Abstract
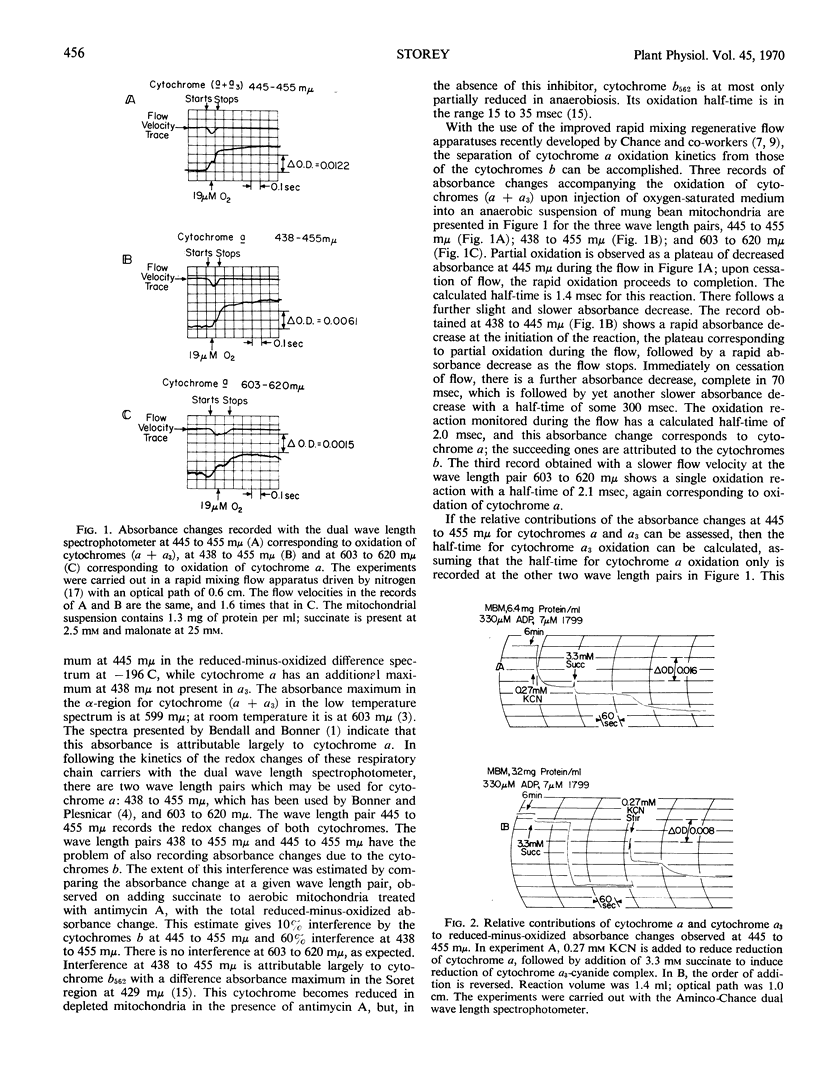
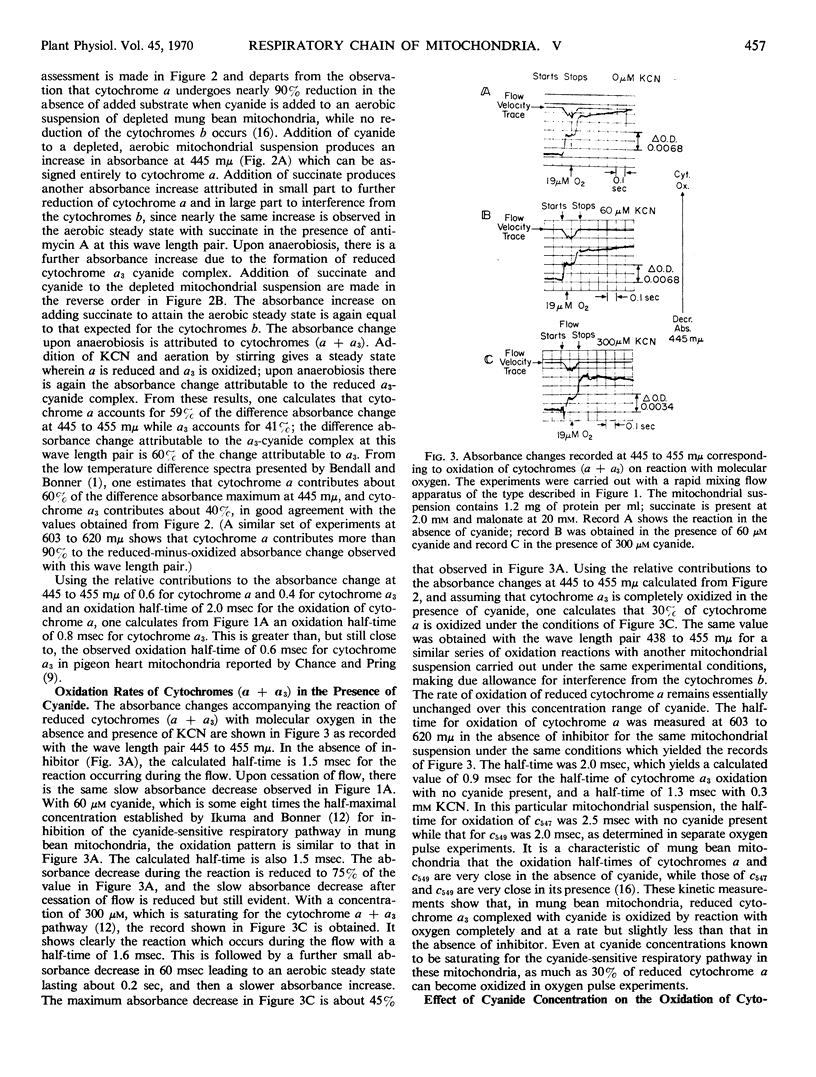
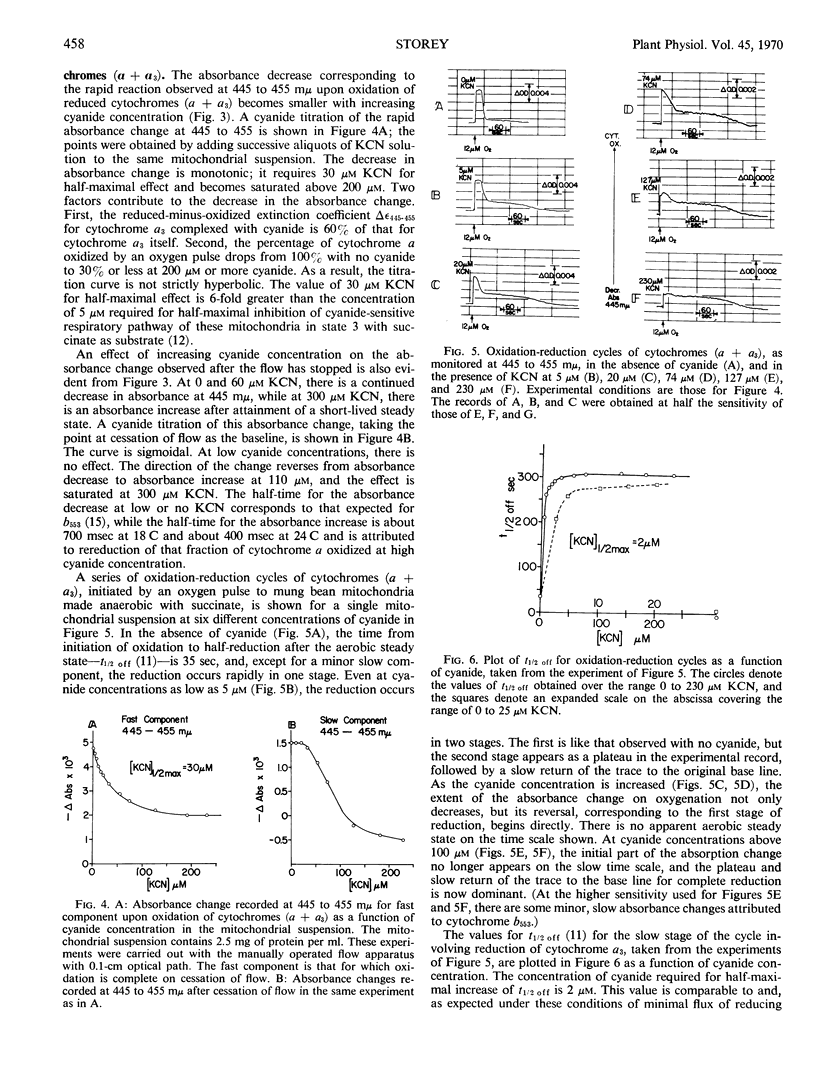
The half-times of oxidation by oxygen pulses of reduced cytochromes a and a3 in mung bean mitochondria made anaerobic with succinate have been measured by means of a rapid mixing flow apparatus coupled to a dual wave length spectrophotometer in the presence and absence of cyanide. The absorbance changes at 438 to 455 millimicrons and 603 to 620 millimicrons are suitable for recording the time course of cytochrome a oxidation; the half-time is 2.0 milliseconds at 24 Celsius. This half-time does not change over the range 0 to 300 μm KCN, but the fraction of cytochrome a oxidized falls to a limiting value of 0.3 at the higher cyanide concentrations. The absorbance changes at 445 to 455 millimicrons record the time course of both cytochrome a and cytochrome a3 oxidation; the former contributes 60% of the absorbance change and the latter 40%. The half-time for a3 oxidation is calculated as 0.9 milliseconds at 24 Celsius. This half-time increases slightly to 1.3 milliseconds at 300 μm KCN. Reduced cytochrome a3, whether uncomplexed or complexed with cyanide, becomes fully oxidized. The dissociation constant for the reduced cytochrome a3-cyanide complex is estimated to be 30 μm, whereas that for the oxidized a3-cyanide complex which inhibits electron transport is estimated to be 2 μm. This suggests two different binding sites for cyanide on the reduced and oxidized forms of cytochrome a3. The fact that a limiting fraction of reduced cytochrome a can be oxidized at high cyanide concentrations implies that there is no interference by cyanide with electron transport from a to a3, if cyanide remains bound to the site it occupies on reduced a3 after this carrier becomes oxidized on reaction with molecular oxygen. Rearrangement of cyanide from this noninhibitory site to the inhibitory site occurs rapidly enough to compete with cytochrome a oxidation. The half-time for the rearrangement is calculated to be 0.9 milliseconds.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. D., Jr, Plesnicar M. Electron transport carriers in plant mitochondria. Nature. 1967 May 6;214(5088):616–617. doi: 10.1038/214616a0. [DOI] [PubMed] [Google Scholar]
- CHANCE B. Spectra and reaction kinetics of respiratory pigments of homogenized and intact cells. Nature. 1952 Feb 9;169(4293):215–221. doi: 10.1038/169215a0. [DOI] [PubMed] [Google Scholar]
- HIGGINS J. Analysis of sequential reactions. Ann N Y Acad Sci. 1963 May 10;108:305–321. doi: 10.1111/j.1749-6632.1963.tb13382.x. [DOI] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. III. Effects of Respiratory Inhibitors. Plant Physiol. 1967 Nov;42(11):1535–1544. doi: 10.1104/pp.42.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance C., Bonner W. D. The respiratory chain components of higher plant mitochondria. Plant Physiol. 1968 May;43(5):756–766. doi: 10.1104/pp.43.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. I. Electron transport between succinate and oxygen in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):115–125. doi: 10.1104/pp.44.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria. III. Oxidation Rates of the Cytochromes c and b in Mung Bean Mitochondria Reduced With Succinate. Plant Physiol. 1969 Mar;44(3):413–421. doi: 10.1104/pp.44.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. IV. Oxidation rates of the respiratory carriers of mung bean mitochondria in the presence of cyanide. Plant Physiol. 1970 Apr;45(4):447–454. doi: 10.1104/pp.45.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONETANI T., RAY G. S. STUDIES ON CYTOCHROME OXIDASE. VI. KINETICS OF THE AEROBIC OXIDATION OF FERROCYTOCHROME C BY CYTOCHROME OXIDASE. J Biol Chem. 1965 Aug;240:3392–3398. [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. I. Absolute and difference absorption spectra. J Biol Chem. 1960 Mar;235:845–852. [PubMed] [Google Scholar]
- Yocum C. S., Hackett D. P. Participation of Cytochromes in the Respiration of the Aroid Spadix. Plant Physiol. 1957 May;32(3):186–191. doi: 10.1104/pp.32.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]


