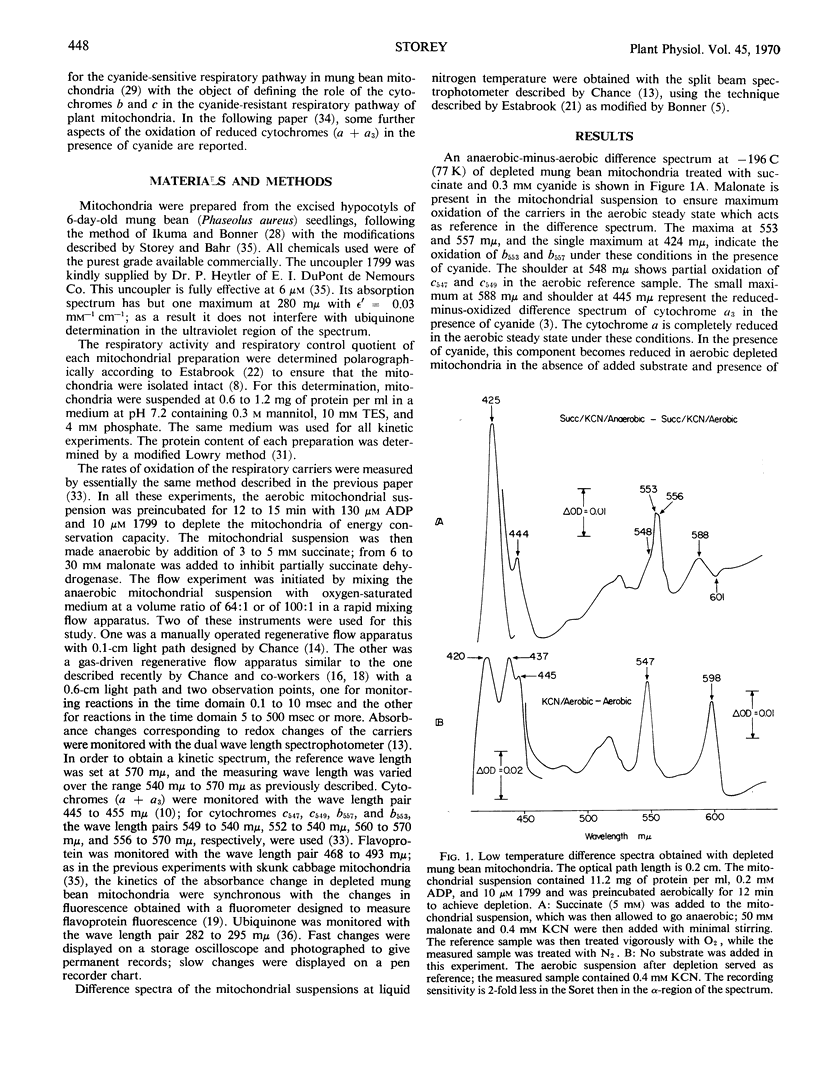
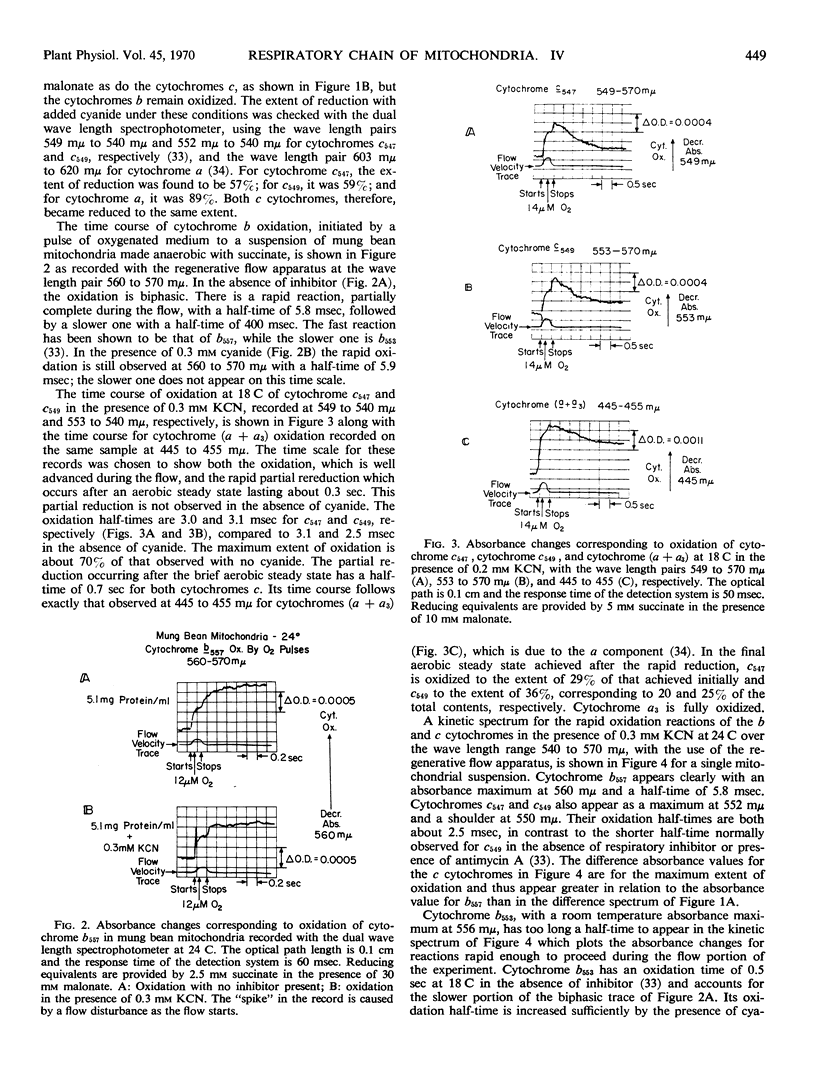
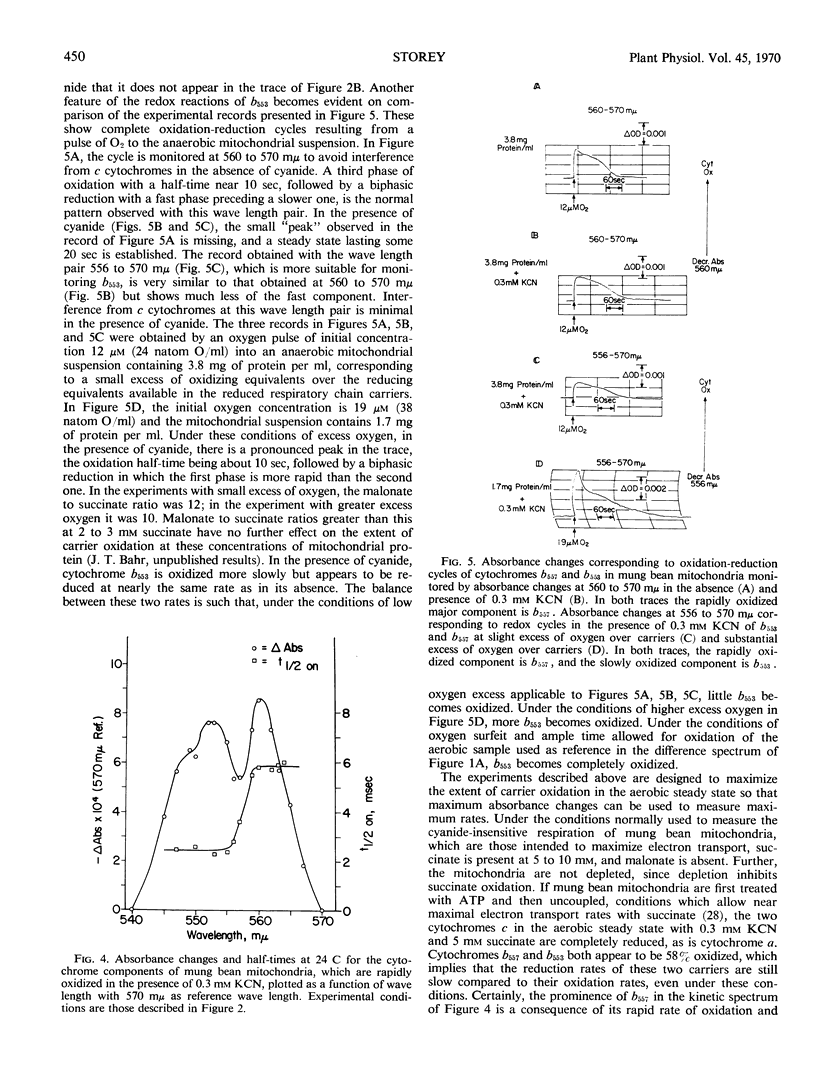
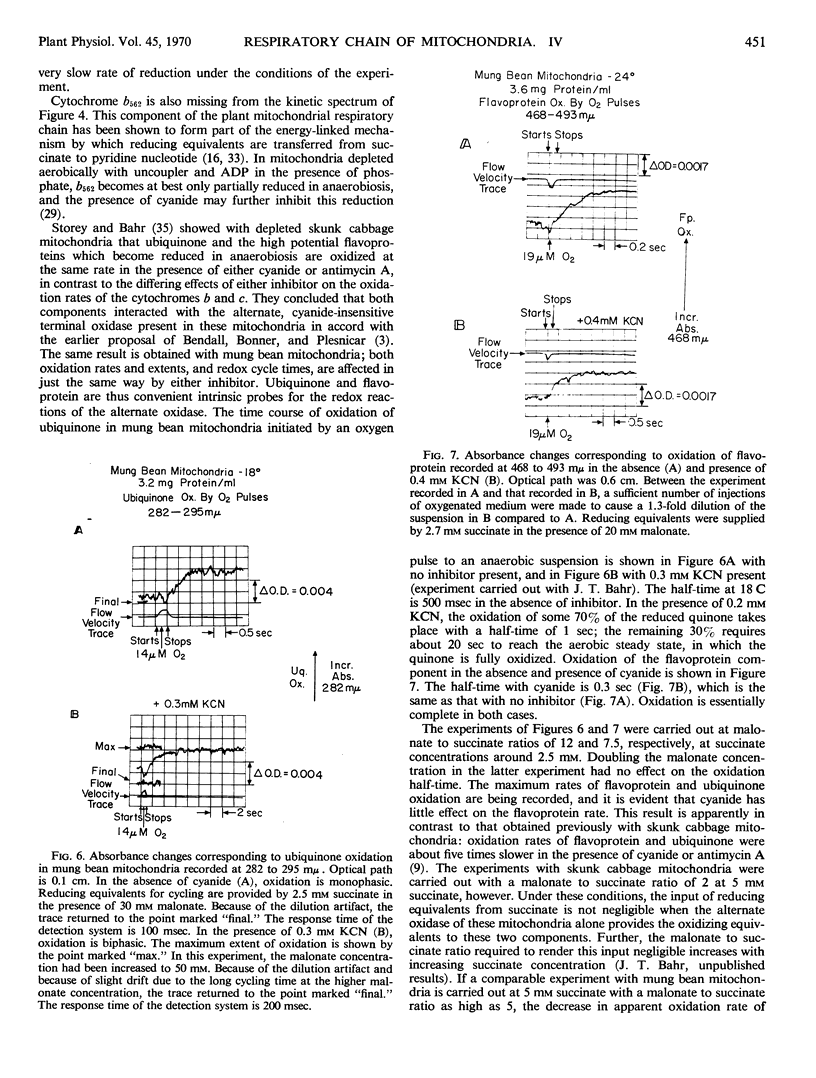
Abstract
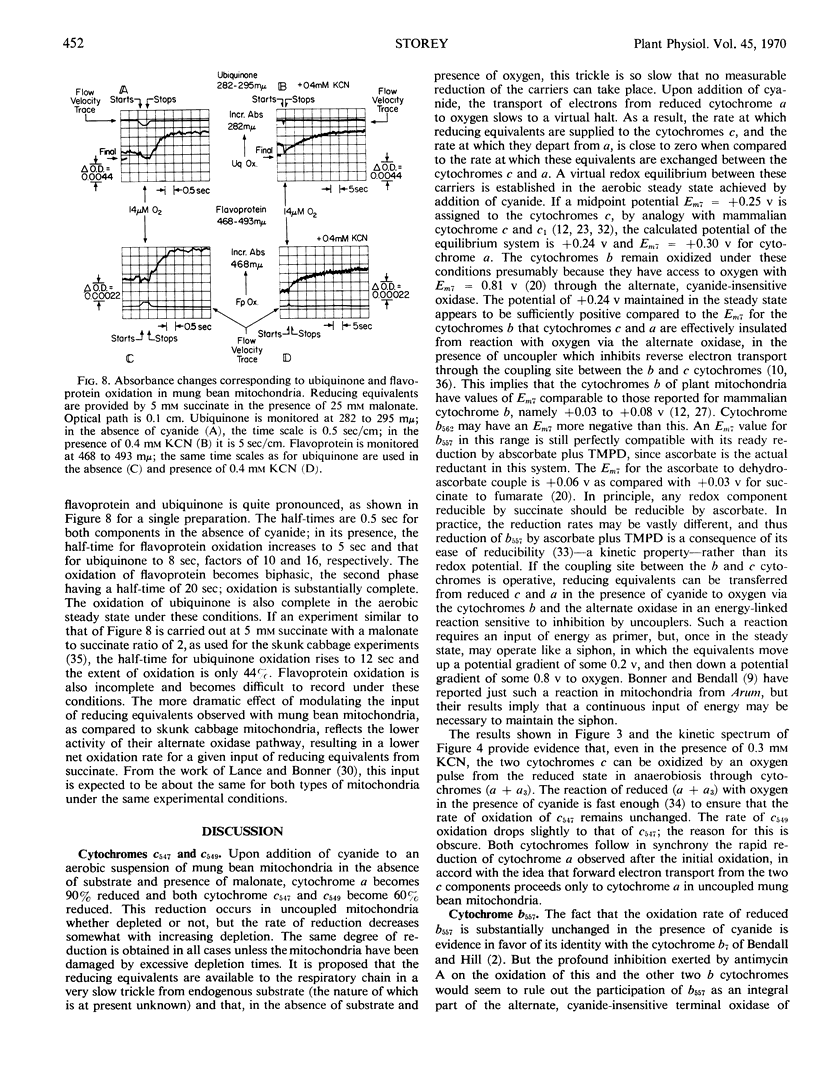
The half-time for oxidation of cytochrome b557 in mitochondria from etiolated mung bean (Phaseolus aureus) hypocotyls is 5.8 milliseconds at 24 Celsius in the absence or presence of 0.3 mm KCN, when the oxidation is carried out by injecting a small amount of oxygenated medium into a suspension of mitochondria made anaerobic in the presence of succinate plus malonate. Since oxygen is consumed by the alternate, cyanide-insensitive respiratory pathway of these mitochondria, cycles of oxidation and reduction can be obtained with the oxygen pulses when cyanide is present. Reduced cytochromes (a + a3) also become oxidized at nearly the uninhibited rate under these conditions, a3 completely and a partially. The half-time for oxidation of c547 is also unaffected by 0.3 mm KCN, but c549 has a half-time equal to that of c547 in the presence of KCN, compared to the shorter one observed in the absence of inhibitor. The maximum extent of oxidation of the cytochromes c is about 70% in the presence of 0.3 mm KCN; this oxidation is rapidly followed by an extensive reduction which is synchronous with the reduction of cytochrome a observed under the same conditions. In the presence of cyanide, it appears likely that the cytochromes c and b557 are oxidized by cytochrome oxidase in oxygen pulse experiments, rather than by the alternate oxidase. The oxidation of cytochrome b553 is partially inhibited by KCN, but complete oxidation is attained in the aerobic steady state with excess oxygen. If the oxygen pulse experiment is carried out in the presence of sufficient malonate so that entry of reducing equivalents into the respiratory chain occurs at a rate negligible compared to inter-carrier electron transport, the half-time for flavoprotein oxidation is unaffected by 0.3 mm KCN while that for ubiquinone oxidation is but 2-fold larger. The observed net oxidation rate of these two carriers in mung bean mitochondria is more sensitive to the entry rate of reducing equivalents, as set by succinate concentration and malonate to succinate ratio, then it is in skunk cabbage (Symplocarpus foetidus) mitochondria. These observations are interpreted in terms of a respiratory carrier Y, placed between flavoprotein plus ubiquinone and the cytochromes, which is the fork in the split respiratory pathway to the two terminal oxidases and which has lower electron transport capacity in mung bean mitochondria than in skunk cabbage mitochondria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDALL D. S. Cytochromes and some respiratory enzymes in mitochondria from the spadix of Arum maculatum. Biochem J. 1958 Nov;70(3):381–390. doi: 10.1042/bj0700381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. D., Jr, Plesnicar M. Electron transport carriers in plant mitochondria. Nature. 1967 May 6;214(5088):616–617. doi: 10.1038/214616a0. [DOI] [PubMed] [Google Scholar]
- Caswell A. H. Potentiometric determination of interrelationships of energy conservation and ion gradients in mitochondria. J Biol Chem. 1968 Nov 25;243(22):5827–5836. [PubMed] [Google Scholar]
- Chance B., Hackett D. P. The Electron Transfer System of Skunk Cabbage Mitochondria. Plant Physiol. 1959 Jan;34(1):33–49. doi: 10.1104/pp.34.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTABROOK R. W. The low temperature spectra of hemoproteins. I. Apparatus and its application to a study of cytochrome c. J Biol Chem. 1956 Dec;223(2):781–794. [PubMed] [Google Scholar]
- GREEN D. E., JARNEFELT J., TISDALE H. D. Studies on the elecron transport system. XIV. The isolation and properties of soluble cytochrome c1. Biochim Biophys Acta. 1959 Jan;31(1):34–46. doi: 10.1016/0006-3002(59)90436-6. [DOI] [PubMed] [Google Scholar]
- HIGGINS J. Analysis of sequential reactions. Ann N Y Acad Sci. 1963 May 10;108:305–321. doi: 10.1111/j.1749-6632.1963.tb13382.x. [DOI] [PubMed] [Google Scholar]
- HOLTON F. A., COLPA-BOONSTRA J. Spectrophotometric observations relating to the oxidation-reduction potential of cytochrome b in non-phosphorylating heart-muscle particles. Biochem J. 1960 Jul;76:179–189. doi: 10.1042/bj0760179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett D. P., Haas D. W. Oxidative Phosphorylation and Functional Cytochromes in Skunk Cabbage Mitochondria. Plant Physiol. 1958 Jan;33(1):27–32. doi: 10.1104/pp.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. III. Effects of Respiratory Inhibitors. Plant Physiol. 1967 Nov;42(11):1535–1544. doi: 10.1104/pp.42.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance C., Bonner W. D. The respiratory chain components of higher plant mitochondria. Plant Physiol. 1968 May;43(5):756–766. doi: 10.1104/pp.43.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. I. Electron transport between succinate and oxygen in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):115–125. doi: 10.1104/pp.44.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. II. Oxidative phosphorylation in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):126–134. doi: 10.1104/pp.44.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria. III. Oxidation Rates of the Cytochromes c and b in Mung Bean Mitochondria Reduced With Succinate. Plant Physiol. 1969 Mar;44(3):413–421. doi: 10.1104/pp.44.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. V. Reaction of reduced cytochromes a and a3 in mung bean mitochondria with oxygen in the presence of cyanide. Plant Physiol. 1970 Apr;45(4):455–460. doi: 10.1104/pp.45.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum C. S., Hackett D. P. Participation of Cytochromes in the Respiration of the Aroid Spadix. Plant Physiol. 1957 May;32(3):186–191. doi: 10.1104/pp.32.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]


