Abstract
The closely related transcription factors (TFs), estrogen receptors ERα and ERβ, regulate divergent gene expression programs and proliferative outcomes in breast cancer. Utilizing breast cancer cells with ERα, ERβ, or both receptors as a model system to define the basis for differing response specification by related TFs, we show that these TFs and their key coregulators, SRC3 and RIP140, generate overlapping as well as unique chromatin-binding and transcription-regulating modules. Cistrome and transcriptome analyses and the use of clustering algorithms delineated 11 clusters representing different chromatin-bound receptor and coregulator assemblies that could be functionally associated through enrichment analysis with distinct patterns of gene regulation and preferential coregulator usage, RIP140 with ERβ and SRC3 with ERα. The receptors modified each other’s transcriptional effect, and ERβ countered the proliferative drive of ERα through several novel mechanisms associated with specific binding-site clusters. Our findings delineate distinct TF-coregulator assemblies that function as control nodes, specifying precise patterns of gene regulation, proliferation, and metabolism, as exemplified by two of the most important nuclear hormone receptors in human breast cancer.
Keywords: coregulator usage, estrogen receptors α and β, gene regulation, metabolism, proliferation
Introduction
Members of the superfamily of nuclear hormone receptors (NHRs) have pivotal roles in almost all aspects of physiology, including reproduction, metabolism, and immune function (Bookout et al, 2006). The estrogen receptor-α (ERα) and -β (ERβ) belong to this superfamily of transcription factors (TFs) and mediate the diverse actions of estrogens, including their involvement in several disease states, such as breast cancer (Katzenellenbogen and Katzenellenbogen, 2000; Nilsson et al, 2001; Deroo and Korach, 2006). Although encoded by different genes, ERα and ERβ share the same general modular protein structure common to the NHR superfamily, and have nearly 100% amino acid sequence homology in their DNA-binding domain. As such, it has been previously shown that ERα and ERβ are able to bind to similar DNA elements. They also show 59% amino acid homology in their ligand-binding domains, and can heterodimerize as well as act as homodimers (Cowley et al, 1997; Pace et al, 1997).
It has been well documented that ERα controls the transcription of hundreds of genes (Frasor et al, 2003; Hah et al, 2011), and that its presence in breast cancer cells drives enhanced proliferation in response to estrogens (Frasor et al, 2003; Chang et al, 2008). Therefore, ERα is the main therapeutic target in ER-positive breast tumors, which represent two-thirds of all breast cancers. The precise role of ERβ and the manner in which it can impact estrogen actions in breast cancer, however, are less clear. ERβ is expressed in ca. 70% of human breast tumors, often along with ERα, with some human breast tumors expressing only ERβ (Kurebayashi et al, 2000; Speirs et al, 2004; Saji et al, 2005; Skliris et al, 2006). Although several reports have implicated ERβ as having net antiproliferative effects in breast cancer cells (Lazennec et al, 2001; Paruthiyil et al, 2004; Strom et al, 2004; Chang et al, 2006; Lin et al, 2007a; Williams et al, 2008), elucidation of the mechanistic basis for the seemingly contrasting actions of ERα and ERβ in breast cancer cells, including delineating the manner in which the genes involved are differentially selected for regulation by ERα and ERβ, and mapping of the signaling pathways utilized, remain critical issues.
When ERα and ERβ bind their ligand, 17β-estradiol (E2), they undergo conformational changes that release heat shock proteins, enhancing receptor dimerization, interactions with coregulators (Skliris et al, 2006; Xu et al, 2009), and binding to the regulatory regions of target genes. ERs can be targeted to chromatin by direct recognition of estrogen response elements (EREs) through the agency of pioneer factors (e.g., FOXA1, GATA3, and PBX1) that modify the chromatin environment to a more permissive state, or via tethering to other TFs (e.g., Sp1 and AP1; Ali and Coombes, 2000; Glass and Rosenfeld, 2000; McKenna and O’Malley, 2002; Fullwood et al, 2009; Stender et al, 2010; Rosell et al, 2011; Jozwik and Carroll, 2012).
Given the fact that both ERs can potentially recognize similar chromatin-binding sites, interact with a largely overlapping set of coregulators, and form both homo- and heterodimers in order to regulate gene expression and cell phenotypic properties, we explored how estradiol can elicit contrasting phenotypic outcomes—proproliferative versus antiproliferative—through these two closely related TFs. In this report, we have undertaken an integrative genomic approach to map in a comprehensive manner the chromatin-binding interactions of ERα and ERβ, and their key coregulators, SRC3 and RIP140 (Cavailles et al, 1995; Glass and Rosenfeld, 2000; Xu et al, 2000; Rosell et al, 2011), in the same cell background when the receptors are present alone or together. The use of novel clustering algorithms enabled us to associate the distinct chromatin-binding landscapes of these receptor and coregulator modules with ER-regulated gene sets that delineate the specific cellular pathways and regulatory programs underlying the distinct phenotypic outcomes induced by hormone working through these two important NHRs in breast cancer cells.
These integrative and clustering approaches, delineating distinct genome-wide patterns of chromatin binding of receptors and coregulators with gene expression behavior and functional outcomes, can be applied broadly to elucidate the molecular underpinnings for the transcriptional regulation and physiological effects of any TF in response to extrinsic or temporally modulated stimuli.
Results
Genome-wide analysis of ERα, ERβ, SRC3 and RIP140 chromatin binding by ChIP-seq
Although ERα and ERβ have high structural and sequence homology, especially in their DNA-binding domains, it is not known whether these closely related receptors, in the same cell background, would substitute for one another when present alone, whether they would synergize or antagonize each other at different regulatory gene sites when present together, and how their utilization of coregulators might contribute to their specification of activities at the many gene regulatory sites to which these ERs bind. To compare genome-wide cartographies of ERα and ERβ, and their modulation of gene expression in these contexts, we utilized MCF-7 breast cancer cells that endogenously express only ERα, or cells expressing only ERβ (adenovirally expressed ERβ with knockdown of ERα via RNAi), or both ERα and ERβ at similar levels (termed ERα/ERβ cells). The presence of ERα and/or ERβ protein in the three cell types used for genome-wide analyses was confirmed by western blot (Supplementary Figure 1). ChIP samples for ChIP-seq analysis and total RNA for cDNA microarray analysis under vehicle control or 10 nM estradiol treatments were prepared in parallel in these three cell contexts (see Figure 1A for the experimental scheme).
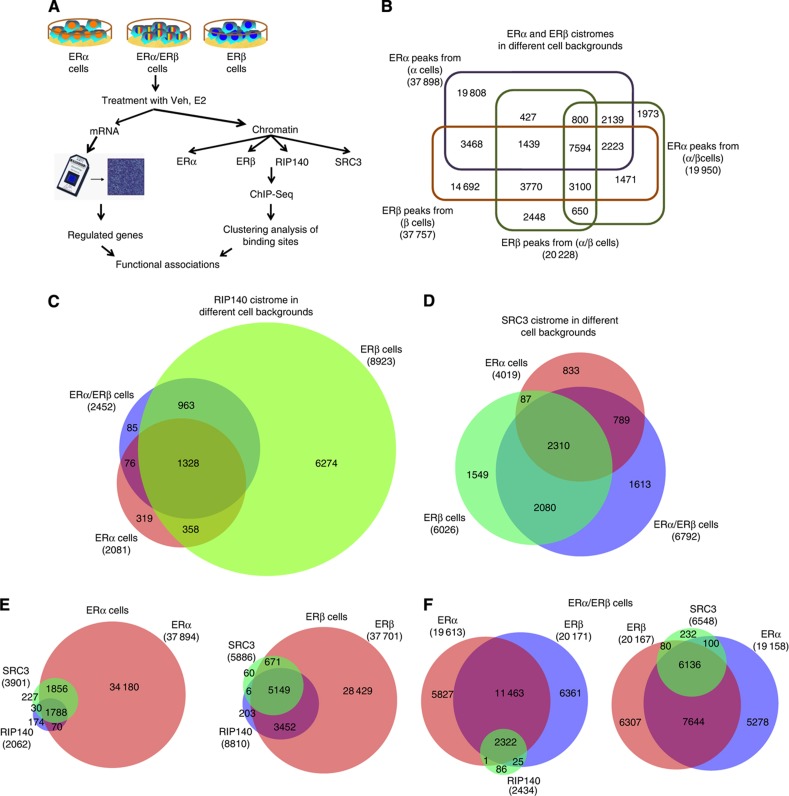
Figure 1.
Genome-wide analysis of ERα, ERβ, SRC3 and RIP140 chromatin binding by ChIP-seq. (A) Experimental design for ChIP-seq, gene microarray, and clustering analyses. Veh, vehicle; E2, 10 nM 17β-estradiol. (B) Comparison of ERα- and ERβ-binding sites in the different cell backgrounds. (C) Comparison of RIP140-binding sites in the different cell backgrounds. (D) Comparison of SRC3-binding sites in the different cell backgrounds. (E) Comparison of the number of ER and coregulator (SRC3, RIP140)-binding sites in ERα cells or ERβ cells. (F) Overlap of RIP140- or SRC3-binding sites with ERs in ERα/ERβ cells.
For cistrome analysis cells were treated with hormone for 45 min, whereas for gene expression studies total RNA was collected after an early (4 h) and a late (24 h) time of hormone treatment to gain insight into both rapid and more delayed responses. Whole-genome binding sites profiled by the ChIP-Seq approach were analyzed using MACS (Model-based Analysis of ChIP-Seq; Zhang et al, 2008), to identify binding sites with high confidence. Multiple rounds of ChIP-seq data analysis were then carried out using the integrated ChIP-seq data interpretation program, seqMINER (Ye et al, 2011), to identify, by clustering algorithms, subgroups of genomic loci for ERα, ERβ, and the coregulators SRC3 and RIP140 having similar compositional features. Further analyses were then done to relate subgroups of genomic binding sites (clusters) with binding sites for other TFs, and with genes regulated by estradiol in the three receptor cell backgrounds.
We first compared ERα and ERβ cistromes when the two receptors were present alone (ERα versus ERβ cells). As shown by the Venn diagram in Figure 1B, out of ca. 38 000 high-confidence binding sites for each receptor in ERα or ERβ cells, only about 40% (15 000) overlapped, indicating that despite homologous DNA-binding domains the two ERs bind to largely distinct chromatin regions. Notably, when both ERs were present together in cells, the number of binding sites for each ER was markedly reduced (to ca. 20 000), suggesting that under this condition a more complex chromatin interplay causes a mutual restriction in the total number of sites accessible to ERα and ERβ. Approximately half (7600) of the DNA regions bound by both ERα and ERβ in the ERα/ERβ cells overlapped with binding sites occupied by the receptors in the ERα- or ERβ-only cells. Moreover, we identified ca. 2500 binding sites for ERα and ca. 3000 sites for ERβ, which were unique to the ERα/ERβ cells, further emphasizing that cells expressing both receptors present a different cistromic landscape.
To examine whether the concurrent presence of key coregulators for ERs would provide better stratification of ER-binding sites, we performed ChIP-seq for the key coregulators SRC3 and RIP140, known to be involved in estrogen-controlled mechanisms of transcriptional regulation (Cavailles et al, 1995; Augereau et al, 2006; Lanz et al, 2010; Rosell et al, 2011; Madak-Erdogan and Katzenellenbogen, 2012). In case of RIP140 (Figure 1C), we observed >2000 binding sites in ERα and ERα/ERβ cells, with an overlap of ca.1400. Interestingly, in the ERβ cells the number of RIP140-binding sites was more than fourfold increased, to ca. 9000, suggestive of a major shift in the role of RIP140 when ERβ is present alone. On the other hand, this major change with ERβ was not seen for SRC3, where only a modest (ca.1.5-fold) increase in the number of SRC3-binding sites was observed in the ERβ cells (Figure 1D).
We then overlaid the coregulator cistromes with the ER cistromes (Figures 1E and F). As apparent from the Venn diagrams, we observed that the great majority of coregulator binding sites (>80%) were associated with genomic regions bound by ERα and/or ERβ, clearly showing that ERs are the major hubs for chromatin localization of these coregulators after hormone treatment.
Categorization of binding-site clusters based on ERα, ERβ, SRC3, and RIP140 cistromes
To parse the wealth of data obtained from ChIP-seq analyses, we examined whether the combinatorial usage of binding sites for ERs and coregulators might contain more physiologically and/or mechanistically relevant information. We therefore utilized a clustering approach using the seqMINER software (Ye et al, 2011), which provided a more comprehensive view of genome occupancy of ERs and coregulators across the different cell backgrounds (Figure 2A). This software compared the presence of the multiple factors at a given chromosomal location within a 300-bp window in both directions and clustered together those binding sites that shared a similar pattern of factor localization.
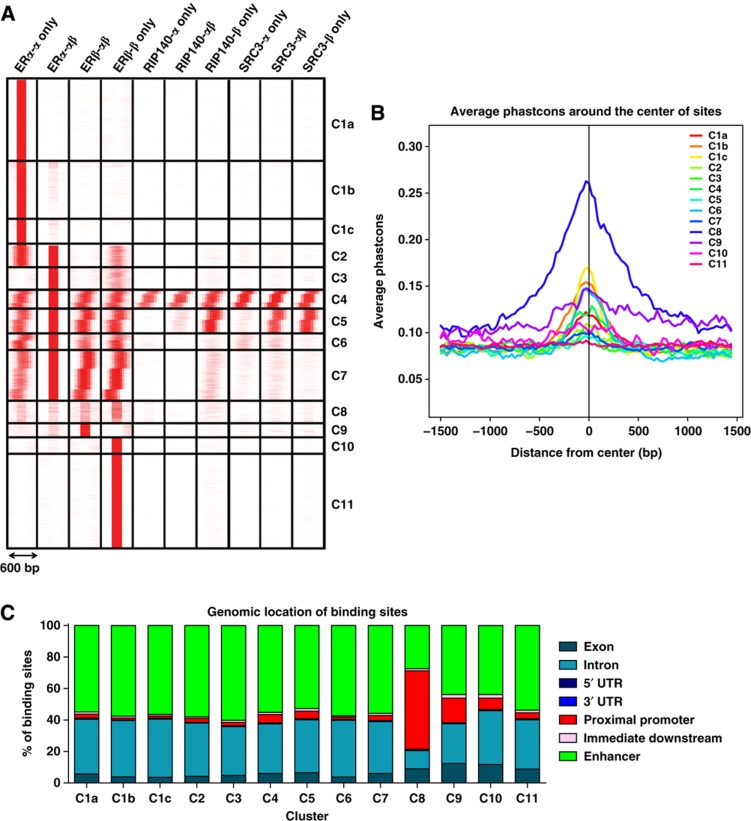
Figure 2.
Categorization of binding sites into clusters based on ERα, ERβ, SRC3, and RIP140 cistromes. (A) Clustering of the binding sites. seqMINER software was used for clustering based on colocalization of different factors in different cell backgrounds within a 300-bp window in both directions. (B) Conservation of binding sites among vertebrates: cistrome conservation tool was used to compare conservation of binding sites from different clusters among vertebrates. (C) Genomic location of binding sites: web-based CEAS tool was used for identifying genomic location of binding sites from each cluster.
In some of the clusters, we observed a consistent set ofca. 100-bp steps in the location of the binding sites for the factors with respect to ER. As the 300-bp window in both directions encompasses nearly three nucleosomes these factors are presumed to be recruited into the same complexes, but might have been crosslinked by the ChIP process to neighboring nucleosomes with respect to ER. These displacements are highly consistent in magnitude among different data sets, even when our data are compared with that of different factors from different laboratories (such as FOXA1 and GATA3; Supplementary Figure 2). This suggests that we are observing a biologically significant phenomenon that is inherent to these complexes and the recruitment process.
From these analyses, we identified 11 distinct clusters representing groups of loci having similar receptor and coregulator composition (Figure 2A). Cluster 1 represents ERα-binding sites observed in ERα cells that were lost upon introduction of ERβ (ERα/ERβ cells) and were not bound by ERβ in ERβ-only cells. Clusters 2 and 3 contained ERα- and/or ERβ-binding sites in three (C2) or two (C3) of the cell contexts. Clusters 4–7 represent ER-binding sites that were occupied by either receptor in all of the cell backgrounds. Clusters 8 and 9 contained binding sites predominantly in cells containing ERβ (ERα/ERβ or ERβ-only), and Clusters 10 and 11 contained binding sites that were occupied by ERβ in ERβ-only cells. A very interesting observation was that the presence of either SRC3 and/or RIP140 indicated that a binding site would be occupied by either ERα and/or ERβ in all the cell backgrounds (Clusters 4 and 5), whereas the two coregulators were virtually absent at binding sites in Clusters 1–3 and Clusters 10 and 11, which represent ERα and ERβ unique sites, respectively, and these coregulators were also reduced in Clusters 6–9. ER bound at sites in these clusters might use alternative coregulators. As discussed below, Clusters 4–7 are associated with many of the classic estrogen-regulated genes, such as TFF1/pS2 (trefoil factor 1) and GREB1.
Associations of clusters with FOXA1- and GATA3-binding sites
With the public availability of other ChIP-seq data sets from MCF-7 ERα cells, we combined our cistrome analysis with genome-wide data on FOXA1 (GSE data set 26 831 (Joseph et al, 2010)) and GATA3 (GSE 29 073 (Kong et al, 2011)), which are reported to be important pioneering TFs for ER-binding site determination and epithelial lineage differentiation (Carroll et al, 2005, 2006; Eeckhoute et al, 2007; Lin et al, 2007b; Lupien et al, 2008; Hurtado et al, 2011; Kong et al, 2011). By doing so, we were able to further stratify Cluster 1 into three smaller clusters (Clusters 1a, 1b, and 1c; Figure 2A) that are distinguished by having (Clusters 1b and 1c) or lacking (Cluster 1a) FOXA1 and/or GATA3 binding, overlapping with ERα-binding sites (Supplementary Figure 2). FOXA1- and GATA3-binding sites were also associated with Clusters 4 and 6 but, interestingly, not with Clusters 2, 3, 5, 7, or 8, despite ERα binding in these clusters. We also observed no association of GATA3 and FOXA1 with Clusters 9, 10, and 11 specific for ERβ DNA binding, implying that FOXA1- and GATA3-binding sites in ERα cells do not predict ERβ binding to chromatin in ERβ cells.
Enriched TF-binding motifs and binding-site mapping in the clusters
To further examine these clusters, we performed TF motif analysis prediction for each cluster in Figure 2A, using Seqpos and CEAS (cis-regulatory element annotation system; Shin et al, 2009) softwares ( http://liulab.dfci.harvard.edu/CEAS/usermanual.html). Table I shows the most highly enriched binding-site motifs for each cluster. As expected, the ER motif was the most highly enriched motif, but other motifs were characteristic of specific clusters (Table I, and see Supplementary Tables 1 and 2 for more detailed analysis of enriched TF motifs in the ER-binding sites in Clusters 1–11, based on SeqPos and CEAS analysis), suggesting a diversity of pathways likely to be represented after Gene Ontology (GO) analysis (described below). Also consistent with our cistromes overlay analysis, FOXA1- and GATA3-binding motifs were enriched only at ERα-binding sites (Table I). Interestingly, motif enrichment analysis failed to show any major differences in the composition of TF-binding motifs or strength of EREs between different clusters, suggesting that the selectivity for binding might be due to certain epigenetic marks or other factors (such as FOXA1 and GATA3) that render these sites accessible for ER binding with or without SRC3 and/or RIP140 in different cell backgrounds (Supplementary Figure 2).
Table 1. Clusters and their associated GO terms and enriched TF-binding motifs.
| Cluster number and receptor sites (and cells) | Associated GO terms | Enriched TF-binding motifs |
|---|---|---|
| Cluster 1ERα (α cells) | Gene associated in ER+ tumors, downregulated in Tam-resistant tumorsAbnormal cell migration, mammary gland epithelium developmentEpithelial cell proliferationMammary gland development | ER, GATA3 (C1c)FOXA1, other FOX family members (C1b–c) |
| Cluster 2ERα (α,α/β)ERβ (β) | Mammary gland developmentAbnormal lactation, mammary gland, liver, and thymus developmentGene downregulated in tamoxifen-resistant breast cancers | ER |
| Cluster 3ERα (α/β)ERβ (β) | Mammary gland alveolar hyperplasiaIncreased cell proliferationEpithelial cell developmentMammary gland epithelium developmentFOXA1 TF network | ER |
| Cluster 4ERα (α,α/β)ERβ (α/β,β)SRC3 (α,α/β,β)RIP140 (α,α/β,β) | Epithelial cell differentiationMammary gland branchingGenes associated with tamoxifen resistance in breast tumors | ER, AP2α, Zic1, VDR |
| Cluster 5ERα (α,α/β)ERβ (α/β,β)SRC3 (α/β,β)RIP140 (β) | Fatty acid metabolismRegulation of NF-κB activityLiver inflammationIGF-1 signaling pathway | ER, AP2α, Snail, SP1 |
| Cluster 6ERα (α,α/β)ERβ (α/β,β) | PI3K bindingEpithelial cell proliferationProstate gland developmentFocal adhesionEndometrial carcinoma | ER, HNF4, AP1 |
| Cluster 7ERα (α,α/β)ERβ (α/β,β)RIP140 (β) | Cellular response to E2Adipose tissue developmentInsulin receptor subunit bindingMammary gland epithelia development | ER, Nurr, ROR, COUP-TF, HNF4 |
| Cluster 8ERβ (β) | Ubiquitin conjugating enzymesCell cycle and apoptosis | ER, E2F, ETS, CREB |
| Cluster 9ERβ (α/β) | Mammary gland alveolus developmentMammary gland epithelial cell proliferationIncrease cell proliferation | ER, AP2α, STAT3, p300, PAX-4 |
| Cluster 10ERβ (β) | Osteoblast developmentMammary gland epithelial developmentResponse to corticosteronep38 signaling pathway | ER, Snail, E2F |
| Cluster 11ERβ (β) | Apoptosis signaling pathwayCell proliferationGenes decreased during metastasisp38 signaling | ER, AP2α, SP1, ERR, LMO2, Hairy |
Web-based GREAT software was used to determine the GO grouping of genes that map to each cluster (20 kb window). Cistrome, Seqpos, and CEAS softwares were utilized to determine enriched TF-binding motifs.
Regarding the genomic location of the receptor binding sites, ERα and ERβ were found to bind preferentially to distal enhancer regions, followed by intronic regions, in almost all clusters (Figure 2B). Notably, however, almost 50% of ER-binding sites in C8, 16% in C9, and 8% in C10 mapped to proximal promoter regions. These clusters showed enrichment for cell cycle-related genes, and E2F predicted binding site motifs (Table I). Another notable feature of cluster 8 was its high sequence conservation among different species, which was the highest of all clusters (Figure 2C).
Patterns of E2-regulated gene expression in ERα, ERα/ERβ, and ERβ cells, and their association with ER and coregulator cistromes and cellular pathways
To understand the relative importance and functional outcomes of each of the identified clusters of ER and coregulator binding sites in each cell background, we compared gene expression cDNA microarrays in the three (ERα, ERβ, and ERα/ERβ) cell backgrounds at two time points (4 h and 24 h). After data normalization and clustering analysis of the three cell transcriptomes (Figure 3A and B), we found that at the 4-h time point estradiol regulated, both up or down, a similar number of genes in ERα cells (657) or ERβ cells (674). In cells with both receptors, there was a greater overlap with ERα cells (∼30%), and more importantly there was a significant number of genes that were uniquely regulated in ERα cells (253), in ERβ cells (555), or in ERα/ERβ cells (243). Hence, ERα and ERβ, alone and together, regulate some common but also a large number of unique transcripts.
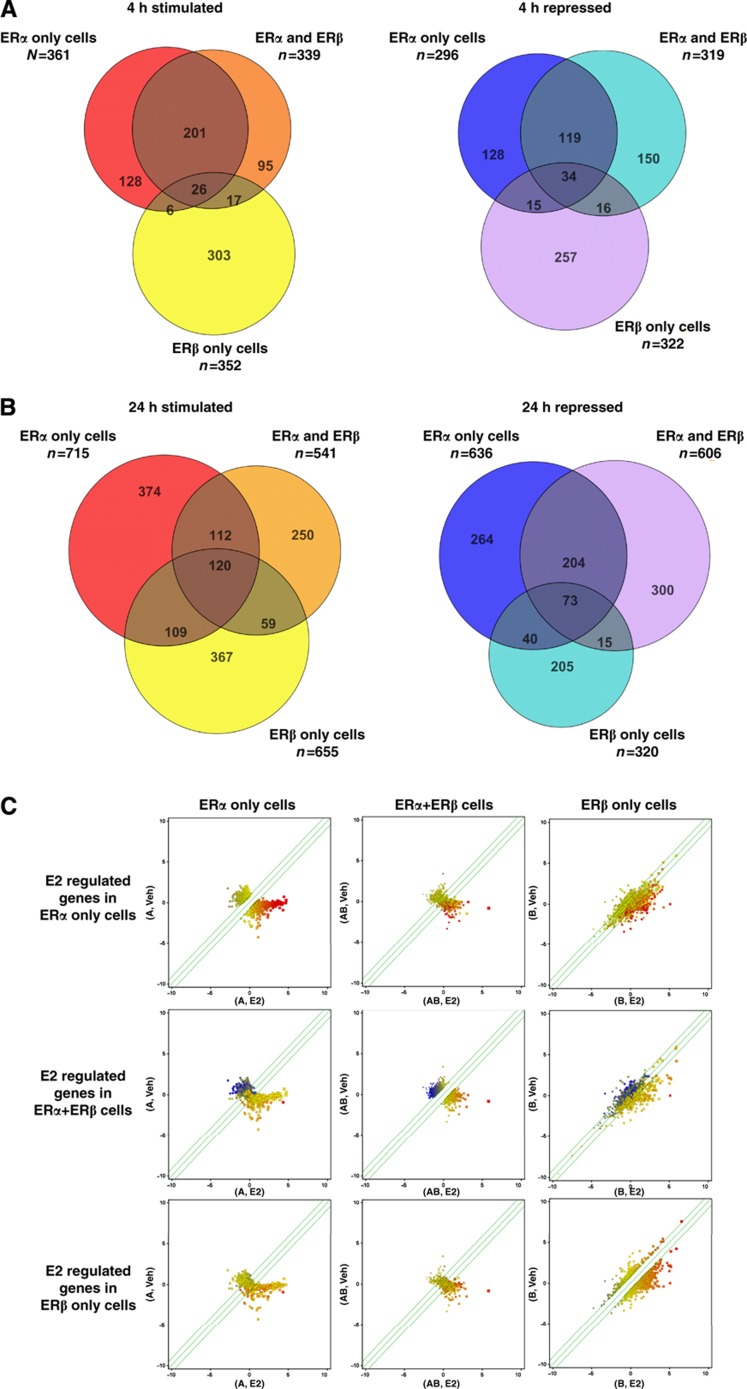
Figure 3.
Patterns of E2-regulated gene expression in ERα, ERα/ERβ, and ERβ cells. (A) Comparison of number of 17β-estradiol (E2) regulated genes after 4 h of ligand treatment in different cell backgrounds. Genespring Venn Diagram tool was used to compare overlap between genes regulated ⩾1.8-fold with FDR of 0.01 in each background. (B) Comparison of number of E2-regulated genes after 24 h of ligand treatment in the different cell backgrounds. (C) Scatter plots for gene regulations in the different cell backgrounds: scatter plots of E2-regulated genes in each cell background were generated using the Genespring scatterplot tool. The top three panels show how genes that are regulated by E2 in ERα cells are also being regulated in ERα/ERβ cells and in ERβ cells. The middle three panels show how genes that are regulated by E2 in ERα/ERβ cells are also being regulated in ERα cells and in ERβ cells. The bottom three panels show how genes that are regulated by E2 in ERβ cells are also being regulated in ERα cells and in ERα/ERβ cells. E2-upregulated genes are shown in red and E2-downregulated genes are shown in blue.
We observed that the number of genes regulated in all cell backgrounds was greater at 24 h versus 4 h (1296 versus 657 in ERα cells, 1133 versus 658 in ERα/ERβ cells, and 988 versus 674 in ERβ cells, respectively). As an alternative way of describing the data, we generated scatter plots of the regulated genes in each cell background (Figure 3C), which highlight two main trends in this data. One is that the direction of regulation by ERα (up (shown in red) or down (shown in blue)) does not change with the copresence of ERβ or in the ERβ-only cells (i.e., if a gene is regulated in all the cell backgrounds, it will always change in the same direction); and two, the magnitude of the E2 response varies among cell backgrounds, generally being highest in ERα cells, followed by ERα/ERβ cells and then ERβ cells.
To investigate how changes in hormone-mediated gene expression are related to receptor and coregulator binding events, we mapped the closest E2-regulated gene that resides within a 20-kb window of an ER-binding site. We found that two-thirds of all of the E2-regulated genes harbored at least one receptor binding site in its proximity (2861/4190 genes; Figure 4A), and often harbored multiple sites for different clusters (Figure 4B and C). This allowed us to use powerful statistical means to evaluate the predictive power of the ER-binding site clusters, described in Figure 2A, in determining the direction and magnitude of E2-dependent gene regulation with different cell background and duration of ligand treatment (4 h and 24 h; Figure 4B). For this, we calculated enrichment of E2-regulated genes relative to all the genes with a binding site in the respective cluster, and then performed clustering.
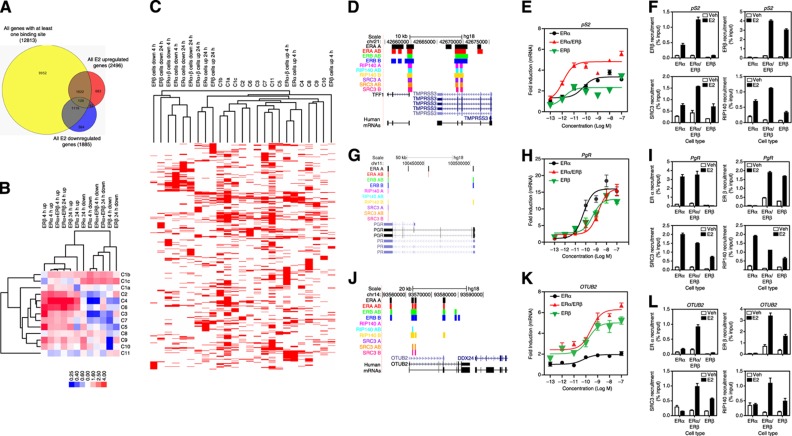
Figure 4.
Association of ER-mediated gene expression with receptor and coregulator cistromes. (A) Comparison of number of genes with at least one binding site within 20 kb and E2-upregulated or -downregulated genes. (B) Association of gene regulations in different cell backgrounds with binding site clusters: enrichment of binding-site clusters (C1–C11) associated with upregulated and downregulated genes in each cell background after 4 or 24 h of estradiol treatment is shown. Level of enrichment is designated by the color scale. Cluster 3.0 was used to cluster the data. Data were visualized using Java Treeview. (C) Clustering of genes that are regulated by E2 treatment and genes that have binding sites from different clusters: BED files for E2-regulated genes were generated using Genespring. The seqMINER enrichment based method was used to calculate the density array for the E2-regulated gene BED files and BED files obtained for each cluster from Figure 2A. Density array was clustered using Cluster 3.0 and data was visualized using Java Treeview. (D) The pS2 gene and associated binding sites for ERα, ERβ, RIP140, and SRC3 in the three cell backgrounds. (E) The pS2 gene regulation by E2 (10−13 to 10−7 M) in the different cell backgrounds. (F) The pS2 gene ChIP for ERα, ERβ, and coregulators in cells treated with control vehicle (0.01% ethanol) or 10 nM E2. (G) The PgR gene and associated binding sites in cells treated with control vehicle (0.01% ethanol) or 10 nM E2. (H) The PgR gene regulation by E2 in the different cell backgrounds. (I) The PgR gene ChIP for ERα, ERβ, and coregulators in cells treated with control vehicle (0.01% ethanol) or 10 nM E2. (J) The OTUB2 gene and associated binding sites in cells treated with control vehicle (0.01% ethanol) or 10 nM E2. (K) The OTUB2 gene regulation by E2 in the different cell backgrounds. (L) The OTUB2 gene ChIP for ERα, ERβ, and coregulators in cells treated with control vehicle (0.01% ethanol) or 10 nM E2.
From this analysis (Figure 4B) we found that, overall, upregulated genes in the three cell contexts at both times were most highly enriched in binding sites from Clusters 2, 4, and 6. By contrast, downregulated genes overall were enriched in sites from Clusters 1b and 1c, and in most cases showed deficits in binding sites from several other clusters (e.g., C4, C3, and C7). Other patterns of binding-site enrichment and deficits could be associated with particular upregulation or downregulation of gene expression in different cell contexts or times. Thus, this clustering approach not only is able to stratify ER-binding sites based upon co-occupancy of these TFs and coregulators, but also has predictive value for the direction and time of E2-mediated gene regulation for any given cell background.
To further examine the relationship between hormone-regulated genes and the binding sites from different clusters, we clustered the BED files for the genes that map to binding sites in each cluster with those for hormone-regulated genes at different times of ligand treatment in different cell backgrounds that contained at least one ER-binding site (Figure 4C). This schematically illustrates that hormone-regulated genes are highly likely to have more than one type of ER-binding site, often having representatives from several different clusters.
Cell receptor background-specific effects on profiles of gene regulation and ERα-, ERβ-, SRC3-, and RIP140-binding sites
We investigated the cell background-specific effects on selected E2-regulated genes and ER and coregulator binding sites. For each example provided, we show a Genome Browser view of the genomic region of interest with the highlightedER-binding site patterns in each cell background, a dose–response curve of mRNA expression, and ChIP assays for ERα, ERβ, SRC3 and RIP140 in the three cell backgrounds. For example, the well-known estrogen-regulated gene TFF1/pS2 has binding sites from Clusters 1, 2, and 4 (Figure 4D); its mRNA (Figure 4E) is upregulated by its natural ligand, E2, in all cell backgrounds, albeit to a different magnitude, with the highest stimulation being in the ERα/ERβ cells. Also, the E2 dose–response analysis reveals a change in sensitivity to E2, evident in the ERα/ERβ cells, where the EC50 for E2 is close to 10−12 M, compared with 10−11 to 10−10 M in ERα or ERβ cells. By ChIP assays (Figure 4F), it is also evident that there is an increase in ERα recruitment when ERβ is present, and a slight increase in ERβ recruitment when present with ERα, and this is mirrored also in elevated SRC3 and RIP140 recruitment in ERα/ERβ cells. For the progesterone receptor (PgR) gene, which harbors binding sites from Clusters 1, 2, and 7 (Figure 4G), there were no major differences in magnitude of response or recruitment of ERs in the three cell backgrounds (Figure 4H and I); however, a change in sensitivity to E2 was observed but opposite in direction to that seen for TFF1 (EC50 10−10 M for ERα cells, 10-9 M for ERα/ERβ or ERβ cells). Finally, for the otubain2 (OTUB2) gene, which we previously identified as an ERβ target gene (Chang et al, 2006, 2008), as seen in Figure 4J–L, we confirmed little regulation of OTUB2 mRNA in ERα cells, and little ERα recruitment and no stimulation of SRC3 or RIP140 recruitment in these cells; by contrast, maximal stimulation of the OTUB2 gene expression was seen in ERβ and ERα/ERβ cells (Figure 4K and Supplementary Figure 3). Interestingly, ChIP assays showed that ERα could be recruited to the gene regulatory site when ERβ was also present (ERα/ERβ cells), and this correlated with increased recruitment of SRC3 and RIP140 in this cell background (Figure 4L). ERβ showed increased recruitment in response to E2 in ERα/ERβ and ERβ cells that was accompanied by increased recruitment of both coregulators in these two cell backgrounds (Figure 4L). At the Ki-67 gene, RIP140 recruitment was strongly associated with E2 stimulation in ERβ-only cells, consistent with our ChIP-Seq data (Supplementary Figure 3).
Pathway analysis of the E2-regulated genes and effects on hormone-regulated proliferation and their association with ERα- and ERβ-binding site clusters
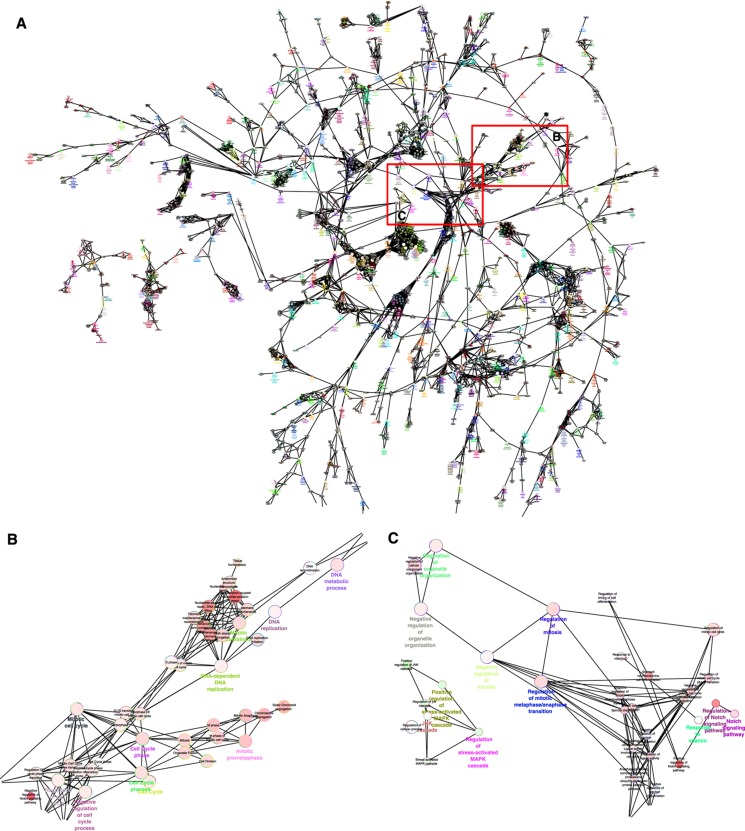
The GO analysis of the binding-site clusters was performed using web-based Metacore, DAVID, Cytoscape ClueGO plugin and Genespring software programs (Dennis et al, 2003; Huang da et al, 2009), and the main results are shown in Figure 5 and Table I. Of note, the major group of genes found to be differentially regulated between ERα and ERβ cells was associated with regulation of the M-phase of the cell cycle (Figure 5A–C) (a complete description of GO terms associated with all of the binding-site clusters is given in Table I).
Figure 5.
Pathway analysis of the E2-regulated genes associated with the ERα and ERβ-binding site clusters. (A) Clustering of gene ontologies that are differentially enriched in ERα cells versus ERβ cells: ClueGO plugin of Cytoscape was used to identify differentially regulated GO terms between ERα and ERβ cells. (B) Expanded view of M-phase-associated clusters. (C) Expanded view of additional M-phase-associated clusters.
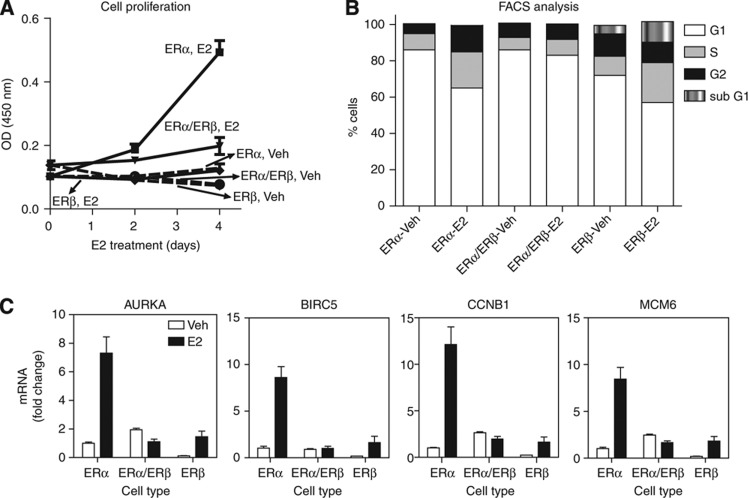
The GO terms ‘cell cycle related’ were associated withE2-stimulated genes selectively in ERα cells, and this was abrogated in ERα/ERβ cells. Interestingly, genes involved in the G1–S transition of the cell cycle were upregulated by E2 in both ERα-only and ERβ-only cells, but not in ERα/ERβ cells, and genes involved in the G2–M transition were upregulated only in ERα-containing cells but not in the other two cell backgrounds, highlighting profound differences in regulation of cell cycle stages by each receptor, either alone or in combination (Figure 5, Table I, and Supplementary Figure 4). We therefore examined in more detail the impact of ERα and ERβ on cell proliferation in the three cell backgrounds, with a specific interest in understanding the role of ERβ when present alone or in combination with ERα.
As seen in Figure 6A, ERα cells showed a stimulation of proliferation in response to E2. The copresence of ERβ greatly reduced proliferation, and cells expressing only ERβ showed little proliferative stimulation by E2. Because of the interesting differences in regulation of cell cycle progression by each ER that were highlighted by the combination of gene expression and cistrome analyses, we went on to validate this also by flow cytometry (Figure 6B). As predicted, we observed anE2-dependent increase in S phase in both ERα and ERβ cells, but not in ERα/ERβ cells. Notably, even though there was a basal increase in G2–M phase in ERβ-containing cells, ligand-dependent changes in the proportion of G2–M phase cells were observed exclusively in ERα cells, and not in the other two cell types, indicating that the entire cell cycle machinery is activated only when ERα is present alone. The changes in cell proliferation and phases of the cell cycle were dependent on the RIP140 coregulator function, as knockdown of RIP140 abrogated the E2-mediated increase in cell number (Supplementary Figure 4A) and percent of cells in G2–M phase in all cell backgrounds (Supplementary Figure 4B). Interestingly, in ERβ-containing cells RIP140 knockdown also increased the percent of S-phase cells, consistent with a report suggesting a corepressor function for RIP140 action with E2F1 (Docquier et al, 2010). There was also an increase in the subG1 population when we introduced ERβ, and this was further enhanced with RIP140 knockdown, implying a corepressor function for RIP140. However, RIP140 overall acted as a coactivator for E2-stimulated cell proliferation mediated by ERα.
Figure 6.
ERα supports strong E2-mediated cell proliferation; ERβ reduces the proliferative stimulation of ERα in ERα/ERβ cells, and ERβ by itself supports very little if any hormone-enhanced proliferation. (A) Cell proliferation: ERα, ERα/ERβ, and ERβ cells in six-well plates were treated with Veh (0.01% EtOH) or 10 nM E2 (day 0). Treatment was repeated at day 2. Cell number was monitored by MTS assay. Treatment groups were monitored in triplicate in two separate experiments. (B) FACS analysis: cell cycle stages were analyzed by flow cytometry using BD-FACS Canto. Cells were fixed in 70% ethanol, stained for 30 min with 20 μg/ml PI in Triton-X in the presence of DNAse-free RNAse A, and PI staining was measured. (C) Gene expression of four M-phase-related genes in cells treated with control vehicle or 10 nM E2 for 24 h. mRNA level in vehicle-treated ERα cells was set at 1.
Our gene expression microarray data suggest that the effect of ERβ might be due to reduced expression of important G2–M phase activators (Figure 6C and Supplementary Figure 5), such as FOXM1, as shown in ERα/ERβ cells previously (Chang et al, 2006), and Ki-67 and BIRC5, which all showed a RIP140-dependent increase in expression level only in ERα cells (Supplementary Figure 4C).
Analysis of gene networks and pathways associated with the influence of ERβ: impact on cell proliferation, MAPK activation, adipogenesis, and the involvement of RIP140
Our examination of the role of ERβ in cell proliferation revealed that ERβ affects cell proliferation in at least three important ways. As demonstrated in Figure 6, ERβ changed the cell cycle profile and was unable to drive the cells into G2/M. This first mechanism could be due to the loss of central factors, such as FOXM1(Supplementary Figure 4C; Chang et al, 2006), and a direct effect on cell cycle genes via the loss of binding of ERα when ERβ is present, as shown by Cluster 1, and gain of genomic binding of ERβ, as shown by Cluster 8, which is enriched for cell cycle and apoptosis-related genes.
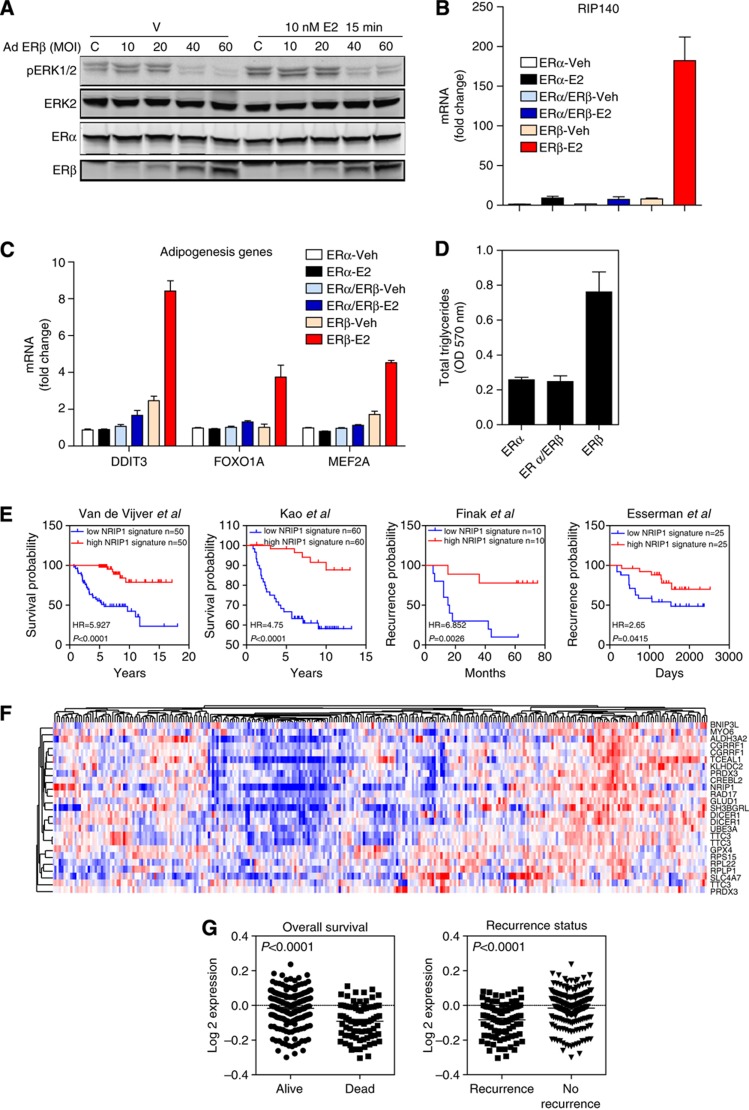
Second, we observed that ERα binds to sites near important pathway modulator genes (such as MAP2K1-MEK1 and MAP3K1-MEKK1). In our previous studies (Madak-Erdogan et al, 2011) we showed that MAPK signaling was essential for E2-mediated cell proliferation. To test the impact of ERβ on MAPK signaling, we monitored ERK1/2 activation after E2 treatment of cells containing both receptors. Our findings (Figure 7A) show that E2 increased pERK1/2 in cells containing ERα only (C, control cells with ERα but no ERβ) and that increasing concentration of ERβ abrogated pERK1/2 activation.
Figure 7.
Impact of ERβ on ERK1/2 activation, RIP140 gene expression, and adipogenesis, and an ERβ and RIP140 gene signature and its association with overall survival and recurrence status of breast cancer patients. (A) Modulation of MAPK pathway activation by ERβ. MCF-7ERα cells were infected with the indicated amounts of ERβ-adenovirus (multiplicity of infection, MOI) and were treated with 10 nM E2 for 15 min. ERK1/2 phosphorylation was monitored using pMAPK antibody (9101; Cell Signaling). Total ERK2 (as loading control), and ERα and ERβ were also monitored. C represents control cells infected with 40 MOI of AdGal and no AdERβ. (B) Analysis of RIP140 mRNA levels in ERα, ERα/ERβ, and ERβ cells treated with control vehicle or 10 nM E2 for 24 h. (C) Regulation of genes involved in adipogenesis in the three cell types treated with vehicle or E2 for 24 h. (D) Total triglyceride levels in cells were measured. Assays were performed twice in triplicate. (E) Patient survival analysis using our 20-gene ERβ and RIP140 signature and van de Vijver et al (2002), Kao et al (2011), Finak et al (2008) and Esserman et al (2012) breast cancer data sets. All the data sets were obtained from the Oncomine database. (F) Hierarchical clustering of our signature genes using expression values obtained from the van de Vijver et al (2002), data set. (G) Comparison of average expression levels of the signature genes based on survival and recurrence data of patients.
A third mechanism we uncovered was the major influence of ERβ on metabolic pathways (Figure 7 B–D) due to its preferential coupling with RIP140. We observed in our cistromes analysis that the number of genomic binding sites for RIP140, a well-known factor in metabolic regulation (Rosell et al, 2011; Siersbaek et al, 2012), was dramatically increased in E2-treated cells containing ERβ only. GO analysis of these binding sites (Table I, and see Supplementary Table 3 for functional enrichment of pathway and process networks for ERβ-modulated genes) revealed enrichment for genes associated with fatty acid metabolism, regulation of NF-κB and inflammation, IGF-1 signaling, and mammary gland epithelial development.
RIP140 is an estrogen-induced protein (Cavailles et al, 1995; Augereau et al, 2006) and is known to be a repressor of metabolic pathways (Christian et al, 2005; Rosell et al, 2011), especially lipolysis in adipocytes. RIP140 was 3-fold upregulated by E2 in ERα and ERα/ERβ cells but, most remarkably, RIP140 was more than 100-fold upregulated by hormone in ERβ cells (Figure 7B). Of note, we observed greatly elevated expression of key adipogenic factors, such as DDIT3, FOXO1A, and MEF2A, in ERβ cells treated with E2, consistent with the binding data for ERs and coregulators (Figure 7C and Supplementary Figure 6). This gene regulation was dependent on RIP140 and SRC3, as knockdown of either coregulator blocked the mRNA induction (Supplementary Figure 7). Interestingly, recruitment of each coregulator was reduced when the other coregulator was depleted, suggesting a cooperative role for each coregulator in the recruitment of a functional complex. When we monitored mRNA expression and coregulator recruitment for other genes not associated with adipogenesis, such as pS2 or OTUB2, we saw that SRC3 was required for mRNA induction and RIP140 recruitment in all cell backgrounds, while RIP140 showed a gene and cell background-specific function. For TFF1, RIP140 knockdown increased mRNA induction and interestingly increased recruitment of SRC3 in all receptor cell backgrounds. For OTUB2, RIP140 knockdown in ERα/ERβ cells increased mRNA induction and SRC3 recruitment, whereas both of these were blocked by the knockdown of RIP140 in ERβ cells. These results suggest that although RIP140 acts as a coactivator or corepressor in a gene and cell background-dependent manner, adipogenesis requires high levels of RIP140 that we observe in ERβ cells.
To further assess the effect of ERβ and RIP140 on adipogenic properties of the breast cancer cells, we monitored the cellular level of triglycerides (Figure 7D), which revealed a threefold increase in triglyceride levels in ERβ cells. This effect was dependent on RIP140.
These findings in MCF-7 cells were also verified in another ERα-positive cell line, T47D, where we introduced ERβ and generated cells with ERα and/or ERβ. Findings from cell proliferation (Supplementary Figure 8A), adipogenesis (Supplementary Figure 8B), and adipogenesis-related gene expression (Supplementary Figure 8C) studies in these T47D cells were consistent with our observations in MCF-7 cells. The results rule out phenotypic differences and transcriptional regulatory effects being unique to the MCF-7 cells. The observations in these MCF-7 and T47D breast cancer models also highlight several novel gene networks involved in delineating the phenotypic properties of the ERβ cells and the suppression of proliferation by this receptor.
A gene signature associated with RIP140 predicts better prognosis in breast cancer patients
To examine possible importance of our gene targets in breast cancer patient prognosis, we used the Oncomine database to obtain a 20-gene signature associated with ERβ- and RIP140-binding sites, and found that this signature predicted better prognosis for patients in several large clinical data sets. The initial gene list that we used to derive the signature included genes whose expression was modulated by ERβ in our gene expression microarrays and harbored binding sites for ERβ and RIP140 within 20 kb of the transcription start site of the gene. The gene signature predicted clinical outcome in 23 data sets from Oncomine (Figure 7E and Supplementary Figure 9). Moreover, this signature was most predictive for outcome in breast cancer as compared with other cancers, as we observed an enrichment of concepts associated with breast cancer but not other cancer types (Supplementary Figure 9). The signature genes included RIP140; oxidoreductases ALDH3A2, GLUD1, PRDX3, and GPX4; ribosomal proteins RPL22, RPLP1, and RPS15; negative regulators of gene transcription BNIP3L, DICER1, and TCEAL1; cell cycle regulators RAD17, CREBL2, and CGRRF1; and ubiquitin modifiers TTC3 and UBE3A. These genes were coexpressed in breast tumors (Figure 7F), and their overexpression marked tumors that had a better prognosis in terms of overall survival and reduced recurrence (Figure 7G and Supplementary Figure 10). Thus, our integrative genomics approach revealed genes that are associated with better prognosis in breast cancer patients.
Discussion
The regulation of transcription in eukaryotes involves the assembly of multiprotein complexes at specific sites on chromosomal DNA that occurs in a temporally coordinated and cell-specific manner in response to specific stimuli (Katzenellenbogen et al, 2000; Katzenellenbogen and Katzenellenbogen, 2002; Welboren et al, 2009; Madak-Erdogan et al, 2011; Ross-Innes et al, 2012). Herein we have shown that multiple rounds of data analysis using clustering algorithms have enabled us to delineate a hierarchy of ER- and coregulator-binding site subgroups that demarcate and govern gene expression programs and lead to the distinct physiological outcomes controlled by the closely related TFs, ERα and ERβ. The two ERs have very specific cistromes and differentially engage SRC3 and RIP140 at different chromatin sites to regulate distinct sets of target genes that can explain the ERα proliferative and ERβ antiproliferative activities of these receptors. In fact, we find that ERα is capable of regulating genes involved with all phases of the cell cycle when expressed alone, whereas ERβ when coexpressed with ERα dampens this response. When ERβ is expressed alone, it appears capable of driving the cells into S phase, but is then incapable of successfully completing the cell cycle. Moreover, this action of ERβ on cell cycle genes is complemented by ERβ cell-selective effects on lipid metabolism genes that can be linked to an ERβ preferential usage of the coregulator RIP140.
Given the clinical importance of ERα and ERβ in breast cancer development and in the response of patients to breast cancer treatments (Ali and Coombes, 2000; Katzenellenbogen and Katzenellenbogen, 2000; Speirs et al, 2004; Saji et al, 2005; Deroo and Korach, 2006; Skliris et al, 2006; Chang et al, 2008; Nilsson and Gustafsson, 2011), these findings illuminate the molecular mechanisms that underlie the distinct differences in the biological phenotypes and in the endocrine therapy responses of ERα- and/or ERβ-positive breast cancers. Our analyses of gene expression data on human breast tumors where we could divide tumors into those expressing ERα or ERβ only, or both receptors, showed distinct gene expression profiles for these tumors that were consistent with our observations in MCF-7 cells having these three different complements of ERs. In this human tumor gene expression microarray study, major differences were observed in cell proliferation genes that were correlated with clinical outcome and patient survival (Gruvberger-Saal et al, 2007). Patients in this cohort, whose tumors were ERα-negative and ERβ-positive, had a better outcome on tamoxifen, consistent with a reduced proliferation status. Moreover, when we combined our gene expression data and binding site analysis to obtain an ERβ-specific gene signature, this included RIP140 as well as other genes whose overexpression predicted better prognosis for breast cancer patients. Although speculative, utilization of ERβ-selective agonists (Meyers et al, 2001; Harris, 2006; Charn et al, 2010) and activation of ERβ gene programs in ERα plus ERβ-positive or ERβ-only-containing breast cancers, or increasing levels of RIP140, which could have a growth suppressive activity through ERβ, might provide a benefit for patients with ERβ-positive breast tumors.
Although there have been reports on the binding of ERα and SRC3 in cells containing only ERα (Lanz et al, 2010), and we and others have shown effects of introduction of ERβ on the expression of some ERα-regulated genes (Paruthiyil et al, 2004; Chang et al, 2006; Lin et al, 2007a; Chang et al, 2008; Charn et al, 2010; Grober et al, 2011; Nassa et al, 2011), this is the first study to comprehensively compare the binding of ERs and coregulators, and the effects of hormone on gene expression in breast cancer cells containing ERα and ERβ alone and together. To the best of our knowledge, this is also the first report of genome-wide ChIP-seq binding of RIP140 in any MCF-7 ER context, and the first for the SRC3 cistrome in ERα and ERβ, and ERβ-only cells. We selected these two central ER coregulators for the study, as SRC3, also known as amplified in breast cancer-1 (Anzick et al, 1997), is a coactivator that promotes estrogen-stimulated transcriptional activity by ERα (Anzick et al, 1997; Suen et al, 1998; Azorsa et al, 2001; Louie et al, 2004); RIP140 is an especially interesting coregulator, as it can act either as a coactivator or a corepressor (Cavailles et al, 1995; Rosell et al, 2011).
ER-positive breast cancers differ in their content of ERα and ERβ, and as the cancer progresses to increasingly malignant character there is usually a decline in the level of ERβ relative to ERα (Speirs et al, 2004; Saji et al, 2005). To examine how estrogen action in breast cancer proceeds through the two ERs, in terms of chromatin-binding site selection and gene regulation, to result in distinct cellular phenotypes, we constructed a model that was based on MCF-7 cells. We chose this as the cell background because it is the most well-studied breast cancer cell line in which much is known about ERα-binding sites as well as cistromes for certain other cofactors, such as FOXA1 and GATA3, providing a broad context for our studies (Carroll et al, 2005, 2006; Eeckhoute et al, 2007; Lupien et al, 2008; Hurtado et al, 2011; Liu et al, 2011; Ross-Innes et al, 2012). In addition, the introduction of ERβ results in a decrease in proliferation and a change in other cellular properties that mirror the effects of ERβ in human breast cancers, based on gene microarray and patient outcome data (Gruvberger-Saal et al, 2007).
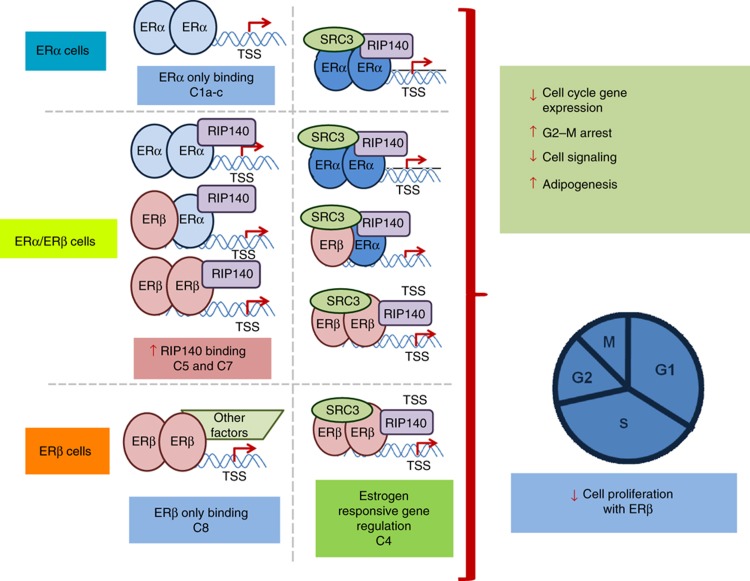
A multilayer model of progressive specification that underlies the distinctive activity of estradiol (E2) through ERα versus ERβ in breast cancer cells
In this study, as schematized in the model in Figure 8, we have examined at multiple levels the ways by which estradiol (E2) acts through its closely related receptors in breast cancer cells to generate distinct phenotypic and proliferative outcomes. At the chromatin-binding level, we show that ERα and ERβ select distinct, only partially overlapping sets of chromatin-binding sites when present alone; when present together, they compete for these sites, and restrict and shift each other’s binding. There are also characteristic differences in the ERα and ERβ sites in terms of TF-binding motif enrichments and genomic location.
Figure 8.
Model depicting modulation of gene expression, metabolism, and proliferative properties of breast cancer cells by ERα and ERβ, based on findings in this report. The three cell contexts are illustrated, ERα at top, ERα/ERβ in middle (as both homo and heterodimers), and ERβ at bottom. Sites are shown with both SRC3 and RIP140 (right), and RIP140 and other factors, but not SRC3 (left). Representative associations between binding site clusters and cellular regulations are illustrated. These include antiproliferative actions of ERβ through utilization of novel mechanisms, including modulation of transcription by ERα, changes in MAPK signaling, and alteration in adipogenesis in breast cancer cells.
At a second level, the coregulators SRC3 and RIP140, known to have important roles in the mammary gland and in breast cancer (Cavailles et al, 1995; Font de Mora and Brown, 2000; Xu et al, 2000; Augereau et al, 2006), become recruited in a distinctive manner to some sites at which ERα and/or ERβ were bound to chromatin, and some hormone-induced genes were strongly associated with ER-binding sites that were also co-occupied by these coregulators. Of note was the increased (ca. fourfold) number of RIP140-binding sites in the ERβ cells, and also the greatly increased expression of RIP140 in ERβ cells, highlighting a potentially preferential action of this coregulator with ERβ. Both coregulators were highly recruited to chromatin together with ER after E2 treatment, as shown by a >80% overlap between the binding sites of the coregulators and the binding sites of the ERs. The majority of ER-binding sites, however, did not show any colocalization with SRC3 or RIP140; at these sites, ER might be using other coregulators (McKenna and O’Malley, 2002; Green and Carroll, 2007). Recent studies have elegantly demonstrated the promoter-specific involvement of distinct coregulators in the regulation of estrogen-responsive genes in MCF-7 cells. Thus, SRC3 was present at nine of the ten genes examined in MCF-7 ERα cells, whereas fewer coregulators were involved in estrogen stimulation of other genes, such as the PgR gene (Won Jeong et al, 2012).
The overlay of our ERα and ERβ cistromes with our SRC3 and RIP140 coregulator cistromes, and with genome-wide cistrome analysis of the FOXA1 and GATA3 pioneering factors by others (Carroll et al, 2005, 2006; Eeckhoute et al, 2007; Lupien et al, 2008), followed by clustering, enabled a stratification of binding-site patterns that predict time and direction of gene expression and phenotypic changes in each cell background. FOXA1 and GATA3 are important DNA-bound cofactors of ERα (Carroll et al, 2005, 2006; Eeckhoute et al, 2007; Lupien et al, 2008), and they have been shown to be necessary for ERα chromatin binding and subsequent activation of many ERα target genes. This additional layer of analysis allowed us to organize the ER-binding sites into 11 clusters having distinct characteristics in terms of predicted TF-binding site motifs, genomic location, conservation, associated genes and enriched pathways. Finally, the direction of E2 regulation (stimulation or repression) of the target genes could be defined based upon clustering analysis of ER and coregulator cistromes. Of note, each cluster of binding sites could be associated with distinctive functional gene ontologies that can explain avenues through which ERα and ERβ modulate cell proliferation and metabolism.
Specification of estrogen action at the chromatin layer
Despite sharing great sequence and structural homologyin the DNA-binding domain (Katzenellenbogen and Katzenellenbogen, 2000; Nilsson et al, 2001; Deroo and Korach, 2006), ERα and ERβ select distinct, only partially overlapping chromatin-binding sites, with more sites being accessed by each ER when present by itself. When both are together, mutual competition between them restricts the number of sites that are occupied by each ER subtype and contracts the number of overlapping sites; moreover, we also observe the appearance of many new binding sites for ERα and ERβ when both are present, which indicates a profound redistribution of cistromes when the receptors are copresent.
Although the two ERs can both homodimerize and heterodimerize, and both homo and heterodimers can be observed in vitro and in cells (Powell and Xu, 2008; Paulmurugan et al, 2011; Powell et al, 2012), the potential number of chromatin-binding sites through which ERα-ERβ heterodimers might function (12 000 shared sites) is only a fraction (43%) of the total number of ER-binding sites that can be occupied by either ER in the ERα/ERβ cells (28 000). In addition, most of the new sites present only in ERα/ERβ cells are associated uniquely with either ERα or ERβ, and thus can be occupied only by homodimers of ERα and ERβ. Clearly then, the context of a chromatin-binding site imposes a significant specification of whether ER homo or heterodimers bind, regardless of their cellular abundance.
Specification of ER action at the gene function–phenotypic layer
Among the nuclear receptor family, it has proved challenging to associate ER chromatin-binding sites with estrogen-regulated genes, because many of the ER regulatory sites are in far-distal enhancer regions rather than in proximal promoters (Carroll et al, 2005, 2006; Lin et al, 2007b; Madak-Erdogan et al, 2011). Nevertheless, through our genome-wide location and expression analyses, we were able to define several novel mechanisms underlying the effects of ERβ on cell proliferation. By analyzing the effect of E2 on the cell cycle, we found that E2 directly regulated genes involved in all the phases of the cell cycle in ERα cells; this was dramatically altered and reduced, however, with ERβ copresence. Very interestingly, ERβ, when present alone, was able to increase the percent of cells in S phase, but cells were then unable to complete the cell cycle because they became arrested at the G2/M transition.
GO analysis of ER-binding site clusters revealed an intriguing model for ERβ antiproliferative action. First, ERβ impinges on cell cycle genes in multiple ways: by reducing ERα binding when ERβ is present (cluster 1), by binding directly to promoters of cell cycle-regulated genes (cluster 8), and by reducing the levels of factors implicated in G2/M transition (e.g., FOXM1). Cluster 8 was particularly interesting, as it was enriched in promoter proximal binding sites that contained overrepresented motifs for E2F TFs. The E2F family members consist of activators and repressors, and the fact that our findings show that ERβ-binding sites in this specific cluster are more enriched with E2F motifs (Table I) raises the possibility that ERβ might be preferentially binding to the chromatin near E2F-binding sites and utilizing some of the E2F members to affect the regulation of its target genes, consistent with their suggested involvement from prior studies (Strom et al, 2004; Stender et al, 2007).
ERβ also exerted an extensive effect on MAPK signaling, which we previously showed to be essential for stimulation of cell proliferation by E2 in ERα-containing breast cancer cells (Madak-Erdogan et al, 2011). Moreover, ERβ altered cell metabolism that is central to cell growth, where it was found to act in several reinforcing ways. ERβ increased the genomic binding and expression level of RIP140, a critical metabolic regulator, in ERβ-only cells. These unique binding sites were related to genes involved in IGF-1 signaling, inflammation, and fatty acid metabolism. Functional outcomes associated with increased binding of RIP140 included the ability of ERβ to directly bind and increase the expression of adipogenesis genes, elevating triglyceride levels in ERβ cells.
An integrative, whole-genome and clustering analysis for elucidating the progressive specification of the biological activities of NHR complexes
Through integration of cistromes and transcriptomes, and by application of clustering analyses, we have been able to define a combinatorial pattern of regulation by these factors, in response to hormone stimulation, that specifies gene regulation across binding site clusters. Importantly, gene expression thereby becomes predictable and allows association of multiple regulatory sequences with a pattern of control defined by the cell’s receptor context and the time of hormonal exposure.
Work in many laboratories, in both mammalian systems (Farnham, 2009) and in yeast (Beer and Tavazoie, 2004), has highlighted the difficulty in associating TF cistrome binding with gene expression outcomes. Our approach using clustering analyses, which we have used to elucidate patterns of binding of multiple regulatory factors and their association with gene regulation by two of the most important NHRs in human breast cancer, should be of help in characterizing the layers of specification that determine the regulation of transcription and distinct functional outcomes by any TF of interest.
Materials and methods
Cell culture, adenovirus, lentivirus, and siRNA, and ligand treatments
MCF-7 cells were grown in minimal essential medium (MEM) (Sigma, St Louis, MO), supplemented with 5% calf serum (HyClone, Logan, UT), and 100 μg/ml penicillin/streptomycin (Invitrogen, Carlsbad, CA) (Chang et al, 2006, 2008). For experiments, the cells were maintained in phenol red-free MEM plus 5% charcoal-dextran-treated calf serum for at least 3 days, and were then seeded at a density of 3 × 105 cells per 10-cm tissue culture dish (Corning, Corning, NY) for 2 days before adenovirus infection. Recombinant adenoviruses were constructed and prepared as described (Chang et al, 2006). Adenoviral infection conditions were those described previously (Chang et al, 2006; Frasor et al, 2006; Charn et al, 2010) and were used to generate MCF-7 cells expressing levels of ERβ equal to that of the endogenously expressed ERα. ERβ-only cells were generated from these cells by knockdown of ERα in parental cells using the following siERα sequences from Dharmacon: forward, 5′-UCAUCGCAUUCCUUGCAAAdTdT-3′, and reverse, 5′-UUUGCAAGGAAUGCGAUGAdTdT-3′. siRNA experiments were performed as previously described, and resulted in knocknown of ERα mRNA and protein by greater than 95% (Chang et al, 2008). Briefly, cells were transfected with 20 nM siCtrl or siERα for 48 h after infection. Then, cells were treated with 0.1% EtOH (Veh) or 10 nM E2 (Sigma-Aldrich) for the indicated times. For coregulator knockdown experiments, 24 h after control or ERβ adenovirus infection, cells were infected with control, SRC3 or RIP140 shRNA lentiviral particles according to the manufacturer’s suggestions (Santa Cruz Biotechnology). All experiments were conducted with three or more replicates.
ChIP assays
ChIP assay was carried out as described (Barnett et al, 2008) for ERα, ERβ, SRC3, and RIP140. Briefly, the different cells were treated with 0.1% EtOH (Veh) or 10 nM E2 for 45 min. Chromatin was crosslinked using 1% formaldehyde for 15 min at room temperature. Cells were washed with PBS and were collected. After three times 10 s sonication in ChIP lysis buffer, the samples were centrifuged for 10 min at 4 °C. The antibodies used for immunoprecipitation of DNA/protein complexes were ERα antibody HC-20 (Santa Cruz Biotechnology); ERβ antibodies were a combination with equal parts of CWK-F12 (produced in our lab (Choi et al, 2001)), GTX70182 (GeneTex), GR40 (Calbiochem), and PA1-311 (Affinity Bioreagents); SRC3 antibody SC-9119 (Santa Cruz Biotechnology); and RIP140 antibody SC-8997 (Santa Cruz Biotechnology). Precipitated complexes were washed with RIPA buffer (three times) and TE buffer (2 times). After overnight incubation at 68 °C, ChIP DNA was isolated using QIAGEN PCR purification kit as per the manufacturer’s suggestions. The ChIP DNA was used forChIP-seq analysis and quantitative real-time PCR.
ChIP-seq analysis and clustering
The ChIP DNA was prepared into libraries according to Illumina Solexa ChIP-seq sample processing methods, and single read sequencing was performed using the Illumina Solexa Genomic Analyzer. Sequences generated were mapped uniquely onto the human genome (hg18) by ELAND. MACS algorithm (Zhang et al, 2008) was used to identify enriched peak regions (default settings) with a P-value cutoff of 6.0e−7 and FDR of 0.01. ChIP-seq data will be available from the Gene Expression Omnibus database.
The seqMINER density array method with a 300-bp window in both directions was used for the generation of clusters, i.e., groups of loci having similar compositional features (Ye et al, 2011). This ChIP-seq data interpretation platform allows the comparison and integration of multiple ChIP-seq data sets, and their extraction and visualization of specific patterns within data sets. A meta binding BED file was generated using ChIP-seq tool set, which included ER-binding sites from all three cell backgrounds (Blahnik et al, 2010). As there were only a relatively small number of coregulator-specific binding sites without any receptor binding, these sites were not included in the clustering step. This reference file was then used to map the binding sites for each factor from different cell backgrounds. BED files for each cluster were used for further analysis with Galaxy Cistrome integrative analysis tools (Venn diagram, conservation, CEAS; Liu et al, 2011).
Gene Chip mRNA transcriptional profiling microarrays
Total RNA was used to generate cRNA, which was labeled with biotin according to techniques recommended by Affymetrix. All analyses were done for three or more samples for each treatment. The biotin-labeled cRNA was then hybridized to Affymetrix U133 plus 2.0 GeneChips, which contain oligonucleotide probe sets for over 47 000 transcripts. After washing, the chips were scanned and analyzed using Affymetrix processing software. CEL files were processed using GeneSpring GX 11.0 software (Agilent) to obtain fold-change and P-value with Benjamini and Hochberg multiple test correction (Hochberg and Benjamini, 1990) for each gene for each treatment relative to the vehicle control. We considered genes with fold-change >1.8 and P-value <0.05 as statistically significant, differentially expressed.
Motif and GO category analysis
Overrepresented GO biological processes were determined by the web-based DAVID Bioinformatics Resources database (Dennis et al, 2003; Huang da et al, 2009) and the GeneSpring and web-based GREAT (Genomic Regions Enrichment of Annotations Tool) software (McLean et al, 2010). Motif-enrichment analysis was done using Seqpos, which uses TRANSFAC PWMs, JASPAR, and de novo motif discovery tools (Liu et al, 2011).
Cell proliferation and flow cytometry
MCF-7 cells were seeded at 30 000 cells/well in six-well plates. On the second day, the cells were infected with AdGal or AdERβ at multiplicity of infection 20 for 4 h and then the medium was changed. After 24 h, the cells were transfected with 20 nM siCtrl or siERα. At 24 h, cells were treated with Veh (0.01% EtOH) or 10 nM E2 (day 0) and again at day 2. Cell number was monitored using the MTS assay (Bergamaschi et al, 2011). Flow cytometry and analysis of cell cycle stage were conducted as described using BD-FACS Canto. Cells were fixed in 70% ethanol, stained for 30 min with 20 μg/ml propidium iodide (PI; Molecular Probe) in Triton-X (Sigma) in the presence of DNAse-free RNAse A (Sigma), and PI staining was measured (Bergamaschi et al, 2011; Bhatt et al, 2012).
Adipogenesis assay
Adipogenesis was monitored in cells grown in full media and used the adipogenesis assay kit from BioVision (Milpitas, CA) that measures total triglycerides. Absorbance was measured at 570 nm using a BioRad 680 Microplate Reader, and all assays were performed in triplicate.
Tumor data sets and data analysis
Gene lists for generation of the ERβ and RIP140 signature were selected based on the modulation of gene expression in ERβ-containing cells, and the presence of ERβ- and RIP140-binding sites within 20 kb of the transcription start site of the genes. This gave us a list of 345 genes. We further examined this list using the Oncomine database to find genes that predicted molecular outcome in breast cancer data sets with an odds ratio of at least 2 and P-value of 0.0001. The log2 median-centered intensity expression values for signature genes were obtained from the Oncomine database. This analysis yielded a 20-gene ERβ and RIP140 signature. Hierarchical clustering of data was performed and displayed using Cluster 3.0 and Java TreeView software for analysis and visualization.
Survival analysis
For breast cancer patient survival analysis, patients were stratified according to average expression value of the genes in the signature, and the top 30% and bottom 30% of patients were used for computation of Kaplan–Meier curves by the Cox–Mantel log-rank test and Gehan–Breslow–Wilcoxon test in Graphpad Prism.
Data availability
Gene expression and ChIP-Seq data will be available from the Gene Expression Omnibus database with the following accession numbers: meta study, GSE42349; gene expression, GSE42347; and ChIP-Seq, GSE42348.
Supplementary Material
Supplementary Figures 1-10 and Supplementary Tables 1-3
CEAS analysis of transcription factor binding motif enrichment for binding site clusters (C1-11) identified through SeqMINER analysis.
SeqPos analysis of transcription factor binding motif enrichment for binding site clusters (C1-11) identified through SeqMINER analysis.
Functional enrichment of categories for ERβ-modulated genes.
Acknowledgments
This study was supported by NIH grants P50 AT006268 from the National Center for Complementary and Alternative Medicine (NCCAM), the Office of Dietary Supplements (ODS), and the National Cancer Institute (NCI) (BSK); also by NIH R37DK015556 (JAK) and a grant from The Breast Cancer Research Foundation (BSK). ZME received partial support from NIH T32 ES07326. THC was supported by an A*STAR graduate fellowship from The Singapore Agency for Science, Technology and Research. ETL was supported by A*STAR of Singapore and EU-grant CRESCENDO (FP6-018652). We thank Luke Petry for technical assistance.
Author contributions: ZME, THC, YJ, ETL, JAK, and BSK conceived and designed the experiments. ZME, THC, and YJ performed the experiments. ZME, THC, YJ, ETL, JAK, and BSK analyzed the data. ZME, THC, YJ, ETL, JAK, and BSK wrote the paper. ZME, JAK, and BSK proofread the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ali S, Coombes RC (2000) Estrogen receptor alpha in human breast cancer: occurrence and significance. J Mammary Gland Biol Neoplasia 5: 271–281 [DOI] [PubMed] [Google Scholar]
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS (1997) AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277: 965–968 [DOI] [PubMed] [Google Scholar]
- Augereau P, Badia E, Fuentes M, Rabenoelina F, Corniou M, Derocq D, Balaguer P, Cavailles V (2006) Transcriptional regulation of the human NRIP1/RIP140 gene by estrogen is modulated by dioxin signalling. Mol Pharmacol 69: 1338–1346 [DOI] [PubMed] [Google Scholar]
- Azorsa DO, Cunliffe HE, Meltzer PS (2001) Association of steroid receptor coactivator AIB1 with estrogen receptor-alpha in breast cancer cells. Breast Cancer Res Treat 70: 89–101 [DOI] [PubMed] [Google Scholar]
- Barnett DH, Sheng S, Charn TH, Waheed A, Sly WS, Lin CY, Liu ET, Katzenellenbogen BS (2008) Estrogen receptor regulation of carbonic anhydrase XII through a distal enhancer in breast cancer. Cancer Res 68: 3505–3515 [DOI] [PubMed] [Google Scholar]
- Beer MA, Tavazoie S (2004) Predicting gene expression from sequence. Cell 117: 185–198 [DOI] [PubMed] [Google Scholar]
- Bergamaschi A, Christensen BL, Katzenellenbogen BS (2011) Reversal of endocrine resistance in breast cancer: interrelationships among 14-3-3zeta, FOXM1, and a gene signature associated with mitosis. Breast Cancer Res 13: R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Xiao Z, Meng Z, Katzenellenbogen BS (2012) Phosphorylation by p38 mitogen-activated protein kinase promotes estrogen receptor alpha turnover and functional activity via the SCF(Skp2) proteasomal complex. Mol Cell Biol 32: 1928–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blahnik KR, Dou L, O’Geen H, McPhillips T, Xu X, Cao AR, Iyengar S, Nicolet CM, Ludascher B, Korf I, Farnham PJ (2010) Sole-Search: an integrated analysis program for peak detection and functional annotation using ChIP-seq data. Nucleic Acids Res 38: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ (2006) Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126: 789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M (2005) Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122: 33–43 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M (2006) Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38: 1289–1297 [DOI] [PubMed] [Google Scholar]
- Cavailles V, Dauvois S, L’Horset F, Lopez G, Hoare S, Kushner PJ, Parker MG (1995) Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J 14: 3741–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EC, Charn TH, Park SH, Helferich WG, Komm B, Katzenellenbogen JA, Katzenellenbogen BS (2008) Estrogen Receptors alpha and beta as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol Endocrinol 22: 1032–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EC, Frasor J, Komm B, Katzenellenbogen BS (2006) Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology 147: 4831–4842 [DOI] [PubMed] [Google Scholar]
- Charn TH, Liu ET, Chang EC, Lee YK, Katzenellenbogen JA, Katzenellenbogen BS (2010) Genome-wide dynamics of chromatin binding of estrogen receptors alpha and beta: mutual restriction and competitive site selection. Mol Endocrinol 24: 47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I, Ko C, Park-Sarge OK, Nie R, Hess RA, Graves C, Katzenellenbogen BS (2001) Human estrogen receptor beta-specific monoclonal antibodies: characterization and use in studies of estrogen receptor beta protein expression in reproductive tissues. Mol Cell Endocrinol 181: 139–150 [DOI] [PubMed] [Google Scholar]
- Christian M, Kiskinis E, Debevec D, Leonardsson G, White R, Parker MG (2005) RIP140-targeted repression of gene expression in adipocytes. Mol Cell Biol 25: 9383–9391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley SM, Hoare S, Mosselman S, Parker MG (1997) Estrogen receptors alpha and beta form heterodimers on DNA. J Biol Chem 272: 19858–19862 [DOI] [PubMed] [Google Scholar]
- Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA (2003) DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 4: P3. [PubMed] [Google Scholar]
- Deroo BJ, Korach KS (2006) Estrogen receptors and human disease. J Clin Invest 116: 561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docquier A, Harmand PO, Fritsch S, Chanrion M, Darbon JM, Cavailles V (2010) The transcriptional coregulator RIP140 represses E2F1 activity and discriminates breast cancer subtypes. Clin Cancer Res 16: 2959–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhoute J, Keeton EK, Lupien M, Krum SA, Carroll JS, Brown M (2007) Positive cross-regulatory loop ties GATA-3 to estrogen receptor alpha expression in breast cancer. Cancer Res 67: 6477–6483 [DOI] [PubMed] [Google Scholar]
- Esserman LJ, Berry DA, Cheang MC, Yau C, Perou CM, Carey L, DeMuchele A, Gray JW, Conway-Dorsey K, Lenburg ME, Buxton MB, Davis SE, van't Veer LJ, Hudis C, Chin K, Wolf D, Krontiras H, Montgomery L, Tripathy D, Lehman C et al. (2012) Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat 132: 1049–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnham PJ (2009) Insights from genomic profiling of transcription factors. Nat Rev Genet 10: 605–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M (2008) Stromal gene expression predicts clinical outcome in breast cancer. Nat Medicine 14: 518–527 [DOI] [PubMed] [Google Scholar]
- Font de Mora J, Brown M (2000) AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol 20: 5041–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasor J, Chang EC, Komm B, Lin CY, Vega VB, Liu ET, Miller LD, Smeds J, Bergh J, Katzenellenbogen BS (2006) Gene expression preferentially regulated by tamoxifen in breast cancer cells and correlations with clinical outcome. Cancer Res 66: 7334–7340 [DOI] [PubMed] [Google Scholar]
- Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS (2003) Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144: 4562–4574 [DOI] [PubMed] [Google Scholar]
- Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, Orlov YL, Velkov S, Ho A, Mei PH, Chew EG, Huang PY, Welboren WJ, Han Y, Ooi HS, Ariyaratne PN, Vega VB, Luo Y, Tan PY, Choy PY et al. (2009) An oestrogen-receptor-alpha-bound human chromatin interactome. Nature 462: 58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Rosenfeld MG (2000) The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14: 121–141 [PubMed] [Google Scholar]
- Green KA, Carroll JS (2007) Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nat Rev Cancer 7: 713–722 [DOI] [PubMed] [Google Scholar]
- Grober OM, Mutarelli M, Giurato G, Ravo M, Cicatiello L, De Filippo MR, Ferraro L, Nassa G, Papa MF, Paris O, Tarallo R, Luo S, Schroth GP, Benes V, Weisz A (2011) Global analysis of estrogen receptor beta binding to breast cancer cell genome reveals an extensive interplay with estrogen receptor alpha for target gene regulation. BMC Genomics 12: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruvberger-Saal SK, Bendahl PO, Saal LH, Laakso M, Hegardt C, Eden P, Peterson C, Malmstrom P, Isola J, Borg A, Ferno M (2007) Estrogen receptor beta expression is associated with tamoxifen response in ERalpha-negative breast carcinoma. Clin Cancer Res 13: 1987–1994 [DOI] [PubMed] [Google Scholar]
- Hah N, Danko CG, Core L, Waterfall JJ, Siepel A, Lis JT, Kraus WL (2011) A rapid, extensive, and transient transcriptional response to estrogen signaling in breast cancer cells. Cell 145: 622–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA (2006) Preclinical characterization of selective estrogen receptor beta agonists: new insights into their therapeutic potential. Ernst Schering Found Symp Proc 1: 149–161 [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y (1990) More powerful procedures for multiple significance testing. Stat Med 9: 811–818 [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57 [DOI] [PubMed] [Google Scholar]
- Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS (2011) FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet 43: 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R, Orlov YL, Huss M, Sun W, Kong SL, Ukil L, Pan YF, Li G, Lim M, Thomsen JS, Ruan Y, Clarke ND, Prabhakar S, Cheung E, Liu ET (2010) Integrative model of genomic factors for determining binding site selection by estrogen receptor-alpha. Mol Syst Biol 6: 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozwik KM, Carroll JS (2012) Pioneer factors in hormone-dependent cancers. Nat Rev Cancer 12: 381–385 [DOI] [PubMed] [Google Scholar]
- Kao KJ, Chang KM, Hsu HC, Huang AT (2011) Correlation of microarray-based breast cancer molecular subtypes and clinical outcomes: implications for treatment optimization. BMC Cancer 11: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Katzenellenbogen JA (2000) Estrogen receptor transcription and transactivation: estrogen receptor alpha and estrogen receptor beta: regulation by selective estrogen receptor modulators and importance in breast cancer. Breast Cancer Res 2: 335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Katzenellenbogen JA (2002) Defining the ‘S’ in SERMs. Science 295: 2380–2381 [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Montano MM, Ediger TR, Sun J, Ekena K, Lazennec G, Martini PG, McInerney EM, Delage-Mourroux R, Weis K, Katzenellenbogen JA (2000) Estrogen receptors: selective ligands, partners, and distinctive pharmacology. Recent Prog Horm Res 55: 163–193 discussion 194–195 [PubMed] [Google Scholar]
- Kong SL, Li G, Loh SL, Sung WK, Liu ET (2011) Cellular reprogramming by the conjoint action of ERalpha, FOXA1, and GATA3 to a ligand-inducible growth state. Mol Syst Biol 7: 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi J, Otsuki T, Kunisue H, Tanaka K, Yamamoto S, Sonoo H (2000) Expression levels of estrogen receptor-alpha, estrogen receptor-beta, coactivators, and corepressors in breast cancer. Clin Cancer Res 6: 512–518 [PubMed] [Google Scholar]
- Lanz RB, Bulynko Y, Malovannaya A, Labhart P, Wang L, Li W, Qin J, Harper M, O’Malley BW (2010) Global characterization of transcriptional impact of the SRC-3 coregulator. Mol Endocrinol 24: 859–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazennec G, Bresson D, Lucas A, Chauveau C, Vignon F (2001) ER beta inhibits proliferation and invasion of breast cancer cells. Endocrinology 142: 4120–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Strom A, Li Kong S, Kietz S, Thomsen JS, Tee JB, Vega VB, Miller LD, Smeds J, Bergh J, Gustafsson JA, Liu ET (2007a) Inhibitory effects of estrogen receptor beta on specific hormone-responsive gene expression and association with disease outcome in primary breast cancer. Breast Cancer Res 9: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, Chiu KP, Lipovich L, Barnett DH, Stossi F, Yeo A, George J, Kuznetsov VA, Lee YK, Charn TH, Palanisamy N, Miller LD, Cheung E, Katzenellenbogen BS, Ruan Y et al. (2007b) Whole-genome cartography of estrogen receptor alpha binding sites. PLoS Genet 3: e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Ortiz JA, Taing L, Meyer CA, Lee B, Zhang Y, Shin H, Wong SS, Ma J, Lei Y, Pape UJ, Poidinger M, Chen Y, Yeung K, Brown M, Turpaz Y, Liu XS (2011) Cistrome: an integrative platform for transcriptional regulation studies. Genome Biol 12: R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie MC, Zou JX, Rabinovich A, Chen HW (2004) ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol Cell Biol 24: 5157–5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M (2008) FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 132: 958–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madak-Erdogan Z, Katzenellenbogen BS (2012) Aryl hydrocarbon receptor modulation of estrogen receptor alpha-mediated gene regulation by a multimeric chromatin complex involving the two receptors and the coregulator RIP140. Toxicol Sci 125: 401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madak-Erdogan Z, Lupien M, Stossi F, Brown M, Katzenellenbogen BS (2011) Genomic collaboration of estrogen receptor alpha and extracellular signal-regulated kinase 2 in regulating gene and proliferation programs. Mol Cell Biol 31: 226–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NJ, O’Malley BW (2002) Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108: 465–474 [DOI] [PubMed] [Google Scholar]
- McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, Wenger AM, Bejerano G (2010) GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol 28: 495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA (2001) Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 44: 4230–4251 [DOI] [PubMed] [Google Scholar]
- Nassa G, Tarallo R, Guzzi PH, Ferraro L, Cirillo F, Ravo M, Nola E, Baumann M, Nyman TA, Cannataro M, Ambrosino C, Weisz A (2011) Comparative analysis of nuclear estrogen receptor alpha and beta interactomes in breast cancer cells. Mol Biosyst 7: 667–676 [DOI] [PubMed] [Google Scholar]
- Nilsson S, Gustafsson JA (2011) Estrogen receptors: therapies targeted to receptor subtypes. Clin Pharmacol Ther 89: 44–55 [DOI] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA (2001) Mechanisms of estrogen action. Physiol Rev 81: 1535–1565 [DOI] [PubMed] [Google Scholar]
- Pace P, Taylor J, Suntharalingam S, Coombes RC, Ali S (1997) Human estrogen receptor beta binds DNA in a manner similar to and dimerizes with estrogen receptor alpha. J Biol Chem 272: 25832–25838 [DOI] [PubMed] [Google Scholar]
- Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC (2004) Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res 64: 423–428 [DOI] [PubMed] [Google Scholar]
- Paulmurugan R, Tamrazi A, Massoud TF, Katzenellenbogen JA, Gambhir SS (2011) In vitro and in vivo molecular imaging of estrogen receptor alpha and beta homo- and heterodimerization: exploration of new modes of receptor regulation. Mol Endocrinol 25: 2029–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell E, Shanle E, Brinkman A, Li J, Keles S, Wisinski KB, Huang W, Xu W (2012) Identification of estrogen receptor dimer selective ligands reveals growth-inhibitory effects on cells that co-express ERalpha and ERbeta. PLoS One 7: e30993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell E, Xu W (2008) Intermolecular interactions identify ligand-selective activity of estrogen receptor alpha/beta dimers. Proc Natl Acad Sci USA 105: 19012–19017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell M, Jones MC, Parker MG (2011) Role of nuclear receptor corepressor RIP140 in metabolic syndrome. Biochim Biophys Acta 1812: 919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Innes CS, Stark R, Teschendorff AE, Holmes KA, Ali HR, Dunning MJ, Brown GD, Gojis O, Ellis IO, Green AR, Ali S, Chin SF, Palmieri C, Caldas C, Carroll JS (2012) Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481: 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji S, Hirose M, Toi M (2005) Clinical significance of estrogen receptor beta in breast cancer. Cancer Chemother Pharmacol 56: Suppl 121–26 [DOI] [PubMed] [Google Scholar]
- Shin H, Liu T, Manrai AK, Liu XS (2009) CEAS: cis-regulatory element annotation system. Bioinformatics 25: 2605–2606 [DOI] [PubMed] [Google Scholar]
- Siersbaek R, Nielsen R, Mandrup S (2012) Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol Metab 23: 56–64 [DOI] [PubMed] [Google Scholar]
- Skliris GP, Leygue E, Curtis-Snell L, Watson PH, Murphy LC (2006) Expression of oestrogen receptor-beta in oestrogen receptor-alpha negative human breast tumours. Br J Cancer 95: 616–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speirs V, Carder PJ, Lane S, Dodwell D, Lansdown MR, Hanby AM (2004) Oestrogen receptor beta: what it means for patients with breast cancer. Lancet Oncol 5: 174–181 [DOI] [PubMed] [Google Scholar]
- Stender JD, Frasor J, Komm B, Chang KC, Kraus WL, Katzenellenbogen BS (2007) Estrogen-regulated gene networks in human breast cancer cells: involvement of E2F1 in the regulation of cell proliferation. Mol Endocrinol 21: 2112–2123 [DOI] [PubMed] [Google Scholar]
- Stender JD, Kim K, Charn TH, Komm B, Chang KC, Kraus WL, Benner C, Glass CK, Katzenellenbogen BS (2010) Genome-wide analysis of estrogen receptor alpha DNA binding and tethering mechanisms identifies Runx1 as a novel tethering factor in receptor-mediated transcriptional activation. Mol Cell Biol 30: 3943–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA (2004) Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci USA 101: 1566–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen CS, Berrodin TJ, Mastroeni R, Cheskis BJ, Lyttle CR, Frail DE (1998) A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. J Biol Chem 273: 27645–27653 [DOI] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH et al. (2002) A gene-expression signature as a predictor of survival in breast cancer. New Engl J Med 347: 1999–2009 [DOI] [PubMed] [Google Scholar]
- Welboren WJ, van Driel MA, Janssen-Megens EM, van Heeringen SJ, Sweep FC, Span PN, Stunnenberg HG (2009) ChIP-Seq of ERalpha and RNA polymerase II defines genes differentially responding to ligands. EMBO J 28: 1418–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Edvardsson K, Lewandowski SA, Strom A, Gustafsson JA (2008) A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene 27: 1019–1032 [DOI] [PubMed] [Google Scholar]
- Won Jeong K, Chodankar R, Purcell DJ, Bittencourt D, Stallcup MR (2012) Gene-specific patterns of coregulator requirements by estrogen receptor-alpha in breast cancer cells. Mol Endocrinol 26: 955–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O’Malley BW (2000) The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci USA 97: 6379–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wu RC, O’Malley BW (2009) Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer 9: 615–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T, Krebs AR, Choukrallah MA, Keime C, Plewniak F, Davidson I, Tora L (2011) seqMINER: an integrated ChIP-seq data interpretation platform. Nucleic Acids Res 39: e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nussbaum C, Myers RM, Brown M, Li W, Liu XS (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol 9: R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-10 and Supplementary Tables 1-3
CEAS analysis of transcription factor binding motif enrichment for binding site clusters (C1-11) identified through SeqMINER analysis.
SeqPos analysis of transcription factor binding motif enrichment for binding site clusters (C1-11) identified through SeqMINER analysis.
Functional enrichment of categories for ERβ-modulated genes.
Data Availability Statement
Gene expression and ChIP-Seq data will be available from the Gene Expression Omnibus database with the following accession numbers: meta study, GSE42349; gene expression, GSE42347; and ChIP-Seq, GSE42348.