Abstract
Background/Aims
Pre-existing diabetes mellitus (DM) has been identified as an adverse prognostic variable associated with increased mortality in various cancers. Although DM and hyperglycemia are considered risk factors for pancreatic cancer (PC), antidiabetic treatments for patients with advanced PC have been overlooked. This study aimed to evaluate the impact of hemoglobin A1c (HbA1c) levels on PC survival.
Methods
We retrospectively reviewed the medical records of first-diagnosed patients with advanced PC who were admitted to Konkuk University Medical Center from 2005 to 2011.
Results
A total of 127 patients were enrolled, and there were 111 deaths (87.4%) within the 7-year observational period. The most common etiology was disease progression (n=108). DM before PC diagnosis was observed in 65 patients (51.1%), including 28 patients with new-onset DM. The overall median survival times in patients with and without DM were 198 and 263 days, respectively (p=0.091). Survival time according to HbA1c was significantly different between the <7.0% and ≥7.0% groups (362 and 144 days, respectively; p=0.038). In the HbA1c ≥7.0% group, the median overall survival time was 273 days for the metformin group and 145 days for the nonmetformin oral agent group; however, there was no significant difference between the two groups (p=0.058).
Conclusions
A high HbA1c level may be associated with worse survival in patients with advanced PC with DM. Antidiabetic treatment, metformin in particular, was associated with an improved outcome.
Keywords: Pancreatic neoplasms, Diabetes mellitus, Glycosylated hemoglobin A, Metformin
INTRODUCTION
Pancreatic cancer (PC) is a devastating disease that poses significant management challenges. Because of its exceptionally high mortality with an overall survival of <5%, PC ranks as the fifth leading cause of cancer-related death in most developed countries.1,2 Currently, there is no effective means to screen for or prevent this cancer.
The association between diabetes mellitus (DM) and PC is well-documented.3-5 However, there has been some concern that DM may be a consequence rather than a cause of this neoplasia.5,6 The precise prevalence of DM in patients with PC has been difficult to establish because studies relying upon self-reports or registry data for diagnosis of DM have largely underestimated its frequency in patients with PC. Recent studies applying objective biochemical criteria showed that while the prevalence of DM in patients with PC is around 40%, as many as 80% of patients with PC may have abnormalities in glucose metabolism.4,7
Pre-existing DM has been identified as an adverse prognostic variable associated with increased mortality in various cancers, including colorectal, prostate, and breast cancers.8,9 Pre-existing DM was also independently associated with reduced survival in patients undergoing resection for PC.10,11 Postload plasma glucose concentration and PC mortality were inversely associated in men with a normal glycemic level.12
A hyperglycemic state has various indicators; of them, hemoglobin A1c (HbA1c) reflects long-term glycemic control and is a more stable measurement than fasting plasma glucose levels.13 Although the associations between HbA1c levels and incidence or mortality for malignancies have been shown in two cohort studies,14,15 no studies have evaluated the impact of HbA1c levels on the survival of advanced PC.
The diagnosis and treatment of cancer may distract both the patient and the health care team from appropriate management of hyperglycemia, which is proven to reduce morbidity and mortality in adult patients with DM. The expected survival of advanced PC is extremely short, and many physicians are not concerned about either DM itself or glycemic control.
In the present study, we conducted a retrospective analysis of the relationship between HbA1c levels and advanced PC with DM survival, and took into consideration antidiabetic therapy, including metformin.
MATERIALS AND METHODS
1. Patients
Data from the Konkuk University Medical Center were used in this study. Institutional Review Board approved retrospective review. The data was collected between May 2005 and April 2011. One hundred and sixty-five patients with advanced PC were evaluated. Thirty-eight patients were excluded because of missing follow-up data, age of >90 years, lack of evaluation for DM, poor performance status, or gastrointestinal obstruction by cancer. Thus, 127 patients underwent analysis. A total of 65 patients with pre-existing DM and 62 patients without pre-existing DM were recruited. Sixty-five patients with pre-existing DM were divided into a low HbA1c group (group A, <7.0% of HbA1c, n=24) and a high HbA1c group (group B, ≥7.0% of HbA1c, n=41).
2. Definition of diabetes
Diagnosis of pre-existing DM was made based on the documented clinical history and/or biochemical findings with application of the diagnostic criteria outlined by the American Diabetes Association in January 2010.16 In brief, patients with a known history of DM, a baseline fasting blood glucose level of ≥126 mg/dL, two or more outpatient random blood glucose levels of ≥200 mg/dL, or an HbA1c level of ≥6.5% were classified into the DM group. Among patients with only inpatient preoperative laboratory results, those with two or more serum measurements of ≥200 mg/dL obtained before 7:00 AM were classified as having DM. The duration of DM before the cancer diagnosis was determined based on the clinical history or first date of documented laboratory abnormalities. New-onset DM was defined as a diagnosis of DM 24 months before the PC diagnosis, and patients with a diagnosis of DM >24 months preceding the cancer diagnosis were classified as having long-standing DM.7,17 DM medication profiles were classified into three groups (metformin with/without other agents, other agents with/without insulin, or no treatment).
3. Measurement of HbA1c values
HbA1c levels were measured by high performance liquid chromatography (ADAMS HA-8180; Arkray Inc., Tokyo, Japan) in 65 patients with unresectable PC who were recruited after 2005. Patients without pre-existing DM were not measured because of their normal fasting glucose level. According to the guidelines of the American Diabetes Association,16 an HbA1c level of 4.0% to 6.0% was considered to be; an HbA1c level of <7.0% was the recommended glycemic control level for adults with DM. Therefore, we divided all patients with PC and DM into two groups according to their HbA1c level: <7.0% and ≥7.0%.
4. Data collection
Initial variables were included age, gender, education, body mass index (BMI), smoking, alcohol consumption, performance status (Karnofsky status),18 family history of PC, and carbohydrate antigen 19-9 (CA 19-9) level. Family history of cancer was restricted to first-degree relatives. The BMI was calculated at admission. According to the World Health Organization standard, BMIs of <24.9, 25 to 29.9, and ≥30 kg/m2 were defined as normal, overweight, and obese, respectively. Education was classified as less than high school and high school graduate or more. Chemotherapy history after diagnosis of PC was evaluated.
Because the number of patients who received monotherapy was small, insulin use was not considered for an individual group of glycemic control. In addition, many patients used the drug or the combinations changed over time; thus, the final analysis used the categorical variables of ever or never use of metformin. Oral medications were categorized into two groups: 1) metformin and 2) other drugs (not including metformin), including insulin secretagogues, thiazolidinediones, dipeptidyl peptidase-4, and glucagon-like peptide 1 analogue.
5. Statistical analysis
Patients were classified into DM and non-DM groups. In the DM group, patients were classified according to HbA1c level: <7.0% (group A) and ≥7.0% (group B). Cigarette smoking status was classified as never and ever (past and current).
Numerical data are presented as the median with interquartile range (IQR). Data for patients with PC with DM and without DM were compared with respect to demographic, clinical, and treatment variables using Pearson chi-square test and Fisher exact test for categorical variables. Among patients with DM, data for patients with ≥7.0% and <7.0% HbA1c were compared using the above methods. Estimates of probabilities of survival for the follow-up study were calculated using the Kaplan-Meier method with the log-rank test. Data were summarized by the median and 95% confidence intervals (CIs). For survival rates, deaths unrelated to PC, such as cardiovascular events, were treated as censored patients. Survival was calculated from the day of diagnosis until death or the last follow-up.
Cox regression was used to determine independent predictors of outcome, using survival as the dependent variable and significant factors (p<0.15) by univariate analysis as the independent variables. The p-values ≤0.05 were deemed to indicate statistical significance. Statistical analyses were performed using the SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Population characteristics
The characteristics of the study population, including risk factors for PC, are shown in Table 1. Baseline clinical and demographic profiles in the DM and non-DM groups were similar with the exception the CA 19-9 level. The median level of CA 19-9 was higher in the DM than in the non-DM group (670.3, IQR=139.4 to 3,955.0 vs 514.4, IQR=99.4 to 3,687.3; p=0.036). In total, the median age was 69 years (range, 43 to 90 years), the median BMI was 22.6 kg/m2 (range, 15.2 to 33.3 kg/m2), and 47.2% of patients were male. A documented history of tobacco use was present in 34.6% of patients. Fifty-eight patients (45.7%) underwent gemcitabine-based chemotherapy. Ninety-two patients (72.4%) had a low education level (less than high school); however, there was no significant difference between the DM and non-DM groups.
Table 1.
Clinicopathological Characteristics of Diabetes and Nondiabetes in Advanced Pancreatic Cancer Patients
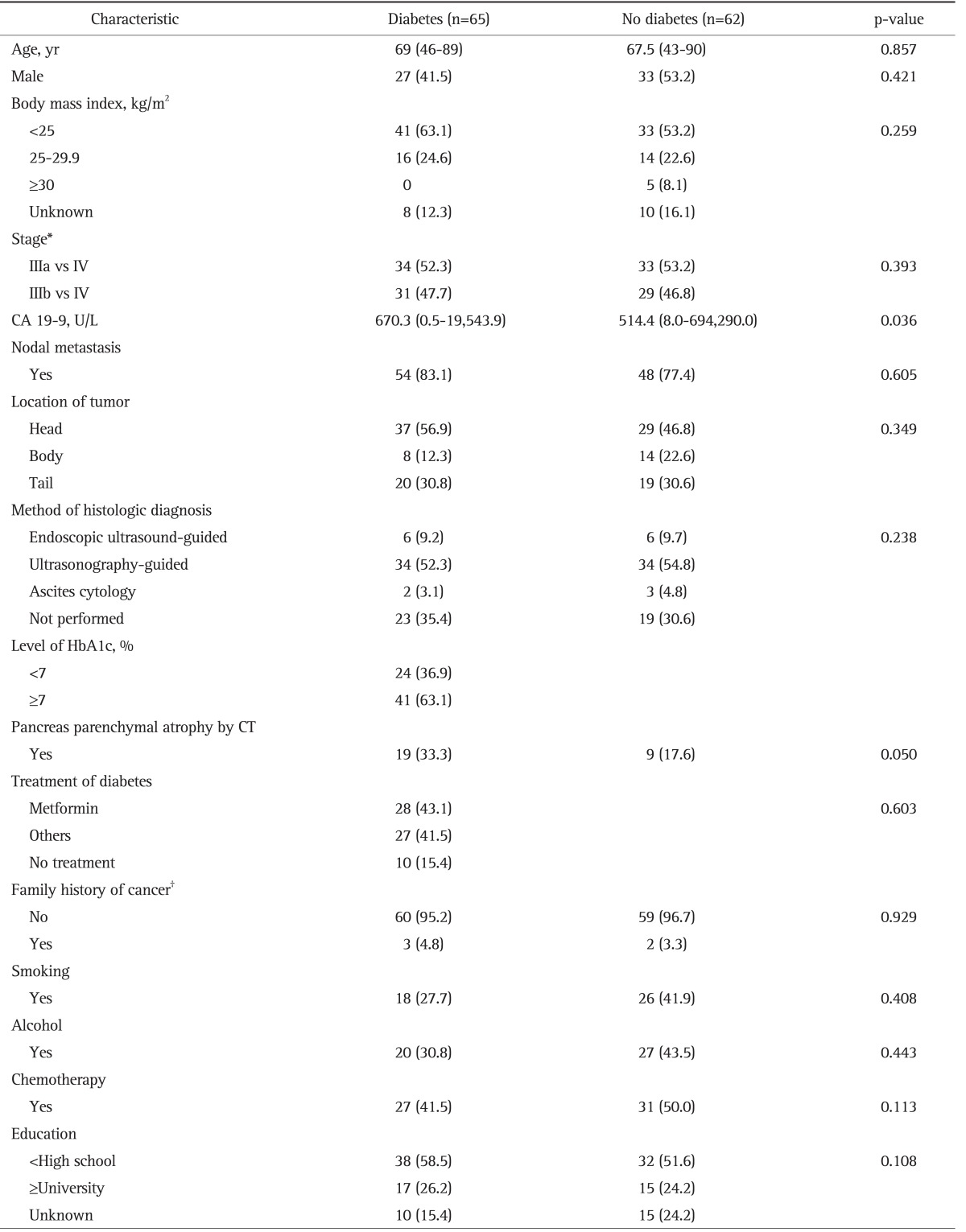
Data are presented as median (range) or number (%).
CA 19-9, carbohydrate antigen 19-9; HbA1c, hemoglobin A1c; CT, computed tomography.
*American Joint Committee Cancer staging 7th edition; †Information on family history was missing for five cases.
DM was documented or diagnosed using initial laboratory results in 65 patients (51.2%). DM was determined to be of new onset in 28 patients (43.1%). Patients with DM showed a higher frequency of pancreatic parenchymal atrophic changes on abdominal computed tomography than did patients without DM (33.3% vs 17.6%, respectively; p=0.05). However, there was no statistically significant difference in the frequency of pancreas parenchymal atrophy between patients with DM in groups A and B (37.5% vs 28.9%, respectively; p=0.378). Fifty-five patients (84.6%)-29 (52.7%), metformin use; 26 (47.3%), metformin nonuse-were taking oral agents for DM, 12 of 55 patients (21.8%) were also being administered insulin, and 10 patients (15.4%) were not on any DM medications. At initial diagnosis of PC, 41 patients (63.1%) had a high HbA1c level (≥7.0%) and 24 patients (30.8%) had a HbA1c level of <7.0%. Four patients (6.2%) with clinical documentation of DM did not undergo evaluation of the HbA1c level.
2. Influence of HbA1c level and antidiabetic treatment on survival of patients with PC and DM
The demographic characteristics of group A (HbA1c of <7.0%) and group B (HbA1c of ≥7.0%) in advanced PC with DM were summarized in Table 2. There was no significant difference in age, sex, BMI, smoking, alcohol consumption, education, tumor location, chemotherapy, or type of antidiabetic therapy between groups A and B. The median level of HbA1c was 6.5% (range, 5.7% to 6.9%) in group A and 8.1% (range, 7.0% to 20.0%) in group B (p=0.003).
Table 2.
Clinicopathological Characteristics according to Hemoglobin A1c Levels in Advanced Pancreatic Cancer with Diabetes
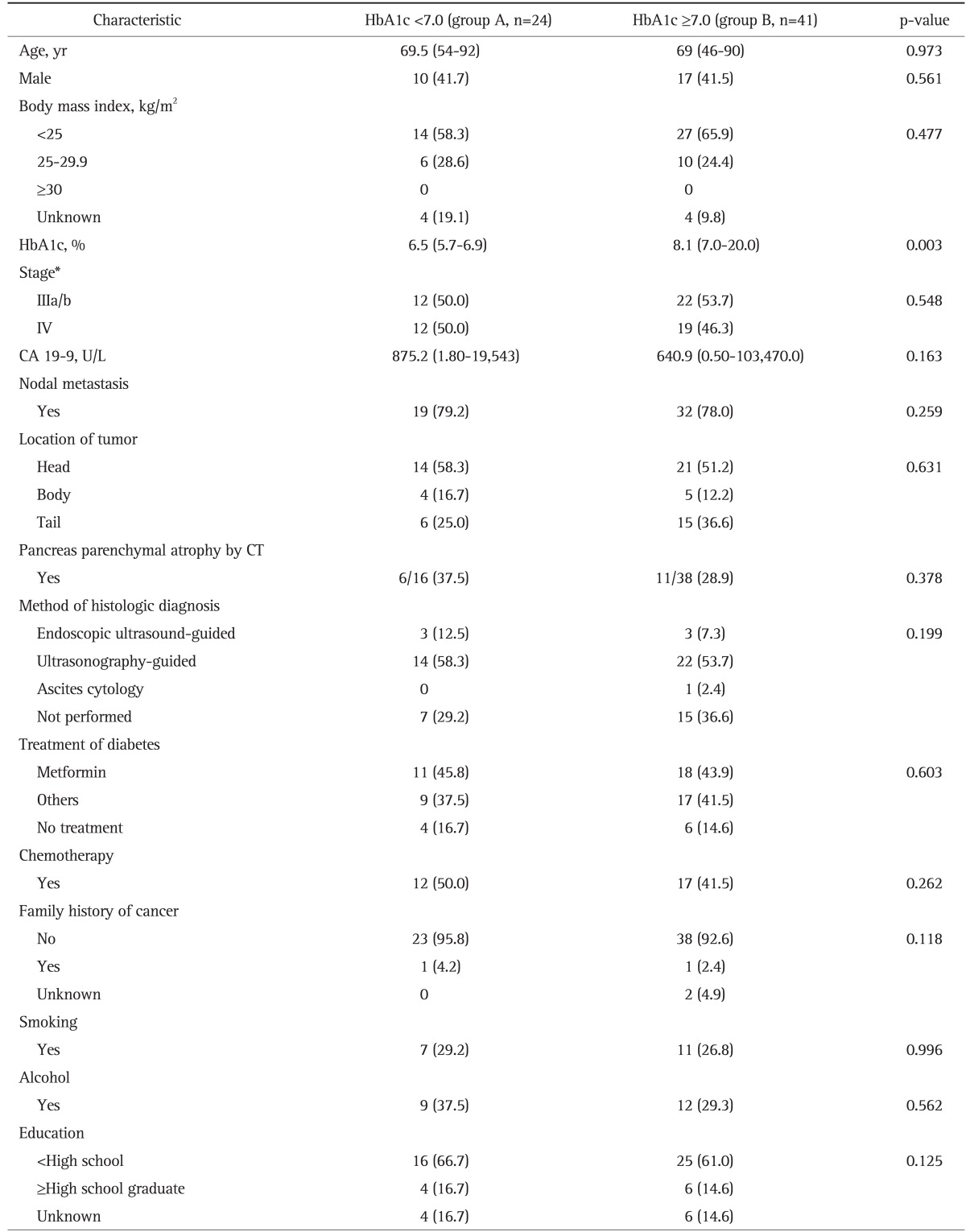
Data are presented as median (range) or number (%).
HbA1c, hemoglobin A1c; CA 19-9, carbohydrate antigen 19-9; CT, computed tomography.
*American Joint Committee Cancer staging 7th edition.
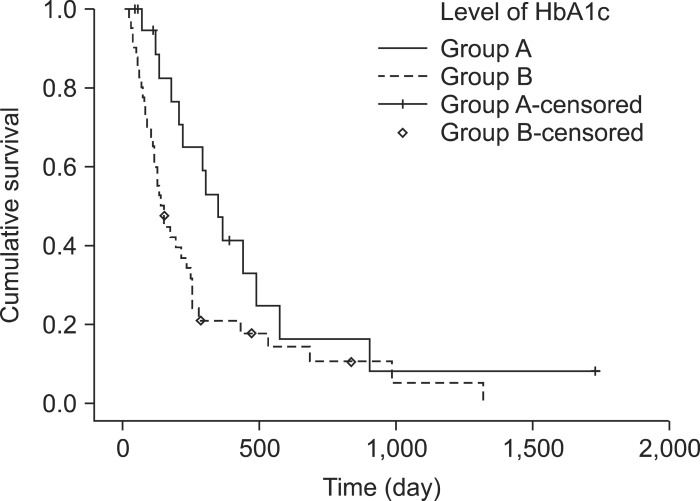
Kaplan-Meier survival analysis showed a slightly longer survival time in patients without DM than in patients with DM (Fig. 1), but the difference was not statistically significant. The median survival was 263 days in patients without DM (95% CI, 114.1 to 372.0) and 198 days in patients with DM (95% CI, 125.4 to 283.5; p=0.091). Among patients with DM, Kaplan-Meier survival analysis showed a significantly longer survival time in group A (HbA1c level of <7.0%) than in group B (HbA1c level of ≥7.0%) (Fig. 2). The median survival was 362 days (95% CI, 246.2 to 461.8) in group A and 145 days (95% CI, 109.0 to 179.0) in group B (p=0.014).
Fig. 1.
Kaplan-Meier estimated survival probability curve for patients with advanced pancreatic cancer with and without diabetes. The median survival of patients with diabetes was 198 days (95% confidence interval [CI], 125.4 to 283.5) compared with 263 days (95% CI, 114.1 to 372.0) in the nondiabetic group (p=0.091).
DM, diabetes mellitus.
Fig. 2.
Kaplan-Meier estimated survival probability curve for patients with advanced pancreatic cancer with diabetes according to the level of hemoglobin A1c (HbA1c; <7.0%, group A) and according to the median survival was 362 days (95% confidence interval [CI], 246.2 to 461.8) in group A and 145 days (95% CI, 109.0 to 179.0) in group B (p=0.014).
Metformin use showed a longer survival than metformin nonuse in patients with DM; however, it did not show a statistically significant difference (median survival, 264 days vs 195 days, respectively; p=0.058). In the subgroup analysis of patients with an HbA1c level of ≥7.0%, metformin use also showed a longer survival compared with other antidiabetic medications; however, the difference was not statistically significant (median survival, 273 days vs 145 days; p=0.058) (Fig. 3). The mean value (standard deviation) of HbA1c was 9.2% (3.2) in metformin use and 8.2% (2.4) metformin nonuse in patients with DM (p=0.342).
Fig. 3.
Kaplan-Meier estimated survival probability curve for patients with hemoglobin A1c levels ≥7.0% according to the antidiabetic treatment (metformin use or metformin nonuse). The median survival time was 273 days (95% confidence interval [CI], 128.2 to 359.8) in the metformin use group and 145 days (95% CI, 39.5 to 256.3) in the metformin nonuse group (p=0.058).
3. Impact of other potential variables on survival
The median survival was 284 days (95% CI, 209.6 to 372.4) in stage IIIa/b and 119 days (95% CI, 93.5 to 144.5) in stage IV (p<0.001). The 1- and 2-year survival rates were 43% and 20% in stage IIIa/b and 21% and 3% in stage IV, respectively. The median survival was 298 days (95% CI, 226.8 to 355.2) in patients with chemotherapy and 166 days (95% CI, 92.0 to 254.0) in patients without chemotherapy (p=0.005). The 1- and 2-year survival rates were 45% and 18% in patients with chemotherapy and 22% and 9% in patients without chemotherapy, respectively.
Table 3 shows the results of univariate and multivariate analyses of all variables associated with survival in all patients. DM (hazard ratio [HR], 1.28; 95% CI, 0.89 to 2.49; p=0.091), CA 19-9 of >150 U/L (HR, 2.11; 95% CI, 1.27 to 3.49; p=0.003), stage IV (HR, 1.77; 95% CI 1.27 to 2.47; p<0.001), lymph node metastasis (HR, 1.46; 95% CI, 1.27 to 2.45; p=0.030), and no chemotherapy (HR, 1.81; 95% CI, 1.19 to 2.77; p=0.005) were statistically significant predictors of survival in the univariate analysis.
Table 3.
Results of Univariate and Multivariate Analysis of All Prognostic Factors Associated with Survival in All Patients
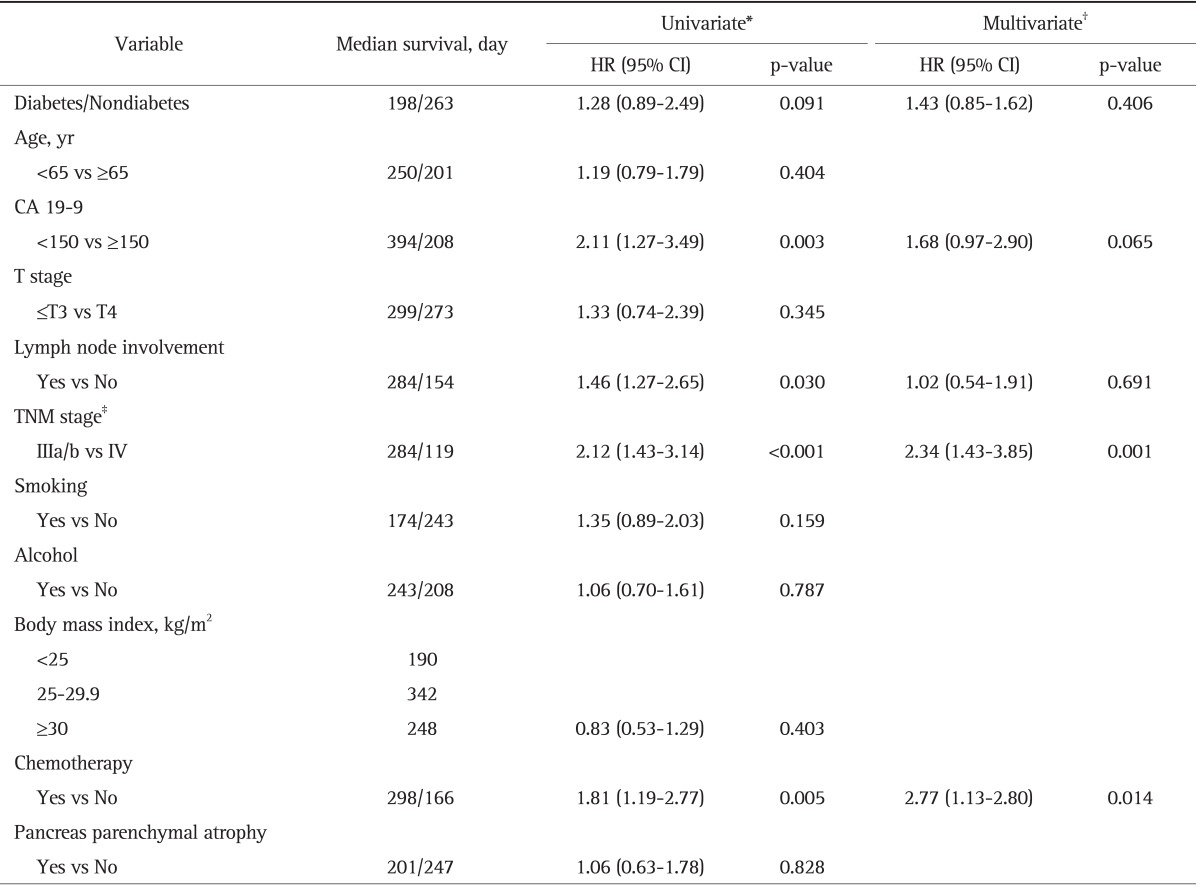
HR, hazard ratio; CI, confidence interval; CA 19-9, carbohydrate antigen 19-9; TNM, tumor, node, metastasis.
*Kaplan-Meier and long rank test; †Cox proportional hazards model; ‡American Joint Committee Cancer staging 7th edition.
In the multivariate analysis using the Cox regression model in all patients, chemotherapy (HR, 2.77; 95% CI, 1.13 to 2.80; p=0.014) and stage III (HR, 2.34; 95% CI, 1.43 to 3.85) were the significant predictor of longer survival in advanced PC.
In subgroup analysis in patients with DM (Table 4), an HbA1c level of ≥7.0% (HR, 2.12; 95% CI, 1.15 to 3.92; p=0.014), CA 19-9 level of >150 U/L (HR, 1.98; 95% CI, 1.08 to 3.95; p=0.043), metformin nonuse (HR, 1.53; 95% CI, 0.93 to 2.94; p=0.058), and no chemotherapy (HR, 2.23; 95% CI, 1.22 to 4.08; p=0.003) were statistically significant predictors of survival in the univariate analysis. Multivariate analysis using the Cox regression model showed that no chemotherapy (HR, 1.98; 95% CI, 1.04 to 4.32; p=0.047) and high level of HbA1c (HR, 2.55; 95% CI, 1.08 to 6.04; p=0.033) were significantly associated with poor survival compared with patients with chemotherapy and low level of HbA1c in advanced PC with DM.
Table 4.
Results of Univariate and Multivariate Analysis of All Prognostic Factors Associated with Survival in Diabetes

HR, hazard ratio; CI, confidence interval; HbA1c, hemoglobin A1c; CA 19-9, carbohydrate antigen 19-9; TNM, tumor, node, metastasis.
*Kaplan-Meier and long rank test; †Cox proportional hazards model; ‡American Joint Committee Cancer staging 7th edition.
At the end of the observation period, 56 patients (86.2%) with DM and 55 patients (88.7%) without DM died. The causes of death in patients with DM were tumor progression (94.6%, n=53) and non-PC-related causes (5.4%, n=3 with heart disease). The causes of death in patients without DM were tumor progression (98.2%, n=54), and biliary sepsis (1.8%, n=1).
DISCUSSION
DM and impaired glucose tolerance are thought to be risk factors for not only cardiovascular events,19 but also malignancies.3,20 Comorbid of DM has been implicated as an adverse prognostic variable for cancer in general. A recent meta-analysis of 23 studies reported that pre-existing DM was associated with increased all cause mortality relative to non-DM cancer patients (HR, 1.41; 95% CI, 1.28 to 1.55).8 Two large cohort studies examined the association of DM for ≥10 years with subsequent PC risk.21,22 In both studies, DM was positively associated with PC risk. Another prospective cohort study reported a 2.5-fold greater risk of PC mortality among men with DM compared with those without DM.23
Most studies that have investigated the association between DM and the cancer morbidity or mortality used the fasting plasma glucose level or postload plasma glucose level. On the other hand, HbA1c is a good time-integrated indicator of blood glucose concentrations over the preceding 1 to 3 months.24 It is also a particularly convenient screening or monitoring tool for DM because it does not require subjects to fast. However, no studies to date have investigated the association between HbA1c levels and the mortality of advanced PC. Because PC has a very rapid clinical course and is almost uniformly fatal, it may distract both the patient and the health care team from the diagnosis of DM and appropriate management of hyperglycemia. Our findings indicate that an elevated HbA1c level (≥7.0%) was associated with decreased survival in patients with advanced PC with DM.
Another possibility is that DM and hyperglycemia affect tumor behavior by increasing proliferation and dissemination. There are several potential explanations for the observed association between increased all cause mortality and pre-existing DM in patients with cancer. First, patients with both cancer and DM may have increased tumor cell proliferation and metastases in a physiologic environment of hyperinsulinemia and hyperglycemia.25 Insulin has been shown to have a direct, dose-dependent, growth-promoting effect on PC cell lines in vitro.26 Moreover, high concentrations of insulin are able to bind to and activate the insulin-like growth factor 1 (IGF-1) receptor.27 Activation of this receptor is known to have growth-promoting effects, including modulation of cell cycle progression. Excess insulin could also indirectly affect the development of PC through down-regulation of IGF-binding protein 1.28 Second, patients with preexisting DM may have a poor response to cancer treatment, including increased risk and intraoperative mortality.29 In this study, subgroup analysis of the patients with chemotherapy showed that survival was shorter in patients with an HbA1c level of ≥7.0% than in patients with an HbA1c level of <7.0%, although the difference was not statistically significant (median survival, 394 days, 95% CI, 271.7 to 516.4 vs 227 days, 95% CI, 80.8 to 373.2; p=0.093). Finally, DM is a well-established risk factor for cardiovascular mortality in adults without cancer,30 and the microvascular and macrovascular damage it causes likely accumulates to some extent regardless of cancer status. In this study, while three patients (7.3%) died of cardiovascular events in the high HbA1c group, there was no death related to cardiovascular events in the low HbA1c group.
Because PC has a very rapid clinical course and is almost uniformly fatal, many physicians are not concerned with either DM itself or glycemic control. There are no studies of the impact of DM or high levels of glucose on mortality in patients with advanced PC with DM. Our findings revealed that DM does not have an impact on the prognosis of advanced PC compared with previous studies, which showed an association between DM and cancer mortality. However, in patients with DM, a high HbA1c level was associated with poor survival compared with a low level of HbA1c in PC with DM. The median survival was 362 days (95% CI, 246.2 to 461.8) in group A and 145 days (95% CI, 109.0 to 179.0) in group B (p=0.014). Appropriate antidiabetic treatment is required in advanced PC if the patients do not have a poor performance status or advanced stage. Among antidiabetic therapeutic agents, metformin was associated with a longer survival of PC in this study (p=0.058). However, the statistical power was limited because this observation was made from a small number of study subjects (25 metformin and 28 other antidiabetics). Two recent epidemiologic studies reported that patients with DM treated with metformin were less likely to develop cancer, but those treated with insulin or sulfonylurea were more likely to die of cancer.31,32 The molecular target of metformin is unknown, but it acts to inhibit complex I of the mitochondrial electron transport chain to block oxidative respiration.33 This results in increased cellular adenosine monophosphate (AMP) to adenosine triphosphate ratios and activates AMP-activated protein kinase (AMPK).34 AMPK coordinates the activity of a plethora of key metabolic and growth pathways that together act to restore the cellular energy balance.35 Furthermore, AMPK has been found to play a role in cell polarity and cell division.36 Therefore, in addition to amelioration of hyperglycemia and hyperinsulinemia, which are factors that mediate the adverse impact of type 2 DM on cancer, metformin has direct effects on cancer cells, blocking the mitogenic effects of insulin and IGF-1.
This study has several major limitations. First, it was retrospective in design and included a single tertiary referral center. Second, the statistical power was limited because these observations were made from a small number of study subjects. The association between high HbA1c levels and shorter survival of advanced PC must be further investigated in larger studies. However, this cannot be investigated in a prospective randomized controlled study due to ethical and moral issues. Third, the change in the HbA1c level after antidiabetic treatment was not evaluated.
In conclusion, high HbA1c is associated with poor survival in patients with PC. Antidiabetic treatment, metformin in particular, use is associated with an improved outcome.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis. 2010;28:645–656. doi: 10.1159/000320068. [DOI] [PubMed] [Google Scholar]
- 3.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273:1605–1609. [PubMed] [Google Scholar]
- 4.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–987. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F, Gupta S, Holly EA. Diabetes mellitus and pancreatic cancer in a population-based case-control study in the San Francisco Bay Area, California. Cancer Epidemiol Biomarkers Prev. 2006;15:1458–1463. doi: 10.1158/1055-9965.EPI-06-0188. [DOI] [PubMed] [Google Scholar]
- 6.Noy A, Bilezikian JP. Clinical review 63: diabetes and pancreatic cancer: clues to the early diagnosis of pancreatic malignancy. J Clin Endocrinol Metab. 1994;79:1223–1231. doi: 10.1210/jcem.79.5.7962312. [DOI] [PubMed] [Google Scholar]
- 7.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. 2007;120:1986–1992. doi: 10.1002/ijc.22532. [DOI] [PubMed] [Google Scholar]
- 10.Chu CK, Mazo AE, Goodman M, et al. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2010;17:502–513. doi: 10.1245/s10434-009-0789-6. [DOI] [PubMed] [Google Scholar]
- 11.Cannon RM, LeGrand R, Chagpar RB, et al. Multi-institutional analysis of pancreatic adenocarcinoma demonstrating the effect of diabetes status on survival after resection. HPB (Oxford) 2012;14:228–235. doi: 10.1111/j.1477-2574.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith GD, Egger M, Shipley MJ, Marmot MG. Post-challenge glucose concentration, impaired glucose tolerance, diabetes, and cancer mortality in men. Am J Epidemiol. 1992;136:1110–1114. doi: 10.1093/oxfordjournals.aje.a116576. [DOI] [PubMed] [Google Scholar]
- 13.Rohlfing CL, Little RR, Wiedmeyer HM, et al. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000;23:187–191. doi: 10.2337/diacare.23.2.187. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi S, Yamada M, Hattori N, Suzuki G. Relationship between HbA(1)c and mortality in a Japanese population. Diabetologia. 2005;48:230–234. doi: 10.1007/s00125-004-1643-9. [DOI] [PubMed] [Google Scholar]
- 15.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Preliminary communication: glycated hemoglobin, diabetes, and incident colorectal cancer in men and women: a prospective analysis from the European prospective investigation into cancer-Norfolk study. Cancer Epidemiol Biomarkers Prev. 2004;13:915–919. [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes: 2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gullo L, Pezzilli R, Morselli-Labate AM Italian Pancreatic Cancer Study Group. Diabetes and the risk of pancreatic cancer. N Engl J Med. 1994;331:81–84. doi: 10.1056/NEJM199407143310203. [DOI] [PubMed] [Google Scholar]
- 18.Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. 1980;45:2220–2224. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 20.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 21.Chow WH, Gridley G, Nyrén O, et al. Risk of pancreatic cancer following diabetes mellitus: a nationwide cohort study in Sweden. J Natl Cancer Inst. 1995;87:930–931. doi: 10.1093/jnci/87.12.930. [DOI] [PubMed] [Google Scholar]
- 22.Calle EE, Murphy TK, Rodriguez C, Thun MJ, Heath CW., Jr Diabetes mellitus and pancreatic cancer mortality in a prospective cohort of United States adults. Cancer Causes Control. 1998;9:403–410. doi: 10.1023/a:1008819701485. [DOI] [PubMed] [Google Scholar]
- 23.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283:2552–2558. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27:1761–1773. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
- 25.Richardson LC, Pollack LA. Therapy insight: influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol. 2005;2:48–53. doi: 10.1038/ncponc0062. [DOI] [PubMed] [Google Scholar]
- 26.Fisher WE, Boros LG, Schirmer WJ. Insulin promotes pancreatic cancer: evidence for endocrine influence on exocrine pancreatic tumors. J Surg Res. 1996;63:310–313. doi: 10.1006/jsre.1996.0266. [DOI] [PubMed] [Google Scholar]
- 27.Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med. 1997;336:633–640. doi: 10.1056/NEJM199702273360907. [DOI] [PubMed] [Google Scholar]
- 28.Suikkari AM, Koivisto VA, Rutanen EM, Yki-Järvinen H, Karonen SL, Seppälä M. Insulin regulates the serum levels of low molecular weight insulin-like growth factor-binding protein. J Clin Endocrinol Metab. 1988;66:266–272. doi: 10.1210/jcem-66-2-266. [DOI] [PubMed] [Google Scholar]
- 29.Meyerhardt JA, Catalano PJ, Haller DG, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;21:433–440. doi: 10.1200/JCO.2003.07.125. [DOI] [PubMed] [Google Scholar]
- 30.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S14–S21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- 31.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 33.El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 34.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin M, Marais R. Metformin: a diabetes drug for cancer, or a cancer drug for diabetics? J Clin Oncol. 2012;30:2698–2700. doi: 10.1200/JCO.2012.42.1677. [DOI] [PubMed] [Google Scholar]
- 36.Williams T, Brenman JE. LKB1 and AMPK in cell polarity and division. Trends Cell Biol. 2008;18:193–198. doi: 10.1016/j.tcb.2008.01.008. [DOI] [PubMed] [Google Scholar]