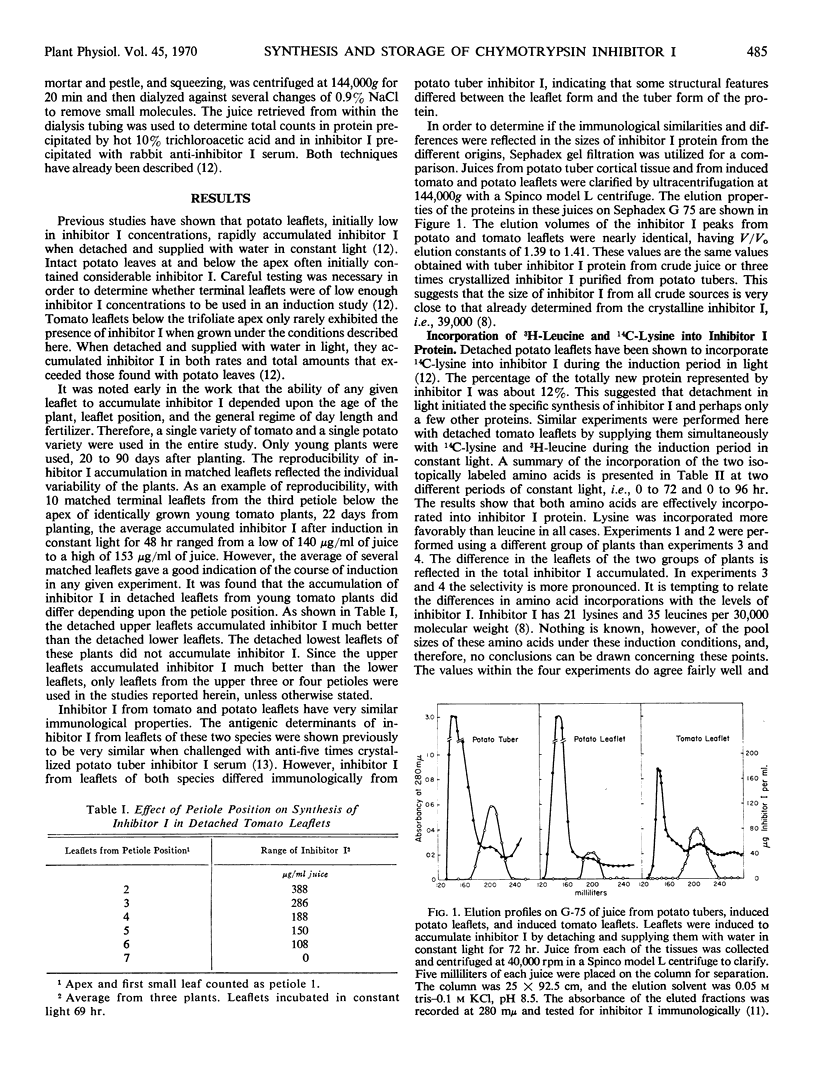
Abstract
The synthesis and accumulation of chymotrypsin inhibitor I in tomato leaflets is induced by detachment, or by destruction of petiole phloem by steam when followed by incubation of the leaflets in light. The induction process with detached tomato leaflets is similar to that found with detached potato leaflets. The large amount of inhibitor I synthesized per leaflet cell per unit time suggests either that the structural gene is redundant or that an unusually stable messenger RNA is present. In both tomato and potato leaflets the accumulation of inhibitor I is potently inhibited by actinomycin D, puromycin, and cycloheximide, but not by chloramphenicol. Indoleacetic acid is moderately inhibitory, as is 3-(3,4-dichlorophenyl)-1,1-dimethylurea. Glutamine and asparagine are both markedly stimulating. The cumulative data suggest that inhibitor I is a major depot or interim storage protein and that its existence in any particular tissue is under complex controls by both the internal and external environments of the plants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLS A. K., RYAN C. A. CONCERNING A CRYSTALLINE CHYMOTRYPTIC INHIBITOR FROM POTATOES, AND ITS BINDING CAPACITY FOR THE ENZYME. J Biol Chem. 1963 Sep;238:2976–2982. [PubMed] [Google Scholar]
- Bassham J. A., Kirk M., Jensen R. G. Photosynthesis by isolated chloroplasts. I. Diffusion of labeled photosynthetic intermediates between isolated chloroplasts and suspending medium. Biochim Biophys Acta. 1968 Jan 15;153(1):211–218. doi: 10.1016/0005-2728(68)90162-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loeb J. N., Hubby B. G. Amino acid incorporation by isolated mitochondria in the presence of cycloheximide. Biochim Biophys Acta. 1968 Oct 29;166(3):745–748. doi: 10.1016/0005-2787(68)90392-4. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Baker R. F., Yanofsky C. Translation of the tryptophan messenger RNA of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1428–1435. doi: 10.1073/pnas.60.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYAN C. A., BALLS A. K. An inhibitor of chymotrypsin from Solanum tuberosm and its behavior toward trypsin. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1839–1844. doi: 10.1073/pnas.48.10.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. An inducible protein in potato and tomato leaflets. Plant Physiol. 1968 Nov;43(11):1880–1881. doi: 10.1104/pp.43.11.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. Chymotrypsin inhibitor I from potatoes: reactivity with mammalian, plant, bacterial, and fungal proteinases. Biochemistry. 1966 May;5(5):1592–1596. doi: 10.1021/bi00869a020. [DOI] [PubMed] [Google Scholar]
- Ryan C. A., Huisman O. C. Chymotrypsin inhibitor I from potatoes: a transient protein component in leaves of young potato plants. Nature. 1967 Jun 3;214(5092):1047–1049. doi: 10.1038/2141047a0. [DOI] [PubMed] [Google Scholar]
- Ryan C. A., Huisman O. C., Van Denburgh R. W. Transitory Aspects of a Single Protein in Tissues of Solanum tuberosum and Its Coincidence With the Establishment of New Growth. Plant Physiol. 1968 Apr;43(4):589–596. doi: 10.1104/pp.43.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. Quantitative determination of soluble cellular proteins by radial diffusion in agar gels containing antibodies. Anal Biochem. 1967 Jun;19(3):434–440. doi: 10.1016/0003-2697(67)90233-3. [DOI] [PubMed] [Google Scholar]
- Ryan C. A., Shumway L. K. Differential synthesis of chymotrypsin inhibitor I in variegated leaves of a cytoplasmic mutant of tobacco (Dp1). Plant Physiol. 1970 Apr;45(4):512–514. doi: 10.1104/pp.45.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. Synthesis of chymotrypsin inhibitor I protein in potato leaflets induced by detachment. Plant Physiol. 1968 Nov;43(11):1859–1865. doi: 10.1104/pp.43.11.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff J. A., Zeldin M. H., Rubman J. Chlorophyll Formation and Photosynthetic Competence in Euglena During Light-Induced Chloroplast Development in the Presence of 3, (3,4-dichlorophenyl) 1,1-Dimethyl Urea (DCMU). Plant Physiol. 1967 Dec;42(12):1716–1725. doi: 10.1104/pp.42.12.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M., Graham D., Dwyer M. R., Grieve A., Tobin N. F. Evidence for the synthesis in vivo of proteins of the Calvin cycle and of the photosynthetic electron-transfer pathway on chloroplast ribosomes. Biochem Biophys Res Commun. 1967 Aug 23;28(4):604–610. doi: 10.1016/0006-291x(67)90356-7. [DOI] [PubMed] [Google Scholar]
- Steward F. C., Street H. E. THE SOLUBLE NITROGEN FRACTIONS OF POTATO TUBERS; THE AMIDES. Plant Physiol. 1946 Apr;21(2):155–193. doi: 10.1104/pp.21.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMSON A. R., EVELEIGH J. W., MILES B. J. AMINO-ACID SEQUENCE AROUND THE REACTIVE THIOL GROUPS OF ADENOSINE TRIPHOSPHATE--CREATINE PHOSPHOTRANSFERASE. Nature. 1964 Jul 18;203:267–269. doi: 10.1038/203267a0. [DOI] [PubMed] [Google Scholar]
- Zucker M. Induction of phenylalanine ammonia-lyase in Xanthium leaf disks. Photosynthetic requirement and effect of daylength. Plant Physiol. 1969 Jun;44(6):912–922. doi: 10.1104/pp.44.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]


