Abstract
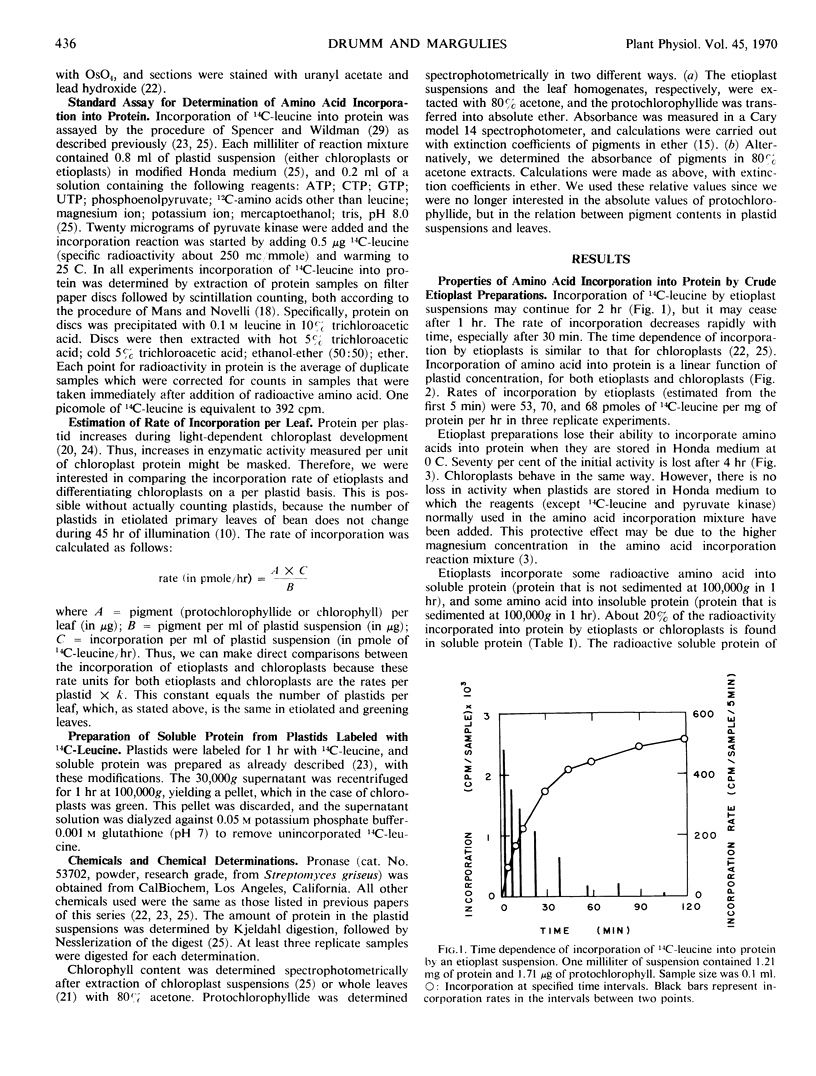
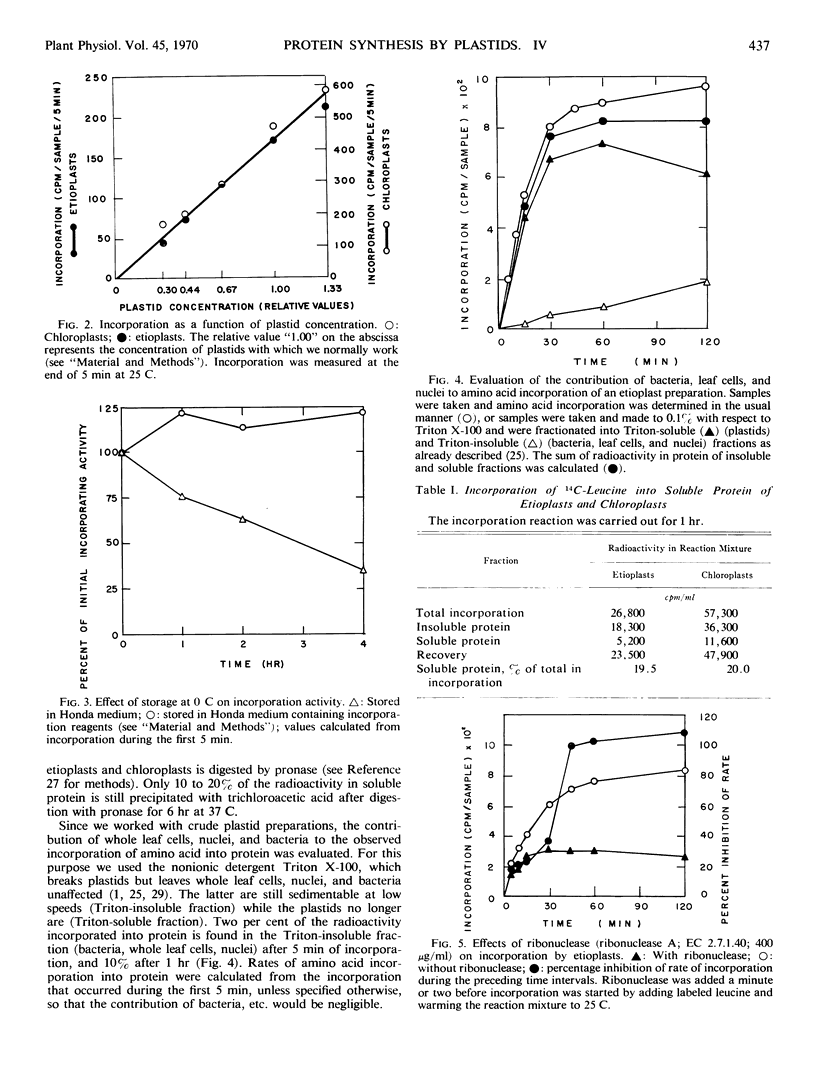
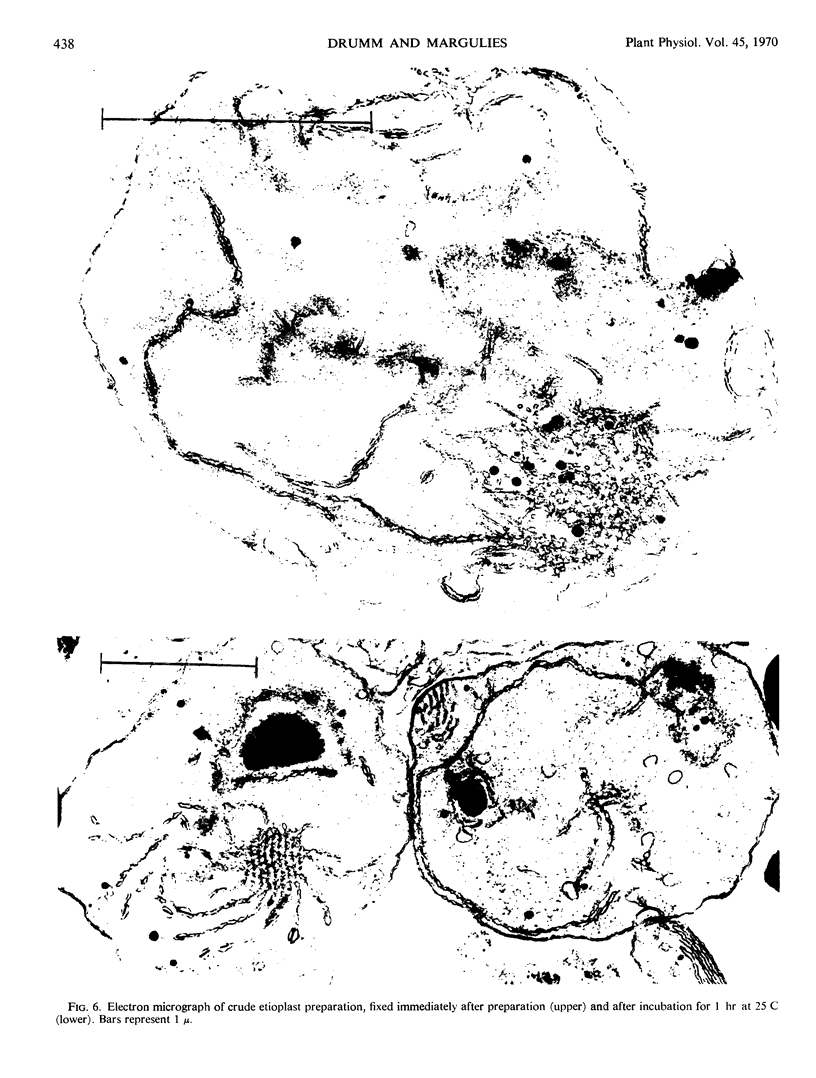
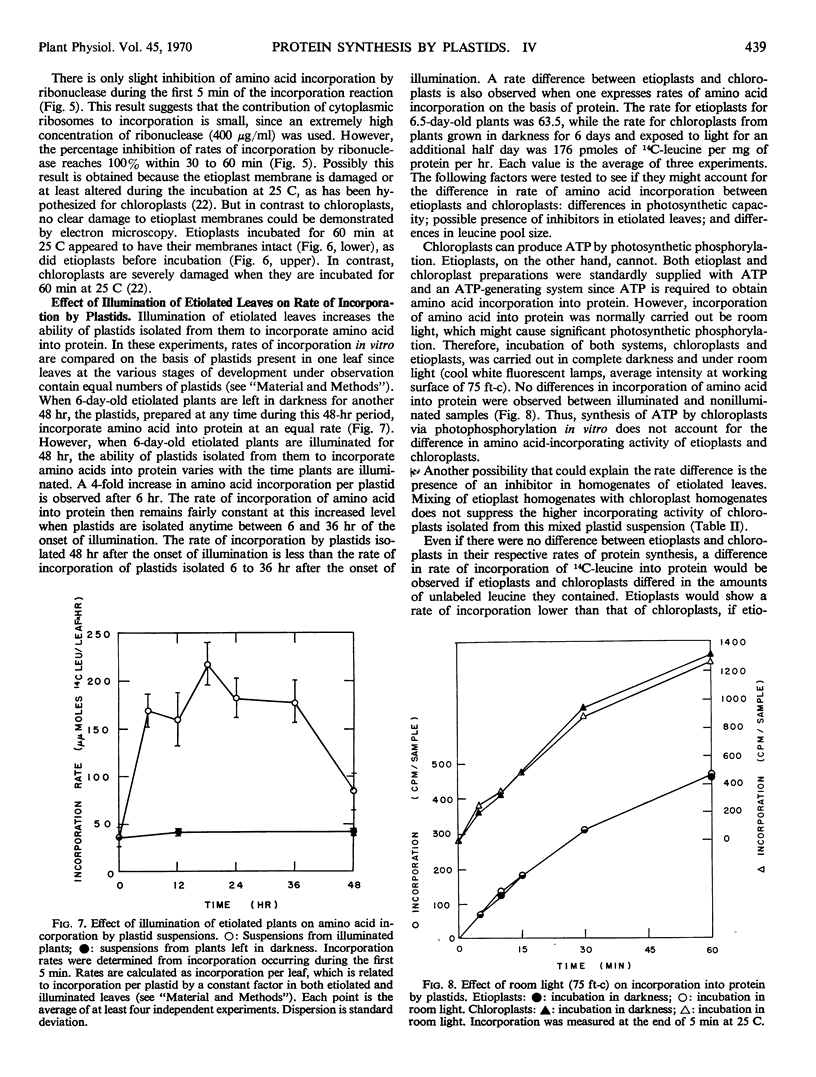
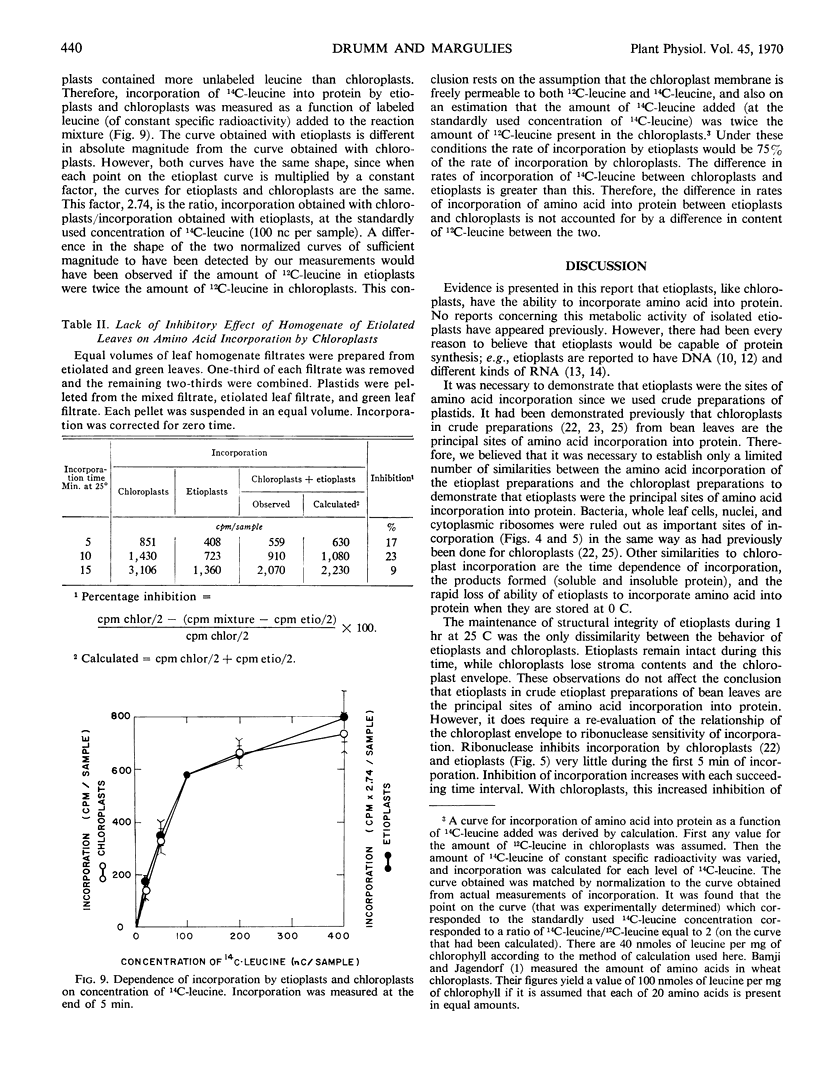
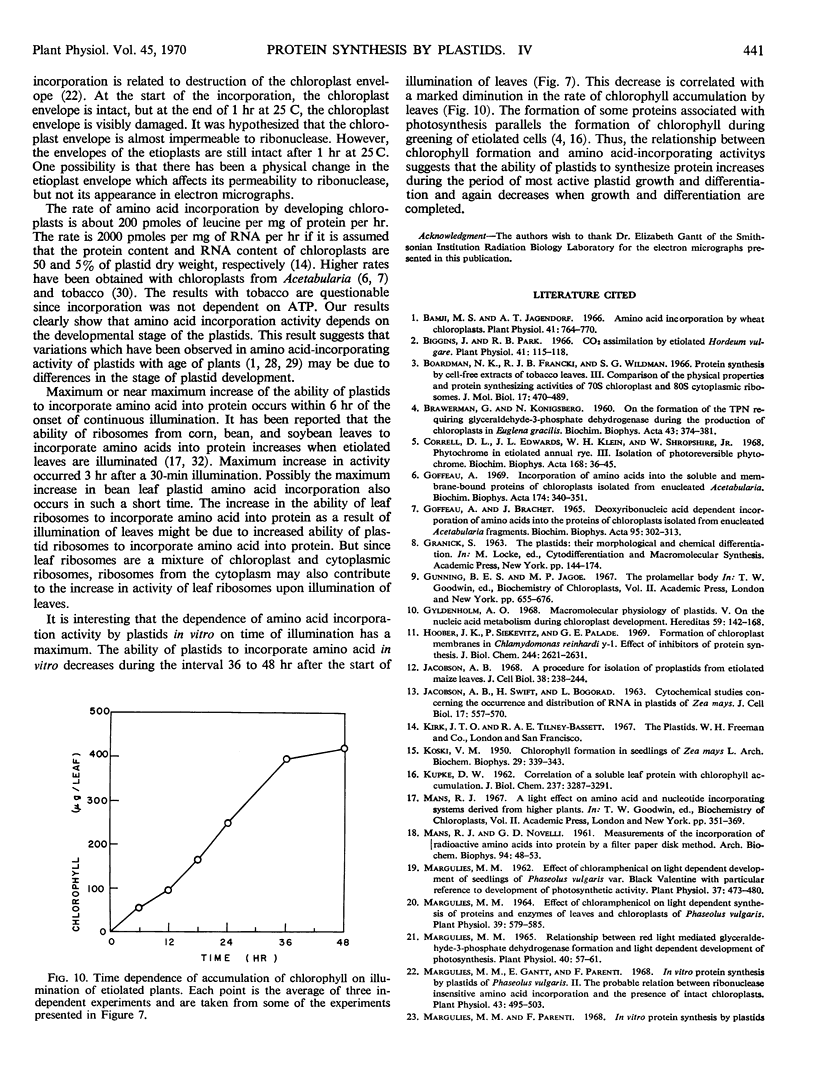
Protein synthesis in vitro by etioplasts and chloroplasts from Phaseolus vulgaris was examined to study the factors regulating the development of etioplasts into chloroplasts. The properties of incorporation of 14C-leucine into protein by etioplasts from plants grown 6.5 days in darkness are similar to those of chloroplasts from plants of the same age that were illuminated for 12 hours. However, the rate of incorporation per plastid by chloroplasts is 4 times higher than the rate of amino acid incorporation by etioplasts. When 6-day-old plants are placed in light, this 4-fold increase occurs within 6 hours and is maintained up to 36 hours. The difference in rate of amino acid incorporation into protein between etioplasts and chloroplasts represents a real difference in the ability of etioplasts and chloroplasts to synthesize protein. A difference in pool size of leucine between etioplasts and chloroplasts does not account for the difference in amino acid incorporation between etioplasts and chloroplasts. Also the difference in photosynthetic capabilities of etioplasts and chloroplasts does not account for the difference in the ability to incorporate amino acid into protein. Furthermore, there are no factors in homogenates of etiolated leaves which inactivate amino acid incorporation into protein by chloroplasts. The difference in rates of amino acid incorporation between etioplasts and chloroplasts is correlated with the state of development of the plastids. The plastids have increased ability to incorporate amino acid into protein when the plastids are undergoing growth and differentiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamji M. S., Jagendorf A. T. Amino Acid incorporation by wheat chloroplasts. Plant Physiol. 1966 May;41(5):764–770. doi: 10.1104/pp.41.5.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins J., Park R. B. CO(2) Assimilation by Etiolated Hordeum vulgare Seedlings during the Onset of Photosynthesis. Plant Physiol. 1966 Jan;41(1):115–118. doi: 10.1104/pp.41.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman N. K., Francki R. I., Wildman S. G. Protein synthesis by cell-free extracts of tobacco leaves. 3. Comparison of the physical properties and protein synthesizing activities of 70 s chloroplast and 80 s cytoplasmic ribosomes. J Mol Biol. 1966 Jun;17(2):470–487. doi: 10.1016/s0022-2836(66)80157-2. [DOI] [PubMed] [Google Scholar]
- Correll D. L., Edwards J. L., Klein W. H., Shropshire W., Jr Phytochrome in etiolated annual rye. 3. Isolation of photoreversible phytochrome. Biochim Biophys Acta. 1968 Sep 10;168(1):36–45. doi: 10.1016/0005-2795(68)90231-6. [DOI] [PubMed] [Google Scholar]
- GOFFEAU A., BRACHET J. DEOXYRIBONUCLEIC ACID-DEPENDENT INCORPORATION OF AMINO ACIDS INTO THE PROTEINS OF CHLOROPLASTS ISOLATED FROM ANUCLEATE ACETABULARIA FRAGMENTS. Biochim Biophys Acta. 1965 Feb 8;95:302–313. doi: 10.1016/0005-2787(65)90494-6. [DOI] [PubMed] [Google Scholar]
- Goffeau A. Incorporation of amino acids into the soluble and membrane-bound proteins of chloroplasts isolated from enucleated Acetabularia. Biochim Biophys Acta. 1969 Jan 21;174(1):340–350. doi: 10.1016/0005-2787(69)90259-7. [DOI] [PubMed] [Google Scholar]
- Gyldenholm A. O. Macromolecular physiology of plastids V. On the nucleic acid metabolism during chloroplast development. Hereditas. 1968;59(1):142–168. doi: 10.1111/j.1601-5223.1968.tb02168.x. [DOI] [PubMed] [Google Scholar]
- Hoober J. K., Siekevitz P., Palade G. E. Formation of chloroplast membranes in Chlamydomonas reinhardi y-1. Effects of inhibitors of protein synthesis. J Biol Chem. 1969 May 25;244(10):2621–2631. [PubMed] [Google Scholar]
- Jacobson A. B. A procedure for isolation of proplastids from etiolated maize leaves. J Cell Biol. 1968 Jul;38(1):238–244. doi: 10.1083/jcb.38.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A. B., Swift H., Bogorad L. Cytochemical studies concerning the occurrence and distribution of RNA in plastids of Zea mays. J Cell Biol. 1963 Jun;17:557–570. doi: 10.1083/jcb.17.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSKI V. M. Chlorophyll formation in seedlings of Zea mays L. Arch Biochem. 1950 Dec;29(2):339–343. [PubMed] [Google Scholar]
- KUPKE D. W. Correlation of a soluble leaf protein with chlorophyll accumulation. J Biol Chem. 1962 Oct;237:3287–3291. [PubMed] [Google Scholar]
- MEGO J. L., JAGENDORF A. T. Effect of light on growth of Black Valentine bean plastids. Biochim Biophys Acta. 1961 Oct 28;53:237–254. doi: 10.1016/0006-3002(61)90437-1. [DOI] [PubMed] [Google Scholar]
- Margulies M. M. Effect of Chloramphenicol on Light Dependent Development of Seedlings of Phaseolus vulgaris var. Black Valentine, With Particular Reference to Development of Photosynthetic Activity. Plant Physiol. 1962 Jul;37(4):473–480. doi: 10.1104/pp.37.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M. Effect of Chloramphenicol on Light-Dependent Synthesis of Proteins and Enzymes of Leaves and Chloroplasts of Phaseolus vulgaris. Plant Physiol. 1964 Jul;39(4):579–585. doi: 10.1104/pp.39.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M., Gantt E., Parenti F. In vitro Protein Synthesis by Plastids of Phaseolus vulgaris. II. The Probable Relation Between Ribonuclease Insensitive Amino Acid Incorporation and the Presence of Intact Chloroplasts. Plant Physiol. 1968 Apr;43(4):495–503. doi: 10.1104/pp.43.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M. Relationship Between Red Light Mediated Glyceraldehyde-3-Phosphate Dehydrogenase Formation and Light Dependent Development of Photosynthesis. Plant Physiol. 1965 Jan;40(1):57–61. doi: 10.1104/pp.40.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti F., Margulies M. M. In Vitro Protein Synthesis by Plastids of Phaseolus vulgaris. I. Localization of Activity in the Chloroplasts of a Chloroplast Containing Fraction from Developing Leaves. Plant Physiol. 1967 Sep;42(9):1179–1186. doi: 10.1104/pp.42.9.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER D., WILDMAN S. G. THE INCORPORATION OF AMINO ACIDS INTO PROTEIN BY CELL-FREE EXTRACTS FROM TOBACCO LEAVES. Biochemistry. 1964 Jul;3:954–959. doi: 10.1021/bi00895a019. [DOI] [PubMed] [Google Scholar]
- STEPHENSON M. L., THIMANN K. V., ZAMECNIK P. C. Incorporation of C14-amino acids into proteins of leaf disks and cell-free fractions of tobacco leaves. Arch Biochem Biophys. 1956 Nov;65(1):194–209. doi: 10.1016/0003-9861(56)90187-4. [DOI] [PubMed] [Google Scholar]
- Spencer D. Protein synthesis by isolated spinach chloroplasts. Arch Biochem Biophys. 1965 Aug;111(2):381–390. doi: 10.1016/0003-9861(65)90200-6. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Gailey F. B. Carbon Dioxide Fixation by Etiolated Plants after Exposure to White Light. Plant Physiol. 1955 Nov;30(6):491–499. doi: 10.1104/pp.30.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]