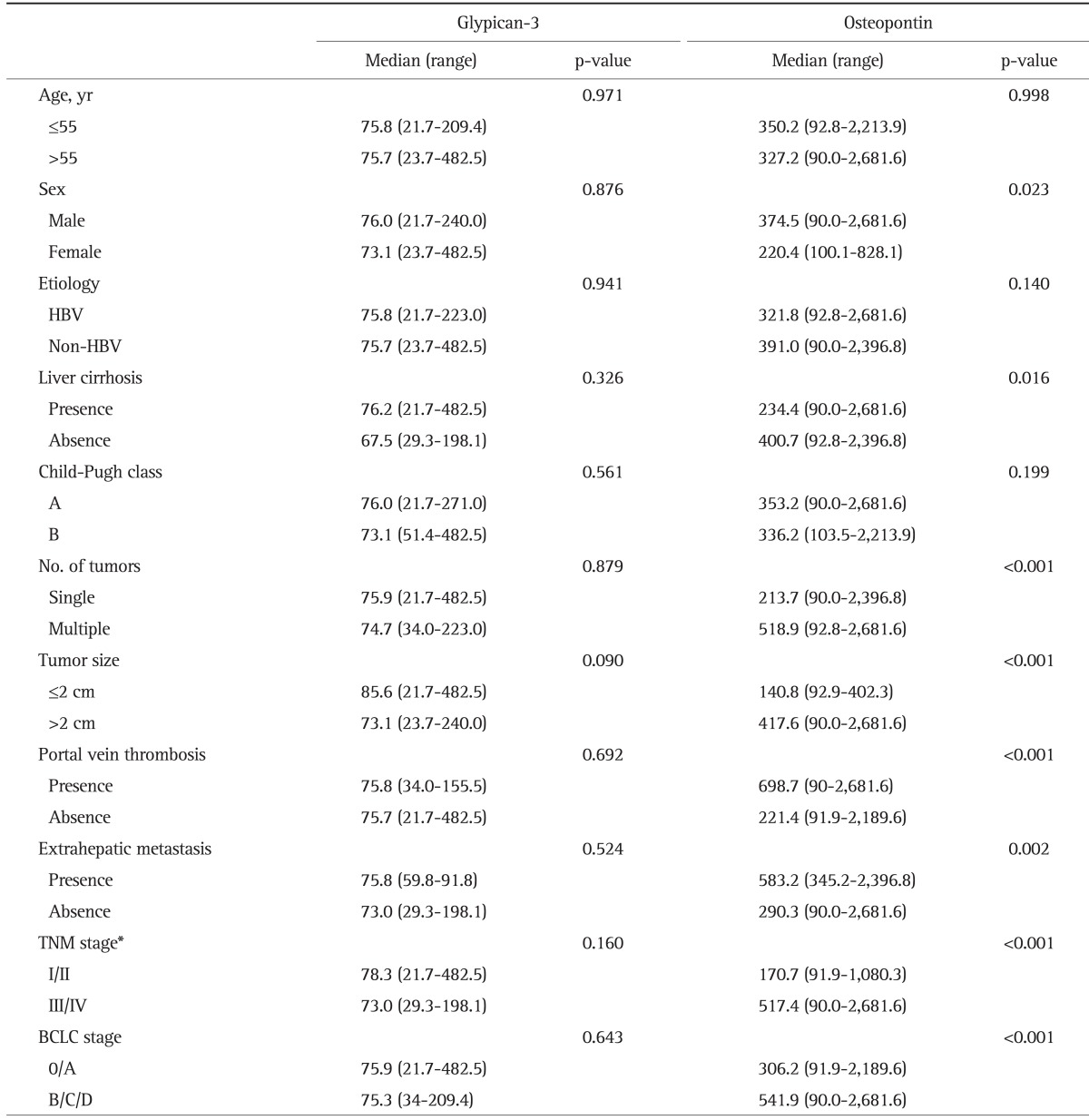
HBV, hepatitis B virus; TNM, tumor node metastasis; BCLC, Barcelona Clinic Liver Cancer.
*TNM stage for hepatocellular carcinoma was determined according to the American Joint Committee on Cancer/United International Consensus Committee, 6th edition.
3. Plasma GPC3 and OPN as diagnostic markers for HCC
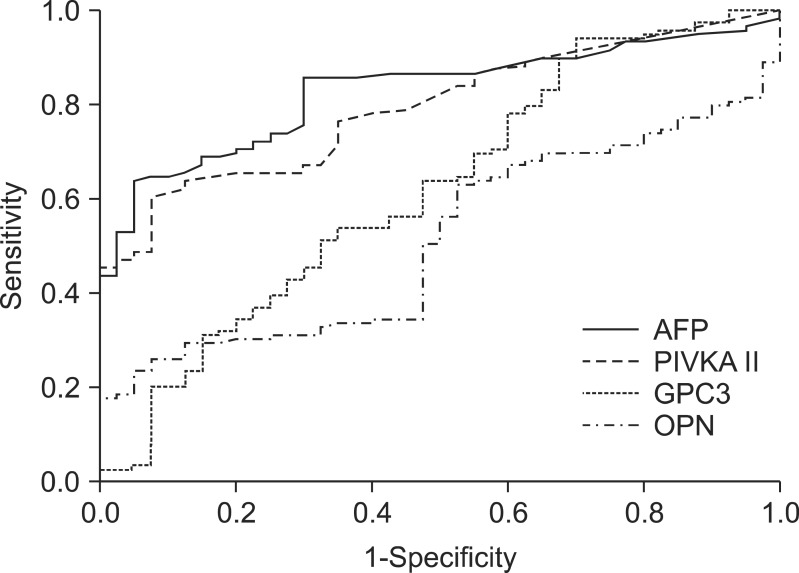
The area under the ROC curve (AUROC) was calculated to compare the accuracies achieved when using GPC3, OPN, AFP, or PIVKA II for diagnosis of HCC (Fig. 2). The AUROC values for AFP (0.83; 95% confidence interval [CI], 0.77 to 0.89), and PIVKA II (0.80; 95% CI, 0.73 to 0.86) were higher than those for GPC3 (0.62; 95% CI, 0.52 to 0.73) and OPN (0.51; 95% CI, 0.42 to 0.61). These results suggest that in the selected study population, the accuracy achieved by using plasma GPC3 or OPN levels for diagnosis of HCC was inferior to the accuracy achieved using AFP or PIVKA II. We also analyzed the complementary properties of using GPC3 or OPN in combination with AFP or PIVKA II for the diagnosis of HCC, using a logistic regression model. The combined use of GPC3 and PIVKA II further increased the AUROC (0.83; 95% CI, 0.76 to 0.89). The combined use of OPN and PIVKA II also produced a higher AUROC than when using PIVKA II alone (0.83; 95% CI, 0.76 to 0.89); however, the AUROCs of these two combinations did not exceed the value obtained when using AFP alone.
Fig. 2.
Area under the receiver-operating curve (AUROC) for glypican-3 (GPC3), osteopontin (OPN), α-fetoprotein (AFP), and prothrombin-induced vitamin K absence II (PIVKA II). The AUROC was 0.62 for GPC3, 0.51 for OPN, 0.83 for AFP, and 0.80 for PIVKA II, respectively.
Table 5 shows the sensitivity and specificity of tumor markers differentiating HCC cases from CLD cases. For each marker, cutoff values were chosen based on the ROC analysis by using the maximum sum of sensitivity and specificity. The sensitivity and specificity of plasma GPC3 levels in HCC patients relative to the CLD group were 53.8% and 65.0%, respectively, at a cutoff value of 73 ng/mL. In this study group, GPC did not perform better than AFP or PIVKA II. At a cutoff value of 557 ng/mL, plasma OPN showed a high specificity (92.5%) but a lower sensitivity (26.1%). Using a combination of GPC3 at 73 ng/mL and AFP at 6 ng/mL, increased the sensitivity to 84.2%, but the specificity decreased to 62.5%. Similar results were observed when GPC3 and PIVKA II were used in combination, (the sensitivity was 84.2% and the specificity was 57.5%). The combination of OPN and AFP/PIVKA II also showed an increased sensitivity and a decreased specificity compared to using AFP or PIVKA II alone.
Table 5.
Sensitivity and Specificity of Tumor Markers and Their Combination for Hepatocellular Carcinoma and Chronic Liver Disease
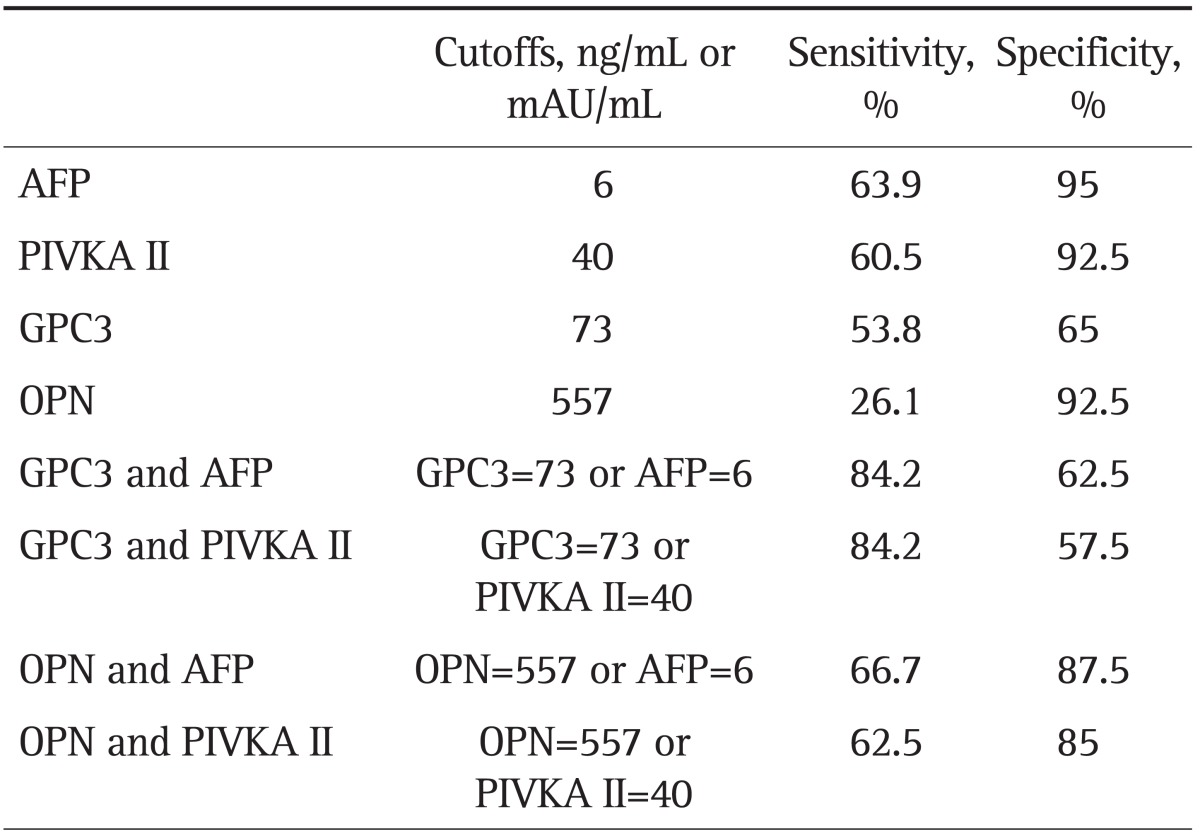
AFP, α-fetoprotein; PIVKA II, prothrombin-induced vitamin K absence II; GPC3, glypican-3; OPN, osteopontin.
We analyzed the sensitivity of tumor markers in relation to the size of the HCC tumor (Table 6). Regarding small HCCs (tumor size <2 cm in diameter), we found that 18 of 29 patients (62.1%) showed elevated levels of plasma GPC3. However, OPN, AFP, and PIVKA II had relatively low sensitivities compared to GPC3 in patients with small-sized HCCs (0%, 10.3%, and 0%, respectively).
Table 6.
Sensitivity of Tumor Markers in Relation to the Size of the Hepatocellular Carcinoma
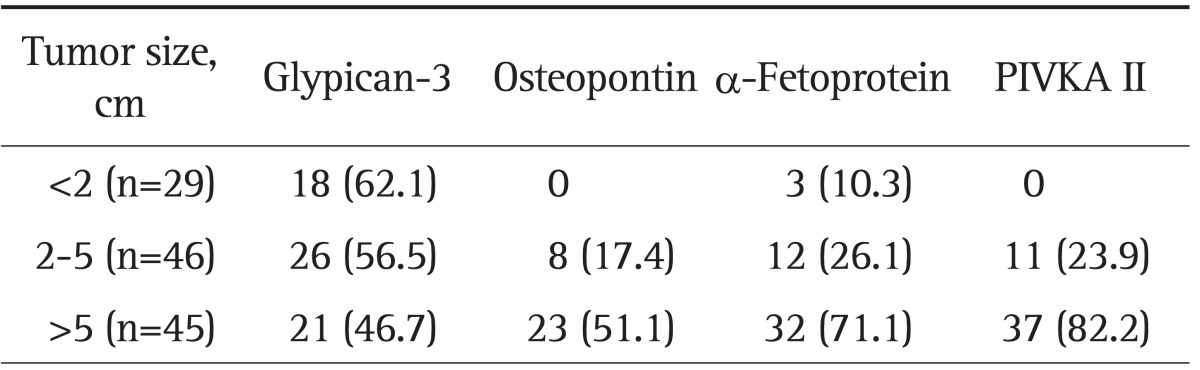
Data are presented as number (%). Glypican-3 at a cutoff value of 73 ng/mL; osteopontin at a cutoff value of 557 ng/mL; α-fetoprotein at a cutoff value of 6 ng/mL; PIVKA II at a cutoff value of 40 mAU/mL. PIVKA II, prothrombin induced vitamin K absence II.
4. Plasma GPC3 and OPN in HCC patients with low AFP and PIVKA II levels
We separately analyzed the AFP and PIVKA II levels in HCC patients. Low AFP and PIVKA II patients were defined as having serum AFP concentrations of ≤20 ng/mL and PIVKA II concentrations of ≤100 mAU/mL. In this study, 57 of 120 patients (47.5%) with HCC had low levels of serum AFP and PIVKA II. The remaining 63 HCC patients (52.5%) were included in the high AFP and PIVKA II group. Demographic data regarding levels of AFP and PIVKA II are shown in Table 7. There were no differences in age, sex, etiology, or Child-Pugh class between the two groups. The prevalence of cirrhosis was higher in the low AFP and PIVKA II group (p=0.022). Also, significant differences were documented regarding tumor multiplicity, size, portal vein thrombosis, or extrahepatic metastasis. Low levels of AFP and PIVKA II were significantly associated with early stage tumors as described by the TNM and BCLC systems (p<0.001).
Table 7.
Demographic Data of Patients with Hepatocellular Carcinoma with High and Low α-Fetoprotein (Cutoff Value of 20 ng/mL) and Prothrombin Induced Vitamin K Absence II (Cutoff Value of 100 mAU/mL) Levels
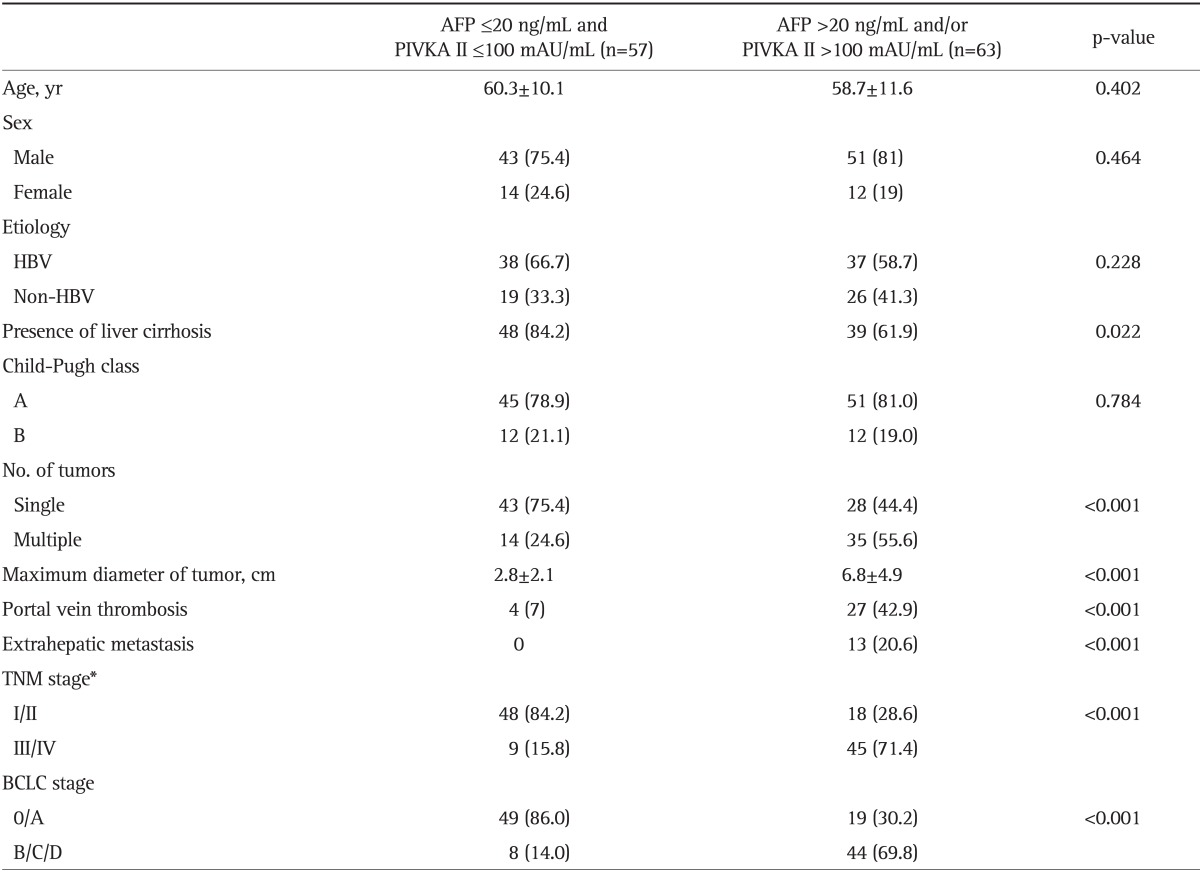
AFP, α-fetoprotein; PIVKA II, prothrombin-induced vitamin K absence II; HBV, hepatitis B virus; TNM, tumor node metastasis; BCLC, Barcelona Clinic Liver Cancer.
*TNM stage for hepatocellular carcinoma was determined according to the American Joint Committee on Cancer/United International Consensus Committee, 6th edition.
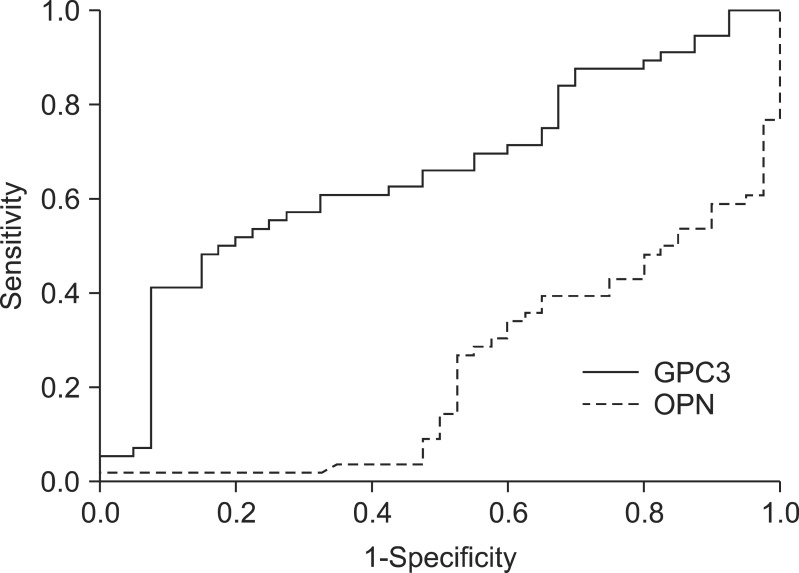
Plasma GPC3 and OPN levels in the low AFP and PIVKA II group were 86.3 and 136.8 ng/mL, respectively. Plasma GPC3 values in the low AFP and PIVKA II group were higher than in the CLD group, but plasma OPN levels in the same group were lower than in the CLD group. When only HCC patients with low AFP and PIVKA II levels were included, the GPC AUROC remained relatively high (0.66; 95% CI, 0.55 to 0.77), while the OPN AUROC remained very low (0.23; 95% CI, 0.14 to 0.33) (Fig. 3).
Fig. 3.
Area under the receiver-operating curve for glypican-3 (GPC3) and osteopontin (OPN) for chronic liver disease and hepatocellular carcinoma patients with low α-fetoprotein and protein-induced vitamin K absence II levels (0.66 vs 0.23).
DISCUSSION
In the present study, we evaluated whether levels of GPC3 and OPN could be used for the diagnosis of HCC, along with other tumor markers (AFP and PIVKA II). We observed higher levels of plasma GPC3 in patients with HCC than in patients without HCC. Plasma OPN levels in the HCC group were numerically higher than those in the CLD group, but these differences were not statistically significant. In addition, the diagnostic accuracies of GPC3 and OPN were not superior to accuracies for AFP or PIVKA II in terms of the AUROC. As a result, GPC3 or OPN would not be adequate candidates to substitute for the conventional tumor markers.
GPC3 is a member of the heparan sulfate proteoglycan family and is anchored to the plasma membrane by glycosylphosphatidylinositol. Whereas GPC3 is normally involved in cell growth, differentiation, and migration, changes in glypican expression may be associated with tumorigenesis.20 It has been reported that GPC3 is downregulated in ovarian cancer and mesothelioma, but is upregulated in HCC.21-23 Hsu et al.23 reported that GPC3 is more frequently upregulated in HCC than AFP, which is another oncofetal protein that has been widely used as tumor marker, and GPC3 may serve as a sensitive tumor marker for HCC. In some clinical studies, GPC3 could be detected in ~30% to 50% of HCC patients who were negative for AFP and/or PIVKA II. Moreover, using the combination of both GPC3 and AFP could significantly increase the sensitivity of each agent for HCC diagnosis.11,24 In the current study, GPC3 did not show better sensitivity or diagnostic accuracy compared to AFP or PIVKA II. When GPC3 was used in combination with AFP or PIVKA II, sensitivity increased, but specificity decreased. In terms of the AUROC, the diagnostic accuracy achieved by using GPC3 combined with other tumor makers did not exceed that achieved by using AFP.
On the other hand, plasma GPC3 levels were not significantly affected by almost all clinical parameters analyzed, including age, sex, the etiology of HCC, the presence of cirrhosis, or the HCC tumor stage. Until now, GPC3 has been considered to be a promising immunohistochemical marker for the differential diagnosis of HCC;10,11 however, whether it can be used as a serologic marker for HCC is still questionable. Recently, several subsequent studies have verified the value of measuring circulating GPC3 for HCC diagnosis.24-26 Tangkijvanich et al.25 reported that similar to our results, there was no correlation between serum GPC3 levels and tumor size or tumor stage. The requirements for a good biomarker may include that its levels increase according to tumor burden. This might be a limitation of using GPC3 as a serologic marker for HCC. However, further studies including large numbers of patients will certainly be required to confirm this observation.
This study also evaluated whether GPC3 could be used as a marker for the detection of small HCCs. Generally, ultrasonography (US) is the method most widely used for HCC surveillance and has an acceptable diagnostic accuracy. However, US was less effective for detecting early stage HCC, with a sensitivity of 63%. In a meta-analysis study, AFP provided no additional benefit to US.27 Our data showed that plasma GPC3 levels in patients with small-sized HCC tumors (<2 cm) provided a sensitivity of >60%, while the sensitivities of other tumor markers were extremely low (<10%). In agreement with our data, another study concerning the immunohistochemistry of GPC3 in HCC tissue showed that the expression of GPC3 in small-sized HCCs (<3 cm) was significantly greater than the expression of AFP.10 In this study, the sensitivity of GPC3 for detecting small HCCs was not superior to that of US. However, the data suggested that use of combined US and GPC3, instead of AFP, may provide additional benefits for surveillance of small-sized HCCs. Future studies are necessary to determine the optimal surveillance methods for small HCCs.
In the current study population, 57 of 120 patients with HCC (47.5%) had AFP levels <20 ng/mL and PIVKA II levels <100 mAU/mL. Serum AFP concentrations at a cutoff value of 20 ng/mL showed a sensitivity of 60%, although this sensitivity decreased for the detection of small tumors.28 When plasma levels were determined in these patients, the diagnostic accuracy of GPC3 was similar in all HCC patients. Therefore, plasma GPC3 levels might be helpful for the diagnosis of HCC in the patients with low levels of AFP and PIVKA.
OPN is a glycophosphoprotein that has a pivotal role in the regulation of cellular signaling that controls neoplastic and malignant transformation.14 Elevated OPN expression has been observed in a variety of cancers, and OPN has been linked to tumor invasion, progression, or metastasis in breast, gastric, lung, prostate, and colon cancer.14,29 In this study, plasma OPN levels were not statistically elevated in patients with HCC compared to levels in patients without HCC, showing a low diagnostic accuracy in terms of AUROC. However, within the HCC patient group, plasma OPN levels increased significantly in patients with large and multiple tumors, portal vein thrombosis, and distant metastasis. This data was in agreement with several previous reports concerning the use of OPN for diagnosis of HCC. Pan et al.15 demonstrated that overexpression of OPN mRNA correlated with high grade, late-stage, and early tumor recurrence. Similarly, Zhang et al.30 demonstrated that OPN overexpression was associated with invasion by tumor blood vessels and advanced tumor grade. In addition, plasma OPN levels were shown to increase significantly with advancement of tumor stage and tumor recurrence.31,32 These studies suggested that plasma OPN levels could be used as a prognostic and predictive marker for HCC. In the current study population, most of the HCC patients had an early stage tumor (55%, TNM stage I or II), and did not have vascular invasion (74.2%) or metastases (89.2%). In other studies, a relatively higher proportion of HCC patients had advanced tumor stage and invasive tumor characteristics compared to our study population. This could explain the statistically non-significant elevation of OPN levels found in HCC patients in this study. On the other hand, a recent study using plasma proteomic profiling suggested OPN could be a marker for early-stage HCC. Moreover, in a pilot prospective study, almost 87% of patients had OPN levels greater than the cutoff point preceding HCC diagnosis.33 This finding is in conflict with previous studies and the present study. Further studies with large numbers of patients are needed to confirm this result.
Several recent studies have suggested a potential role for OPN in liver fibrosis;34-36 however, in our study, OPN levels were negatively associated with the presence of cirrhosis in HCC patients. On the other hand, in control patients without HCC, OPN levels were significantly higher in patients with cirrhosis than in patients without cirrhosis (486.3 ng/mL vs 158.4 ng/mL, p<0.001). This lack of concordance may be due to a role of OPN in tumorigenesis rather than in fibrosis. In our study population, HCC patients without cirrhosis had a greater incidence of having an advanced stage of tumor than HCC patients with cirrhosis (66.6% vs 36.8%); further investigations are warranted to elucidate these findings.
Our current study has a limitation concerning the methodology used. We measured levels of GPC3 and OPN using stored plasma samples, but levels of AFP and PIVKA II were measured using serum samples taken for routine laboratory examinations. Ideally, all biomarkers should have been measured using the same samples. Further evaluations using a unified measuring protocol and the same plasma or serum samples will be needed in the future.
In conclusion, our study showed that plasma GPC3 levels were significantly elevated in patients with HCC compared to patients with CLD, but plasma OPN levels did not show statistically significant differences between the two groups. Furthermore, plasma GPC3 and OPN levels had low diagnostic accuracies for HCC compared with the accuracies achieved with AFP and PIVKA II. However, GPC3 may have a complementary role in diagnosing HCC in patients with low serum levels of AFP and PIVKA II, and also for diagnosing the presence of small-sized HCC tumors. Further analysis is needed to determine the utility of OPN in diagnosing HCC, and the search for novel biomarkers for HCC will continue.
ACKNOWLEDGEMENTS
This work was supported by a Korea University Grant and the Jeil Pharmaceutical Co., Ltd., Korea.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121:119–126. doi: 10.1016/j.amjmed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMahon BJ, Bulkow L, Harpster A, et al. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology. 2000;32(4 Pt 1):842–846. doi: 10.1053/jhep.2000.17914. [DOI] [PubMed] [Google Scholar]
- 6.Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432–438. [PubMed] [Google Scholar]
- 7.Kasahara A, Hayashi N, Fusamoto H, et al. Clinical evaluation of plasma des-gamma-carboxy prothrombin as a marker protein of hepatocellular carcinoma in patients with tumors of various sizes. Dig Dis Sci. 1993;38:2170–2176. doi: 10.1007/BF01299891. [DOI] [PubMed] [Google Scholar]
- 8.Ikoma J, Kaito M, Ishihara T, et al. Early diagnosis of hepatocellular carcinoma using a sensitive assay for serum des-gamma-carboxy prothrombin: a prospective study. Hepatogastroenterology. 2002;49:235–238. [PubMed] [Google Scholar]
- 9.Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capurro M, Wanless IR, Sherman M, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 11.Nakatsura T, Yoshitake Y, Senju S, et al. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 2003;306:16–25. doi: 10.1016/s0006-291x(03)00908-2. [DOI] [PubMed] [Google Scholar]
- 12.Anborgh PH, Mutrie JC, Tuck AB, Chambers AF. Role of the metastasis-promoting protein osteopontin in the tumour microenvironment. J Cell Mol Med. 2010;14:2037–2044. doi: 10.1111/j.1582-4934.2010.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Tanani MK. Role of osteopontin in cellular signaling and metastatic phenotype. Front Biosci. 2008;13:4276–4284. doi: 10.2741/3004. [DOI] [PubMed] [Google Scholar]
- 14.Johnston NI, Gunasekharan VK, Ravindranath A, O'Connell C, Johnston PG, El-Tanani MK. Osteopontin as a target for cancer therapy. Front Biosci. 2008;13:4361–4372. doi: 10.2741/3009. [DOI] [PubMed] [Google Scholar]
- 15.Pan HW, Ou YH, Peng SY, et al. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer. 2003;98:119–127. doi: 10.1002/cncr.11487. [DOI] [PubMed] [Google Scholar]
- 16.Gotoh M, Sakamoto M, Kanetaka K, Chuuma M, Hirohashi S. Overexpression of osteopontin in hepatocellular carcinoma. Pathol Int. 2002;52:19–24. doi: 10.1046/j.1440-1827.2002.01316.x. [DOI] [PubMed] [Google Scholar]
- 17.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 18.Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 19.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 20.Filmus J, Selleck SB. Glypicans: proteoglycans with a surprise. J Clin Invest. 2001;108:497–501. doi: 10.1172/JCI13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin H, Huber R, Schlessinger D, Morin PJ. Frequent silencing of the GPC3 gene in ovarian cancer cell lines. Cancer Res. 1999;59:807–810. [PubMed] [Google Scholar]
- 22.Murthy SS, Shen T, De Rienzo A, et al. Expression of GPC3, an X-linked recessive overgrowth gene, is silenced in malignant mesothelioma. Oncogene. 2000;19:410–416. doi: 10.1038/sj.onc.1203322. [DOI] [PubMed] [Google Scholar]
- 23.Hsu HC, Cheng W, Lai PL. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Cancer Res. 1997;57:5179–5184. [PubMed] [Google Scholar]
- 24.Liu H, Li P, Zhai Y, et al. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J Gastroenterol. 2010;16:4410–4415. doi: 10.3748/wjg.v16.i35.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tangkijvanich P, Chanmee T, Komtong S, et al. Diagnostic role of serum glypican-3 in differentiating hepatocellular carcinoma from non-malignant chronic liver disease and other liver cancers. J Gastroenterol Hepatol. 2010;25:129–137. doi: 10.1111/j.1440-1746.2009.05988.x. [DOI] [PubMed] [Google Scholar]
- 26.Qiao SS, Cui ZQ, Gong L, et al. Simultaneous measurements of serum AFP, GPC-3 and HCCR for diagnosing hepatocellular carcinoma. Hepatogastroenterology. 2011;58:1718–1724. doi: 10.5754/hge11124. [DOI] [PubMed] [Google Scholar]
- 27.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trevisani F, D'Intino PE, Morselli-Labate AM, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570–575. doi: 10.1016/s0168-8278(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 29.Wai PY, Kuo PC. The role of Osteopontin in tumor metastasis. J Surg Res. 2004;121:228–241. doi: 10.1016/j.jss.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 30.Zhang CH, Xu GL, Jia WD, et al. Prognostic significance of osteopontin in hepatocellular carcinoma: a meta-analysis. Int J Cancer. 2012;130:2685–2692. doi: 10.1002/ijc.26301. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Ki SS, Lee SD, et al. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. Am J Gastroenterol. 2006;101:2051–2059. doi: 10.1111/j.1572-0241.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Ye QH, Ren N, et al. The prognostic significance of preoperative plasma levels of osteopontin in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2006;132:709–717. doi: 10.1007/s00432-006-0119-3. [DOI] [PubMed] [Google Scholar]
- 33.Shang S, Plymoth A, Ge S, et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology. 2012;55:483–490. doi: 10.1002/hep.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorena D, Darby IA, Gadeau AP, et al. Osteopontin expression in normal and fibrotic liver altered liver healing in osteopontin-deficient mice. J Hepatol. 2006;44:383–390. doi: 10.1016/j.jhep.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Syn WK, Choi SS, Liaskou E, et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53:106–115. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urtasun R, Lopategi A, George J, et al. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin α(V) β(3) engagement and PI3K/pAkt/NFκB signaling. Hepatology. 2012;55:594–608. doi: 10.1002/hep.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]