Summary
For cell-based treatments of myocardial infarction, a better understanding of key developmental signaling pathways and more robust techniques for producing cardiomyocytes are required. Manipulation of Notch signaling has promise as it plays an important role during cardiovascular development, but previous studies presented conflicting results that Notch activation both positively and negatively regulates cardiogenesis. We developed surface- and microparticle-based Notch-signaling biomaterials that function in a time-specific activation-tunable manner, enabling precise investigation of Notch activation at specific developmental stages. Using our technologies, a biphasic effect of Notch activation on cardiac differentiation was found: early activation in undifferentiated human embryonic stem cells (hESCs) promotes ectodermal differentiation, activation in specified cardiovascular progenitor cells increases cardiac differentiation. Signaling also induces cardiomyocyte proliferation, and repeated doses of Notch-signaling microparticles further enhance cardiomyocyte population size. These results highlight the diverse effects of Notch activation during cardiac development and provide approaches for generating large quantities of cardiomyocytes.
Highlights
-
•
Oriented Jagged-1-modified biomaterials controllably activate Notch signaling
-
•
Activation of Notch in pluripotent hESCs increases ectodermal differentiation
-
•
Notch activation in cardiovascular progenitor cells increases cardiac differentiation
-
•
hESC-derived cardiomyocyte proliferation is induced by Notch activation
Murry, Giachelli, and colleagues engineer Notch-signaling biomaterials to investigate Notch signaling during cardiac development. Early Notch activation in undifferentiated human embryonic stem cells promotes ectodermal differentiation, whereas late activation in specified cardiovascular progenitor cells increases cardiac differentiation. Activation also induces proliferation of differentiated cardiomyocytes with repeated activation further enhancing population size.
Introduction
Specific control of cellular fate by biological surface modification has garnered recent attention for the ability to create biomimetic microenvironments (Lutolf and Hubbell, 2005). Normally, the body contains stem cell niches composed of complex, spatially and temporally controlled mixtures of soluble chemokines, insoluble extracellular matrix molecules, and cells expressing transmembrane receptor ligands that direct cell fate. Much focus has been given to modifying surfaces to mimic these stem cell niche microenvironments in order to control cellular fate (Lutolf and Hubbell, 2005; Keselowsky et al., 2005; Hoffman and Hubbell, 2004). In these studies, molecular immobilization is proposed to have a critical role by increasing protein stability, promoting persistent signaling, and inducing receptor clustering (Irvine et al., 2002). Despite the attention given to mimicking stem cell niches via surface modifications, few studies have utilized cell-cell surface-ligand-receptor interactions for controlling cellular fate.
One particularly promising cell-surface pathway is the Notch pathway, which has been shown to play an important role in development and normal cell function, regulating such events as cell growth, proliferation, survival, migration, and differentiation (Artavanis-Tsakonas et al., 1999). The Notch pathway is initiated upon binding of a cell-surface-bound Notch ligand with a Notch receptor on a second cell, triggering two proteolytic cleavages that release the Notch intracellular domain (NICD) from the plasma membrane. Once released, the NICD translocates to the nucleus where it binds to and converts the CSL transcription factor from a transcriptional repressor to an activator, allowing for Notch target-gene transcription (Bray, 2006; Mumm and Kopan, 2000). Activation of the pathway contributes to numerous cell-fate decisions including maintenance of hematopoietic stem cells in an undifferentiated state (Varnum-Finney et al., 2000b), induction of endothelial-to-mesenchymal transformation (Noseda et al., 2004), expansion of neural precursors (Oishi et al., 2004), and inhibition of differentiation toward an osteoblastic phenotype (Sciaudone et al., 2003). During cardiac morphogenesis, the Notch signaling pathway is crucial as Notch perturbation has been implicated in the pathogenesis of various human cardiovascular diseases (Nemir and Pedrazzini, 2008; Joutel and Tournier-Lasserve, 1998). However, past studies have presented conflicting conclusions, stating that Notch activation can both promote and inhibit cardiac differentiation (Schroeder et al., 2003; Nemir et al., 2006; Noggle et al., 2006; Jang et al., 2008; Lowell et al., 2006; Chen et al., 2008; Fox et al., 2008; Yu et al., 2008). Thus, we hypothesized that Notch signaling plays multiple roles in cardiac development from human embryonic stem cells, with the precise effect on cellular fate being highly context-dependent.
Because the Notch pathway is a cell-cell signaling pathway, unique approaches must be taken to successfully activate signaling. Common approaches include in vitro coculture with Notch-ligand-presenting cells (Neves et al., 2006) and transfection with constitutively active forms of the NICD. Unfortunately, these approaches possess several disadvantages. Coculture systems result in unrelated cell-to-cell interactions, and heterogeneity between cell lines and cell-culture conditions may induce varying levels of ligand expression (Sokolova and Epple, 2008). Overexpression of the NICD results in the pathway being permanently activated, when often only transient activation is desired. Gene transfection also results in heterogeneous conditions, whereas transfection efficiency and cytotoxicity may compromise cell viability and normal gene expression. In addition, because of the ability of Notch ligands to bind with multiple Notch receptors, genetic modifications that serve to overactivate single Notch receptors may fail to properly address the complexity of Notch activation. The use of genetically modified Notch receptors can also result in the expression of Notch receptors at nonphysiologic levels. Notch-activating surface modifications avoid these issues through the engineering of homogenous surfaces that allow for precise activation of the Notch signaling pathway. As previously published by our group, compared to soluble treatment and nonspecific immobilization of a Notch ligand, oriented ligand immobilization results in a more potent induction of Notch-mediated epithelial differentiation in vitro and in a neonatal foreskin-rafted organ culture model (Beckstead et al., 2006, 2009). Compared to use of a soluble ligand that can act as a Notch agonist or antagonist depending on the context (Artavanis-Tsakonas et al., 1999; Hicks et al., 2002; Varnum-Finney et al., 2000a; Small et al., 2001), these Notch-signaling surfaces provide an approach for precisely and reliably controlling Notch activation for use in guiding cellular fate.
In the present study, we have engineered oriented Notch-signaling biomaterials capable of precisely controlling stem cell fate. In order to determine the context-dependent effect of Notch activation on cardiac development, Notch activation was precisely targeted at several developmental stages. The effect of oriented Jagged-1 surfaces on cardiomyocyte differentiation at the pluripotent embryonic stem cell and cardiovascular progenitor cell developmental stage were assessed, and changes in terminally differentiated human embryonic stem cell (hESC)-derived cardiomyocyte proliferation following Notch activation were also determined.
Results
Oriented Jagged-1 Immobilization on Surfaces and Microparticles Activates Notch Signaling in a Controllable Manner
An indirect immobilization scheme was used to control Notch ligand surface orientation and increase Notch-signaling activity. 125I radioactivity experiments demonstrate that Jagged-1 can be indirectly immobilized on a tissue culture polystyrene (TCPS) surface (Figure 1A). By increasing precoat concentration, we observed dose-dependent increases in Jagged-1 surface concentration, eventually reaching a maximum surface concentration of ∼120 ng/cm2, an amount that corresponds to levels expected for a theoretical Jagged-1 protein monolayer. Activation of Notch signaling through the CSL-dependent pathway in human embryonic kidney, neonatal (HEKn) cells plated on our oriented Notch ligand surfaces was analyzed via a CBF-1 luciferase assay. Oriented Jagged-1 surfaces incubated in at least 7 nM Jagged-1 precoat solutions resulted in a 3-fold or greater increase in Notch/CBF-1 signaling at 24 hr postplating compared to TCPS control (Figure 1B). By further increasing the Jagged-1 incubation solution concentration, a greater than 4-fold increase could be observed.
Figure 1.
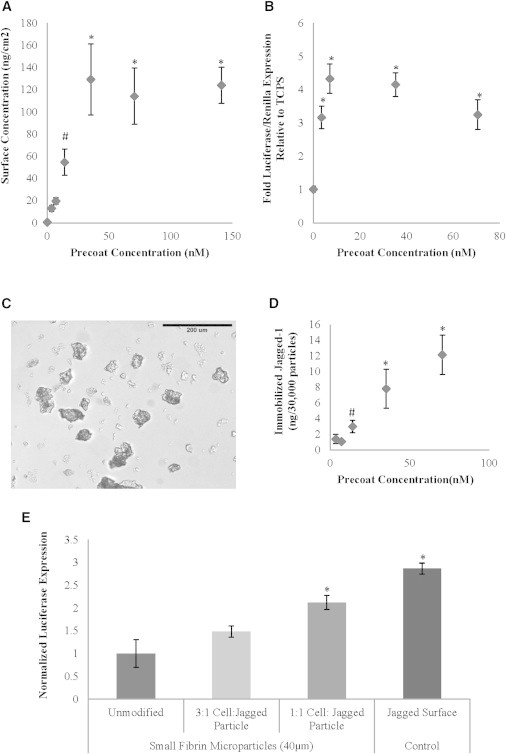
Characterization of Oriented Jagged-1 Biomaterials
(A) 125I radiolabeling shows dose-dependent increases in surface concentration of Jagged-1 indirectly immobilized on a TCPS surface. A maximum surface concentration corresponding to levels expected for a theoretical Jagged-1 protein monolayer was achieved.
(B) CBF-1 luciferase assays measuring Notch/CSL signaling in HEKn cells plated on oriented Jagged-1 surfaces show a dose-dependent increase in signal activation.
(C) A stirred emulsion reaction mixture generates 40 μm diameter fibrin microparticles.
(D) 125I radiolabeling showing the dose-dependent indirect immobilization of Jagged-1 on fibrin microparticles.
(E) CBF-1 luciferase assay results measuring Notch/CSL signaling in HEKn cells treated with oriented Jagged-1-modified fibrin microparticles at various ratios, unmodified fibrin microparticles, or oriented Jagged-1-positive control surface. Cells treated with oriented Jagged-1-modified fibrin microparticles showed a dose-dependent activation of Notch/CBF-1 signaling. Results are representative of three independent experiments and are mean ± SD, n = 3. Points marked by a given symbol denote statistical significance (p < 0.05 compared to points that are unmarked or marked by a different symbol).
In order to repeatedly activate Notch signaling without the need to replate cells, an oriented Jagged-1-modified fibrin microparticle signaling platform was developed. Using an aqueous in oil stirred emulsion approach, fibrin microparticles with an approximate diameter of 40 μm were synthesized (Figure 1C). As was done with our surfaces, Jagged-1 was bound to fibrin microparticles via an indirect immobilization approach. Utilizing free terminal carboxyl groups on the fibrin microparticles, 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride/N-hydroxysulfosuccinimide (EDC/NHS) chemistry was used to create amine-reactive sulfo-NHS esters, allowing covalent attachment of antipolyhistidine molecules via their free primary amines and subsequent formation of amide bonds. Subsequent incubation in Jagged-1 solutions resulted in the affinity immobilization of Jagged-1 on antipolyhistidine-modified fibrin microparticles. 125I radio-labeled Jagged-1 served to confirm immobilization to fibrin microparticles (Figure 1D). Additionally, the quantity of Jagged-1 immobilized to fibrin microparticles could be fine-tuned in a dose-dependent manner by varying Jagged-1 precoat solution concentration. The ability to activate Notch signaling through the CSL-dependent pathway was again determined via a HEKn CBF-1 luciferase assay. Cells treated with oriented Jagged-1-modified fibrin microparticles showed a dose-dependent activation of Notch/CBF-1 signaling (Figure 1E), whereas no Notch activation was observed in the unmodified fibrin microparticle control group. These results demonstrate that both oriented Jagged-1 surface- and microparticle-based signaling platforms not only activate Notch signaling but do so in a highly controllable and predictable manner.
Jagged-1-Mediated Notch Activation in Undifferentiated hESCs Increases Ectodermal Gene Expression
Because we believed the role of Notch activation in cardiogenesis to be time-specific, we began by investigating the role of Notch activation in the differentiation of pluripotent embryonic stem cells (ESCs). To determine this, undifferentiated hESCs maintained in mouse-embryonic-fibroblast-conditioned media were replated in serum-containing media on either control surfaces or oriented Jagged-1 surfaces. As displayed in our control samples without Notch activation, these conditions initiated spontaneous differentiation and departure of ESCs from the pluripotent state. In hESCs plated on oriented Jagged-1 surfaces, expression of the Notch target gene, HES1, was significantly increased compared to control surfaces, demonstrating Notch-signaling activation (Figure 2A). All experimental groups downregulated expression of the OCT4 pluripotency gene and upregulated markers of mesoderm formation (BRACHYURYT) and endoderm formation (FOXA2) in a comparable manner (Figures 2B–2D). In contrast, a sustained increase (>2-fold) expression of the ectodermal gene, SOX1, was observed on oriented Jagged-1 surfaces compared to control surfaces (Figure 2E). Thus, Jagged-1-mediated Notch activation encourages ectodermal gene expression in undifferentiated hESCs, demonstrating a relationship between Jagged-1-mediated Notch activation and ectoderm formation at this early developmental time point.
Figure 2.
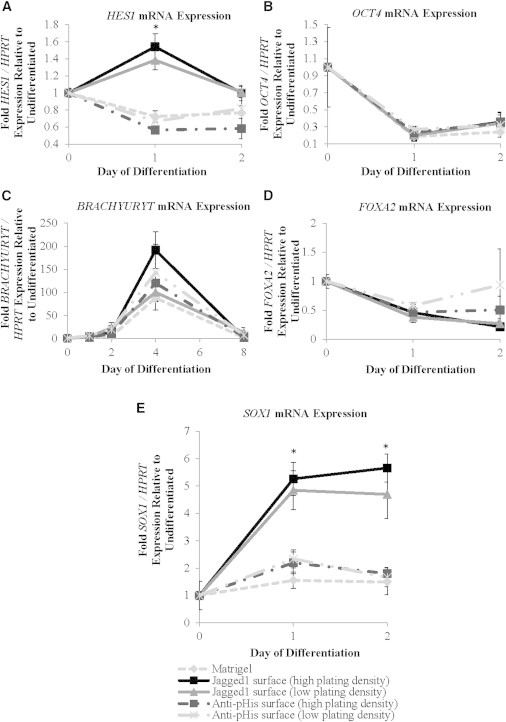
The Role of Notch Signaling Activation in the Differentiation of Human Pluripotent Embryonic Stem Cells
Undifferentiated human embryonic stem cells were plated on control or oriented Jagged-1 surfaces, and gene expression was analyzed via quantitative PCR (qPCR) up to 8 days after initial plating. Notch gene: HES1; pluripotency gene: OCT4; mesodermal gene: BRACHYURYT; endodermal gene: FOXA2; ectodermal gene: SOX1.
(A) hESCs plated on oriented Jagged-1 surfaces display increased expression of the Notch target gene, HES1, peaking 1 day after plating, indicating successful activation of the Notch pathway via oriented Jagged-1 surfaces.
(B–E) Activation of Notch signaling does not alter pluripotency, mesoderm, or endoderm gene expression compared to controls but does increase ectodermal gene expression. Results are representative of three independent experiments and are mean ± SD, n = 3; symbols denote p < 0.05 compared to controls.
Oriented Jagged-1 Surfaces Promote Cardiomyocyte Differentiation of KDR+ Progenitor Cells
Next, we sought to determine the effect of Notch activation at a later multipotent cardiac progenitor developmental stage. KDR+ cardiovascular progenitor cells are a multipotent cell population with the capacity to differentiate into endothelial, smooth muscle, and cardiac cell types (Yang et al., 2008). For our experiments, we generated populations enriched for KDR+/platelet-derived-growth-factor-receptor-α-positive progenitor cells (>90%). This cell population actively expresses mRNA transcripts of the Notch ligand, JAGGED-1, and the Notch target gene, HES1, compared to an undifferentiated hESC population (Figure 3A), suggesting that Jagged-1-mediated Notch activation can occur in this cell population.
Figure 3.
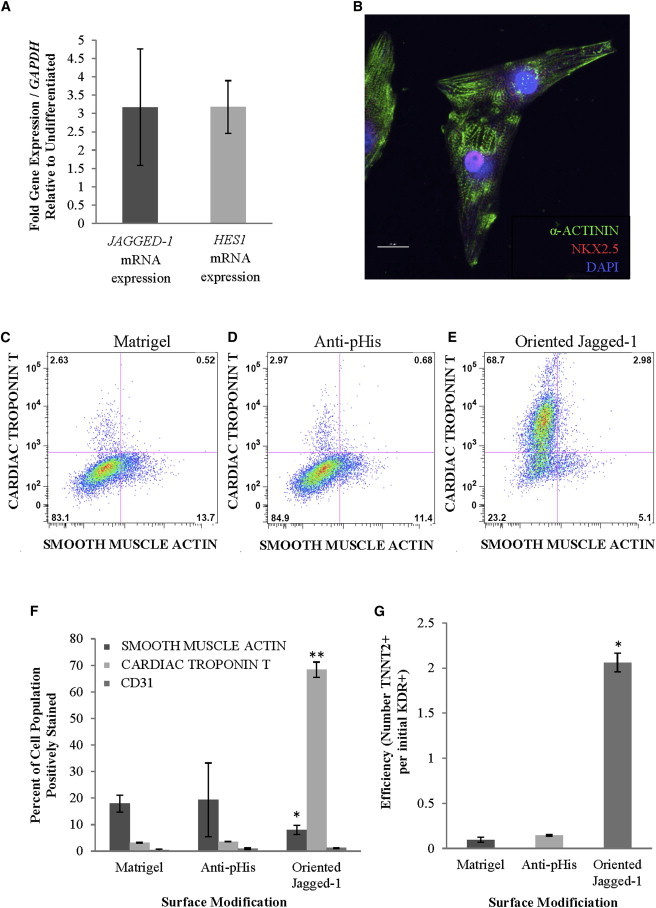
The Role of Notch Signaling Activation in the Differentiation of KDR+ Cardiovascular Progenitor Cells
For analysis of differentiation, KDR+ cardiovascular progenitor cells were plated on control or oriented Jagged-1 surfaces in defined medium. Following 12 days in culture, cells were analyzed for cardiac differentiation.
(A) mRNA gene expression demonstrates transcription of the Notch ligand, JAGGED-1, and the Notch target gene, HES1, in KDR+ cells.
(B) Immunofluorescent staining illustrates the presence of α-ACTININ and NKX2.5 in KDR+ cells plated on oriented Jagged-1surfaces. Cells stained positively for α-ACTININ and display striations characteristic of sarcomeric formation. Cells also costain positively for the cardiac transcription factor NKX2.5, with staining localized primarily in the nucleus. (60× magnification).
(C–E) FACS analysis displays that plating KDR+ cells on oriented Jagged-1 surfaces (compared to control surfaces) results in a >60% increase in cardiomyocyte differentiation and 10% decrease in smooth muscle differentiation after 12 days in culture. Representative plots shown.
(F) Summary of FACS analysis demonstrates that, after plating on oriented Jagged-1 surfaces, KDR+ cells show no significant change in endothelial differentiation (CD31), but significant decreases in smooth muscle cell differentiation (SMOOTH MUSCLE ACTIN) and increases in cardiomyocyte (CARDIAC TROPONIN T, TNNT2) differentiation are observed.
(G) The efficiency of cardiomyocyte differentiation increases on oriented Jagged-1 surfaces as defined by the number of KDR+ cardiovascular progenitor cells required to generate TNNT2+ cardiomyocytes. Results are representative of three independent experiments and are mean ± SD, n = 3; symbols denote p < 0.05 compared to controls.
To determine whether Jagged-1-mediated Notch activation influences their differentiation, KDR+ progenitors (>90% purity) were plated on oriented Jagged-1 surfaces. In culture, beating loci were observed on oriented Jagged-1 surfaces 8 days after plating, whereas cells on control surfaces did not exhibit any contractions until 12 days after plating. At 12 days after plating, fluorescence-activated cell sorting (FACS) analysis demonstrated that Jagged-1-induced Notch activation in KDR+ progenitor cells resulted in a >20-fold increase in the percent of cells expressing cardiac troponin T (TNNT2) (from ∼3% to ∼70%), roughly a 50% decrease in the population of smooth muscle actin+ cells (from ∼14% to ∼7%), and no significant change in CD31+ endothelial cells (Figures 3C–3F). This Notch-mediated increase in cardiac differentiation resulted in an 8-fold increase in cardiac differentiation efficiency (Figure 3G), as every KDR+ progenitor cell plated on an oriented Jagged-1 surface generated roughly two TNNT2+ cardiomyocytes, whereas control surfaces required more than four KDR+ progenitors to generate a single TNNT2+ cardiomyocyte. Additionally, cardiomyocytes expressed the cardiac transcription factor NKX2.5 and the cytoskeletal protein α-actinin, with α-actinin staining displaying the striations characteristic of sarcomeric formation (Figure 3B). Perhaps the most striking difference in these differentiated cardiomyocytes is observed in culture, as these differentiated cardiomyocytes form sheets of spontaneously beating cells, with synchronous waves of contraction propagating throughout the entire culture (Movie S1 available online). These results demonstrate that Jagged-1-mediated Notch activation through oriented Jagged-1 surfaces provides a nongenetic approach capable of inducing highly efficient cardiac differentiation in human KDR+ progenitor cells. Combined with the results from our undifferentiated hESC studies, these findings demonstrate a biphasic effect of Notch activation during cardiac differentiation and highlight the temporally dependent nature of Notch signaling.
Oriented Jagged-1 Surfaces Induce hESC-derived Cardiomyocyte Proliferation
Upon determining context-specific effects of Notch activation on cardiac differentiation, we sought to determine the role of Notch activation in guiding the development of terminally differentiated cardiomyocytes. hESC-derived MYH7+ cardiomyocytes exhibit significant increases in mRNA levels of the Notch ligand, JAGGED-1, and the Notch signaling target gene, HES1, compared to an undifferentiated hESC population (Figure 4A). These results demonstrate JAGGED-1 expression and HES1 transcription in hESC-derived MYH7+ cardiomyocytes, suggesting that, like the KDR+ progenitor cells, Jagged-1-mediated Notch activation can occur in definitive cardiomyocytes.
Figure 4.
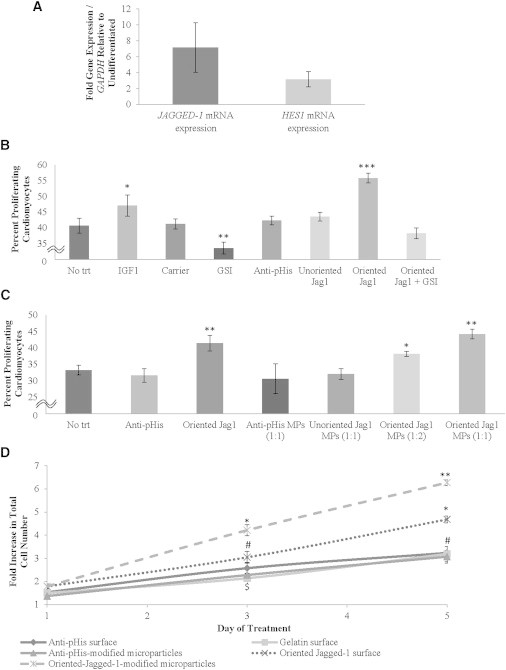
Effects of Oriented Jagged-1 Biomaterial-Mediated Notch-Signaling Activation on the Induction of Cardiomyocyte Proliferation
After attachment, differentiated MYH7+ cardiomyocytes were stimulated as described for 48 hr in serum-free media and labeled with BrdU during the last 24 hr of treatment, at which point induction of proliferation was determined via immunostaining.
(A) qPCR analysis of mRNA gene expression demonstrates transcription of the Notch ligand, JAGGED-1, and the Notch-signaling target gene, HES1, in hESC-derived MYH7+ cardiomyocytes.
(B) BrdU proliferation assays demonstrate induction of hESC-derived cardiomyocyte proliferation. MYH7+ cardiomyocytes plated on oriented Jagged-1 surfaces display statistically significant greater levels of proliferation compared to controls. ɣ-secretase inhibitor treatment reverses the proliferative effects of oriented Jagged-1 surfaces, verifying that oriented Jagged-1 surface bioactivity is Notch specific. The mode of Jagged-1 immobilization also plays a considerable role in the effectiveness of Jagged-1 surfaces to induce proliferation, as randomly oriented Jagged-1 surfaces are unable to induce cardiomyocyte proliferation.
(C) Treatment with oriented Jagged-1-modified fibrin microparticles results in a significant increase in cardiomyocyte proliferation as measured by BrdU incorporation. Method of Jagged-1 immobilization influences the microparticle bioactivity as oriented Jagged-1-modified fibrin microparticles successfully induce cardiomyocyte proliferation, whereas cardiomyocytes treated with unoriented Jagged-1-modified fibrin microparticles display proliferation levels similar to negative controls. The degree of cardiomyocyte proliferation is further controlled in a dose-dependent manner by oriented Jagged-1-modified fibrin microparticles through adjustment of the particle:cell ratio.
(D) Oriented Jagged-1-modified fibrin microparticles can repeatedly induce cardiomyocyte proliferation to further heighten population growth. Oriented Jagged-1 surfaces accelerate cardiomyocyte population growth compared to controls, whereas repeated treatment with oriented Jagged-1-modified fibrin microparticles further increases the rate of cardiomyocyte population growth. Results are representative of three independent experiments and are mean ± SD, n = 7 (A–C) or 3 (D). Conditions marked by a given symbol denote statistical significance (p < 0.05 compared to experimental groups that are unmarked or marked by a different symbol).
hESC-derived MYH7+ cardiomyocytes were plated on several surfaces to determine whether Notch-signaling activation could promote cardiac proliferation. We found that 55% of cardiomyocytes plated on oriented Jagged-1 surfaces exhibited positive staining for bromodeoxyuridine (BrdU) incorporation, a significant increase compared to 42% of cardiomyocytes plated on control surfaces (Figures 4B and S1). This is the most robust mitogen we have found to date for hESC-derived cardiomyocytes, significantly surpassing the 47% BrdU incorporation rates observed with insulin growth factor 1 (IGF-1) (McDevitt et al., 2005). Additionally, ɣ-secretase inhibitor treatment, which blocks the proteolytic activation of the Notch intracellular domain, inhibited this increase in proliferation, significantly decreasing the proliferative cardiomyocyte population to 38%, and verifying that the proliferative effects of oriented Jagged-1 surfaces are indeed Notch mediated. Interestingly, ɣ-secretase inhibitor treatment of cardiomyocytes plated on gelatin control surfaces served to further decrease the proliferative population to 33%, suggesting that basal hESC-derived cardiomyocyte proliferation is partially a result of endogenous levels of Notch signaling. Lastly, unoriented Jagged-1 surfaces synthesized by nonspecific protein adsorption displayed proliferative levels (43%) significantly lower than oriented Jagged-1 surfaces and more analogous to control surfaces, demonstrating that the Jagged-1 immobilization technique influences the capacity of Notch-signaling surfaces to induce proliferation in hESC-derived cardiomyocytes. These results highlight the ability of oriented Jagged-1 surfaces to induce Notch-mediated proliferation of hESC-derived cardiomyocytes, highlighting yet another distinct developmental stage at which Notch signaling displays potent effects.
Oriented Jagged-1-Modified Fibrin Microparticles Induce the Proliferation of hESC-Derived Cardiomyocytes
While effective for inducing Notch signaling at a singular time point, oriented Jagged-1 surfaces only result in an initial spike in Notch activation. To achieve greater versatility of Notch activation, we developed a microparticle-based Notch-signaling platform, which through repeated addition of modified microparticles allows for sustained signaling activation or activation at multiple distinct time points. hESC-derived MYH7+ cardiomyocytes were treated with oriented Jagged-1-modified fibrin microparticles to determine if this technology could successfully induce proliferation in differentiated cardiomyocytes. We found that 44% of cardiomyocytes treated with oriented Jagged-1-modified fibrin microparticles at a 1:1 cell:particle ratio incorporated BrdU, a statistically significant increase from the 32% incorporation rate observed in controls exposed to particles containing only the antipolyhistidine modification (Figure 4C). In addition, this increase in cardiomyocyte proliferation matched that of cardiomyocytes plated on oriented Jagged-1 surfaces, indicating that our microparticle-based signaling platform is an equally effective activator of Notch signaling. Importantly, no statistically significant difference was observed in cardiomyocytes treated with antipolyhistidine-modified fibrin microparticles compared to cells plated on a gelatin surface, demonstrating that the increase in cardiomyocyte proliferation is specifically due to the immobilization of Jagged-1 on fibrin microparticles and not due to a nonspecific effect of the particles. Cells treated with unoriented Jagged-1-modified fibrin microparticles at a 1:1 cell:particle ratio exhibited only 32% BrdU incorporation, indicating that unoriented Jagged-1-modified fibrin microparticles are unable to induce cardiomyocyte proliferation. A dose-response curve showed that cardiomyocytes treated with one particle per two cells had significantly increased proliferation compared to controls (38% versus 32%), and still higher proliferation rates were observed with a 1:1 particle:cell ratio (44%). These studies demonstrate that oriented Jagged-1-modified fibrin microparticles are capable of inducing proliferation in hESC-derived cardiomyocytes in a dose-dependent manner.
As our Notch-signaling microparticles possess the ability to repeatedly activate Notch signaling, we sought to demonstrate the utility of this technology by attempting to further heighten cardiomyocyte population growth via the administration of multiple treatments. hESC-derived cardiomyocytes were subjected to multiple doses of modified microparticles, and cell population growth was measured over a 5-day time span. Compared to oriented Jagged-1 surfaces, which served to increase cell numbers by about 4-fold after 5 days (3-fold increase in controls), cardiomyocytes receiving two 1:1 cell:particle doses of oriented Jagged-1-modified fibrin microparticles 2 days and 4 days after plating displayed >6-fold increase in cell number (Figure 4D). These results demonstrate that repeated dosing with Notch-signaling microparticles has potential for the large-scale generation of hESC-derived cardiomyocytes.
Discussion
In the present study, we describe the development of several oriented Notch-signaling biomaterials and their use in generating hESC-derived cardiomyocytes. Our results indicate that Notch activation has a biphasic effect over the course of human cardiac differentiation, favoring ectodermal differentiation at an undifferentiated pluripotent stem cell stage and increasing cardiac differentiation at the KDR+ cardiovascular progenitor stage. Additionally, Notch activation induces proliferation in the differentiated cardiomyocyte cell population. Importantly, by taking a physiologically relevant approach and triggering Notch activation through the presentation of a Notch ligand and not through the use of constitutively active or drug-inducible Notch receptors, these findings demonstrate the natural potential of Notch activation to influence multiple stages of human cardiac development. These results highlight the temporally dependent potent effects of Notch activation and suggest that Notch-signaling technologies may be of value for generating differentiated cardiomyocytes for clinical applications.
Recent studies have begun to investigate the role of Notch signaling in guiding cardiogenic fate decisions. Several studies have demonstrated that Notch inhibition through either expression of defective RBP-Jκ (Schroeder et al., 2003), Notch1-deficient cells (Nemir et al., 2006), or treatment with the gamma secretase inhibitors (Noggle et al., 2006; Jang et al., 2008) can both promote or inhibit cardiomyocyte differentiation, whereas Notch activation in cells as observed through reporter gene activation (Nemir et al., 2006) or activation through DLL1-expressing OP9 feeder layers (Lowell et al., 2006) leads to reduced cardiac differentiation in favor of neuroectoderm formation. At a more specified developmental stage, timed activation of Notch signaling in cardiac mesoderm promotes cardiac development, whereas Notch activation in mouse-derived hemangioblasts respecifies them to a cardiac cell fate (Chen et al., 2008). In addition, the Notch signaling pathway has been shown to be active in proliferating neonatal rat cardiomyocytes, with exogenous Notch activation serving to stimulate cardiomyocyte proliferation and cell-cycle re-entry (Collesi et al., 2008; Campa et al., 2008). Our results help to resolve some of the discrepancies between these studies, showing that not only does Notch signaling play a significant role in the differentiation and proliferation of human cardiomyocytes, but the timing of Notch activation is also critical, as pathway activation can have positive or negative effects on the generation of human-derived cardiomyocytes depending on the developmental stage at which it is triggered.
In this study, we have shown that oriented Jagged-1 biomaterials, immobilized on either a solid surface or microparticles, are able to precisely activate the Notch signaling pathway for use in controlling cellular fate. In guiding cardiomyocyte development, we have demonstrated multiple temporally distinct effects, where Notch activation at the pluripotent state acts in an anticardiogenic manner by favoring ectodermal differentiation and is procardiogenic at later stages, serving to increase cardiac differentiation in KDR+ progenitors and induce proliferation in differentiated cardiomyocytes. Whereas the exact mechanism by which the Notch signaling pathway exerts these effects on differentiation in our system is unclear, reports have suggested significant interaction between Notch and other signaling pathways, including the transforming growth factor β (TGF-β)/bone morphogenetic protein (BMP) (Guo and Wang, 2009) and Wnt pathways (Hayward et al., 2008). Interestingly, our results appear to coincide with previous work that suggested that the Notch and TGF-β/BMP pathways work synergistically, whereas Notch inhibits the Wnt pathway during the process of cardiomyocyte-directed differentiation (Chen et al., 2008). These results demonstrate the importance of timing Notch activation, as pathway activation can have positive or negative effects on cardiomyocyte differentiation depending on the developmental stage at which signaling is activated.
Our studies utilized the Notch ligand Jagged-1 for inducing Notch activation. Previous studies have highlighted the contrasting effects stemming from activating the Notch signaling pathway via the Delta versus the Jagged ligand family in various organs and tissues, including the developing nervous and immune systems (Amsen et al., 2004; Brooker et al., 2006). In addition, a family of glycosyltransferases called Fringe proteins are known to modify O-fucose on Notch receptors through the addition of N-acetylglucosamine, a modification that serves to restrict activation of Notch receptors to only Delta ligands, inhibiting Jagged-mediated Notch activation (Haines and Irvine, 2003). In future studies, investigating the effects of Fringe proteins or Delta-mediated Notch activation in our systems would serve to provide even further clarity on the precise role of Notch signaling in guiding cellular fate decisions at these developmental stages.
Our data suggest that surfaces remain active primarily over the course of 24 hr. Decreased activity after this time period may result from either inadequate amounts of ligand remaining on the surfaces or self-regulation of the Notch pathway, as most Notch-mediated processes require only a transient pulse of activity that in some cases lasts only a fraction of the cell cycle (Ambros, 1999). We were able to surpass the effects of oriented Jagged-1 surfaces through treatment with multiple doses of Notch-signaling microparticles, demonstrating the promise of using this technology to repeatedly activate or maintain Notch signaling activation. Though useful, this technology may possess several limitations. Previous studies have demonstrated that, during neonatal rat cardiomyocyte maturation, expression levels of Notch-signaling components are actually downregulated over time (Collesi et al., 2008), serving to limit the proliferation of the maturing cardiomyocyte population. As both of our Notch-signaling platforms require expression of endogenous Notch receptors in our cells of interest in order to achieve successful Notch activation, the aging of hESC-derived cardiomyocytes in culture may limit their responsiveness to our Notch-signaling technologies. In addition, as neonatal rat cardiomyocytes mature and decrease Notch pathway activity, forced activation of the pathway results in pronounced activation of DNA damage checkpoint, eventually leading to mitotic arrest and cell apoptosis (Campa et al., 2008). These findings again highlight the important role our engineered signaling platforms can play in activating Notch signaling in a physiologically relevant manner.
In conclusion, we have expanded on previous work by demonstrating the potential application of Notch-signaling surface modifications for use in precisely controlling cell-fate decisions at several developmental stages of human cardiomyocyte development. Our findings suggest several approaches that allow for more efficient hESC-derived cardiomyocyte generation. Furthermore, because the Notch signaling pathway plays a key context-dependent role in numerous developmental processes, these Notch-signaling biomaterials have the potential for widespread application in directing cell-fate decisions in a variety of cell systems.
Experimental Procedures
Preparation of Oriented JAG1 Biomaterials
Oriented JAG1 surfaces were fabricated using an indirect immobilization scheme, and for antipolyhistidine control surfaces and unoriented JAG1 surfaces, proteins were attached using a direct immobilization scheme (Beckstead et al., 2006, 2009). Fibrin microparticles were fabricated using a stirred-emulsion approach and JAG1 indirectly immobilized using antipolyhistidine and EDC/NHS chemistry.
Human Embryonic Stem Cell Differentiation Assay
Undifferentiated hESCs were grown as described by Xu et al. (2001) and seeded onto oriented JAG1 surfaces, antipolyhistidine, or Matrigel with differentiation analyzed via quantitative RT-PCR.
Cardiovascular Progenitor Cell Differentiation Assay
KDR+ cardiovascular progenitor cells were generated via an embryoid-body-based differentiation system (Yang et al., 2008) and plated on either Matrigel, antipolyhistidine, or oriented JAG1 surfaces. Differentiation was characterized via FACS and immunofluorescent staining.
Human-Embryonic-Stem-Cell-Derived Cardiomyocyte Proliferation Assay
hESC-derived MYH7+ cardiomyocytes were generated from KDR+ progenitors as described previously using oriented JAG1 surfaces. Dissociated cardiomyocytes were replated in chamber slides and treated as described. Proliferation was characterized via AlamarBlue and immunostaining.
Statistical Analysis
Data were analyzed for significance using a one-way ANOVA and a Tukey’s post hoc test for pairwise comparison. The software used was SPSS for Windows and significance set at 95% confidence.
Detailed methods can be found in the Supplemental Information.
Acknowledgments
This work was supported by P01 HL094374, R01 HL084642, P01 GM081719, U01 HL100405 (to C.E.M.), R01 HL64387 (to C.E.M. and B.D.R.), T32 EB001650 (to J.C.T.), and unrestricted research funds (to C.M.G.).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Charles E. Murry, Email: murry@uw.edu.
Cecilia M. Giachelli, Email: ceci@u.washington.edu.
Supplemental Information
After 2 weeks in culture, waves of contraction are observed propagating through entire sheets of cells.
References
- Ambros V. Cell cycle-dependent sequencing of cell fate decisions in Caenorhabditis elegans vulva precursor cells. Development. 1999;126:1947–1956. doi: 10.1242/dev.126.9.1947. [DOI] [PubMed] [Google Scholar]
- Amsen D., Blander J.M., Lee G.R., Tanigaki K., Honjo T., Flavell R.A. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Rand M.D., Lake R.J. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Beckstead B.L., Santosa D.M., Giachelli C.M. Mimicking cell-cell interactions at the biomaterial-cell interface for control of stem cell differentiation. J. Biomed. Mater. Res. A. 2006;79:94–103. doi: 10.1002/jbm.a.30760. [DOI] [PubMed] [Google Scholar]
- Beckstead B.L., Tung J.C., Liang K.J., Tavakkol Z., Usui M.L., Olerud J.E., Giachelli C.M. Methods to promote Notch signaling at the biomaterial interface and evaluation in a rafted organ culture model. J. Biomed. Mater. Res. A. 2009;91:436–446. doi: 10.1002/jbm.a.32214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S.J. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Brooker R., Hozumi K., Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Campa V.M., Gutiérrez-Lanza R., Cerignoli F., Díaz-Trelles R., Nelson B., Tsuji T., Barcova M., Jiang W., Mercola M. Notch activates cell cycle reentry and progression in quiescent cardiomyocytes. J. Cell Biol. 2008;183:129–141. doi: 10.1083/jcb.200806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen V.C., Stull R., Joo D., Cheng X., Keller G. Notch signaling respecifies the hemangioblast to a cardiac fate. Nat. Biotechnol. 2008;26:1169–1178. doi: 10.1038/nbt.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collesi C., Zentilin L., Sinagra G., Giacca M. Notch1 signaling stimulates proliferation of immature cardiomyocytes. J. Cell Biol. 2008;183:117–128. doi: 10.1083/jcb.200806091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox V., Gokhale P.J., Walsh J.R., Matin M., Jones M., Andrews P.W. Cell-cell signaling through NOTCH regulates human embryonic stem cell proliferation. Stem Cells. 2008;26:715–723. doi: 10.1634/stemcells.2007-0368. [DOI] [PubMed] [Google Scholar]
- Guo X., Wang X.F. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines N., Irvine K.D. Glycosylation regulates Notch signalling. Nat. Rev. Mol. Cell Biol. 2003;4:786–797. doi: 10.1038/nrm1228. [DOI] [PubMed] [Google Scholar]
- Hayward P., Kalmar T., Arias A.M. Wnt/Notch signalling and information processing during development. Development. 2008;135:411–424. doi: 10.1242/dev.000505. [DOI] [PubMed] [Google Scholar]
- Hicks C., Ladi E., Lindsell C., Hsieh J.J., Hayward S.D., Collazo A., Weinmaster G. A secreted Delta1-Fc fusion protein functions both as an activator and inhibitor of Notch1 signaling. J. Neurosci. Res. 2002;68:655–667. doi: 10.1002/jnr.10263. [DOI] [PubMed] [Google Scholar]
- Hoffman A.S., Hubbell J.A. Surface-immobilized biomolecules. In: Ratner B.D., Hoffman A.S., Schoen F.J., Lemons J.E., editors. Biomaterials Science: An Introduction to Materials in Medicine. Elsevier; San Diego: 2004. pp. 225–233. [Google Scholar]
- Irvine D.J., Hue K.A., Mayes A.M., Griffith L.G. Simulations of cell-surface integrin binding to nanoscale-clustered adhesion ligands. Biophys. J. 2002;82:120–132. doi: 10.1016/S0006-3495(02)75379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J., Ku S.Y., Kim J.E., Choi K., Kim Y.Y., Kim H.S., Oh S.K., Lee E.J., Cho H.J., Song Y.H. Notch inhibition promotes human embryonic stem cell-derived cardiac mesoderm differentiation. Stem Cells. 2008;26:2782–2790. doi: 10.1634/stemcells.2007-1053. [DOI] [PubMed] [Google Scholar]
- Joutel A., Tournier-Lasserve E. Notch signalling pathway and human diseases. Semin. Cell Dev. Biol. 1998;9:619–625. doi: 10.1006/scdb.1998.0261. [DOI] [PubMed] [Google Scholar]
- Keselowsky B.G., Collard D.M., García A.J. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc. Natl. Acad. Sci. USA. 2005;102:5953–5957. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell S., Benchoua A., Heavey B., Smith A.G. Notch promotes neural lineage entry by pluripotent embryonic stem cells. PLoS Biol. 2006;4:e121. doi: 10.1371/journal.pbio.0040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf M.P., Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- McDevitt T.C., Laflamme M.A., Murry C.E. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. J. Mol. Cell. Cardiol. 2005;39:865–873. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm J.S., Kopan R. Notch signaling: from the outside in. Dev. Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- Nemir M., Pedrazzini T. Functional role of Notch signaling in the developing and postnatal heart. J. Mol. Cell. Cardiol. 2008;45:495–504. doi: 10.1016/j.yjmcc.2008.02.273. [DOI] [PubMed] [Google Scholar]
- Nemir M., Croquelois A., Pedrazzini T., Radtke F. Induction of cardiogenesis in embryonic stem cells via downregulation of Notch1 signaling. Circ. Res. 2006;98:1471–1478. doi: 10.1161/01.RES.0000226497.52052.2a. [DOI] [PubMed] [Google Scholar]
- Neves H., Weerkamp F., Gomes A.C., Naber B.A., Gameiro P., Becker J.D., Lúcio P., Clode N., van Dongen J.J., Staal F.J., Parreira L. Effects of Delta1 and Jagged1 on early human hematopoiesis: correlation with expression of notch signaling-related genes in CD34+ cells. Stem Cells. 2006;24:1328–1337. doi: 10.1634/stemcells.2005-0207. [DOI] [PubMed] [Google Scholar]
- Noggle S.A., Weiler D., Condie B.G. Notch signaling is inactive but inducible in human embryonic stem cells. Stem Cells. 2006;24:1646–1653. doi: 10.1634/stemcells.2005-0314. [DOI] [PubMed] [Google Scholar]
- Noseda M., McLean G., Niessen K., Chang L., Pollet I., Montpetit R., Shahidi R., Dorovini-Zis K., Li L., Beckstead B. Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ. Res. 2004;94:910–917. doi: 10.1161/01.RES.0000124300.76171.C9. [DOI] [PubMed] [Google Scholar]
- Oishi K., Kamakura S., Isazawa Y., Yoshimatsu T., Kuida K., Nakafuku M., Masuyama N., Gotoh Y. Notch promotes survival of neural precursor cells via mechanisms distinct from those regulating neurogenesis. Dev. Biol. 2004;276:172–184. doi: 10.1016/j.ydbio.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Schroeder T., Fraser S.T., Ogawa M., Nishikawa S., Oka C., Bornkamm G.W., Nishikawa S., Honjo T., Just U. Recombination signal sequence-binding protein Jkappa alters mesodermal cell fate decisions by suppressing cardiomyogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:4018–4023. doi: 10.1073/pnas.0438008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaudone M., Gazzerro E., Priest L., Delany A.M., Canalis E. Notch 1 impairs osteoblastic cell differentiation. Endocrinology. 2003;144:5631–5639. doi: 10.1210/en.2003-0463. [DOI] [PubMed] [Google Scholar]
- Small D., Kovalenko D., Kacer D., Liaw L., Landriscina M., Di Serio C., Prudovsky I., Maciag T. Soluble Jagged 1 represses the function of its transmembrane form to induce the formation of the Src-dependent chord-like phenotype. J. Biol. Chem. 2001;276:32022–32030. doi: 10.1074/jbc.M100933200. [DOI] [PubMed] [Google Scholar]
- Sokolova V., Epple M. Inorganic nanoparticles as carriers of nucleic acids into cells. Angew. Chem. Int. Ed. Engl. 2008;47:1382–1395. doi: 10.1002/anie.200703039. [DOI] [PubMed] [Google Scholar]
- Varnum-Finney B., Wu L., Yu M., Brashem-Stein C., Staats S., Flowers D., Griffin J.D., Bernstein I.D. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J. Cell Sci. 2000;113:4313–4318. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- Varnum-Finney B., Xu L., Brashem-Stein C., Nourigat C., Flowers D., Bakkour S., Pear W.S., Bernstein I.D. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat. Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- Xu C., Inokuma M.S., Denham J., Golds K., Kundu P., Gold J.D., Carpenter M.K. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Yang L., Soonpaa M.H., Adler E.D., Roepke T.K., Kattman S.J., Kennedy M., Henckaerts E., Bonham K., Abbott G.W., Linden R.M. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- Yu X., Zou J., Ye Z., Hammond H., Chen G., Tokunaga A., Mali P., Li Y.M., Civin C., Gaiano N., Cheng L. Notch signaling activation in human embryonic stem cells is required for embryonic, but not trophoblastic, lineage commitment. Cell Stem Cell. 2008;2:461–471. doi: 10.1016/j.stem.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
After 2 weeks in culture, waves of contraction are observed propagating through entire sheets of cells.


