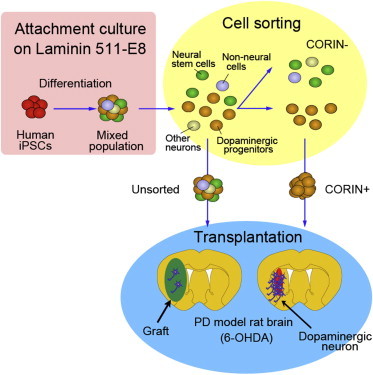
Summary
Human induced pluripotent stem cells (iPSCs) can provide a promising source of midbrain dopaminergic (DA) neurons for cell replacement therapy for Parkinson’s disease. However, iPSC-derived donor cells inevitably contain tumorigenic or inappropriate cells. Here, we show that human iPSC-derived DA progenitor cells can be efficiently isolated by cell sorting using a floor plate marker, CORIN. We induced DA neurons using scalable culture conditions on human laminin fragment, and the sorted CORIN+ cells expressed the midbrain DA progenitor markers, FOXA2 and LMX1A. When transplanted into 6-OHDA-lesioned rats, the CORIN+ cells survived and differentiated into midbrain DA neurons in vivo, resulting in significant improvement of the motor behavior, without tumor formation. In particular, the CORIN+ cells in a NURR1+ cell-dominant stage exhibited the best survival and function as DA neurons. Our method is a favorable strategy in terms of scalability, safety, and efficiency and may be advantageous for clinical application.
Graphical Abstract
Highlights
-
•
DA neurons were highly efficiently induced from iPSCs on xeno-free laminin fragment
-
•
DA progenitors were enriched by sorting of CORIN+ cells
-
•
CORIN+ cell grafts resulted in good DA neuron survival without tumor formation
-
•
NURR1+ cell-dominant stage exhibited the best survival and function as DA neurons
Takahashi and colleagues have described a protocol for generating transplantable dopaminergic neurons from human iPSCs for clinical application and addressed that enriched dopaminergic progenitors by cell sorting with CORIN survived well and functioned as midbrain dopaminergic neurons in the 6-OHDA-lesioned rats and showed minimal risk of tumor formation.
Introduction
The transplantation of dopaminergic (DA) neurons represents one of the potential strategies for the treatment of Parkinson’s disease (PD), and human pluripotent stem cells (PSCs) are expected to be a promising source of such cells. Residual undifferentiated stem cells or proliferating neural progenitor cells (NPCs) with rostral identity, however, may cause tumor formation (Roy et al., 2006; Brederlau et al., 2006; Elkabetz et al., 2008; Doi et al., 2012). In addition, previous clinical trials using fetal mesencephalic cells suggested that contaminated serotonergic neurons may cause graft-induced dyskinesia (Carlsson et al., 2007; Politis et al., 2010). To avoid these adverse outcomes, it is critical to eliminate the unwanted cells from the donor cell population. As another issue, the timing of transplantation of the donor DA neurons has to be optimized in the differentiation stage in order to obtain the best survival of the midbrain DA neurons in the brain.
To address these issues, the purification of NPCs (Fukuda et al., 2006; Pruszak et al., 2009; Sundberg et al., 2013) and premature or postmitotic DA neurons (Jönsson et al., 2009; Ganat et al., 2012) has been attempted. In the studies with murine cells, premature DA neurons were found to most effectively reduce the risk of tumor formation, as well as to improve the motor deficits in hemiparkinsonian rodents. Prior to the clinical application of human PSCs, it is critical to be able to purify or enrich the DA progenitor cells, which can be achieved by cell sorting using a cell surface antigen. A cell surface marker specific for DA progenitor cells, however, has not been identified so far.
CORIN is a serine protease, initially found in the heart, which converts proatrial natriuretic peptide (ANP) into ANP (Yan et al., 2000). In the developing brain, it is specifically expressed in the floor plate where DA progenitor cells are located, and mouse embryonic stem cell (ESC)-derived DA progenitor cells could be enriched by cell sorting using an anti-CORIN antibody (Ono et al., 2007). More recently, mouse ESC-derived CORIN+OTX2+ cells were found to coexpress midbrain DA progenitor markers, such as FOXA2 and LMX1B (Chung et al., 2011). These cells survived without tumor formation in the 6-OHDA-lesioned rats and improved their motor dysfunction. These results were obtained using murine ESCs, and for the clinical application, it is critical to determine whether the same strategy can be applied to human PSCs.
In the present study, we show that purification of the CORIN+ cells generated from human induced pluripotent stem cells (iPSCs) could enrich midbrain DA progenitor cells. Transplantation of both the CORIN+ and the unsorted cells improved the behavior of 6-OHDA-lesioned rats. However, the CORIN+ cell-derived grafts contained more TH+ cells (a DA neuron marker) than those derived from unsorted cells. Furthermore, the CORIN+ cell-derived grafts had a smaller volume and contained only a few number of KI67+ cells (a marker of proliferating cells) compared to those from unsorted cells. In addition, we have optimized the timing of cell sorting and transplantation.
We also developed a scalable neural induction method by using a xeno-free, chemically defined matrix. Taken together, our findings provide an efficient protocol for the clinical application of human iPSCs to treat PD.
Results
Midbrain DA Neurons Are Efficiently Generated by Attachment Culture on Human Laminin Fragments
We generated midbrain DA neurons from human iPSCs (836B3) based on a dual SMAD inhibition and floor plate induction protocol (Chambers et al., 2009; Fasano et al., 2010; Figure 1A), with modification for the initial attachment culture. We used recombinant E8 fragments of human laminin 511 (LM511-E8; Miyazaki et al., 2012) instead of the mouse sarcoma cell-derived matrix, Matrigel (MG). This xeno-free, chemically defined matrix supported the neural differentiation and cell survival more efficiently than MG or another xeno-free matrix, CELLstart (CS) (Figures 1B and 1C; Figures S1A and S1B available online). In our protocol, a GSK3β inhibitor (CHIR99021) that strongly activates wnt signaling (Ying et al., 2008) promoted midbrain specification, as reported previously by Kriks et al. (2011), Kirkeby et al. (2012), and Xi et al. (2012) (Figure S1C).
Figure 1.
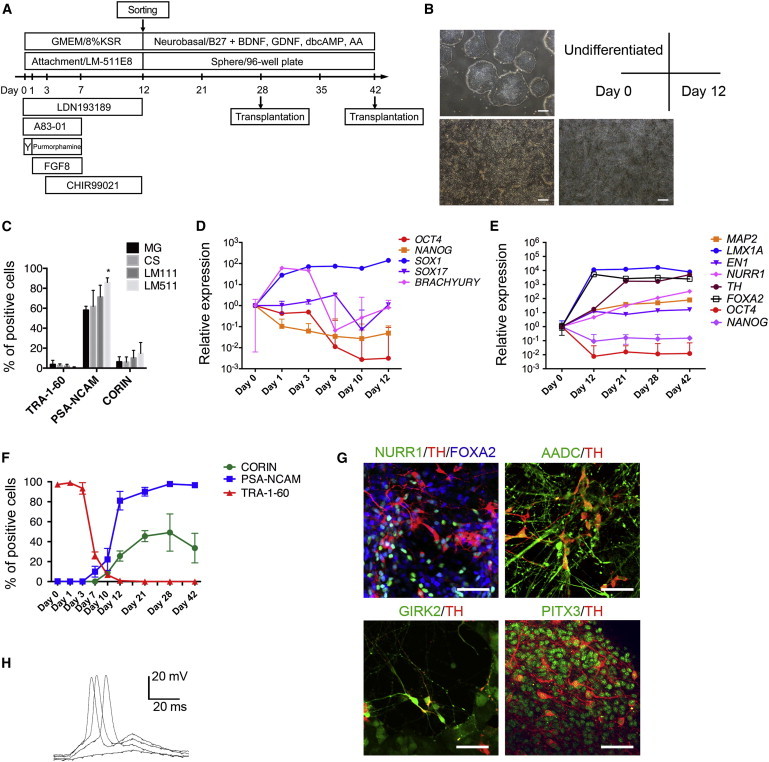
Differentiation of Human iPSCs by Attachment Culture on LM511-E8
(A) An overview of the culture protocol.
(B) Phase-contrast images of the undifferentiated iPSCs on LM511-E8 on differentiation culture day 0 and day 12. Scale bars, 200 μm.
(C) The results of the flow cytometric analysis of differentiating cells on MG, CS, Laminin 111-E8 fragment (LM111), and Laminin 511-E8 fragment (LM511) on day 12. The values are the mean ± SD. ∗p = 0.0459 by a one-way ANOVA with Tukey’s multiple comparison test (n = 3 independent experiments).
(D and E) The results of the gene expression analysis by quantitative RT-PCR. The expression level of the undifferentiated cells (day 0) was set to 1. The values are the mean ± SD (n = 3 independent experiments).
(F) The results of the temporal expression analysis by flow cytometry. The values are the mean ± SD (n = 4 independent experiments).
(G) Immunofluorescence images of the differentiated cells on poly-L-O/F/L-coated dishes on day 42. Scale bars, 50 μm.
(H) Current clamp recordings of induced action potentials by brief current pulses from an iPSC-derived neuron on day 42.
A comparative temporal gene expression analysis revealed that the expression of pluripotent cell markers (OCT3/4, NANOG) gradually decreased, whereas that of a neural cell marker (SOX1) increased until day 12 (Figure 1D). The expression of a mesodermal cell marker (BRACHYURY) transiently increased but eventually became equivalent to that of undifferentiated iPSCs. The expression level of an endodermal cell marker (SOX17) remained low. In the expression level analysis performed for a longer time period, the expression levels of OCT3/4 and NANOG remained low after day 12. In contrast, a midbrain marker (LMX1A) and a basal and floor plate marker (FOXA2) reached a plateau on day 12 (Figure 1E). The expression levels of other DA neuron markers gradually increased during the course of differentiation. A flow cytometric analysis revealed that TRA-1-60+ cells (a PSC marker) had almost disappeared on day 12, whereas PSA-NCAM+ cells (a neural cell marker) emerged on day 7 and became the major population around day 12 (Figure 1F). The CORIN+ cells (a floor plate marker) emerged after day 10 and peaked around day 21–28.
Similar results with regard to the expression of these markers were obtained with another iPSC line, 404C2 (Figures S1D–S1F). To confirm the potential of the differentiated cells to become midbrain DA neurons, the floating spheres were replated on poly-ornithine/laminin/fibronectin (O/F/L)-coated 8-well chamber slides on day 28, then incubated for 14 days. An immunofluorescence study performed on day 42 showed that about 40% of the total cells expressed TH (a DA neuron marker), and most of them coexpressed the midbrain markers, FOXA2 and NURR1 (Figure 1G). The TH+ cells also expressed markers for mature midbrain DA neurons, such as AADC, PITX3, and GIRK2, and the action potentials of the neurons were recorded by an electrophysiological analysis (Figure 1H).
Sorting of CORIN+ Cells Enriches the DA Progenitor Cells
Based on the above results, we purified the CORIN+ cells using fluorescence-activated cell sorting (FACS) on days 12 (Figures 2A and 2B) and 21 (Figure S2A). A quantitative PCR analysis of the day 12 CORIN+ cells revealed that the expression levels of midbrain markers (LMX1A and EN1) and a basal and floor plate marker (FOXA2) were higher in the CORIN+ cells, whereas those of a hindbrain marker (GBX2) and a forebrain marker (SIX3) were lower compared to those in the unsorted and CORIN− cells (Figure 2C). Again, similar results were obtained using another iPSC line, 404C2 (Figure S1G). A global transcriptome analysis of the sorted and unsorted cells on day 12 disclosed that the expression levels of rostral (PAX6), caudal (HOXA2), early neural (NEUROG2), postmitotic neuron (NEFM), and nonneural cell (DLK1, CYP1B1) markers were higher in the unsorted cells (Figures S2E and S2F; Table S1). Midbrain DA progenitor cells can be defined as those having dual expression of LMX1A and FOXA2 (Kriks et al., 2011; Kirkeby et al., 2012). A double-labeled immunofluorescence study revealed that the percentage of LMX1A+/FOXA2+ cells was higher in the CORIN+ cell population compared to the unsorted cells (75.5% ± 8.2% versus 47.3% ± 6.6%; Figures 2D–2E). OTX2 is a transcription factor expressed at the rostral to midbrain/hindbrain boundary. An immunofluorescence study revealed that the percentage of OTX2+/LMX1A− cells (namely, rostral cells) was decreased in the sorted CORIN+ cells (Figures 2D and 2F).
Figure 2.
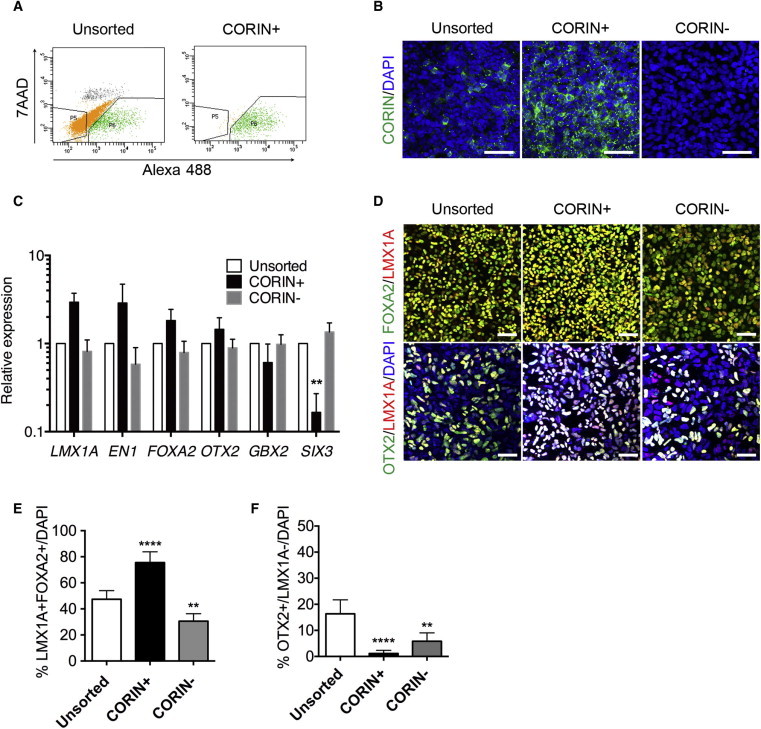
Purification and Characterization of iPSC-Derived CORIN+ Cells on Day 12
(A) Dot plots of the FACS analysis of the unsorted and the reanalyzed sorted cells. The x-y grid indicates the intensity of 7-AAD and Alexa 488 staining, respectively.
(B) Immunostaining for CORIN (green) and DAPI (blue). Scale bars, 50 μm.
(C) The results of the gene expression analysis by quantitative RT-PCR. The expression level of the unsorted cells was set to 1. The values are the mean ± SD. ∗∗p = 0.0080 by a one-way ANOVA with Dunnett’s multiple comparison test (n = 3 independent experiments).
(D) Immunostaining for FOXA2/LMX1A and OTX2/LMX1A. Scale bars, 100 μm.
(E and F) The percentages of LMX1A+/FOXA2+ cells (E) and OTX2+/LMX1A− cells (F) per the total number of cells. The values are the mean ± SD. ∗∗p = 0.0046 (E), ∗∗p = 0.0013 (F), and ∗∗∗∗p < 0.0001 by a one-way ANOVA with Dunnett’s multiple comparison test (n = 5 different experiments).
See also Figures S1 and S2 and Tables S5 and S6.
On day 21, the percentage of CORIN+ cells was higher compared to that on day 12 (45.4% ± 14.6% versus 18.9% ± 15.4%). In this case, the expression levels of LMX1A and FOXA2 did not change regardless of sorting (Figures S2A and S2B). Regarding the percentage of the LMX1A+/FOXA2+ cells, there were no significant differences between the unsorted and the CORIN+ cells (Figures S3C and S3D).
CORIN+ Cells Exhibit Reduced Proliferation and Differentiate into Midbrain DA Neurons
In order to investigate the characteristics of the day 12 CORIN+ cells, we continued to perform sphere culture of the sorted cells in the presence of brain-derived neurotrophic factor (BDNF) and glial cell-derived neurotrophic factor (GDNF), which support the maturation toward DA neurons. The CORIN+ cells exhibited reduced proliferation, and the average diameter of the spheres was smaller than that of the unsorted cells on both days 28 and 42 (Figures S3A and S3B). Consistently, the sorted CORIN+ cells contained a lower percentage of proliferating (KI67+) cells compared to the unsorted cells on day 28 (Figures S3C and S3D). There were no Oct3/4+ or Nanog+ cells in these spheres (data not shown). An immunofluorescence study of the cultured day 12 CORIN+ cells disclosed that the percentage of FOXA2+ cells was 70%–75%, which was higher than that of the unsorted cells on both days 28 and 42 (Figures 3A–3D). The NURR1+ cells were dominant on day 28 (27.3% ± 5.5% and 2.1% ± 1.2% for NURR1+ and TH+ cells, respectively), whereas the ratio of the TH+ cells increased on day 42 (19.9% ± 6.9% and 42.0% ± 4.4% for NURR1+ and TH+ cells, respectively). Next, we compared the dopamine secretion between the cultured day 12 CORIN+ cells and the unsorted cells. A high-performance liquid chromatography (HPLC) analysis on day 56 revealed that dopamine and its metabolite, DOPAC, were more abundantly released in the cultures of the CORIN+ cells (Figures 3E and 3F) than of the unsorted cells. The secretion of serotonin was not detected in either of the cultures (data not shown). These in vitro results suggested that the day 12 CORIN+ cells were less proliferative and more efficiently gave rise to mature midbrain DA neurons compared to the unsorted cells.
Figure 3.
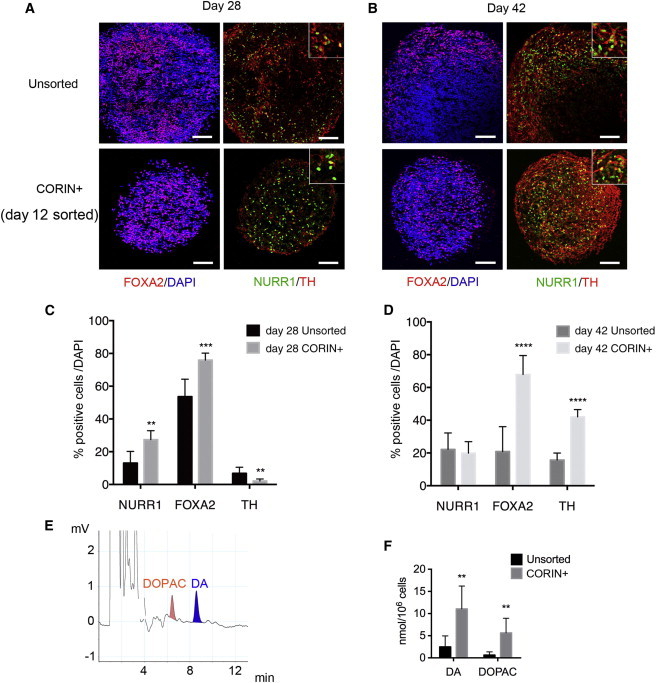
DA Differentiation of the Unsorted and the Day 12 CORIN+ Cells
(A and B) Double-labeled immunostaining of the spheres on day 28 and day 42. FOXA2/DAPI (left) and NURR1/TH (right). Insets indicate magnified images of double-positive cells. Scale bars, 100 μm.
(C and D) The percentages of NURR1+, FOXA2+, and TH+ cells per total cells on day 28 (C) or day 42 (D). The values are the mean ± SD. (C) ∗∗p = 0.0011 for NURR1, ∗∗∗p = 0.0002 for FOXA2, and ∗∗p = 0.0058 for TH, and (D) ∗∗∗∗p < 0.0001 for FOXA2 and TH by the unpaired t test (n = 6 independent experiments).
(E and F) The results of the HPLC analysis of the dopamine and DOPAC releases by the day 56 cultured cells under high potassium stimulation. The values are the mean ± SD. ∗∗p = 0.0043 for DA and ∗∗p = 0.0045 for DOPAC by an unpaired t test (n = 3 independent experiments).
Grafts Derived from the CORIN+ Cells Are Less Expansive
In order to investigate the survival, proliferation, and function of the cultured CORIN+ cells in vivo, we injected the unsorted and the CORIN+ cells (4 × 105 cells in 2 μl) into the putamen of 6-OHDA-lesioned rats. To find the optimal differentiation stage of the donor cells, we sorted the CORIN+ cells on day 12, continued the sphere culture, and grafted the cells on day 28 or 42. When the cells were grafted on day 28, the graft sizes of unsorted cells showed variation from 88.4 to 0.5 mm3 (n = 11) at 16 weeks after transplantation, whereas those of the CORIN+ cells were more evenly distributed, ranging in size from 8.5 to 1.5 mm3 (n = 7, Figure 4A). Furthermore, there were significant differences between the average sizes (35.0 ± 37.5 mm3 versus 3.4 ± 2.9 mm3 for the unsorted and the CORIN+ cells, respectively). The percentage of proliferating (KI67+) cells was less than 1% in both cases but was significantly lower in the grafts derived from the CORIN+ cells (0.06% ± 0.07%) than in those derived from the unsorted cells (0.86% ± 0.53%; Figures 4C–4E). When the cells were grafted on day 42, the average graft sizes were less than 1 mm3 in both cases (n = 6 or 7, Figure 4B), and we could not find any KI67+ cells in these grafts.
Figure 4.
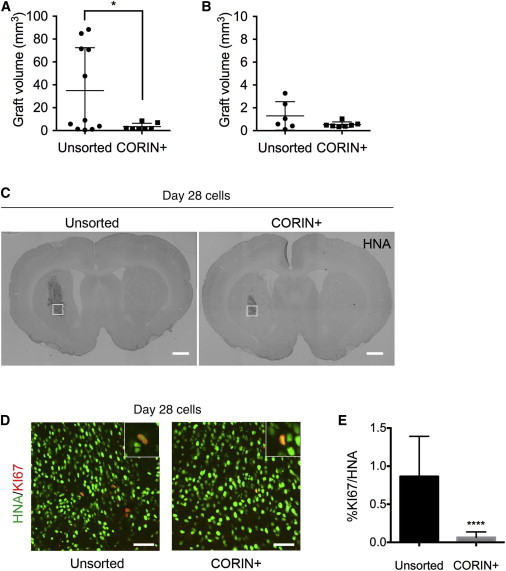
The Proliferation of the Unsorted or the Day 12 CORIN+ Cells In Vivo
(A and B) The graft volumes at 16 weeks. The day 12 CORIN+ cells were grafted on day 28 (n = 11 for unsorted, n = 7 for CORIN+) (A) or day 42 (n = 6 for unsorted, n = 7 for CORIN+) (B). Values are the mean ± SD. ∗p = 0.0435 by the unpaired t test.
(C) Immunohistochemistry for the human nuclear antigen (HNA). Scale bars, 1 mm.
(D) Immunofluorescence images of the squares in (C) for HNA (green) and KI67 (red). Scale bars, 50 μm. Insets show magnified images of KI67+ cells.
(E) The percentages of proliferating (KI67+) cells per donor cells (HNA+). ∗∗∗∗p < 0.0001 by the unpaired t test. The values are the mean ± SD (n = 5 animals).
See also Table S6.
Cultured Day 12 CORIN+ Cells Resulted in Better Survival of DA Neurons and Greater Behavioral Improvement
Next, in order to evaluate the function of the grafted cells, we analyzed the amphetamine-induced rotation of 6-OHDA-lesioned rats, which is caused by an imbalance of dopamine concentration between the right and left putamen (Ungerstedt and Arbuthnott, 1970). When the day 12 CORIN+ or the unsorted cells were grafted on day 28, the abnormal rotations were reduced by both of the cell types at 16 weeks (Figures 5A and S4A). An immunofluorescence study at 16 weeks showed that more TH+ cells survived and exhibited neurite outgrowth in the grafts derived from the day 12 CORIN+ cells compared to those from the unsorted cells (6,747 ± 2,341 versus 3,436 ± 2,384 cells/graft; Figures 5C–5G and S4B). The percentages of TH+ cells per total neurons (NEUN+) or total surviving cells (HNA+) were also higher in the grafts derived from the day 12 CORIN+ cells than those from the unsorted cells (Figures 5I and 5J), consistent with the in vitro data indicating that DA progenitor cells were enriched in the sorted CORIN+ cells. These TH+ cells coexpressed markers for midbrain DA neurons, including FOXA2, NURR1, and PITX3 (Figure 5K). About half of the TH+ cells also expressed GIRK2 (a marker for A9 DA neurons that project from the substantia nigra to the striatum). Serotonergic neurons, which may cause graft-induced dyskinesia, are generated in the ventral basal plate of the rostral hindbrain outside the floor plate. Therefore, the sorting of the CORIN+ cells can exclude serotonergic neurons. The percentages of serotonin+ cells per total neurons were very low: 2.5% ± 2.5% and 1.2% ± 0.8% for the unsorted and the day 12 CORIN+ cells, respectively (Figures 5L and 5M).
Figure 5.
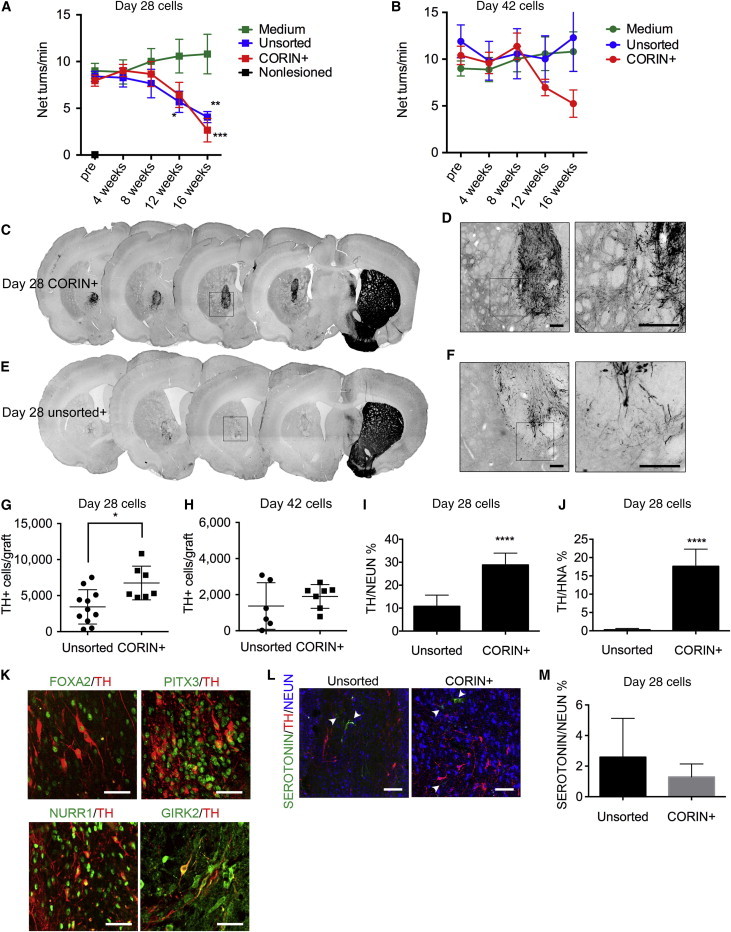
The Survival and Function of DA Neurons Derived from the Unsorted or the Day 12 CORIN+ Cells In Vivo, which Were Grafted on Day 28 or Day 42
(A and B) The methamphetamine-induced rotation of the rats with the grafts. The values are the mean ± SEM. ∗p = 0.0482 (12 weeks), ∗∗p = 0.0017 (unsorted, 16 weeks), and ∗∗∗p = 0.0003 (CORIN+, 16 weeks) by a two-way ANOVA with Dunnett’s multiple comparisons test: n = 6, medium injection and nonlesioned; n = 11, day 28 unsorted; n = 6, day 42-unsorted; and n = 7, day 28- and day 42 CORIN+, respectively.
(C–F) DAB staining of the representative grafts derived from day 28 CORIN+ or day 28-unsorted cells stained for TH. (C and E) The low-power images of consecutive sections throughout the striatum are shown. (D and F) The magnified images of flames in (C) and (E) are shown. The right panels are the magnified images of the flames in the left panels. Scale bars, 200 μm.
(G and H) The number of TH+ cells in each graft. The values are the mean ± SD. ∗p = 0.0106 by the unpaired t test.
(I and J) The rates of TH+ cells per neuronal cells (NEUN+) (I) and TH+ cells per surviving donor cells (HNA+) (J). ∗∗∗∗p < 0.0001 by the unpaired t test. The values are the mean ± SD.
(K) Immunostaining of the grafts derived from day 12 CORIN+ cells for FOXA2/TH, PITX3/TH, NURR1/TH, and GIRK2/TH. Scale bars, 50 μm.
(L) Immunofluorescent images for SEROTONIN(green)/TH(red)/NEUN(blue). Arrowheads indicate SEROTONIN+ cells. Scale bars, 100 μm.
(M) The percentages of the SEROTONIN+ cells per surviving neurons (NEUN+). The values are the mean ± SD. There were no significant differences.
In contrast, when the cells were grafted on day 42, significant behavioral improvement was not observed with either the unsorted or day 12 CORIN+ cells (Figures 5B and S4C). There were no differences in the number of the TH+ cells between the grafts derived from the unsorted or the CORIN+ cells at 16 weeks (Figures 5H). It is worth noting that when the day 12 CORIN+ cells were grafted on day 42, the average numbers of surviving TH+ cells were lower compared to those grafted on day 28: 6,747 ± 2,341 versus 1,900 ± 658 cells/graft for day 28 and 42, respectively (Figures 5G and 5H).
When the CORIN+ cells were sorted on day 21 and grafted on day 28 or 42, we could not observe a reduction of the amphetamine-induced rotations of the 6-OHDA-lesioned rats in either case (n = 4 or 5, Figures S4D–S4G). The numbers of the TH+ cells in these grafts were lower compared to the grafts derived from the day 12 CORIN+ cells (Figure S4H).
Cultured Day 12 CORIN+ Cells on Day 28 Are in the Premature Stage of Development into Midbrain DA Neurons
As shown above, the best survival of the TH+ cells was obtained when the CORIN+ cells were sorted on day 12 and grafted on day 28. In order to assess the identity of the donor cells, we performed the microarray analyses to compare the gene expression profiles between the day 12 CORIN+ cells on day 28 and the unsorted cells on day 28 as well as the day 12 CORIN+ cells on day 28 and the day 12 CORIN+ cells on day 42. The comparison between the day 12 CORIN+ cells and unsorted cells on day 28 revealed a higher level of expression of a forebrain marker, PAX6, in the unsorted cells (Figures 6A and 6B; Table S2). In contrast, higher expression of ALCAM, as well as CORIN, was observed in the CORIN+ cells. The comparison between the day 28 cells and the day 42 cells revealed higher expression of SHH, WNT5A, and CORIN in the day 28 cells and higher expression of TH in the day 42 cells (Figures 6C and 6D; Table S3).
Figure 6.
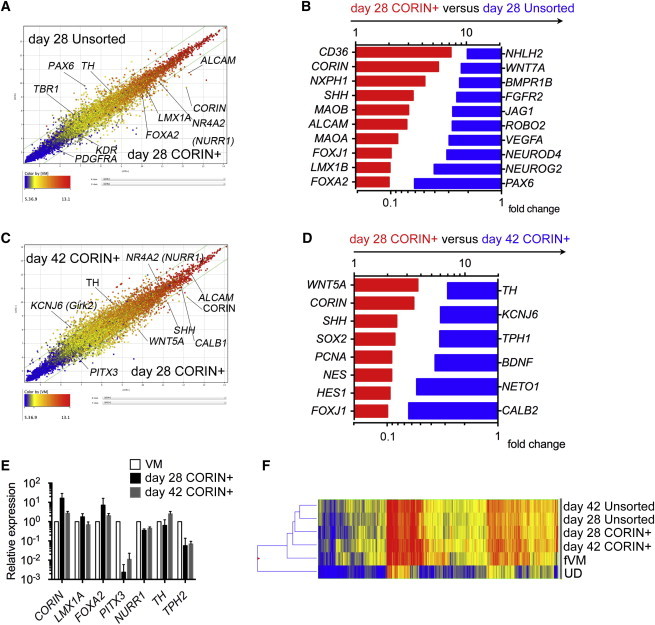
The Results of the Gene Expression Analysis of the iPSC-Derived Cells and Human Fetal Cells
(A–D) The results of the microarray analysis comparing the unsorted versus CORIN+ cells on day 28 (A and B) and the CORIN+ cells on day 28 versus on day 42 (C and D). (A and C) Scatterplots. (B and D) Selected genes of differentially expressed transcripts comparing (B) day 28 CORIN+ versus day 28 unsorted cells and (D) day 28 CORIN+ versus day 42 CORIN+ cells are shown.
(E) The results of the quantitative RT-PCR analysis in the CORIN+ cells and human fetal VM tissue. The expression of the VM tissue was set to 1. The values are the mean ± SD (n = 3 independent experiments).
(F) The hierarchical clustering of the global gene expression data obtained from the iPSC-derived cells and human fetal VM tissue.
In previous clinical trials, transplantation of fetal ventro-mesencephalic (VM) cells improved the neurological deficits of patients with PD (Lindvall et al., 1990; Piccini et al., 1999; Brundin et al., 2000; Mendez et al., 2005; Kefalopoulou et al., 2014). Therefore, we compared the gene expression between the cultured day 12 CORIN+ cells and the VM tissue of a human 7.5-week-old fetus. A quantitative PCR analysis revealed that there was higher expression of mature DA neuron markers, such as TH and PITX3, in the fetal tissue compared to the day 12 CORIN+ cells on day 28 (Figure 6E). It should be noted that the expression level of TPH2, a marker of serotonergic neurons, was much lower in the CORIN+ cells. A microarray analysis revealed that the cultured day 12 CORIN+ cells on day 28 were in the earlier developmental stage than the human fetal VM tissue, which expressed genes related to axon guidance, such as SPON1 and SLIT2 (Figure S5; Table S4). Hierarchical clustering of the global gene expression disclosed that fetal VM tissue is more closely related to the day 12 CORIN+ cells on day 42 than those on day 28 (Figure 6F).
Discussion
In this study, we demonstrated that DA progenitor cells are enriched in the sorted CORIN+ cells derived from human iPSCs. A higher number of midbrain DA neurons survived in the CORIN+ cell-derived grafts compared to those from the unsorted cells and improved the behavior of the 6-OHDA-lesioned rats. The CORIN+ cell-derived grafts contained only a few (<0.1%) proliferating cells, suggesting that they are associated with minimal risk of tumor formation. In addition, we have developed a highly efficient method for DA neuron induction on xeno-free, chemically defined human laminin fragment.
We induced midbrain DA neurons by attachment culture on LM511-E8. Laminin is a heterotrimeric glycoprotein composed of three covalently linked chains that are termed α, β, and γ (Miner et al., 2004); specifically α5, β1, and γ1 for LM511. The full-length (Rodin et al., 2010; Miyazaki et al., 2008) and short fragment (Miyazaki et al., 2012) of laminin-511 supports the stable culture of PSCs. The adhesion of human PSCs to LM511-E8 is primarily α6β1-integrin dependent (Miyazaki et al., 2012), and the laminin interactions with the α6β1-integrin play an important role as an early inductive signal to regulate the proliferation, migration, and fate decision of ESC-derived neural cells (Ma et al., 2008) and the differentiating neural cells in the embryonic brain (Lathia et al., 2007). Consistently, LM511-E8 efficiently supported the neural induction from human iPSCs and the survival of the differentiated cells. Furthermore, we were able to obtain more than 24 times the number of differentiated neural cells per dish compared to our previous feeder-free floating method (Morizane et al., 2011). The use of xeno-free matrix (Swistowski et al., 2009) and the floating culture of cell aggregations (Eiraku et al., 2008; Morizane et al., 2011; Kirkeby et al., 2012) have both been introduced to avoid animal-derived feeder cells in order to facilitate the future clinical application of the PSCs. Our high-density attachment culture on LM511-E8 yielded a higher number of neural cells more easily and seems to be suitable for clinical application.
With regard to safety issues, prolonged differentiation (Brederlau et al., 2006; Doi et al., 2012) and purification of neurally committed cells (Fukuda et al., 2006; Pruszak et al., 2009; Sundberg et al., 2013) have been attempted to reduce the risk of tumor formation. Recently, a dual SMAD inhibition and floor plate induction protocol was shown to reduce the induction of PAX6+ progenitor cells with a rostral identity, which may have a potential of continuous proliferation in the brain (Fasano et al., 2010; Kriks et al., 2011; Figure S1C). The risk of proliferation of the early neural or nonneural cells, however, cannot be ignored. Consistently, the graft sizes derived from the unsorted cells on day 28 widely ranged from 88.4 to 0.5 mm3 at 16 weeks. In contrast, the graft sizes from the day 12 CORIN+ cells were relatively constant (8.5–1.5 mm3), and the average size was significantly smaller. In addition, the percentage of proliferating cells was much lower in the CORIN+ cell-derived grafts, indicating that the purification of CORIN+ cells contributes to the safety of the cell replacement therapy.
The purity of midbrain DA neurons among the donor cells is one of the keys to the functional recovery after transplantation. The sorting of CORIN+ cells on day 12 could enrich the LMX1A+/FOXA2+ DA progenitor cells from 47% to 76% of the total cells. This increase in the number of DA neurons was also confirmed in vivo when the day 12 CORIN+ cells were grafted on day 28. Behavioral improvement was observed by the transplantation of both the unsorted and the cultured CORIN+ cells, probably because the 300 surviving TH+ cells in the unsorted cell grafts were sufficient to exert such an effect (Brundin et al., 1988). However, the ratio of TH+ cells to the total surviving cells was much higher in the CORIN+ cell-derived grafts: at 0.3% versus 18% for the unsorted and the CORIN+ cells, respectively. Recently, two studies of transplantation of human ESC-derived DA progenitor cells into rat brains have been reported, in which a dual SMAD inhibition and floor plate induction protocol was applied (Kriks et al., 2011; Kirkeby et al., 2012). Both studies showed that about 80% of the total cells on day 11 were LMX1A+/FOXA2+, but the ratios of TH+ cells in the grafts at 16 weeks (Kriks et al., 2011) and 18 weeks (Kirkeby et al., 2012) were 6% and 54%, respectively. This decreased ratio of DA neurons in the graft may be due to the vulnerability of DA neurons, or it may take a longer time period for grafted progenitor cells to become fully mature DA neurons. Optimization of the host brain environment might improve the survival or maturation of the grafted DA progenitor cells.
It is noteworthy that the number of TH+ cells per graft decreased when the day 12 CORIN+ cells were engrafted on day 42 compared to day 28. A recent analysis using transgenic mouse ESC reporter lines concluded that the Nurr1+ stage (middle stage) of neuronal differentiation is particularly suitable and led to the best survival of the ESC-derived DA neurons (Ganat et al., 2012). In our study with human iPSCs, the NURR1+ cells were dominant compared to the TH+ cells on day 28. Intriguingly, a comparison of the gene expression profiles revealed that the cultured day 12 CORIN+ cells on day 42 were more closely related to the human 7.5-week fetal VM tissue, and mature DA neuron markers, such as TH and PITX3, were more abundantly expressed in both of these. Taken together, our results suggest that the cultured day 12 CORIN+ cells on day 28 are in the premature stage of the midbrain DA neurons and may be suitable donor cells for the treatment of PD.
In conclusion, we have developed a method for (1) scalable DA neuron induction on human laminin fragments, and (2) sorting DA progenitor cells using an anti-CORIN antibody. Furthermore, we determined the optimal timing for the cell sorting and transplantation. The grafted CORIN+ cells survived well and functioned as midbrain DA neurons in the 6-OHDA-lesioned rats and showed minimal risk of tumor formation. For the clinical application of iPSCs, the scalability and reproducibility of the donor cell preparation are critical. These results suggest that our strategy is advantageous in terms of scalability, safety, and efficiency and can be applied to the cell replacement therapy for PD.
Experimental Procedures
Coating of Extracellular Matrices
The 24-well or 6-well cell culture plates (BD Falcon) were precoated with extracellular matrices (ECMs) at a concentration of 1:20 for MG, 1:50 for CS (Ausubel et al., 2011; Bergström et al., 2011), 1 μg/cm2 for LM111, and 0.5 μg/cm2 for LM511-E8 and were incubated for 2 hr at 37°C just before cell seeding. To dilute the ECMs, Dulbecco’s PBS(+) was used for CS, PBS(−) for LM111 and LM511-E8, and the culture media were used for MG.
Human iPSC Culture
This study was approved by the ethical committees of Kyoto University, Kyoto. Human iPSCs (404C2 and 836B3) were initially maintained on SNL feeder layers in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 (Sigma-Aldrich) supplemented with 0.1 mM 2-mercaptoethanol (Wako Pure Chemicals Industries), 20% knockout serum replacement (KSR; Invitrogen), 0.1 mM nonessential amino acids (Invitrogen), 2 mM L-glutamine, and 5 ng/ml bFGF (Invitrogen). When passaging the cells to LM511-E8-coated dishes, the iPSCs were dissociated into single cells with TrypLE select (Invitrogen) and replated at a density of 1.5 × 104 cells per 6-well plate with StemFit media (Ajinomoto). After they were passaged three times to remove the feeder cells, the iPSCs were used for experiments.
Neural Induction from Human iPSCs
Human iPSCs were dissociated into single cells after 10 min of incubation with TrypLE select and were plated on LM511-E8-coated 6-well plates at a density of 4 × 105 cells. After 4 days of culture, the iPSCs reached a confluent state, and the maintenance media (the StemFit media) were changed to the differentiation media containing GMEM supplemented with 8% KSR, 0.1 mM MEM nonessential amino acids (all Invitrogen), sodium pyruvate (Sigma-Aldrich), and 0.1 mM 2-mercaptoethanol. We added LDN193189 (STEMGENT) and A83-01 (Wako) to efficiently induce neuronal differentiation (Morizane et al., 2011). We also added purmorphamine and FGF8 (Wako) from day 1 to day 7, and CHIR99021 (Wako/STEMGENT) from day 3 to day 12, to induce floor plate cells (Kriks et al., 2011).
After cell sorting on culture day 12, the sorted cells were replated on low cell adhesion 96-well plates (Lipidure-Coat Plate A-96U; NOF CORPORATION) at a density of 2 × 104 cells/well, in the neural differentiation media containing Neurobasal medium supplemented with B27 supplement, 2 mM L-glutamine (all Invitrogen), 10 ng/ml GDNF, 200 mM ascorbic acid, 20 ng/ml BDNF (all Wako), and 400 μM dbcAMP (Sigma-Aldrich). We added 30 μM of Y-27632 (Wako) in the first medium to avoid apoptosis at the initial plating. After that, we changed the media every 3 days. For in vitro studies in Figure 1, the differentiating cells on LM511-E8 were detached and dissociated on day 12, replated on low cell adhesion 96-well plates in the neural differentiation media. The floating spheres were replated on O/F/L-coated plates or 8-well chamber slides on day 28, then cultured with the neural differentiation media until day 42.
Quantitative RT-PCR
Total RNA was isolated using an RNeasy Mini or Micro Kit (QIAGEN), and cDNA was synthesized from 1 μg of RNA using a SuperScript III First-Strand Synthesis System (Invitrogen). Quantitative PCRs were carried out with SYBR Premix Ex Taq (TaKaRa) and the Thermal Cycler Dice Real Time System (TaKaRa). The data were assessed using a delta-Ct method and normalized by the GAPDH expression. The primer sequences used are shown in Table S5.
Immunofluorescence Studies
Double- or triple-labeled immunohistochemical analyses were carried out after permeabilization and blocking with 0.3% Triton X-100 and 2% skim milk. The immunoreactive cells were visualized using a fluorescent microscope (BZ-9000; Keyence) and a confocal laser microscope (Fluoview FV1000D; Olympus). The primary antibodies used are described in Table S6.
Dopamine Release Assay
Human iPSC-derived neural cells (culture day 28) were differentiated on O/F/L-coated 24-well plates for 28 days, then were washed twice with a low KCl (4.7 mM) solution and incubated in the low KCl solution for 15 min. The medium was subsequently replaced with a high KCl solution (60 mM) for 15 min. The solution was then collected, and the concentration of dopamine was determined by HPLC using a reverse-phase column and an electrochemical detector system (HTEC-500; Eicom).
Electrophysiological Analysis
Whole-cell patch-clamp recordings were performed on 42- to 84-day cultured neurons grown on O/F/L-coated glass coverslips. Neurons with a large cell body and neurite-like structures were chosen for examination. The cells were maintained in a physiological saline solution with the following composition: 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 26 mM NaHCO3, 1.25 mM NaH2PO4, and 17 mM glucose. Patch pipettes were made from borosilicate glass capillaries (GC150TF-10; Clark) and had a resistance of 3–4 MW when filled with an internal solution composed of 140 mM KCl, 10 mM HEPES, and 0.2 mM EGTA (pH 7.3). Recordings with a voltage clamp and current clamp were done with a patch-clamp amplifier (EPC-8; HEKA). The gigaseal resistances were in the range of 10–20 GW. The current signals from the amplifier were filtered at 5 kHz through a four-pole low-pass filter with Bessel characteristics (UF-BL2; NF), sampled with a 12-bit A/D converter, and were stored on a 32-bit computer (PC-9821Ra333; NEC). All experiments were performed at room temperature.
Cell Sorting
For the analysis, cells were harvested using Accumax, were gently dissociated into a single cell suspension, and resuspended in phenol-free, Ca2+Mg2+-free Hank’s balanced salt solution (HBSS; Invitrogen) containing 2% FBS, 10 μM Y-27632 (Wako), 20 mM D-glucose (Wako), and 50 μg/ml penicillin/streptomycin (Invitrogen). Samples were filtered through cell-strainer caps (35 μm mesh; BD Biosciences) and then subjected to surface marker staining using a PE-conjugated anti-TRA-1-60 antibody (BD PharMingen), anti-PSA-NCAM antibody (1:100; Millipore), and Alexa 488-conjugated anti-mouse IgM antibody (1:400; Invitrogen), or an anti-CORIN antibody (1:200; a gift from the KAN laboratory) and Alexa 488-conjugated anti-mouse IgG or IgM antibodies (1:400; Invitrogen). The antibodies were added and incubated at 4°C for 20 min, and then cells were washed twice with HBSS buffer. Dead cells and debris were excluded by 7-AAD staining. The analysis was performed using a FACSAria II cell sorter or a BD LSRFortessa flow cytometer and the FACSDiva software program (BD Biosciences). A 100 μm ceramic nozzle (BD Biosciences), with a sheath pressure of 20–25 psi and an acquisition rate of 3,000–5,000 events/s, was used for sorting. Positive staining was set so that less than 0.1% of events exceeded the threshold in samples lacking primary antibodies. The sorted cells were collected and replated in U-shaped 96-well plates (Lipidure-Coat Plate A-96U; 2 × 104 cells/well) with culture medium containing 30 μM Y-27632.
Animals and Cell Transplantation
Animals were cared for and handled according to the Guidelines for Animal Experiments of Kyoto University and the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources (ILAR; Washington, DCA). Adult female Sprague-Dawley rats (SHIMIZU LABORATORY SUPPLIES) weighing 200–250 g were used for the 6-OHDA-lesioned model. The rats were injected with 6-OHDA into the medial forebrain bundle in the right hemisphere. The coordinates were calculated with reference to the bregma: anterior (A), −4.4; lateral (L), −1.2; ventral (V), −7.8; and tooth bar (TB), −2.4. A total of 16 μg of 6-OHDA was injected per rat in 2.5 μl of saline with 0.02% ascorbic acid. Cell transplantation was performed with stereotactic injection of 4 × 105 cells in 2 μl (200,000 cells/μl, 1 μl/10 s) through a 22G needle into the right side of the striatum (from the bregma: A, +1.0; L, −3.0; V, −5.0 and −4.0; and TB, 0). Sixteen weeks after transplantation, the animals were euthanized with pentobarbital and perfused transcardially with 4% paraformaldehyde. The brains were cut with a cryostat at 35 μm thickness and mounted. A total of 12 sections containing the graft region from each rat were chosen for the immunofluorescence study. The graft size was calculated from the area stained for the anti-HNA antibody, using the Bioanalyzer software program (Keyence).
Behavioral Analysis
The methamphetamine-induced rotation assay was performed pretransplantation and every 4 weeks after transplantation using video-monitored rotational bowls. A dose of 2.5 mg/kg of methamphetamine (Dainippon Sumitomo Pharma) was injected intraperitoneally, and the rotations were recorded for 90 min. Only the rats that showed more than six rotations per minute were used as graft recipients. Nonlesioned rats showed no induced net turns (Figure 5A, pre).
Microarray Analyses
For the microarray analyses, the total RNA was processed using an Ambion WT Expression Kit and Affymetrix GeneChip Whole-Transcript (WT) Expression Arrays (Ambion, Life Technologies). Comparisons were performed among the unsorted, sorted, and the human fetal VM sample (gestational age of 7.5 weeks) using the GeneSpring software program (version 12.5; Agilent Technologies). Genes found to have an adjusted value of p < 0.05 and a fold change greater than two were considered to be significant.
Statistical Analysis
The statistical analyses were performed using a commercially available software package (GraphPad Prism 6; GraphPad Software). Data from the in vitro and in vivo experiments were analyzed by a one-factor ANOVA and Tukey’s post hoc analysis (Figures 1C and S1A) or Dunnett’s multiple comparisons test (Figures 2E, 2F, and S2D) or an unpaired t test (Figures 3C, 3D, 3F, 4A, 4B, 4E, 5G–5J, 5M, and S3B). The behavioral data were analyzed by a two-way ANOVA with Dunnett’s multiple comparisons test (Figures 5A and 5B). The differences were considered to be statistically significant for values of p < 0.05. The data are presented as the mean ± SD, except for the behavioral data (mean ± SEM).
Author Contributions
D.D. designed the study, collected and assembled data, performed data analysis and interpretation, and wrote the manuscript. B.S., M.K., T.K., and A.M. collected and/or assembled data. Y.O., K.S., M.N., and M.P. performed data analysis and interpretation. J.T. conceived and designed the study, assembled data, carried out data analysis and interpretation, wrote the manuscript, and made a final approval of the manuscript.
Acknowledgments
The authors thank Drs. J. Toga and E. Yagi (Osaka University) for expression and purification of the recombinant LM511-E8 and Professor H. Ohmori (Kyoto University) for an electrophysiological study. We also thank Dr. E. Yamasaki, Mr. K. Kubota, Ms. A. Fuke, and Ms. Y. Konoshima (CiRA, Kyoto University) for their technical assistance. This study was supported by a grant from the Highway Project for Realization of Regenerative Medicine from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), a grant from the Network Program for Realization of Regenerative Medicine from the Japan Science and Technology Agency, and a grant from the Translational Research Network Program from the MEXT (to K.S.).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Accession Numbers
The GEO (Gene Expression Omnibus) accession number for the microarray data reported in this paper is GSE51214.
Supplemental Information
References
- Ausubel L.J., Lopez P.M., Couture L.A. GMP scale-up and banking of pluripotent stem cells for cellular therapy applications. Methods Mol. Biol. 2011;767:147–159. doi: 10.1007/978-1-61779-201-4_11. [DOI] [PubMed] [Google Scholar]
- Bergström R., Ström S., Holm F., Feki A., Hovatta O. Xeno-free culture of human pluripotent stem cells. Methods Mol. Biol. 2011;767:125–136. doi: 10.1007/978-1-61779-201-4_9. [DOI] [PubMed] [Google Scholar]
- Brederlau A., Correia A.S., Anisimov S.V., Elmi M., Paul G., Roybon L., Morizane A., Bergquist F., Riebe I., Nannmark U. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24:1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- Brundin P., Barbin G., Strecker R.E., Isacson O., Prochiantz A., Björklund A. Survival and function of dissociated rat dopamine neurones grafted at different developmental stages or after being cultured in vitro. Brain Res. 1988;467:233–243. doi: 10.1016/0165-3806(88)90027-2. [DOI] [PubMed] [Google Scholar]
- Brundin P., Pogarell O., Hagell P., Piccini P., Widner H., Schrag A., Kupsch A., Crabb L., Odin P., Gustavii B. Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson’s disease. Brain. 2000;123:1380–1390. doi: 10.1093/brain/123.7.1380. [DOI] [PubMed] [Google Scholar]
- Carlsson T., Carta M., Winkler C., Björklund A., Kirik D. Serotonin neuron transplants exacerbate L-DOPA-induced dyskinesias in a rat model of Parkinson’s disease. J. Neurosci. 2007;27:8011–8022. doi: 10.1523/JNEUROSCI.2079-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Moon J.I., Leung A., Aldrich D., Lukianov S., Kitayama Y., Park S., Li Y., Bolshakov V.Y., Lamonerie T., Kim K.S. ES cell-derived renewable and functional midbrain dopaminergic progenitors. Proc. Natl. Acad. Sci. USA. 2011;108:9703–9708. doi: 10.1073/pnas.1016443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi D., Morizane A., Kikuchi T., Onoe H., Hayashi T., Kawasaki T., Motono M., Sasai Y., Saiki H., Gomi M. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson’s disease. Stem Cells. 2012;30:935–945. doi: 10.1002/stem.1060. [DOI] [PubMed] [Google Scholar]
- Eiraku M., Watanabe K., Matsuo-Takasaki M., Kawada M., Yonemura S., Matsumura M., Wataya T., Nishiyama A., Muguruma K., Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Elkabetz Y., Panagiotakos G., Al Shamy G., Socci N.D., Tabar V., Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22:152–165. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano C.A., Chambers S.M., Lee G., Tomishima M.J., Studer L. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell Stem Cell. 2010;6:336–347. doi: 10.1016/j.stem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fukuda H., Takahashi J., Watanabe K., Hayashi H., Morizane A., Koyanagi M., Sasai Y., Hashimoto N. Fluorescence-activated cell sorting-based purification of embryonic stem cell-derived neural precursors averts tumor formation after transplantation. Stem Cells. 2006;24:763–771. doi: 10.1634/stemcells.2005-0137. [DOI] [PubMed] [Google Scholar]
- Ganat Y.M., Calder E.L., Kriks S., Nelander J., Tu E.Y., Jia F., Battista D., Harrison N., Parmar M., Tomishima M.J. Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. J. Clin. Invest. 2012;122:2928–2939. doi: 10.1172/JCI58767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson M.E., Ono Y., Björklund A., Thompson L.H. Identification of transplantable dopamine neuron precursors at different stages of midbrain neurogenesis. Exp. Neurol. 2009;219:341–354. doi: 10.1016/j.expneurol.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Kefalopoulou Z., Politis M., Piccini P., Mencacci N., Bhatia K., Jahanshahi M., Widner H., Rehncrona S., Brundin P., Björklund A. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 2014;71:83–87. doi: 10.1001/jamaneurol.2013.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkeby A., Grealish S., Wolf D.A., Nelander J., Wood J., Lundblad M., Lindvall O., Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Kriks S., Shim J.W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia J.D., Patton B., Eckley D.M., Magnus T., Mughal M.R., Sasaki T., Caldwell M.A., Rao M.S., Mattson M.P., ffrench-Constant C. Patterns of laminins and integrins in the embryonic ventricular zone of the CNS. J. Comp. Neurol. 2007;505:630–643. doi: 10.1002/cne.21520. [DOI] [PubMed] [Google Scholar]
- Lindvall O., Brundin P., Widner H., Rehncrona S., Gustavii B., Frackowiak R., Leenders K.L., Sawle G., Rothwell J.C., Marsden C.D. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science. 1990;247:574–577. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- Ma W., Tavakoli T., Derby E., Serebryakova Y., Rao M.S., Mattson M.P. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev. Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez I., Sanchez-Pernaute R., Cooper O., Viñuela A., Ferrari D., Björklund L., Dagher A., Isacson O. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J.H., Li C., Mudd J.L., Go G., Sutherland A.E. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131:2247–2256. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Futaki S., Hasegawa K., Kawasaki M., Sanzen N., Hayashi M., Kawase E., Sekiguchi K., Nakatsuji N., Suemori H. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2008;375:27–32. doi: 10.1016/j.bbrc.2008.07.111. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Futaki S., Suemori H., Taniguchi Y., Yamada M., Kawasaki M., Hayashi M., Kumagai H., Nakatsuji N., Sekiguchi K., Kawase E. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat. Commun. 2012;3:1236. doi: 10.1038/ncomms2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane A., Doi D., Kikuchi T., Nishimura K., Takahashi J. Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J. Neurosci. Res. 2011;89:117–126. doi: 10.1002/jnr.22547. [DOI] [PubMed] [Google Scholar]
- Ono Y., Nakatani T., Sakamoto Y., Mizuhara E., Minaki Y., Kumai M., Hamaguchi A., Nishimura M., Inoue Y., Hayashi H. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development. 2007;134:3213–3225. doi: 10.1242/dev.02879. [DOI] [PubMed] [Google Scholar]
- Piccini P., Brooks D.J., Björklund A., Gunn R.N., Grasby P.M., Rimoldi O., Brundin P., Hagell P., Rehncrona S., Widner H. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat. Neurosci. 1999;2:1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- Politis M., Wu K., Loane C., Quinn N.P., Brooks D.J., Rehncrona S., Bjorklund A., Lindvall O., Piccini P. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci. Transl. Med. 2010;2:38ra46. doi: 10.1126/scitranslmed.3000976. [DOI] [PubMed] [Google Scholar]
- Pruszak J., Ludwig W., Blak A., Alavian K., Isacson O. CD15, CD24, and CD29 define a surface biomarker code for neural lineage differentiation of stem cells. Stem Cells. 2009;27:2928–2940. doi: 10.1002/stem.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodin S., Domogatskaya A., Ström S., Hansson E.M., Chien K.R., Inzunza J., Hovatta O., Tryggvason K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 2010;28:611–615. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- Roy N.S., Cleren C., Singh S.K., Yang L., Beal M.F., Goldman S.A. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat. Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Sundberg M., Bogetofte H., Lawson T., Jansson J., Smith G., Astradsson A., Moore M., Osborn T., Cooper O., Spealman R. Improved cell therapy protocols for Parkinson’s disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem Cells. 2013;31:1548–1562. doi: 10.1002/stem.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swistowski A., Peng J., Han Y., Swistowska A.M., Rao M.S., Zeng X. Xeno-free defined conditions for culture of human embryonic stem cells, neural stem cells and dopaminergic neurons derived from them. PLoS One. 2009;4:e6233. doi: 10.1371/journal.pone.0006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U., Arbuthnott G.W. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970;24:485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- Xi J., Liu Y., Liu H., Chen H., Emborg M.E., Zhang S.C. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells. 2012;30:1655–1663. doi: 10.1002/stem.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Wu F., Morser J., Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc. Natl. Acad. Sci. USA. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


