Abstract
Key message
Identification of resistance genes to potato wart disease caused by Synchytrium endobioticum is the key for developing diagnostic markers for breeding resistant cultivars. We present an overview on the current knowledge of this host-pathogen system and molecular advances while highlighting future research focus.
Abstract
Potato wart is a quarantined disease of cultivated potato (Solanum tuberosum L.) caused by the obligate biotrophic, soil-borne fungus Synchytrium endobioticum (Schilb.) Perc. Since its discovery by Schilberszky in 1896, the management of wart disease was enabled by research efforts focusing on understanding and classifying the causative agent, its mode of infection, pathogenesis, geographical distribution, detection and chemical control, on developing screening methods for host resistance and on genetic analyses, which led to the development of resistant cultivars. These early successes are currently challenged by new S. endobioticum pathotypes evolving and the increased risk of dissemination by potato tuber trade. New research efforts are therefore required to ensure continuation of effective and sustainable management of the potato wart disease. Advances in molecular biology and genomic tools offer potential for innovations. This review presents an overview on what we know about this complex host-pathogen interaction, highlights recent molecular work and embarks on an outlook towards future research directions.
Introduction
Potato wart is an important disease of the cultivated potato (Solanum tuberosum L.) known by various names like black wart, black scab, potato tumor, potato cancer or canker, cauliflower disease, warty disease and many other descriptive terms in diverse languages and cultural backgrounds where potato is grown and the disease is present (Frank 2007). The causative agent of wart is the obligate biotrophic, soil-borne fungus Synchytrium endobioticum (Schilb.) Perc. Upon infection, S. endobioticum induces cell divisions in the host proliferating into tumor-like tissues, which provide a nutrient sink (Hampson and Coombes 1985). The tumors progressively increase in size at the expense of tubers, resulting in yield losses in the range of 50–100 % (Hampson 1993; Melnik 1998). The major agricultural problem is the contamination of soil with persistent resting spores that remain infectious for more than 20 years. S. endobioticum is currently considered the most important quarantine pathogen of cultivated potato (Smith et al. 1997). Its occurrence is reported in Latin America, Europe, North America, Asia, Africa and Oceania (Smith et al. 1997). Chemical control is not a practical and sustainable approach to managing the pathogen (Hodgson et al. 1974; O’Brien and Rich 1976). During the first half of the twentieth century, conventional potato breeding schemes were successful in developing resistant varieties. Cultivation of resistant varieties and strict quarantine measures effectively curtailed the spreading of potato wart. This success was made possible by the pioneering work of the early scientists, who discovered and classified the causal pathogen (Schilberszky 1896; Percival 1910), described its life cycle and pathogenesis (Curtis 1921; Glynne 1926), developed methods for resistance screening (Spieckermann and Kothoff 1924; Glynne 1925; Lemmerzahl 1930) and studied the inheritance of resistance to wart (Salaman and Lesly 1923; Lunden and Jørstad 1934; Black 1935).
Early breeding efforts resulted in the development of potato cultivars resistant to wart in the second half of the twentieth century and subsequent breeding focused on traits other than resistance to wart. Now the disease is back on stage. Until 1941, a singular pathotype of S. endobioticum was known (pathotype 1 or the ‘common’ pathotype), to which most varieties were resistant. Since then, more than 30 new pathotypes have been identified (Baayen et al. 2006), some of which occur most frequently in Europe (pathotypes 2, 6 and 18). Resistance to pathotype 1 is not effective against these new pathotypes, which are also favored by lack of crop rotation in regions, where, for example, potatoes are grown for industrial starch production year after year. The few varieties that are resistant to the new pathotypes as well as old resistance sources do not have a comparative advantage with respect to other important agronomic and industrial traits. Moreover, the joining of Eastern European countries to the European Union (EU) increased the trade of tubers. Dissemination of tubers increases risk of pathogen spread and necessitates renewed interest in the breeding of potato cultivars with high resistance to all relevant pathotypes of S. endobioticum. This will be facilitated by molecular genetic tools such as DNA markers diagnostic for host resistance as well as markers diagnostic for the biodiversity of the pathogen. Nothing is known about the molecular basis of the induction of neoplastic growth by the fungus and very little is known about the identity and function of host genes conferring resistance to wart.
We think it therefore timely to review past and recent research on potato wart and to highlight possibilities for future work using genomic tools.
Causative agent, taxonomy and disease symptoms
The microorganism causing potato wart disease was first discovered by Schilberszky from Budapest University, Hungary (1896). He observed the pathogen growing on potatoes in Hornany (today located in Slovakia) and designated it as Chrysophylyctis endobiotica. He included it in the Chytridinea after 8 years of investigation. Percival (1910) had a different view on the pathogen’s taxonomic nomenclature and thus reassigned it as Synchytrium endobioticum (Schilberszky.) Percival. The fungal pathogen Synchytrium endobioticum (Schilb.) Perc. is a member of the order Chytridiales in the phylum Chytridiomycota (Abdullahi et al. 2005). Chytridiales do not form hyphae but sporangia that produce about 200–300 motile zoospores 35–80 μm in diameter. The genus Synchytrium includes endobiotic holocarpic organisms that have inoperculate sporangia. The colonial thallus divides into several sporangia or gametangia which are always enclosed within one membrane forming sorus (Alexopoulos et al. 1996). The fungus is obligate soil-borne (Karling 1964) and produces persistent resting (winter) sporangia (sori) with a long life span of more than 30 years (Hooker 1981; Anon 2004) at depths of 50 cm (Anon 1980). The sori are released into the soil from the decomposition of warts. Each sorus consists of an outer, brittle membrane of disorganized host cells surrounding two inner membranes, the innermost of which is thin and transparent. Summer sporangia develop in infected potato tissue leading to secondary zoospore infections. They are characteristically thin-walled and short-lived (Anon 2004). The long survival time of winter sori and lack of effective chemical control measures made the fungus a target of quarantine programs to limit its distribution. The primary host of S. endobioticum is potato (S. tuberosum) but it also infects roots of tomato (Lycopersicon esculentum Mill.) and other solanaceous species without inducing gall formation (Przetakiewicz 2008). Under experimental conditions the pathogen can infect species in the genera Lycium, Nicandria, Schizanthus, Duboisia, Capsicastrum, Physalis, Nicotiana and Hyoscyamus (Hampson 1986).
The disease caused by S. endobioticum was named as potato wart, due to the warty exudations produced on tubers and occasionally on stems, leaves and flowers (Hooker 1981). Typical symptoms are galls that may vary from the size of a pea to outgrowths as large as or larger than the tubers from which they emerged. The shape is usually spherical but can be irregular. The galls above ground are colored green to brown while subterranean warts are colored white to brown. At maturity wart tissue becomes colored black and leads to total tuber decay (Melodie and Sindermann 1994). Early infection of young developing tubers results in distortions and sponginess and makes them unrecognizable. In older tubers, the eyes are infected and develop into warty, cauliflower-like protuberances. Warts develop on stolons whilst roots are not infected. Green warts of limited size can form in the aerial buds located at the stem basis. Sometimes leaves are attacked. These warts look pulpy to touch and are softer than the tuber warts. Morphologically they consist of distorted, proliferated branches and leaves mixed together in a mass of hyperplastic tissue. Potato wart does not kill its host plant. Below ground infection symptoms may not be evident until harvest. A slight reduction of plant vigor may be noticed (Anon 2004). The Department for Environment Food and Rural Affairs (2011) of the United Kingdom and National Diagnostic Protocol (2011) of Australia showed that symptoms of powdery scab caused by Spongospora subterranea f.sp. Subterranean, can be mistaken for wart occurrence. It is important to note that powdery scab spore balls look different from winter sporangia of S. endobioticum. A view under the microscope reveals the spongy appearance of the ovoid, irregular or elongate spore balls of S. subterranean, which are composed of multiple single spores. National Diagnostic Protocol (2011) of Australia reported that potato smut caused by Thecaphora solani also causes warty swellings on potato tubers, but is distinguishable from wart tissues by the fact that warts contain black spores.
Geographical distribution and dissemination
The origin of S. endobioticum is in the Andean mountains of Latin America, where it co-evolved with potato (Przetakiewicz 2008). It is assumed that S. endobioticum spread at the end of the nineteenth century from the center of origin in the Andes first to Europe and North America, and subsequently across the whole potato growing regions of Asia, Africa and Oceania (Smith et al. 1997). The pathogen is believed to have been introduced to Europe through potato breeding materials from Latin America during the aftermath of the 1840s potato late blight havoc. Historic account has it that potato wart entered England in 1876 or 1878 while another view upholds that the disease has been present in the Liverpool province of England before 1893. The first tuber showing the wart symptoms in Europe was found in England (Taylor 1920). Prior to its discovery potatoes were cultivated in Europe for over 150 years (Weiss and Hartman 1923). Salaman (1989) reported that the disease was probably present in isolated gardens in the Northern English Midlands as far back as 1876, but this did not get attention until some 20 years later when Schilberszky (1896) described the disease and its causative pathogen. Potato wart disease spread rapidly within Europe between 1891 and 1920 and was reported with the highest incidence in the United Kingdom (Moore 1957). The United Kingdom was strategic in the introduction of potato to the European mainland having had an early expertise in potato breeding and hence exported elite cultivars to various parts of the world (Bojnansky 1984). The conditions for disease development and pathogenicity were not very favorable then and not even to date because the warm Gulf stream conditioning mild winters promotes the germination of a high percentage of perennial zoosporangia in potato fields, which tends to reduce the pathogenicity of S. endobioticum (Bojnansky 1960). It is of interest to mention that S. endobioticum created havoc in cultivars carrying the pathogen and introduced into Central Europe from United Kingdom (Bojnansky 1984). This resulted in a ban of potato imports from United Kingdom to the USA and Canada in 1912 causing a reduction of British exports of 20 % and a loss of £1 million (Pratt 1979). The distribution is mostly through human migration. Hilli (1932) described potato wart as a “social disease”. After the pathogen was discovered by Schilberszky (1896) in Hornany, it is of great interest to note that the disease vanished from this region and did not return due to unfavorable climatic conditions (Petras 1969). Conspicuously potato wart was found to occur in mountain regions and under adverse climatic scenarios (Bojnansky 1968), in isolated environments (Sanders 1919), in the Carpathian Mountains (Pidoplichko 1959), in domestic kitchen gardens (Moore 1957), in the Falkland islands (Gibbs 1947), in the Southern islands of New Zealand (Dingley 1970; Anon 1977), and in the Peruvian highlands (Torres et al. 1970).
An overview on the global distribution of potato wart is shown in Fig. 1. Anon (2005) reported that S. endobioticum occurred sporadic in the following European countries: Austria, Belarus, Czech Republic, Denmark, Estonia, Faroe Islands, Finland, Germany, Ireland, Italy, Latvia, Lithuania, Netherlands, Norway, Poland, Romania, Russia, Slovenia, Sweden, Switzerland, United Kingdom, Ukraine and former Yugoslavia (Montenegro). Distributions are fragmentary as a result of strict controls (Anon 1954–1968). Some national publications state that S. endobioticum has been found but not established in France, Belgium and Luxembourg with an unconfirmed report coming from Portugal (Smith et al. 1997). Potato wart was first observed in Germany in 1908 by Spieckermann in Westphalia (Köhler 1931; Langerfeld 1984) and in 1915 in The Netherlands (Anon 1921). The first report from Poland was 1917 (Grabowski 1925), from Belarus 1939 (Sereda et al. 2008), from Bulgaria 2004 (Dimitrova et al. 2011) and from Turkey 2003 (Çakır 2005). Ganguly and Paul (1953) reported the disease for the first time in India while in Finland it was officially detected in Kirkkonummi in 1924 (Liro 1925). The first recorded occurrence in Ukraine was in Zakarpate province in 1963 (Matskiv et al. 1998).
Fig. 1.
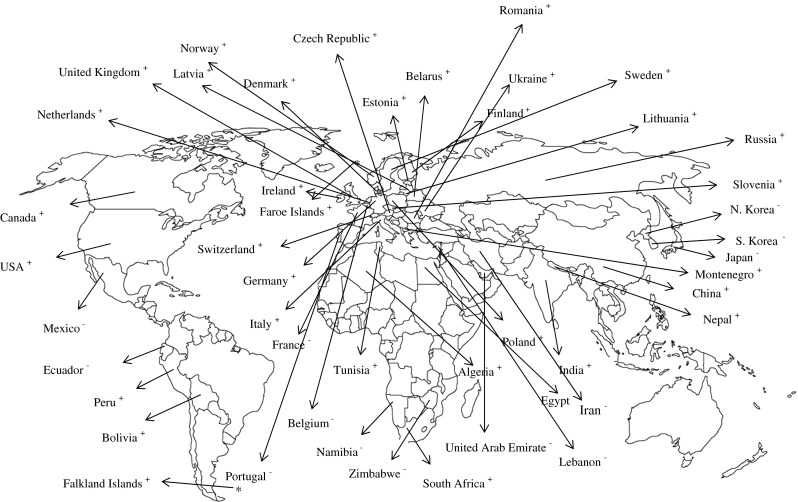
Global distribution of potato wart disease (plus confirmed reports, minus unconfirmed reports)
Potato wart presumably reached North America via Newfoundland with Scottish potatoes in the beginning of the twentieth century (Hampson and Proudfoot 1974). It was first recorded in insular Newfoundland (Canada) by Gussow (1909). According to a myth, potato wart was first carried from Europe to the USA with tubers hidden in the bundles of immigrating miners (Gram 1955). The first report of potato wart in United States of America was in 1918 when it was found in small garden plots located in 27 communities near Pennsylvania (Kunkel 1919). S. endobioticum has been found in Mexico on wild Solanum species with no confirmation from Mexican authorities. On the African continent potato wart was first reported 1947 in South Africa in the town lands of Belfast, Carolina, Handrina and Ermelo (Dyer 1947). According to Anon (1982a, b) South Africa and Tunisia documented the disease, whereas reports from Rhodesia (present Namibia) are unconfirmed. Smith et al. (1997) reported confirmed reports from Algeria and unconfirmed reports from Zimbabwe and Egypt. In Asia, documented reports are from India (Assam, Sikkim, West Bengal), China and Nepal and unconfirmed reports from Japan, Korea Democratic People’s Republic, and Korea Republic. Reports from South America include Bolivia, Chile (found in the past but presently eradicated), Peru, Uruguay (early recorded but now highly disputed by Uruguayan government) and Falkland islands. Reports from Ecuador are unconfirmed (Smith et al. 1997) as well as reports of the pathogen in countries like Iran, Lebanon and United Arab Republic in the Middle East (Anon 1982a). Potato wart was sporadically reported worldwide in Asia, Africa, Europe, Oceania, North America and South America (Anon 2006). Different sources seem to have different data for wart incidence. This requires a careful review of conflicting bits of information. Most reports seem fragmented in terms of distribution pattern in many countries. This is partly explained by the early recognition that potato wart can be devastating which led to prompt and strict regulatory strategies (Anon 1992; Kunkel 1919).
The major route for dissemination of S. endobioticum remains trading across nations and continents of internally infected tubers or tubers with adhering infested soil particles, because S. endobioticum has limited capacity for natural dispersion. Other ways of dispersion include commercial exchange of plants from wart infested farmlands through adhering soil particles, transport of soils from wart infested constructing sites, infested soil particles on farm machinery, irrigation water runoff and windblown dust from wart infested fields (Jöestring 1909; Hilli 1932; Langerfeld 1984; Hampson 1981, 1993, 1996; Smith et al. 1997; Stachewicz and Langerfeld 1998).
Mode of infection and pathogenesis
The first description of the pathogenic pathway of S. endobioticum was given by Schilberszky (1896) when he discovered the discharge of zoospores from the summer sporangia. In his view the zoospores were responsible for further distribution of the pathogen through the tumor, aided by an ability of boring through the walls of the host cell into the adjoining cell. This finding stimulated the curiosity of scientists like Potter (1902–1903), Weiss (1908–1909), Massee (1908–1909), Johnson (1909–1910), Percival (1910), Cotton (1916) and Curtis (1921). They all agreed with Schilberszky (1896) on the observed germination and zoospore motility after using drops of distilled water, potato juice, sugars or acids to monitor sporangia germination. These findings were supported by further studies of Esmarch (1924, 1926, 1927, 1928) whose experimental protocol was based on separating sporangia from tumor pieces on petri dishes and counting empty sporangia to obtain an index of germination. This helped him to determine conditions for zoospore emergence and response to chemotactic stimuli of the host. Schilberszky (1930) reported that spores behaved as a sporangium, bursting open and allowing zoospores to escape. Around 200–300 haploid, uninucleate motile zoospores (Hampson 1986; Franc 2001) are released that have a tail like single flagellum which facilitates its movement through wet soil and helps to reach the epidermal cells of meristematic tissues of buds, stolon tips or young leaf primordia, thus infecting susceptible potato tissues at the point of contact. Upon successful infection host cells grow in size with an uninucleate, intracellular thallus developing, which leads to the formation of haploid sori inside the host cell and causes the proliferation of adjacent host cells, giving rise to warty exudates and hypertrophic growth. The increasing amount of the meristematic tissues provides a favorable environment for the pathogen to thrive. The repetition of this secondary stage of infection gives rise to massive engulfment of host cells thus setting the pace for gall formation, which increase in size of up to 1,800 fold within 16 days (Weiss 1925). This process continues throughout the growing season under favorable environmental conditions like cool to warm temperature (10°–27 °C), annual rainfall of 70 cm (28 in.) or during irrigation and soil pH of 3.9–8.5 (Weiss 1925). Field and laboratory investigations by Gedz (1957) showed that mineral salts like Mn, Cu and Mo reduced infection while Tarasova (1969) found that B, Zn and Cu enhanced sporangium germination. The author stated that soil temperature and moisture play a role in wart tumor decay leading to sporangia release and germination. In dry warm soil types from certain regions, disease progress is slow while piedmont and mountain area soils promote aggressiveness of the fungus. Light, sandy soils have been observed to favor disease development while the reverse was seen on clay and muddy soils (Fedotova 1970; Kharitonova and Tarasova 1971). Curtis (1921) reported that under conditions of water stress, the haploid zoospores fuse in pairs to form an uninucleate, diploid and biflagellate zygote which invades host tissue and develops into thick walled winter sporangia or resting spores which are released into the soil upon the decay of warty growths. These are the source of primary inoculum and dormant with the potential of retaining viability for 40–50 years at soil depths of up to 50 cm (Arora and Khurana 2004).
Pathogen detection and identification of pathotypes
Several experimental approaches have been undertaken by many researchers to extract resting sporangia of S. endobioticum from soil samples, which are summarized in Table 1. The quest for a precise, reproducible and reliable detection protocol was promoted with advances in molecular biology. Niepold and Stachewicz (2004) used an internal transcribed spacer (ITS)-DNA region of S. endobioticum for molecular diagnosis by the polymerase chain reaction (PCR). The source of DNA was wart galls of four pathotypes (1, 2, 6 and 18). Using the universal ITS primer # 4 and the primer Kbr 1 the authors generated a 543 bp PCR fragment from the four fungal pathotypes. They showed that this PCR could discriminate between weakly resistant and moderately susceptible responses of potato cultivars in addition to routine visual inspection. Van den Boogert et al. (2005) developed PCR-based methods for the accurate detection and quantification of S. endobioticum in soil extracts and in planta. The PCR primers were based on the internal transcribed spacer sequence of the multi-copy rDNA genes and tested for specificity, sensitivity and reproducibility in conventional and real-time PCR assays. The primers amplified a 472 bp product of S. endobioticum. The improved real-time PCR assay was used for quantification of S. endobioticum in different substrates like zonal centrifuge extracts, warts and different parts of potato plants (van Gent-Pelzer et al. 2010). Co-amplification of target DNA along with the potato cytochrome oxidase gene as endogenous control made the diagnostic assay more reliable, guarded against false negative results and improved sensitivity of the assay at least 100-fold (van den Boogert et al. 2005). Abdullahi et al. (2005) demonstrated the potential of microarray-based hybridization for the identification of multiple pathogen targets including S. endobioticum. The authors identified oligonucleotide probes exhibiting high specificity for S. endobioticum with good reproducibility of hybridization signals within the same and between different hybridization experiments.
Table 1.
Extraction and detection methods for S. endobioticum
| Method | Citation |
|---|---|
| Separation of sporangia from soil particles based on different specific gravity in chloroform | Glynne (1926), Pratt (1974, 1976a), Hampson and Thompson (1977), Hampson and Robertson (1995) |
| Wet sieving using electromagnetic shaker aided by chloroform floatation | Hampson and Coombes (1996) |
| Soil extraction with the non ionic detergent Triton X100 | Laidlaw (1985) |
| Substitution of chloroform by dibromomethane and oil, use of fluoric acid for sand removal | Nelson and Olsen (1964) |
| Extraction in water and sedimentation using sodium sulfate | Marcus (1969), Mygind (1954; 1961) |
| Extraction with potassium iodide from air dried soil after sieving | Putnam and Sindermann (1994) |
| Density gradients with potassium/sodium salt on air dried soil after sieving | Zeyla and Melnik (1998) |
| Extraction with chloroform, calcium chloride and zinc sulfate | van Leeuwen et al. (2005) |
| Zonal centrifugation | Wander et al. (2007) |
| Microscopic examination | National Diagnostic Protocol (2011) |
| Polymerase chain reaction (PCR) using species-specific primers | Niepold and Stachewicz (2004), Van den Boogert et al. (2005), van Gent-Pelzer et al. (2010) |
| Microarray-based hybridization | Abdullahi et al. (2005) |
Molecular diagnostic tools to discriminate between S. endobioticum pathotypes are currently not available. Biotests using differential cultivars are therefore still the method of choice. Pathotype identification is possible according to the ‘Diagnostic protocols for regulated pests: S. endobioticum’ EPPO PM 7/28 (Anon 2004) using the Spieckermann method (Spieckermann and Kothoff 1924), the Glynne–Lemmerzahl method (Glynne 1925; Lemmerzahl 1930; Noble and Glynne 1970), and field tests. The most important pathotypes in Europe 1, 2, 6, 8 and 18 can be differentiated using ten differential potato cultivars (Table 2). It should be emphasized that pathotypes 6 and 8 only differ in their reaction to the cultivar Delcora. The present differential set used by the majority of the EU countries consists of 10 potato cultivars of which only four are registered and commercially available. Therefore, the development of a new core differential set is needed.
Table 2.
Differential potato cultivars for the identification of pathotypes of S. endobioticum (Anon 2004)
| Differential cultivar | Pathotype 1 | Pathotype 2 | Pathotype 6 | Pathotype 8 | Pathotype 18 |
|---|---|---|---|---|---|
| Tomensa, Deodara | S | S | S | S | S |
| Producent, Combi | R | S | S | S | S |
| Saphir | R | S | R | R | R |
| Delcora | R | R | R | S | S |
| Miriam | R | R | R | R | S |
| Karolin, Ulme, Belita | R | R | R | R | R |
R resistant, S susceptible reaction to pathotypes 1, 2, 6, 8 and 18
Control measures
S. endobioticum is considered an A2 pest by the European and Mediterranean Plant Protection Organization (EPPO) and is classified as a quarantine pest under European Union Directive (Anon 2000). This awareness has led to enacting legislative policies which reduced considerably the worldwide spread of the pathogen. The European Union has set out specific requirements in the ‘Council Directive 69/464/EEC of 8 December 1969 on control of Potato Wart Disease’ (Anon 1969) and the ‘Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community’ (Anon 2000). The main requirements are the demarcation of contaminated plots and of safety zones and the disposal of infected potato material. The member states shall provide that no potatoes and no plants for transplanting in contaminated plots may be grown until the plot is free from potato wart disease. In safety zones only varieties resistant to the pathotype found on the plot may be grown. According to the ‘EPPO specific quarantine requirements’ (Anon 1990) potatoes should not be grown in fields where S. endobioticum has occurred. In addition, there is a zero tolerance for potato wart disease in seed potato production and the trade of infected potatoes is not allowed. When S. endobioticum is diagnosed in a field, potato production is forbidden in that site until the absence of sporangia is ascertained. This is common practice in the European Union member states while European and Mediterranean Plant Protection Organization (EPPO) insists in scheduling infected environment and farm locations for 20 years minimum while further cultivation of potato is subject to soil tests (Anon 1999). The test consists of subjecting soil samples to direct microscopic examination for sporangia and testing for infestation using a bioassay. Sites may be partially scheduled for a shorter period of 10 years allowing resistant potato cultivars to be grown. The plot may not be used for growing other types of potatoes until complete descheduling after about 20 years as discussed above (Anon 1999).
According to the Council Directive 69/464/EEC (Anon 1969), the Member States may adopt additional or stricter provisions as may be required to control potato wart disease or to prevent it from spreading. These national regulations include, for example, the reporting obligation for owners, Plant Protection Service, inspectors and official or private laboratories. In addition, they regulate the responsibility of state institutions in the examination and announcement of wart-resistant potato varieties and the identification of the occurring pathotypes.
Chemical control measures have been explored for some 80 years (Table 3). Treatments were however not successful in eliminating S. endobioticum completely from the soil. Hampson (1977) reported that more than 120 inorganic or organic chemicals, singly or in combination have been evaluated but the only successful treatments reported were either phytotoxic or acted as soil sterilants. The ecological impact of the type and high rates of chemical treatments (mercury, sulfur, copper, chlorine-based chemicals and formaldehyde) that were used left the soil barren (De Boer 2005; Reigner 2006) and till date no chemical is recommended for managing the disease (Hampson 1993). Mirzabekian et al. (1961) focused on a biological control solution through culturing actinomycetes on potato slices, diluting the cultures in sand and including the culture subsequently in infested pot soil mixes. The authors found that the treatment reduces wart incidence from 97 to 25 %. Simultaneous treatment of soils produced better results and they proposed that a 3 year treatment program might eliminate soil infestation. Roach et al. (1925) used Thiobacillus thiooxydans as a biological control of potato wart. This trial was largely effective but its repetition had insignificant effect. Intercropping potato with other crops like maize and crop rotation has been found to reduce the population of viable S. endobioticum resting spores in soil (Singh and Shekhawat 2000).
Table 3.
Chemicals explored for the control of S. endobioticum
| Chemical | Citation |
|---|---|
| Acetic acid | Glynne (1928) |
| Allyspol | Potoček (1991) |
| Ammonia | Dykstra (1940) |
| Ammonium hydroxide | Hampson (1985), Potoček (1991) |
| Ammonium nitrate | Glynne (1928) |
| Ammonium thiocyanate | Hampson (1985) |
| Basamid | Potoček (1991) |
| Bordeaux | Hunt et al. (1925) |
| Bleaching powder, powdered chalk and cymene | Gimingham and Spinks (1919) |
| Calcium | Roach et al. (1925) |
| Calcium cyanamide | Tarasova and Beskorovainy (1973), Efremenko (1980), Efremenko and Yakovleva (1981), Potoček (1991) |
| Carbamide | Tarasova and Beskorovainy (1973) |
| Carbamide, nitrafen | Efremenko (1980) |
| Copper sulfate | Potter (1909), Johnson (1909–1910), Malthouse (1910), Gimingham and Spinks (1919), Dykstra (1940), Hartman (1955) |
| Chloroform | Glynne (1928) |
| Chloro-picrin | Gimingham and Spinks (1919) |
| Chlordinitro-benzene and nitrobenzene | Roach et al. (1925) |
| Creosote | Gimingham and Spinks (1919) |
| Dichlorcresol | Roach et al. (1925) |
| Dichlorpropene | Potoček (1991) |
| Dinitro-orthocresol | Zakopal (1950a), Pidoplichko (1959), Sedivy (1975) |
| Ethyl alcohol | Glynne (1928) |
| Formalin | Gimingham and Spinks (1919) |
| Formaldehyde | Potter (1909), Johnson (1909–1910), Ericksson (1914), Roach et al. (1925), Hunt et al. (1925),Glynne (1928), Hartman (1955) |
| Lime | Malthouse (1910), Schaffnit and Voss (1918), Hampson (1985) |
| Methyl bromide | Rasmussen and Mygind (1977) |
| Mercuric chloride | Hunt et al. (1925), Weiss and Brierley (1928) |
| Nematin | Potoček (1991) |
| Nitraphen | Kharitonova and Tarasova (1971) |
| Nitrosan | Potoček (1991) |
| Perocid | Knorr (1922) |
| Phenol | Glynne (1928) |
| Sodium chloride | Malthouse (1910) |
| Sodium hydroxide | Glynne (1926) |
| Soot | Malthouse (1910) |
| Sulphuric compounds | Malthouse (1910), Gimingham and Spinks (1919), Roach et al. (1925), Crowther et al. (1927), Roach and Glynne (1928) |
| Thiabendazole | Hampson (1977), Gunacti and Erkiliç (2013) |
| Triforine, thiophanate methyl, cypendazole, cyclafuramid, carbathiin, and benomyl | Hampson (1977) |
| Tebuconazole, Pomarsol Forte, İmazalil, Fludioxonil, Metalaxyl, and Azoxystrobin | Gunacti and Erkiliç (2013) |
| Urea | Efremenko and Yakovleva (1981), Potoček (1991) |
Resistance screening
As chemical control is unreliable, incomplete and toxic to the environment, the cultivation of wart-resistant varieties is the best available option. Breeding for resistance requires methods for resistance screening. Traditionally, screening for resistance to potato wart was carried out on naturally or artificially contaminated testing fields. Weather-related variations in the infection rate, different levels of soil contamination in the fields, and the relatively low test capacity within a season were the reasons for developing tests under laboratory conditions. Currently, resistance tests are performed in most countries with the methods of Spieckermann and Kothoff (1924) and Glynne–Lemmerzahl (Glynne 1925; Lemmerzahl 1930; Noble and Glynne 1970). Testing according to Spieckermann involves the preparation of compost containing winter sporangia that are liberated from warts collected from tubers. This can be done at any time of the year. The warty plant material is broken into pieces of around 1 cm and thoroughly mixed with river sand at a ratio of 3 kg sand to 1 kg wart tissue. This mixture is incubated between 18 and 25 °C. Controlled daily watering helps the mixture to rot and increase the acidity. After 4 months the mixture undergoes air drying at temperatures of 8–25 °C for another 2 months, bringing the total number of months to 6. At this point the composting mixture becomes ready to use as a source of inoculum in resistance and pathotype testing. The compost mixture retains full aggressiveness for approximately 2 years. Aggressiveness of inoculum tends to decrease in subsequent years of maintenance (KF, personal observation). Frey (1980) proposed a modified Spieckermann protocol, which uses the plant growth regulator Cycocel® to induce early tuber initiation on potato seedlings. Inoculated plants are maintained in small containers in moist, dark environments until disease symptoms are expressed. This method has the advantage of requiring less space, time and inoculum. The Spieckermann method which uses winter sporangia is very suitable for pathotype identification of old wart tubers with an added advantage of using it as a reference compost mixture of different pathotypes. Anon (1999) recommended that the sporangium density should be ascertained, while infection tests are done using the diagnostic protocol of Anon (2004). In contrast, the Glynne–Lemmerzahl method uses fresh wart tissue kept in close contact with emerging sprouts on whole tubers or cut tuber eye fields (Fig. 2). Young sprouts are infected by zoospores originating from summer sporangia of fresh wart tissue. This method is widely used in European laboratories because of its high reliability and efficiency (Stachewicz 1980; Stachewicz et al. 2005; Przetakiewicz and Kopera 2007; Przetakiewicz 2008). Details of the Glynne–Lemmerzahl diagnostic protocol are available in Anon (2004). Both infection protocols are recommended by Anon (2004) and it is left to the various member countries to decide which one to use. The original protocols have been modified in particular countries. Typical examples are the Polish and German versions of the Glynne–Lemmerzahl method (Fig. 2). Both versions differ in the plant material used (entire tubers or eye fields), the treatment of tubers/eye fields (copper oxychloride after inoculation or pencycuron before inoculation), the temperatures during incubation (12/22 °C alternately or 16–18 °C continuously) and the period of incubation (2–3 weeks or 3–4 weeks). The reaction types of the sprouts are assessed in both methods using a modified scheme of Langerfeld et al. (1994) summarized in Table 4 and shown in Fig. 3. The sprouts are examined two to 4 weeks after inoculation using a stereo microscope.
Fig. 2.
Step of the Glynne–Lemmerzahl method used in Germany (a) and Poland (b). Step 1: Inoculation with fresh wart tissues. Step 2: Warts developing after removal of inoculum. Step 3: Final stage used for resistance scoring
Table 4.
Classification of reaction types according to (Langerfeld and Stachewicz 1994)
| Reaction type | Group | Classification | Description |
|---|---|---|---|
| A | R1 | Extremely resistant | Early defense necrosis; no visible sorus formation |
| B | R1 | Resistant | Late defense necrosis; single necrotic sori visible |
| C | R2 | Weakly resistant | Very late defense necrosis; up to five non-necrotic sori |
| D | S1 | Slightly susceptible | Scattered infections; sorus fields, sprout can be malformed |
| E | S2 | Extremely susceptible | Dense infection fields, numerous ripe sori and sorus fields, predominant tumor formation |
Fig. 3.
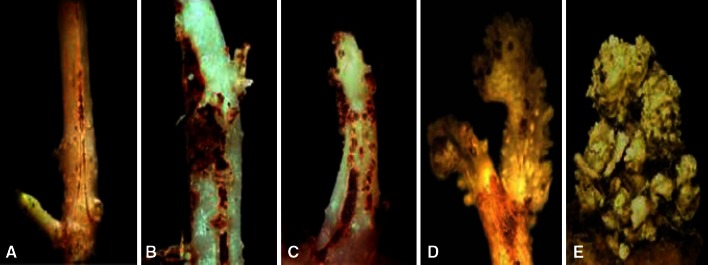
Symptoms of the reaction types a to e obtained with the German version of the Glynne–Lemmerzahl method (from Ballvora et al. 2011)
Glynne (1925) did not specify the temperature for incubation whilst Lemmerzahl (1930) performed his experiments at incubation temperatures of 15–16 °C. Anon (1963) specified an incubation temperature of 10 °C. Sharma and Cammack (1976) proposed another modification of the Glynne–Lemmerzahl protocol, which allowed clearer distinction between different degrees of susceptibility and reduced the time required for testing from 20 to 15 days. The tubers are not incubated in peat or sand but in cotton wool, which reduces interference through sprout infection by other fungi like Verticillium spp and Rhizoctonia solani and allows continuous observation. Langerfeld (1984) believed that the Glynne–Lemmerzahl protocol is more sensitive than the Spieckermann protocol. Browning and Darling (1995) obtained similar results with both methods. Przetakiewicz and Kopera (2007) compared the two principal methods of Glynne–Lemmerzahl and Spieckermann in assessing resistance of some potato cultivars susceptible to S. endobioticum pathotype 1 (D1). The results showed a high degree of susceptibility of all cultivars when using both methods.
Wart resistance testing is done under field or greenhouse conditions to complement the laboratory bioassays. Poland, Germany, England, Hungary, The Netherlands, Scotland, and Norway limit testing to laboratory conditions while Finland and Northern Ireland carry out field tests (Malakhanova et al. 1998). Zakopal (1950b) stated that laboratory tests indicating different biotypes of S. endobioticum are unreliable and should be supported by comparative field evaluation is diverse geographical sites. Anon (1982b) established that the main criterion for resistance assessment should be the capability of a cultivar to prevent S. endobioticum from the completion of its life cycle thus blocking secondary infections. Field assessment of potato cultivars in pots has been carried out by Malec (1972) and Malec and Lubiewska (1979). The reliability of the results was doubtful owing to environmental variation, especially low precipitation. Pot tests in a green house were undertaken by Rintelen et al. (1983) and Dimitrova et al. (2011). Potoček et al. (1991a) prescribed the use of Potoček’s test tubes. Tests for susceptibility were performed using soil samples originating from trial fields and a differential set of cultivars. The control of experimental conditions such as inoculum density and soil moisture is better when performing pot experiments (Malec 1980). Langerfeld (1984) stated that laboratory tests done under controlled environment are more reproducible than field tests, as they are independent of yearly weather situations and differences between test fields with respect to infestation status. Pratt (1976b) studied the relationship between wart susceptibility of potato cultivars in field and laboratory. She demonstrated that moderately resistant cultivars grown in pots avoid infection and show no infection when grown in three soil types with heavy watering. Thus cultivars with intermediate resistance which showed variation of susceptibility in laboratory testing were resistant in the field. A comparison of laboratory and field screening for resistance showed that both methods are correlated and some cultivars found to be susceptible in the laboratory tests produced winter spores under field conditions (Browning, 1995). Browning (1996) and Baayen et al. (2005) observed that cultivars responding to infection in laboratory tests with no more than small warts remained free of infection in the field. Baayen et al. (2005) conducted a systematic comparison of resistance levels in field and laboratory tests. Their findings showed that stable results could be obtained in field tests when considering pathotypes 1 (D1) and 6 (O1), provided that year and location effects are corrected by statistical computing, thus disputing the previous objection to field screening by Langerfeld (1984). Baayen et al. (2005) did show that field resistance levels ≥7 provide adequate protection against secondary infection, as recommended by EU Directive 69/464. Overall, cultivars that did not produce large warts under laboratory conditions showed similar results when tested in the field.
Resistance breeding and S. endobioticum pathotype evolution
After the initial discovery of S. endobioticum pathotype 1 (D1), conventional breeding programs were successful in controlling this devastating disease through the development of resistant varieties early in the twentieth century. Resistance to pathotype 1 was found in old cultivars such as Snowdrop and Flourball, which facilitated resistance breeding. Another source of resistance was the wild potato species Solanum acaule (Frandsen 1958; Ross 1986; Langerfeld et al. 1994). Then, new pathotypes were discovered, first in the German towns Gießübel (pathotype 2 (G19)) and Silberhütte (pathotype 3 (S1)) in 1941 (Braun 1942). At the same time, Blattny (1942) reported the presence of a new pathotype at Budweis in South Bohemia in Czechoslovakia. Hey (1959) reported the occurrence of yet another pathotype in East Germany. The pathotypes included 4 (P1) from Papenheim in 1943, pathotype 9 (R1) from Rudolstadt in 1950, pathotype 5 (K1) from Koppatz in 1951 and pathotype 10 (E1) from Eulendorf in 1956. These pathotypes were quite distinct according to their differential infection profile on six potato cultivars. In former West Germany pathotype 6 (O1) was discovered in Olpe in 1952, and pathotype 8 (F1) in Fulda in 1954 (Ullrich 1958). Pathotype 18 (T1) was reported in The Netherlands. Potoček et al. (1991b) identified 16 new pathotypes of S. endobioticum in the Czech Republic. Their study confirmed previous reports that pathotype 1 (D1) was not identical in all locations in the Czech Republic. Malinowska and Butrymowicz (2007) reported that potato cultivars resistant to S. endobioticum pathotype 1 (D1) were grown in Poland as far back as 1955 while in 1961 and 1965, two other pathotypes were detected. These pathotypes named 2 (Ch1) and 3 (M1) were specific for Poland and differed from other pathotypes present in North Western Europe. Çakır (2008) determined pathotypes of S. endobioticum in Turkey based on 18 isolates collected between 2005 and 2008 in four provinces. Isolates obtained from the provinces Ordu and Nevşehir were tested in Germany by the Glynne–Lemmerzahl method. The isolates from Ordu belonged to pathotype 1 present in Europe while the isolates from Nevşehir did not match any of the known pathotypes. Isolates collected in Nevşehir and Niğde were equally tested in The Netherlands using Spieckermann’s methodology. The results showed that the isolates did not belong to any of the European pathotypes. Fourteen isolates collected from Nevşehir (7), Niğde (5) and Kayseri (2) regions were tested using nine Ukrainian differentials. None of them matched any of the western European, Czech and Ukrainian pathotypes. The 14 isolates corresponded to seven new distinct pathotypes (Çakır et al. 2009). Khiutti et al. (2012) studied the resistance reaction of a well-characterized subset of the collection at the Vavilov Institute of Plant Industry in Russia. No predictive association was found between wart resistance to pathotype 1 and species taxonomy, ploidy level, geographical origin or the molecular marker Nl25-1400 linked to the Sen1 resistance locus (Gebhardt et al. 2006). The emerging new pathotypes are more difficult to manage. Breeding for resistance is hampered by the lack of single genes for resistance and the complexity of resistance screening with several pathotypes (Maris 1961).
Genetics of resistance
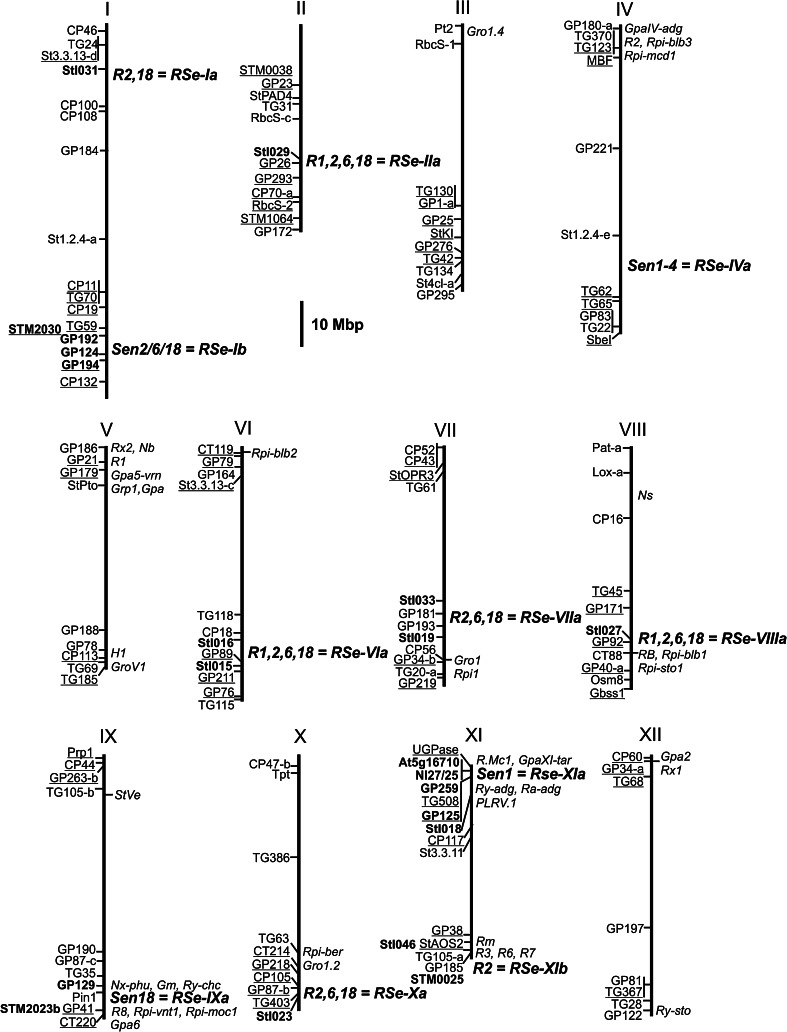
Owing to the importance of wart in potato cultivation 100 years ago, resistance to wart was among the first traits studied in plants for Mendelian inheritance (Salaman and Lesly 1923). However, segregation ratios were variable, difficult to interpret and no consistent genetic model was obtained. Part of the problem was and still is the reliability and reproducibility of the resistance assessment. Surprisingly, wart-resistant genotypes were found among the progeny of certain susceptible parents. Salaman and Lesly (1923) and Black (1935) hypothesized that at least two genes were responsible for resistance. Other studies (Lunden and Jørstad 1934; Maris 1973; Lellbach and Effmert 1990) concluded that a single dominant gene controls resistance, sometimes in combination with other, the resistance phenotype modifying or suppressing genes. In the earliest genetic studies, segregation ratios were observed in tetraploid individuals exhibiting tetrasomic inheritance whilst using genetic models based on disomic inheritance. More clarity was introduced by molecular mapping of genes for wart resistance. Hehl et al. (1999) discovered and mapped the single dominant gene Sen1 for resistance to S. endobioticum pathotype 1 in a diploid mapping population. Figure 4 shows that Sen 1 is located on potato chromosome XI in a genomic region that is syntenic with the tobacco genome region containing the N gene for resistance to tobacco mosaic virus (TMV). DNA markers derived from two potato homologues of N (N-like genes Nl25 and Nl27) were closely linked with Sen1. The same genomic region is a hot spot for genes conferring qualitative and quantitative resistance to various pathogens. Besides Sen1 it contains genes for resistance to Potato Virus Y (PVY), Potato Virus A (PVA), Potato Leaf Role Virus (PLRV), the root cyst nematode Globodera pallida, the root-knot nematode Meloidogyne chitwoodi and quantitative resistance loci (QRL) against the oomycete Phytophthora infestans and the bacterium Erwinia carotovora (Pectobacterium carotovorum) (Fig. 4). Brugmans et al. (2006) also used a diploid potato linkage map to locate Sen1-4, a second dominant gene for resistance to S. endobioticum pathotype 1. Sen1-4 maps on the long arm of chromosome IV in a region containing clusters of genes with the structural signature of plant resistance genes (Michelmore and Meyers 1998). These molecular mappings studies provided evidence that wart resistance can be conferred by a single locus but that there are different sources for resistance to S. endobioticum pathotype 1. Gebhardt et al. (2006) noted that resistance against pathotypes 2 and 6 did not co-segregate with Sen 1. Ballvora et al. (2011) identified the first loci for pathotypes 2, 6 and 18 in two tetraploid half-sib families, in which resistance to pathotype 1 also segregated. The authors found that resistance to pathotypes 2, 6 and 18 was correlated with but also different from resistance to pathotype 1. The phenotypic distribution of wart resistance appeared quantitative in the two mapping populations analyzed (Ballvora et al. 2011), in contrast to the earlier studies in diploid populations (Hehl et al. 1999; Brugmans et al. 2006), in which resistance segregated as a monogenic character. Bulked segregant analysis resulted in the identification of SSR and SNP markers linked to three S. endobioticum (Sen) quantitative resistance loci (QRL). The QRL Sen2/6/18 on chromosome I expressed resistance to pathotypes 2, 6 and 18, and the QRL Sen18 on chromosome IX to pathoype 18. The third QRL co-localized with Sen 1 on chromosome XI and expressed resistance mainly to pathoype 1 (Ballvora et al. 2011). The authors concluded that resistance to S. endobioticum is the result of the interaction of multiple alleles at three loci minimum, which in turn depends on the genetic background. Groth et al. (2013) mapped QTL for resistance to S. endobioticum pathotypes 1, 2, 6 and 18 in progeny of a cross between the varieties Saturna (resistant to pathotype 1) and Panda (resistant to pathotypes 1, 2, 6 and 18) based on AFLP and some SSR markers. With the exception of a major QRL for pathotype 1, which again co-localized with the Sen1 locus on chromosome XI, the QRL detected in this study were different from the ones in Ballvora et al. (2011). QRL for all four pathotypes were located on chromosomes II, VI, VIII and XI, and QRL for pathotypes 2, 6 and 18 on chromosomes VII and X. Figure 4 shows, alongside with other potato loci for resistance to various pathogens, the approximate genomic position of all currently known S. endobioticum resistance loci that can be anchored to the potato genome sequence (PGSC 2011) via linked DNA markers with available sequence information. We propose a new nomenclature for S. endobioticum resistance loci, which allows a better distinction between the known and the integration of new loci: RSe (Resistance to Synchytrium endobioticum) followed by the chromosome number (I to XII) and an arbitrary small letter (Fig. 4). The genetic architecture of resistance to wart as revealed by molecular mapping does not contradict the earlier genetic studies (Salaman and Lesly 1923; Lunden and Jørstad 1934; Black 1935; Maris 1973; Lellbach and Effmert 1990). Discrepancies between phenotypic distributions observed (monogenic, polygenic) and genetic models proposed (one, two or more loci) by different researchers are most likely the result of the fact that wart resistance has several sources and diverse genetic backgrounds were used for genetic analysis.
Fig. 4.
Approximate genomic positions of S. endobioticum resistance loci (RSe) alongside qualitative and quantitative potato loci for resistance to other pathogens. Markers anchored to the twelve pseudomolecule assembled sequences (PGSC version 4.03 at http://potato.plantbiology.msu.edu/cgi-bin/gbrowse/potato/) corresponding to the twelve potato chromosomes are shown on the left of the chromosome. Markers linked to potato QRL other than RSe loci (Danan et al. 2011; Zimnoch-Guzowska et al. 2000; Leonards-Schippers et al. 1994) are underlined. Markers linked to Rse loci (Hehl et al. 1999; Brugmans et al. 2006; Ballvora et al. 2011; Groth et al. 2013) are shown in bold letters. RSe and other potato resistance loci are shown on the right of the chromosome. For details of markers and resistance loci see the molecular linkage and function maps of potato at http://www.gabipd.org/database/maps.shtml
Future research directions
Phenotypic assessment of resistance to S. endobioticum is laborious, time-consuming and therefore costly. The reliable assessment of resistance requires the inoculation of at least 20 tubers per pathotype, which become available only after several years of vegetative multiplication, preventing identification of resistant plants early in the breeding cycle. Diagnostic DNA-based markers closely linked with or, even better, located within wart resistance genes would greatly facilitate the early detection and combination of different resistance sources and are therefore highly desirable. Molecular mapping of several Sen loci conferring qualitative or quantitative resistance to S. endobioticum provide the foundation for marker-assisted, pedigree-based combination of genes for wart resistance and for their eventual positional cloning (Hehl et al. 1999; Brugmans et al. 2006; Gebhardt et al. 2006; Ballvora et al. 2011; Groth et al. 2013). Development of mapping populations for linkage or association analysis, which have good agronomic qualities will be very strategic for the development of truly diagnostic markers. However, the genetic studies also demonstrate that the genetic architecture of wart resistance is complicated, particularly in tetraploid potato, and involves several loci with multiple resistance and susceptibility alleles. Combinations of markers are required to achieve high levels of resistance (Groth et al. 2013). Markers linked to resistance or susceptibility alleles in specific cultivars need to be evaluated for diagnostic value in diverse genetic backgrounds (Khiutti et al. 2012). Identification of the causal resistance genes will be most powerful for developing the ultimate diagnostic markers. This task is now facilitated by the availability of a draft potato genome sequence developed by the Potato Genome Sequencing Consortium (PGSC 2011). Reliable, cost-effective screening methods suitable for commercial breeding programs need to be developed for truly diagnostic markers. Most suitable for this purpose are SNP and SSR markers.
New genomic resources arise from high-throughput genotyping, next generation sequencing (NGS) technologies, transcriptomics and functional genomics. These technologies will aid in quantitative trait locus (QTL) analysis, association mapping and finally in the isolation and functional characterization of genes controlling wart resistance. Optimal utilization of these genomic resources in molecular breeding efforts is anchored on reliable phenotyping protocols. Cloning of the sequences modulating QTLs presents a promising route in marker development with an alternative of selecting markers linked to Sen Loci. This suggests that several QTLs need to be considered and efforts need to be intensified in localizing these regions. The candidate gene approach represents an interesting short cut.
The identification of S. endobioticum pathotypes based on differentials is as tedious as the resistance screening. The quarantine control and management of wart infested sites and materials would greatly benefit from molecular markers that are diagnostic for specific S. endobioticum pathotypes, in addition to the available sequence-based detection methods of the species S. endobioticum (Abdullahi et al. 2005; Niepold and Stachewicz 2004; van den Boogert et al. 2005). The annotated genome sequence of S. endobioticum would be the optimal resource for this application as well as for studies of the molecular evolution and biology of this fungus. The obstacle here is the purification of genomic DNA for sequencing, as axenic culture of the fungus has not been achieved so far. Next generation sequencing of transcripts isolated from wart tissues and searches for homology with sequenced, related fungal genomes might be an alternative possibility to identify S. endobioticum and pathotype specific genes.
In addition to its agronomic importance, the interaction of S. endobioticum and S. tuberosum represents an intriguing but, due to its obligate biotrophic lifestyle, challenging pathosystem. S. endobioticum induces tumors in the plant host like Agrobacterium tumefaciens (Escobar and Dandekar 2003) or Ustilago maydis (Banuett 1995). Wart-resistant plants suppress tumor formation and mount instead a hypersensitive response. In contrast to Agrobacterium and Ustilago, nothing is known about the molecular mechanisms controlling tumorigenesis and resistance to S. endobioticum. Next generation sequencing approaches on appropriate genetic materials combined with bioinformatics for sequence analysis are new possibilities to study this pathosystem at the molecular level. Most plant responses to biotic stress involve programmed cell death (PCD) mediated by signal transduction pathways (Turner et al. 2002; Suzuki 2002; Townley et al. 2005; Mase et al. 2012; Gruska 2013). Dissecting the signal transduction pathways controlling wart disease in potato represents a promising direction.
Concluding remarks
Potato wart is at present the most important quarantine disease in potato production. The development of cultivars with resistance to a broad spectrum of current and emerging pathotypes remains the most appropriate control measure. Natural DNA variation in wild and cultivated potato germplasm provides an excellent platform for the discovery of diagnostic tools for marker-assisted selection and resistance gene cloning. Molecular markers linked to loci conferring resistance to different wart pathotypes offer potentials for the efficient selection of new commercial cultivars that are resistant to multiple S. endobioticum pathotypes. Investigation into the molecular basis of wart formation and resistance via genomic tools such as expression profiling will broaden the knowledge base of this peculiar host-pathogen interaction. To streamline scientific communication among stakeholder’s efforts need to be intensified towards standardizing numerous pathotype reports from many countries while developing a common protocol and cultivar differentials for resistance testing. Collaboration between breeders, agronomists, pathologists, molecular geneticists and policy experts is most critical in combating the menace posed by this disease.
Acknowledgments
This review was compiled under the Georg Forster fellowship granted to JEO by the Alexander von Humboldt foundation. The Max Planck Institute for Plant Breeding Research, Cologne, Germany, and the National Root Crops Research Institute Umudike, Nigeria, are gratefully acknowledged for their support.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abdullahi I, Koerbler M, Stachewicz H, Winter S. The 18S rDNA sequence of Synchytrium endobioticum and its utility in microarrays for the simultaneous detection of fungal and viral pathogens of potato. Appl Microbiol Biotechnol. 2005;68:368–375. doi: 10.1007/s00253-005-1952-z. [DOI] [PubMed] [Google Scholar]
- Alexopoulos CJ, Mims CW, Blackwell M. Introductory mycology. 4. New York: Wiley; 1996. [Google Scholar]
- Anon (1921) Der Kartoffelkrebs in den Niederlanden/Black Scab (Wart disease) in the Netherlands. Verslagen en Mededeelingen van den Phytopathologischen Dienst te Wageningen No. 16a-c
- Anon (1954-1968) Potato wart disease in Europe. EPPO Publication Series B No 8, 48, 52, 63, 65
- Anon (1963) Potato wart disease, 1962. EPPO publications Series B; no 48, EPPO Paris
- Anon (1969) Council Directive 69/464/EEC on control of potato wart disease. Off J Eur Commun L323/1
- Anon (1977) New records. Quarantine newsl, Plant Prot Comm for the S.E Asia and Pacific Region 20:5–7
- Anon (1980) Hojas de datos sobre organismos para los paises miembros de COSAVE: Synchytrium endobioticum
- Anon (1982a) Data sheets on quarantine organisms. 12(1):129–134
- Anon (1982b) Report of the second meeting of the EPPO panel on Potato Wart Disease. EPPO Paris
- Anon (1990) Specific qarantine requirements. EPPO Technical Documents. EPPO Technical Documents No. 1008
- Anon . Synchytrium endobioticum. Qurantine pest for Europe. Wallingford: CAB International; 1992. pp. 638–642. [Google Scholar]
- Anon EPPO Standard PM 3/59 (1) Synchytrium endobioticum: soil tests and descheduling of previously infested plots. OEPP/EPPO Bull. 1999;29:225–231. [Google Scholar]
- Anon Council Directive 2000/29/EC on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the community. Off J Eur Commun. 2000;169:1–112. [Google Scholar]
- Anon EPPO Standards. Synchytrium endobioticum: diagnostic protocols for regulated pests. OEPP/EPPO Bull. 2004;34:213–218. [Google Scholar]
- Anon . Pest quarantine database, ver 4.4. Paris: EPPO; 2005. [Google Scholar]
- Anon (2006) A2 list of pests recommended for regulation as quarantine pests (ver 2006-09). Online. Quarantine Info. Paris France
- Arora RK, Khurana SMP. Major fungal and bacterial diseases of potato and their management. Dis Manag Fruits Veg. 2004;1:189–231. [Google Scholar]
- Baayen HP, Bonthuis H, Withagen JCM, Wander JGN, Lamers JL, Meffert JL, Cochius G, van Leeuwen GCM, Hendriks H, Heerink BGJ, van Den Boogert PHJF, van De Griend P, Bosch RA. Resistance of potato cultivars to Synchytrium endobioticum in field and laboratory tests, risk of secondary infection, and implications for phytosanitary regulations. EPPO Bull. 2005;35(1):9–23. [Google Scholar]
- Baayen R, Cochius G, Hendriks H, Meffert J, Bakker J, Bekker M, van den Boogert P, Stachewicz H, van Leeuwen G. History of potato wart disease in Europe—a proposal for harmonisation in defining pathotypes. Eur J Plant Pathol. 2006;116:21–23. [Google Scholar]
- Ballvora A, Flath K, Lübeck J, Strahwald J, Tacke E, Hofferbert H-R, Gebhardt C. Multiple alleles for resistance and susceptiblity modulate the defence response in the interaction of tetraploid potato (Solanum tuberosum) with Synchytrium endobioticum pathotypes 1, 2, 6 and 18. Theor Appl Genet. 2011;123:1281–1292. doi: 10.1007/s00122-011-1666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F. Genetics of Ustilago maydis, a fungal pathogen that induces tumors in maize. Annu Rev Genet. 1995;29:179–208. doi: 10.1146/annurev.ge.29.120195.001143. [DOI] [PubMed] [Google Scholar]
- Black W. Studies on the inheritance of resistance to wart disease (Synchytrium endobioticum (Schilb.) Perc.) in potatoes. J Genet. 1935;30:127–146. [Google Scholar]
- Blattny C. Vorläufige Mitteilung über die Rassen des Kartoffelkrebses, Synchytrium endobioticum (Schilb.) Perc. Sbornik Ceske Akadamie Zemedelske. 1942;17:40–46. [Google Scholar]
- Bojnansky V. Ecology and prognosis of potato wart disease Synchytrium endobioticum (Schilb.) Perc. Bratislava: House Slovak Academy of Science; 1960. p. 280. [Google Scholar]
- Bojnansky V. Effect of irrigation on the development and harmfulness of the potato wart disease (Synchytrium endobioticum (Schilb.) Perc.) in warmer and drier regions (in Czechoslovak, English summary) Sb, UVTI (Ustav, Vedeckotech. Inf) Ochr. Rostl. 1968;4:133–140. [Google Scholar]
- Bojnansky V. Potato wart pathotypes in Europe from the ecological point of view. EPPO Bull. 1984;14(2):141–146. [Google Scholar]
- Braun H. Biologische Spezialisierung bei Synchytrium endobioticum (Schilb.) Perc. (Vorläufige Mitteilung) Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz. 1942;52:481–486. [Google Scholar]
- Browning IA. A comparison of laboratory and field reactions of a range of potato cultivars to infection with Synchytrium endobioticum (Schilb.) Perc. Potato Res. 1995;38:281–289. [Google Scholar]
- Browning IA. Reactions of potato cultivars to Synchytrium endobioticum and implications for minimum tuber numbers in wart susceptibility tests. OEPP/EPPO Bull. 1996;26:135–140. [Google Scholar]
- Browning IA, Darling M. Development of potato wart susceptibility testing in Scotland. Pot Res. 1995;38:363–370. [Google Scholar]
- Brugmans B, Hutten RGB, Rookmaker N, Visser RGF, van Eck HJ. Exploitation of a marker dense linkage map of potato for positional cloning of a wart disease resistance gene. Theor Appl Genet. 2006;112:269–277. doi: 10.1007/s00122-005-0125-x. [DOI] [PubMed] [Google Scholar]
- Çakır E. First report of potato wart disease in Turkey. Plant Pathol. 2005;54(4):584. [Google Scholar]
- Çakır E (2008) Identification of pathotypes of Synchytrium endobioticum, the causal agent of potato wart disease in Turkey. MSc dissertation, Graduate School of Natural and Applied Sciences, University of Ankara, Turkey
- Çakır E, van Leeuwen GCM, Flath K, Meffert JP, Janssen WAP, Maden S. Identification of pathotypes of Synchytrium endobioticum found in infested fields in Turkey. OEPP/EPPO Bull. 2009;39:175–178. [Google Scholar]
- Cotton AD (1916) Host plant of Synchytrium endobioticum. Kew Roy Bot Gard Bull Misc Inform, 272–275
- Crowther EM, Glynne MD, Roach WA. Sulphur treatment of soil and the control of wart disease of potatoes in pot experiments. Ann Appl Biol. 1927;14:422–427. [Google Scholar]
- Curtis M. The life-history and cytology of Synchytrium endobioticum (Schilb) Perc the cause of wart disease in potato. Philos Trans R Soc Lond (B) 1921;210:409–478. [Google Scholar]
- Danan S, Veyrieras J-B, Lefebvre V. Construction of a potato consensus map and QTL meta-analysis offer new insights into the genetic architecture of late blight resistance and plant maturity traits. BMC Plant Biol. 2011;11:1–16. doi: 10.1186/1471-2229-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer S (2005) Emerging potato disease challenges: an overview. Online International Potato Newsletter. http://www.potatoreporteronline.com
- Department for Environment, Food and Rural Affairs (2011) Potato wart disease. http://www.fera.defra.gov.uk/plants/publications/documents/factsheets/pwd.pdf
- Dimitrova L, Laginova M, Becheva A, van Leeuwen GCM. Occurrence of potato wart disease (Synchytrium endobioticum) in Bulgaria. Bull OEPP/EPPO. 2011;41(2):195–202. [Google Scholar]
- Dingley JM. An outbreak of Potato wart disease in New Zealand. Commonwealth Phytopath News. 1970;5:2. [Google Scholar]
- Dyer RA. Investigation of plant diseases, and botanical surveys. J Farming South Africa. 1947;26:332. [Google Scholar]
- Dykstra TP. The Potato wart eradication program in Pennsylvania. Plant Dis Rep. 1940;24(1):7–8. [Google Scholar]
- Efremenko TS. The decrease of harmfulness of potato wart. Zashchita Rastenii. 1980;3:44. [Google Scholar]
- Efremenko TS, Yakovleva VA (1981) Destruction of Synchytrium endobioticum (Schilb.) Perc. in waste products of potato processing industries. Russ Mikologija I Fitopatologija 15:501–504
- Ericksson J. Wart disease of potatoes. J Board Agric. 1914;21:135–136. [Google Scholar]
- Escobar MA, Dandekar AM. Agrobacterium tumefaciens as an agent of disease. Trends Plant Sci. 2003;8:380–386. doi: 10.1016/S1360-1385(03)00162-6. [DOI] [PubMed] [Google Scholar]
- Esmarch F. On the biology of potato wart (in German) Deut Landw Presse. 1924;51:11–19. [Google Scholar]
- Esmarch F. Studies on potato wart fungus biology. 1. (in German) Angew Bot. 1926;8:102–135. [Google Scholar]
- Esmarch F. Studies on potato wart fungus physiology. 2. (in German) Angew Bot. 1927;9:88–124. [Google Scholar]
- Esmarch F. Studies on potato wart fungus biology. 3. (in German) Angew Bot. 1928;10:280–304. [Google Scholar]
- Fedotova TI (1970) Zones of potential distribution of the potato wart pathogen (in Russian, English summary). Byul VN 1 1 Zashchity rastenii 15:15–17
- Franc GD. Wart. In: Stevenson WR, Loria R, Franc GD, Weingartner DP, editors. Compendium of potato diseases. St. Paul: American Phytopath Society; 2001. pp. 46–47. [Google Scholar]
- Frandsen NO. Resistenzzüchtung gegen pilzliche und bakterielle Krankheiten der Kartoffel. In: Kappert H, Rudorf H, editors. Handbuch der Pflanzenzüchtung. Berlin: Paul Parey; 1958. pp. 71–97. [Google Scholar]
- Frank G (2007) Potato Wart. Online. APSnet Features. doi:10.1094/APSnetFeature-2007-0607
- Frey F. Screening for resistance against wart fungus Synchytrium endobioticum in potato seedlings with a modified Spieckermann method. Potato Res. 1980;23:303–310. [Google Scholar]
- Ganguly A, Paul DK. Wart disease of potatoes in India. Sci Culture. 1953;18:605–606. [Google Scholar]
- Gebhardt C, Bellin D, Henselewski H, Lehmann W, Schwarzfischer J, Valkonen JP. Marker assisted combination of major genes for pathogen resistance in potato. Theor Appl Genet. 2006;112:1458–1464. doi: 10.1007/s00122-006-0248-8. [DOI] [PubMed] [Google Scholar]
- Gedz SM. Effect of Manganese, Boron, Molybdenum and Copper micro-elements on the rise of canker immunity in potatoes (in Russia, English summary) Rept Acad Sci Ukraine. 1957;6:605–608. [Google Scholar]
- Gibbs JG (1947) Abstract on report on the work and findings of the Department of Agriculture, Falkland Islands, 1937–1946:14
- Gimingham CT, Spinks GT (1919) Soil sterilization. Univ Bristol Agr Hort Res Sta Annu Rep, 37–42
- Glynne MD. Infection experiments with wart disease of potato. Synchytrium endobioticum. Ann Appl Biol. 1925;12:34–60. [Google Scholar]
- Glynne MD. Wart disease of potatoes: the development of Synchytrium endobioticum (Schilb.) Perc. In “immune” varieties. Ann Appl Biol. 1926;13(3):358–359. [Google Scholar]
- Glynne MD. The viability of winter sporangium of Synchytrium endobioticum, the organism causing wart disease in potato. Ann Appl Biol. 1928;13:19–36. [Google Scholar]
- Grabowski L. Potato wart (Synchytrium endobioticum (Schilb.) Perc.) in Poland (in Polish) Choroby I szkodniki roslin. 1925;2:1–14. [Google Scholar]
- Gram E. Barriers and by-passes in plant trade. Ann Appl Biol. 1955;42:76–81. [Google Scholar]
- Groth J, Song Y, Kellermann A, Schwarzfischer A. Molecular characterisation of resistance against potato wart races 1, 2, 6 and 18 in a tetraploid population of potato (Solanum tuberosum subsp. tuberosum) J Appl Genet. 2013;54:169–178. doi: 10.1007/s13353-013-0141-5. [DOI] [PubMed] [Google Scholar]
- Gruska D. The brassinosteroid signaling pathway- New key players and interconnections with other signaling networks crucial for plant development and stress tolerance. Int J Mol Sci. 2013;14:8740–8774. doi: 10.3390/ijms14058740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunacti H, Erkiliç A. Developing control strategies of potato wart disease (Synchytrium endobioticum) in Turkey. ESci J of Plant Pathol. 2013;2(2):76–83. [Google Scholar]
- Gussow HT. A serious potato disease occurring in Newfoundland. Can Dept Agric Bull. 1909;63:8. [Google Scholar]
- Hampson MC. Screening systemic fungicides for potato wart disease. Can Plant Dis Surv. 1977;57:75–78. [Google Scholar]
- Hampson MC. Infection of additional hosts of Synchytrium endobioticum, the causal agent of potato wart disease. 3. Tomato as an assay tool in potato wart disease. Can Plant Dis Surv. 1981;61:15–18. [Google Scholar]
- Hampson MC. Pathogenesis of Synchitrium. endobioticum V. Wart disease suppression in potato in soils amended with urea and/or ammonium nitrate in relation to soil pH. Plant Soil. 1985;87:241–250. [Google Scholar]
- Hampson MC. Sequence of events in the germination of the resting spore of Synchytrium endobioticum, European pathotype 2, the causal agent of potato wart disease. Can J Bot. 1986;64:2144–2150. [Google Scholar]
- Hampson MC. History, biology and control of potato wart disease in Canada. Can J Plant Pathol. 1993;15:223–244. [Google Scholar]
- Hampson MC. A qualitative assessment of wind dispersal of resting spores of Synchytrium endobioticum, the causal agent of wart disease of potato. Plant Dis. 1996;80:779–782. [Google Scholar]
- Hampson MC, Coombes JW. Stress and stimulus modifications of disease severity in the wart disease of potato. Phytopath. 1985;75:817–820. [Google Scholar]
- Hampson MC, Coombes JW. Spatial distribution of Synchytrium endobioticum, the cause of potato wart, in field soil. Plant Dis. 1996;80:1006–1010. [Google Scholar]
- Hampson MC, Proudfoot KG. Potato wart disease, its introduction to North America, distribution and control problems in Newfoundland. FAO Plant Prot Bull. 1974;22:53–64. [Google Scholar]
- Hampson MC, Robertson A. Distribution of fungal spores and fractal diversity of quadrats on memebrane filters. J Food Prot. 1995;58:1038–1041. doi: 10.4315/0362-028X-58.9.1038. [DOI] [PubMed] [Google Scholar]
- Hampson MC, Thompson PR. Quantitative method to examine large numbers of soil samples for Synchytrium endobioticum, the cause of potato wart disease. Plant Soil. 1977;46:659–664. [Google Scholar]
- Hartman RE. Potato wart eradication program in Pennsylvania. Am Potato J. 1955;32:317–326. [Google Scholar]
- Hehl R, Faurie E, Hesselbach J, Salamani F, Whitham S, Baker B, Gebhardt C. TMV resistance gene N homologues are linked to Synchytrium endobioticum resistance in potato. Theor Appl Genet. 1999;98:379–386. [Google Scholar]
- Hey A (1959) Die Kartoffelkrebsforschung in der Deutschen Demokratischen Republik und ihre praktische Auswertung. In: Proceedings of the international conference of potato wart disease, Prague. Annals CAAS-Plant Prod 32(6):59–68
- Hilli A. The reasons of the spread of potato wart (Synchytrium endobioticum (Schilb.) Perc.) in England and abroad (in Finnish, English summary) Valt Maatalouskoetiminnan Julk. 1932;46:1–249. [Google Scholar]
- Hodgson WA, Pond DD, Munro J (1974) Diseases and pests of potatoes. Canadaian Department of Agriculture Publication, No 1492
- Hooker WJ. Compendium of potato diseases. St Paul: American Phytopathology Society; 1981. p. 125. [Google Scholar]
- Hunt HR, O’Donnel FG, Marshall RP. Steam and chemical soil disinfection with special reference to potato wart. J Agric Res. 1925;31:301–363. [Google Scholar]
- Jöestring (1909) The prevention and control of potato wart disease (in German). Deut Landwirtsch Presse 36(88):941
- Johnson T (1909-10) Chrysophlyctis endobiotica (potato wart or black scab) and other Chytridiadiaceae. Sci Proc of the Royal Dublin Soc 12:131–144
- Karling JS. Synchytrium. New York: Academic Press; 1964. [Google Scholar]
- Kharitonova ZM, Tarasova VP (1971) Potato wart disease (in Russian). Kolos Publishers, Leningrad, USSR, pp 46
- Khiutti A, Afanasenko O, Antonova O, Shuvalov O, Novikova L, Krylova E, Chalaya N, Mironenko N, Spooner DM, Gavrilenko T. Characterization of resistance to Synchytrium endobioticum in cultivated potato accessions from the collection of Vavilov Institute of Plant Industry. Plant Breeding. 2012;131:744–750. [Google Scholar]
- Knorr P. Potato diseases and their control. Forschungsinst Kartoffelbau. 1922;6:114–121. [Google Scholar]
- Köhler E. Der Kartoffelkrebs und sein Erreger (Synchytrium endobioticum (Schilb.) Perc.) Landwirtschaftliche Jahrbücher. 1931;74:729–806. [Google Scholar]
- Kunkel LO (1919) Wart of potatoes: a disease new to the United States. Office of Cotton, Truck, and Forage Crop Disease Investigations, Circ. 6
- Laidlaw WMR. A method for the detection of resting sporangia of potato wart disease (Synchytrium endobioticum) in the soil of old outbreak sites. Potato Res. 1985;28:223–232. [Google Scholar]
- Langerfeld E (1984) Synchytrium endobioticum (Schilb.) Perc. Zusammenfassende Darstellung des Erregers des Kartoffelkrebses anhand von Literaturberichten. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft, Berlin-Dahlem 219:1–142
- Langerfeld E, Stachewicz H. Assessment of varietal reaction to potato wart (Synchytrium endobioticum) in Germany. EPPO Bull. 1994;24:793–798. [Google Scholar]
- Langerfeld E, Stachewicz H, Rintelen J. Pathotypes of Synchytrium endobioticum in Germany. EPPO Bull. 1994;24:799–804. [Google Scholar]
- Lellbach H, Effmert M. Results of diallel analysis of the genetics of resistance to Synchytrium endobioticum (Schilb.) Perc., pathotype 1(D1) of potato (Solanum tuberosum L.) Potato Res. 1990;33:251–256. [Google Scholar]
- Lemmerzahl J. A new simplified method of inoculation of potato cultivars to test for wart resistance. Züchter. 1930;2:288–297. [Google Scholar]
- Leonards-Schippers C, Gieffers W, Schafer-Pregl R, Ritter E, Knapp SJ, Salamini F, Gebhardt C. Quantitative resistance to Phytophthora infestans in potato: a case study for QTL mapping in an allogamous plant species. Genetics. 1994;137(1):67–77. doi: 10.1093/genetics/137.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liro JI (1925) Maatalouskoelaitoksen Kasvitautiosasto vv. 1924-1925. (Annual Report of Department of plant diseases in agricultural experimental station, in Finnish). Maattalouden Koetoiminnan Keskusvaliokunnan Vuosikertomus, pp 25–31
- Lunden A, Jørstad I. Investigations on the inheritance of immunity to wart disease (Synchytrium endobioticum (Schilb.) Perc.) in the potato. J Genet. 1934;29(3):375–385. [Google Scholar]
- Malakhanova EL, Mel’nik PA, Dmitrashuk VE. Testing of wart resistance in Ukraine. Bull OEPP/EPPO Bull. 1998;28:585–590. [Google Scholar]
- Malec K. Modifications of the method of testing the potato breeding stocks for the resistance of poatato wart Synchytrium endobioticum (Schilb) Perc (in Polish) Biuletyn Instytutu Ziemniaka. 1972;10:5–10. [Google Scholar]
- Malec K. Methodology of testing potato breeding materials for wart resistance used in the laboratory of testing for resistance to quarantine diseases and pests, Institute for Potato Research (in Polish) Biuletyn Instytutu Ziemniaka. 1980;25:125–139. [Google Scholar]
- Malec K, Lubiewska E. Modification of the method of testing the potato breeding stocks for the resistance to potato wart (in Polish) Biuletyn Instytutu Ziemniaka. 1979;23:79–85. [Google Scholar]
- Malinowska E, Butrymowicz J. Pathotypes of Synchytrium endobioticum (Schilb.) Perc. occurring in Poland. Biuletyn Instytutu Hodowli i Aklimatyzacji Roslin. 2007;243:205–218. [Google Scholar]
- Malthouse GT (1910) Wart disease of potatoes (Synchytrium endobioticum). Harper Adams Agricultural College Bull, pp 40
- Marcus O. Studies on counting sporangia of Synchytrium endobioticum in soil samples. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes. 1969;21:153–157. [Google Scholar]
- Maris B. Races of potato wart causing fungus Synchytrium endobioticum (Schilb.) Perc. and some data on the inheritance of resistance to race 6. Euphytica. 1961;10:269–276. [Google Scholar]
- Maris B. Studies with potato dihaploids on the inheritance of resistance to wart disease. Potato Res. 1973;16:324. [Google Scholar]
- Mase K, Mizuno T, Ishihama N, Fujii T, Mori H, Kodama M, Yoshioka H. Ethylene signaling pathway and MAPK cascades are required for AAL toxin-induced programmed cell death. Mol Plant Microbe Interact. 2012;25(8):1015–1025. doi: 10.1094/MPMI-02-12-0036-R. [DOI] [PubMed] [Google Scholar]
- Massee G (1908-1909) Proceedings of meetings: exhibit of black scab with notes. Proc. Linn. Soc. London, 121st session, pp 6–7
- Matskiv TI, Melnik PA, Golik IV. Definition and distribution of aggressive pathotypes of Synchytrium endobioticum in Ukraine. Bull OEPP/EPPO Bull. 1998;28:539–542. [Google Scholar]
- Melnik PA (1998) Wart disease of potato, Synchytrium endobioticum (Schilbersky) Percival. EPPO Technical documents 1032, Paris
- Melodie LP, Sindermann AB. Eradication of Potato wart disease from Maryland. Am Potato J. 1994;71:743–747. [Google Scholar]
- Michelmore RW, Meyers BC. Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Res. 1998;8:1113–1130. doi: 10.1101/gr.8.11.1113. [DOI] [PubMed] [Google Scholar]
- Mirzabekian RO, Sinitsina NV, Belyakova OG. Elaboration of a biological method for the control of potato wart (in Russian) Agrobiologya. 1961;4(130):566–572. [Google Scholar]
- Moore WC. The breakdown of immunity from potato wart disease. Outlook Agric. 1957;1:240–243. [Google Scholar]
- Mygind H. Methods for detection of resting sporangia of potato wart: Synchytrium endobioticum in infested soil. Acta Agric Scand. 1954;4:317–343. [Google Scholar]
- Mygind H. Examination of soil samples for potato wart sporangia. II. Acta Agric Scand. 1961;11:114–120. [Google Scholar]
- National Diagnostic Protocols (2011) Potato wart caused by Synchytrium endobioticum. NDP. 16 Version V1.0
- Nelson GA, Olsen OA. Methods for estimating numbers of resting sporangia of Synchytrium endobioticum in soil. Phytopath. 1964;54:185–186. [Google Scholar]
- Niepold F, Stachewicz H. PCR- detection of Synchytrium endobioticum (Schilb.) Perc. J Plant Dis Prot. 2004;111(4):313–321. [Google Scholar]
- Noble M, Glynne MD. Wart disease of potatoes. FAO Plant Prot Bull. 1970;18:125–135. [Google Scholar]
- O’ Brien MJ, Rich AE (1976) Potato Diseases, United States Department of Agriculture Handbook, pp 474
- Percival J. Potato “wart” disease: the life history and cytology of Synchytrium endobioticum (Schilb.) Percl. Zentralblatt Bakteriol Parasitenkd Infectionskr. 1910;25:440–447. [Google Scholar]
- Petras M. Climatic conditions during the temporary flare-up of potato wart Synchytrium endobioticum (Schilb.) Perc.) in Hornany during the years 1888-1896 (in Czechoslovak) Agrikultura. 1969;8:161–167. [Google Scholar]
- PGSC Genome sequence and analysis of the tuber crop potato. Nature. 2011;475:189–195. doi: 10.1038/nature10158. [DOI] [PubMed] [Google Scholar]
- Pidoplichko NM. Control of potato canker in the Ukranian S.S.R (in Russian, Engl. summary). Sb. Rostlinna Vyroba. 1959;32(6):47–58. [Google Scholar]
- Potoček J. Sanitation of soil infested with Synchytrium endobioticum. Ochrana Rostlin. 1991;27:265–272. [Google Scholar]
- Potoček J, Gaar V, Hnizdil M, Novak F. Protection against spreading of potato wart disease and potato cyst nematode (in Czech) Metodiky UVTIZ. 1991;18:88. [Google Scholar]
- Potoček J, Krajickova K, Klabzubova S, Hnizdil M, Novak F, Perlova V. Identification of new Synchytrium endobioticum pathotypes in the Czech Republic. Ochrana Rostlin UVTIZ. 1991;27(3–4):191–205. [Google Scholar]
- Potter MC (1902-1903) A new potato disease (Chrysophlyctis endobiotica). J Board Agric 9:320–323
- Potter MC (1909) Note on the warty disease and corky scab of the potato. J Newcastle Farmers Club, 65
- Pratt MA (1974) Studies on the effect of biotic and abiotic factors on survival of Synchytrium endobioticum in soil. M.I Biol. Thesis, Plant Pathology Laboratory, Harpenden, now CSL, Sand Hutton, York (GB)
- Pratt MA. A wet-sieving and floatation technique for the detection of resting sporangia of Synchytrium endobioticum in soil. Ann Appl Biol. 1976;82:21–29. [Google Scholar]
- Pratt MA. The relation between field and laboratory susceptibility of potato cultivars to wart disease. EPPO Bull. 1976;6(2):111–117. [Google Scholar]
- Pratt MA. Potato wart disease and its legislative control in England and Wales. In: Ebbels DL, King JE, editors. Plant Health. Oxford: Blackwell; 1979. pp. 199–212. [Google Scholar]
- Przetakiewicz J. Assessment of the resistance of potato cultivars to Synchytrium endobioticum (Schilb.) Per. In Poland. Bull. 2008;38:211–215. [Google Scholar]
- Przetakiewicz J, Kopera K. Comparison of the usefulness of Glynne-Lemmerzahl and Spieckermann methods to assess resistance of potato (Solanum tuberosum L.) to Synchytrium endobioticum (Schilb.) Perc. Pathotype 1 (D1) in mass tests. Biuletyn Instytutu Hodowli i Aklimatyzacji Roslin. 2007;243:235–244. [Google Scholar]
- Putnam ML, Sindermann AB. Eradication of potato wart disease from Maryland. Am Potato J. 1994;71:743–747. [Google Scholar]
- Rasmussen NA, Mygind H. Control of potato wart disease (Synchytrium endobioticum) through methyl bromide soil disinfection. Tidsskrift Planteavl. 1977;81:25–31. [Google Scholar]
- Reigner N (2006) Potential chemical controls and crop protection industry contacts for recovery from establishment of select agent plant diseases in U.S. agriculture. Report from the Croplife Foundation Washington DC
- Rintelen J, Schöner M, Hunnius W. Detection and longevity of potato wart pathogen in once-infected foci (in German) Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz. 1983;90:251–257. [Google Scholar]
- Roach WA, Glynne MD. The toxicity of certain sulphur compounds to Synchytrium endobioticum, the fungus causing wart disease of potatoes. Ann Appl Biol. 1928;15:168–190. [Google Scholar]
- Roach WA, Glynne MD, Brierley WB, Crowther EM. Experiments on the control of wart disease of potatoes by soil treatment with particular reference to the use of sulphur. Ann Appl Biol. 1925;12:152–190. [Google Scholar]
- Ross H (1986) Potato breeding—problems and perspectives. Advances in Plant Breeding, Supplement 13 to Journal of Plant Breeding. P Parey, Berlin, pp 132
- Salaman RN (1989) The history and social influence of the potato (revised edition). Cambridge Universiy Press, UK
- Salaman RN, Lesly JW. Genetic studies in potatoes; inheritance of immunity to wart resistance. J Genet. 1923;13:177–186. [Google Scholar]
- Sanders JG. The discovery of European potato wart disease in Pennsylvania. J Econ Entomol. 1919;12:86–89. [Google Scholar]
- Schaffnit E, Voss G. Experiments on the control of potato wart in the year 1917. Zeitschrift für Planzenkrankheiten und Planzenschutz. 1918;28:111–114. [Google Scholar]
- Schilberszky K. A new parasite causing potato wart disease (in German) Ber Dtsch Bot Ges. 1896;14:36–37. [Google Scholar]
- Schilberszky K. Comprehensive biology of the potato wart (in German) Munich: F.P Datterer and Co., Publ.; 1930. [Google Scholar]
- Sedivy J. In memory of Javoslav Zakopal/Synchytrium endobiticum Phytopathologist (in Russia) Ochr Rostl. 1975;11(3):169–172. [Google Scholar]
- Sereda GM, Zhukova MI, Gurlenya NN (2008) Potato testing for wart resistance in Belarus. Agronomijas Vestis (Latvian J of Agron) No 11, LLU
- Sharma R, Cammack RH. A modification of the Glynne-Lemmerzahl method for testing resistance of potato varieties to wart disease, Synchytrium endobioticum (Schilb.) Perc. Potato Res. 1976;19:165–167. [Google Scholar]
- Singh PH, Shekhawat GS (2000) Wart disease of potato in Darjeeling hills. Technical Bulletin No 19, Central Potato Research Institute, Shimla, pp 73
- Smith IM, McNamara DG, Scott PR, Holderness M, Burger B. Qurantine pests for Europe. Data sheets on quarantine pests for European Union and for the European and Medterranean Plant Protection Organisation. 2. Wallingford: CAB International; 1997. [Google Scholar]
- Spieckermann A, Kothoff P. Testing potatoes for wart resistance. Deutsche Landwirtschaftliche Presse. 1924;51:114–115. [Google Scholar]
- Stachewicz H. Identification of pathotypes of potato wart pathogen - Synchytrium endobioticum (Schilb.) Perc.—by means of test cultivars] Archiv fur Phytopathologie und Pflanzenschutz. 1980;16:1–11. [Google Scholar]
- Stachewicz H, Langerfeld E (1998) Synchytrium endobioticum: on the history of potato wart in Germany) Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 335:38–62. BBA, Berlin (DE)
- Stachewicz H, Flath K, Niepold F. Methods for evaluation of resistance to potato wart disease. Kartoffelbau. 2005;9/10:403–408. [Google Scholar]
- Suzuki K. MAP kinase cascades in elicitor signal transduction. J Plant Res. 2002;115:237–244. doi: 10.1007/s102650200029. [DOI] [PubMed] [Google Scholar]
- Tarasova VP (1969) Role of environment in the decay of warts and germination of zoosporangia of S. endobioticum on Potato. (in Russia, English summary). Byull VN 1 1 Zaschity rastenii 1(13):42–44
- Tarasova VP, Beskorovainy VK. A complex method for controlling potato wart (in Russian) Zaschita rastenii. 1973;11:45. [Google Scholar]
- Taylor HV. The distribution of wart disease. J Minist Agric. 1920;27:733–738. [Google Scholar]
- Torres H, French ER, Nielsen LW. Potato diseases in Peru, 1965–1968. Plant Dis Rep. 1970;54:315–318. [Google Scholar]
- Townley HE, McDonald K, Jenkins DI, Knight MR, Leaver CJ. Ceramides induce programmed cell death in Arabidopsis cells in a calcium-dependent manner. Biol Chem. 2005;386(2):161–166. doi: 10.1515/BC.2005.020. [DOI] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A. The jasmonate signal pathway. Plant Cell. 2002;14(1):153–164. doi: 10.1105/tpc.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich J. Die physiologische Spezialisierung von Synchytrium endobioticum (Schilb.) Perc. in der Bundesrepublik. Phytopathologie Zeitschrift. 1958;31:273–278. [Google Scholar]
- van den Boogert PHJF, van Gent-Pelzer MPE, Bonants PJM, De Boer SH, Wander JGN, Lévesque CA, van Leeuwen GCM, Baayen RP. Development of PCR-based detection methods for the quarantine phytopathogen Synchytrium endobioticum, causal agent of potato wart disease. Eur J Plant Pathol. 2005;113:47–57. [Google Scholar]
- van Gent-Pelzer MPE, Krijger M, Bonants PJM. Improved real-time PCR assay for detection of the quarantine potato pathogen, Synchytrium endobioticum, in zonal centrifuge extracts from soil and in plants. Eur J Plant Pathol. 2010;126(1):129–133. [Google Scholar]
- van Leeuwen GCM, Wander JGN, Lamers J, Meffert JP, van den Boogert PHJF, Baayen RP. Direct examination of soil for sporangia of Synchytrium endobioticum using chloroform, calcium chloride and zinc sulphate as extraction reagents. OEPP/EPPO Bull. 2005;35:25–31. [Google Scholar]
- Wander JGN, van den Berg W, van den Boogert PHJF, Lamers JG, van Leeuwen GCM, Hendrickx G, Bonants P. A novel technique using the Hendrickx centrifuge for extracting winter sporangia of Synchytrium endobioticum from soil. Eur J Plant Pathol. 2007;119:165–174. [Google Scholar]
- Weiss FA (1908-1909) Potato black scab. Nature (London) 79:98–99
- Weiss F. The conditions of infection in potato wart. Am J Bot. 1925;12:413–443. [Google Scholar]
- Weiss F, Brierley P (1928) Factors of spread and repression in Potato wart. Tech Bull 56. USDA, Washington DC
- Weiss FE, Hartman RE (1923) Investigations of potato wart. USDA Bull, No 1156
- Zakopal J (1950a) The possibility of soil disinfection against potato wart disease, (Synchytrium endobioticum (Schilb.) Perc.) with a preparation containing 2, 4 dinitro—orthocresol. Cesk Akad Zemed Ved 23(1/2):132–141
- Zakopal J. The result of tests for resistance to potato wart. Ochrana Rostlin. 1950;23(3):210–215. [Google Scholar]
- Zeyla AG, Melnik PA. Detection methods for Synchytrium endobioticum. OEPP/EPPO Bull. 1998;28:543–544. [Google Scholar]
- Zimnoch-Guzowska E, Marczewski W, Lebecka R, Flis B, Schäfer-Pregl R, Salamini F, Gebhardt C. QTL analysis of new sources of resistance to Erwinia carotovora ssp. atroseptica in potato done by AFLP, RFLP and resistance-gene-like markers. Crop Sci. 2000;40:1156–1167. [Google Scholar]