Abstract
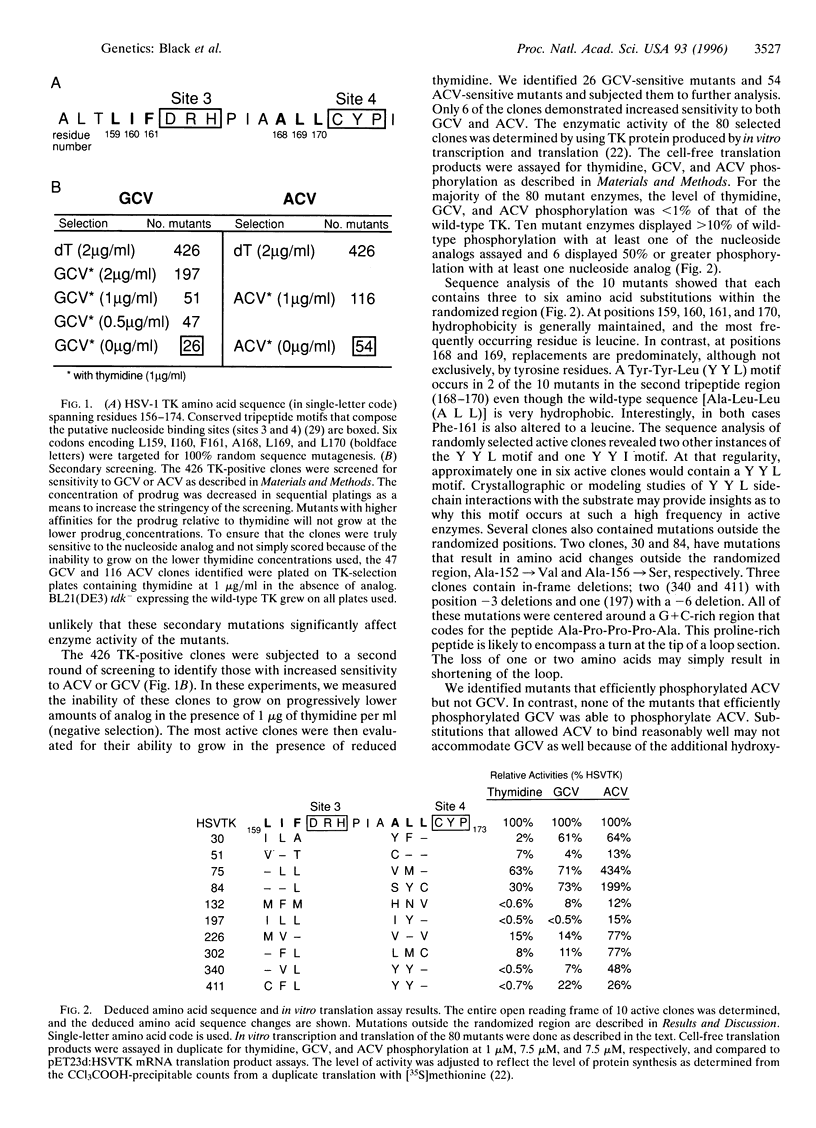
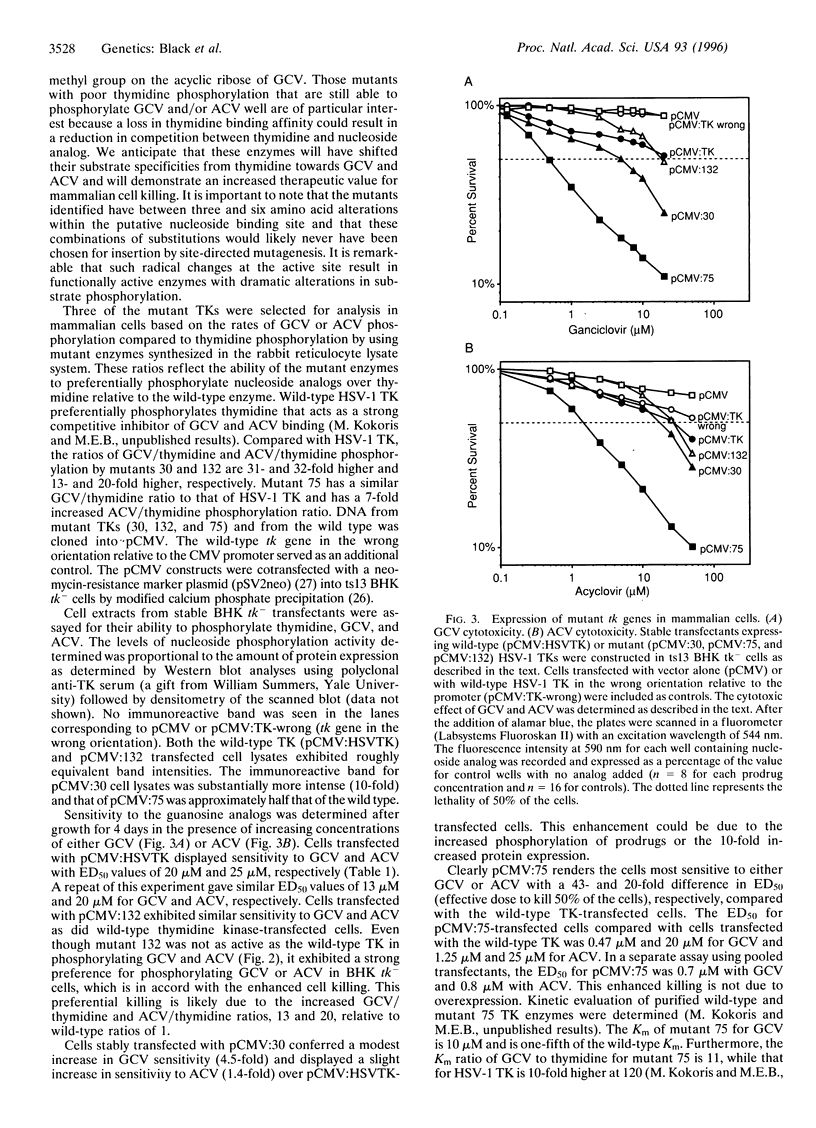
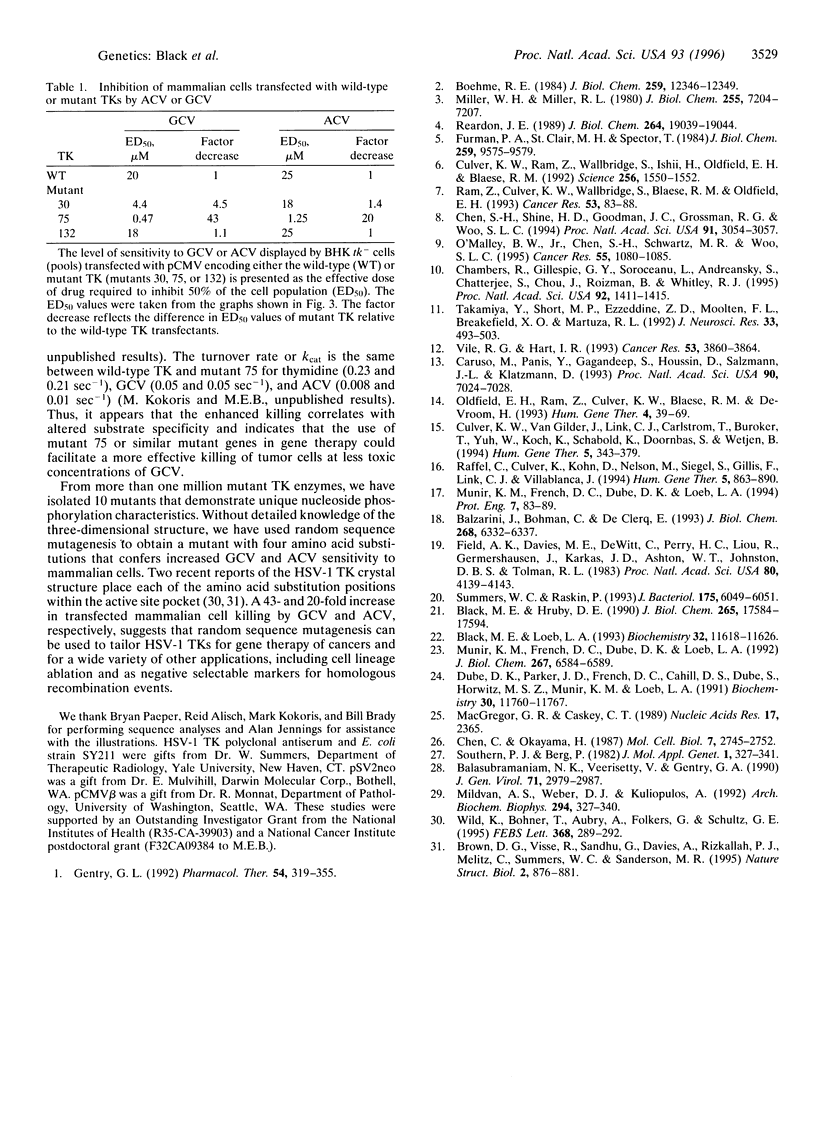
Herpes simplex virus type 1 (HSV-1) thymidine kinase is currently used as a suicide agent in the gene therapy of cancer. This therapy is based on the preferential phosphorylation of nucleoside analogs by tumor cells expressing HSV-1 thymidine kinase. However, the use of HSV-1 thymidine kinase is limited in part by the toxicity of the nucleoside analogs. We have used random sequence mutagenesis to create new HSV-1 thymidine kinases that, compared with wild-type thymidine kinase, render cells much more sensitive to specific nucleoside analogs. A segment of the HSV-1 thymidine kinase gene at the putative nucleoside binding site was substituted with random nucleotide sequences. Mutant enzymes that demonstrate preferential phosphorylation of the nucleoside analogs, ganciclovir or acyclovir, were selected from more than one million Escherichia coli transformants. Among the 426 active mutants we have isolated, 26 demonstrated enhanced sensitivity to ganciclovir, and 54 were more sensitive to acyclovir. Only 6 mutant enzymes displayed sensitivity to both ganciclovir and acyclovir when expressed in E. coli. Analysis of 3 drug-sensitive enzymes demonstrated that 1 produced stable mammalian cell transfectants that are 43-fold more sensitive to ganciclovir and 20-fold more sensitive to acyclovir.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balasubramaniam N. K., Veerisetty V., Gentry G. A. Herpesviral deoxythymidine kinases contain a site analogous to the phosphoryl-binding arginine-rich region of porcine adenylate kinase; comparison of secondary structure predictions and conservation. J Gen Virol. 1990 Dec;71(Pt 12):2979–2987. doi: 10.1099/0022-1317-71-12-2979. [DOI] [PubMed] [Google Scholar]
- Balzarini J., Bohman C., De Clercq E. Differential mechanism of cytostatic effect of (E)-5-(2-bromovinyl)-2'-deoxyuridine, 9-(1,3-dihydroxy-2-propoxymethyl)guanine, and other antiherpetic drugs on tumor cells transfected by the thymidine kinase gene of herpes simplex virus type 1 or type 2. J Biol Chem. 1993 Mar 25;268(9):6332–6337. [PubMed] [Google Scholar]
- Black M. E., Hruby D. E. Identification of the ATP-binding domain of vaccinia virus thymidine kinase. J Biol Chem. 1990 Oct 15;265(29):17584–17592. [PubMed] [Google Scholar]
- Black M. E., Loeb L. A. Identification of important residues within the putative nucleoside binding site of HSV-1 thymidine kinase by random sequence selection: analysis of selected mutants in vitro. Biochemistry. 1993 Nov 2;32(43):11618–11626. doi: 10.1021/bi00094a019. [DOI] [PubMed] [Google Scholar]
- Boehme R. E. Phosphorylation of the antiviral precursor 9-(1,3-dihydroxy-2-propoxymethyl)guanine monophosphate by guanylate kinase isozymes. J Biol Chem. 1984 Oct 25;259(20):12346–12349. [PubMed] [Google Scholar]
- Brown D. G., Visse R., Sandhu G., Davies A., Rizkallah P. J., Melitz C., Summers W. C., Sanderson M. R. Crystal structures of the thymidine kinase from herpes simplex virus type-1 in complex with deoxythymidine and ganciclovir. Nat Struct Biol. 1995 Oct;2(10):876–881. doi: 10.1038/nsb1095-876. [DOI] [PubMed] [Google Scholar]
- Caruso M., Panis Y., Gagandeep S., Houssin D., Salzmann J. L., Klatzmann D. Regression of established macroscopic liver metastases after in situ transduction of a suicide gene. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7024–7028. doi: 10.1073/pnas.90.15.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R., Gillespie G. Y., Soroceanu L., Andreansky S., Chatterjee S., Chou J., Roizman B., Whitley R. J. Comparison of genetically engineered herpes simplex viruses for the treatment of brain tumors in a scid mouse model of human malignant glioma. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1411–1415. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Shine H. D., Goodman J. C., Grossman R. G., Woo S. L. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3054–3057. doi: 10.1073/pnas.91.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver K. W., Ram Z., Wallbridge S., Ishii H., Oldfield E. H., Blaese R. M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992 Jun 12;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- Culver K. W., Van Gilder J., Link C. J., Carlstrom T., Buroker T., Yuh W., Koch K., Schabold K., Doornbas S., Wetjen B. Gene therapy for the treatment of malignant brain tumors with in vivo tumor transduction with the herpes simplex thymidine kinase gene/ganciclovir system. Hum Gene Ther. 1994 Mar;5(3):343–379. doi: 10.1089/hum.1994.5.3-343. [DOI] [PubMed] [Google Scholar]
- Dube D. K., Parker J. D., French D. C., Cahill D. S., Dube S., Horwitz M. S., Munir K. M., Loeb L. A. Artificial mutants generated by the insertion of random oligonucleotides into the putative nucleoside binding site of the HSV-1 thymidine kinase gene. Biochemistry. 1991 Dec 24;30(51):11760–11767. doi: 10.1021/bi00115a004. [DOI] [PubMed] [Google Scholar]
- Field A. K., Davies M. E., DeWitt C., Perry H. C., Liou R., Germershausen J., Karkas J. D., Ashton W. T., Johnston D. B., Tolman R. L. 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine: a selective inhibitor of herpes group virus replication. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4139–4143. doi: 10.1073/pnas.80.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Spector T. Acyclovir triphosphate is a suicide inactivator of the herpes simplex virus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9575–9579. [PubMed] [Google Scholar]
- Gentry G. A. Viral thymidine kinases and their relatives. Pharmacol Ther. 1992;54(3):319–355. doi: 10.1016/0163-7258(92)90006-l. [DOI] [PubMed] [Google Scholar]
- MacGregor G. R., Caskey C. T. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989 Mar 25;17(6):2365–2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildvan A. S., Weber D. J., Kuliopulos A. Quantitative interpretations of double mutations of enzymes. Arch Biochem Biophys. 1992 May 1;294(2):327–340. doi: 10.1016/0003-9861(92)90692-p. [DOI] [PubMed] [Google Scholar]
- Miller W. H., Miller R. L. Phosphorylation of acyclovir (acycloguanosine) monophosphate by GMP kinase. J Biol Chem. 1980 Aug 10;255(15):7204–7207. [PubMed] [Google Scholar]
- Munir K. M., French D. C., Dube D. K., Loeb L. A. Herpes thymidine kinase mutants with altered catalytic efficiencies obtained by random sequence selection. Protein Eng. 1994 Jan;7(1):83–89. doi: 10.1093/protein/7.1.83. [DOI] [PubMed] [Google Scholar]
- Munir K. M., French D. C., Dube D. K., Loeb L. A. Permissible amino acid substitutions within the putative nucleoside binding site of herpes simplex virus type 1 encoded thymidine kinase established by random sequence mutagenesis [corrected]. J Biol Chem. 1992 Apr 5;267(10):6584–6589. [PubMed] [Google Scholar]
- O'Malley B. W., Jr, Chen S. H., Schwartz M. R., Woo S. L. Adenovirus-mediated gene therapy for human head and neck squamous cell cancer in a nude mouse model. Cancer Res. 1995 Mar 1;55(5):1080–1085. [PubMed] [Google Scholar]
- Oldfield E. H., Ram Z., Culver K. W., Blaese R. M., DeVroom H. L., Anderson W. F. Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum Gene Ther. 1993 Feb;4(1):39–69. doi: 10.1089/hum.1993.4.1-39. [DOI] [PubMed] [Google Scholar]
- Raffel C., Culver K., Kohn D., Nelson M., Siegel S., Gillis F., Link C. J., Villablanca J. G., Anderson W. F. Gene therapy for the treatment of recurrent pediatric malignant astrocytomas with in vivo tumor transduction with the herpes simplex thymidine kinase gene/ganciclovir system. Hum Gene Ther. 1994 Jul;5(7):863–890. doi: 10.1089/hum.1994.5.7-863. [DOI] [PubMed] [Google Scholar]
- Ram Z., Culver K. W., Walbridge S., Blaese R. M., Oldfield E. H. In situ retroviral-mediated gene transfer for the treatment of brain tumors in rats. Cancer Res. 1993 Jan 1;53(1):83–88. [PubMed] [Google Scholar]
- Reardon J. E. Herpes simplex virus type 1 and human DNA polymerase interactions with 2'-deoxyguanosine 5'-triphosphate analogues. Kinetics of incorporation into DNA and induction of inhibition. J Biol Chem. 1989 Nov 15;264(32):19039–19044. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Summers W. C., Raksin P. A method for selection of mutations at the tdk locus in Escherichia coli. J Bacteriol. 1993 Sep;175(18):6049–6051. doi: 10.1128/jb.175.18.6049-6051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamiya Y., Short M. P., Ezzeddine Z. D., Moolten F. L., Breakefield X. O., Martuza R. L. Gene therapy of malignant brain tumors: a rat glioma line bearing the herpes simplex virus type 1-thymidine kinase gene and wild type retrovirus kills other tumor cells. J Neurosci Res. 1992 Nov;33(3):493–503. doi: 10.1002/jnr.490330316. [DOI] [PubMed] [Google Scholar]
- Vile R. G., Hart I. R. Use of tissue-specific expression of the herpes simplex virus thymidine kinase gene to inhibit growth of established murine melanomas following direct intratumoral injection of DNA. Cancer Res. 1993 Sep 1;53(17):3860–3864. [PubMed] [Google Scholar]
- Wild K., Bohner T., Aubry A., Folkers G., Schulz G. E. The three-dimensional structure of thymidine kinase from herpes simplex virus type 1. FEBS Lett. 1995 Jul 17;368(2):289–292. doi: 10.1016/0014-5793(95)00680-8. [DOI] [PubMed] [Google Scholar]



