Summary
Tumors escape host immune responses, in part, through the release of immunomodulatory factors and decoy receptors into their microenvironment. Several cancers express surface-bound and soluble members of the tumor necrosis factor (TNF) receptor superfamily, including TNFRSF 11b/osteoprotegerin (OPG). In its physiologic role, OPG regulates bone remodeling through competition for osteoclast-activating cytokines and protects newly-forming bone from T cell-mediated apoptosis. In multiple tumor types, OPG production is associated with an aggressive phenotype and increased metastasis to bone, but no study has examined OPG production in human metastatic melanoma. We demonstrate that a significant proportion of human metastatic melanomas constitutively produce OPG through a mechanism governed by membrane-bound TNF-α signaling through TNF receptor 1 (TNFR1). These observations both define a specific mechanism that regulates melanoma production of OPG and establish a new molecular target for the therapeutic regulation of OPG.
Keywords: melanoma, osteoprotegerin, tumor necrosis factor-alpha, TNFR1
Introduction
Osteoprotegerin (OPG) is a soluble glycoprotein of the tumor necrosis factor (TNF) receptor superfamily (TNFRSF11b) that is expressed in a variety of normal cells and tissues, although expression is targeted to osteoarticular, immune, and vascular systems1. OPG was initially characterized as an inhibitor of osteoclastogenesis2 through its capacity to bind and neutralize the osteoclast activator molecule Receptor Activator of NF-κB Ligand (RANKL/TRANCE)3. OPG was subsequently demonstrated to bind TNF-related apoptosis-inducing ligand (TRAIL), thereby protecting newly-forming bone from apoptotic killing by T cell-derived TRAIL4. The osteo-protective role of OPG is evidenced by homozygous partial deletions of opg allele in patients with juvenile Paget’s disease, characterized by increased bone remodeling, osteopenia, and fractures5. Bone remodeling is also enhanced in OPG-knockout mice6.
OPG expression has been demonstrated in several tumors7; clinically, OPG production is associated with poor prognosis8–10, and circulating OPG was recently described as a potential serum biomarker of colon11 and pancreatic12 cancers. OPG imparts disparate effects on tumors, depending on the source of the OPG; tumor-derived OPG appears to enhance tumor growth, whereas exogenous OPG suppresses the rate of tumor growth13. OPG may serve to enhance bone metastases, although it remains unclear whether OPG mediates enhanced bone-directed migration or prolonged survival of tumors in the bone tissues14. Although melanoma commonly metastasizes to bone15, a lone study has reported the occurrence of OPG gene expression in melanoma cell lines but not melanocytes16, and no study has evaluated OPG production in melanoma cells and patient-derived tumor biopsies.
The mechanisms by which OPG regulation is modulated in melanoma are likewise unknown. The proinflammatory cytokine TNF-α has been demonstrated to drive OPG production in a variety of cell types, including fibroblasts17 and prostate cancers18. Further, signaling through TNFR1 has been implicated in the regulation of OPG17,19,20. We hypothesized that 1) OPG may be produced by human metastatic melanomas, and 2) expression of OPG may be mediated by TNF-α signaling through TNFR1. We evaluated these hypotheses using a panel of patient-derived melanoma cell lines and correlative patient isolates. Collectively, our data demonstrate that OPG production may be differentially regulated in melanoma metastases based on the tumor expression of surface TNF receptors and the availability of TNF-α in the tumor microenvironment. This variability in OPG production may necessitate customized immunotherapy regimens to achieve successful therapeutic outcomes of melanoma and other OPG-producing cancers.
Results
Human metastatic melanomas differentially produce OPG
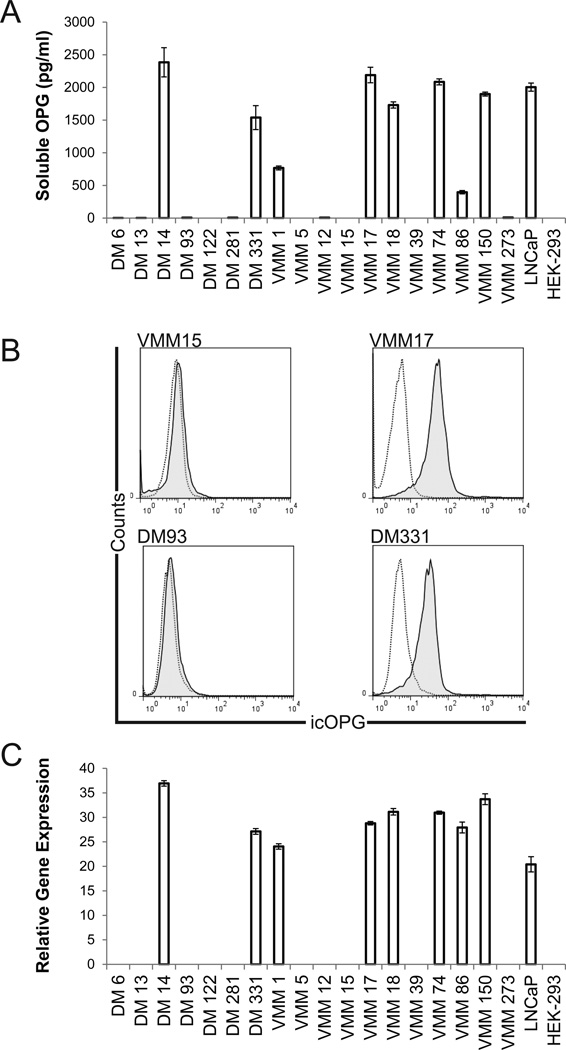
Although absent from melanocytes16, we hypothesized that metastatic human melanomas, which are known to metastasize to bone15, may produce soluble OPG. To evaluate this possibility, 18 human melanoma cell lines derived from infiltrated LN, and two control cell lines (OPG-positive prostate cancer line LNCaP18 and OPG-low human embryonic kidney cell line HEK-29321), were cultured and OPG protein secretion was measured in supernatants. Eight of 18 melanoma cell lines constitutively produced OPG (Fig. 1A), and the accumulated OPG (72h) in these cultures was at least 12-fold greater than the limit of detection for the assay system (~31 pg/ml). The presence or absence of soluble OPG in culture supernatant correlated with intracellular staining for OPG (Fig. 1B) and specific gene message (Fig. 1C), discounting the possibility that the lack of soluble OPG in some melanoma cultures was a consequence of defective extracellular transport or inefficient translation of genetic message.
Figure 1. Differential endogenous OPG status of eighteen human melanoma cell lines.
A) Some melanoma cell lines constitutively produce soluble OPG. Soluble OPG production by human melanoma cells and control (prostate and embryonic kidney) cells after 72 h culture was assessed by specific ELISA. Data are mean ±standard deviation of five replicates in a single experiment; one of 4 representative experiments shown. B) Intracellular staining for OPG correlates with production of soluble OPG. Open histograms, unstained; shaded histograms, stained with specific mAb. Staining with isotype control was not different than unstained (not shown). Data are representative intracellular staining for OPG in four melanoma cell lines, assessed after 72h in culture. Intracellular OPG was detected (>5% positive events in the tumor gate) in all samples that tested positive for OPG by ELISA (> 31pg/ml), and intracellular OPG was absent from cells that were negative for soluble OPG (<31pg/ml). Data from one of three similar experiments. C) OPG gene expression correlates with production of soluble OPG. Expression of OPG genetic message was assessed in 18 human melanoma cell lines, relative to the housekeeping gene GADPH, by real-time PCR. Data are mean ±standard deviation of three replicate measures in one of two representative experiments.
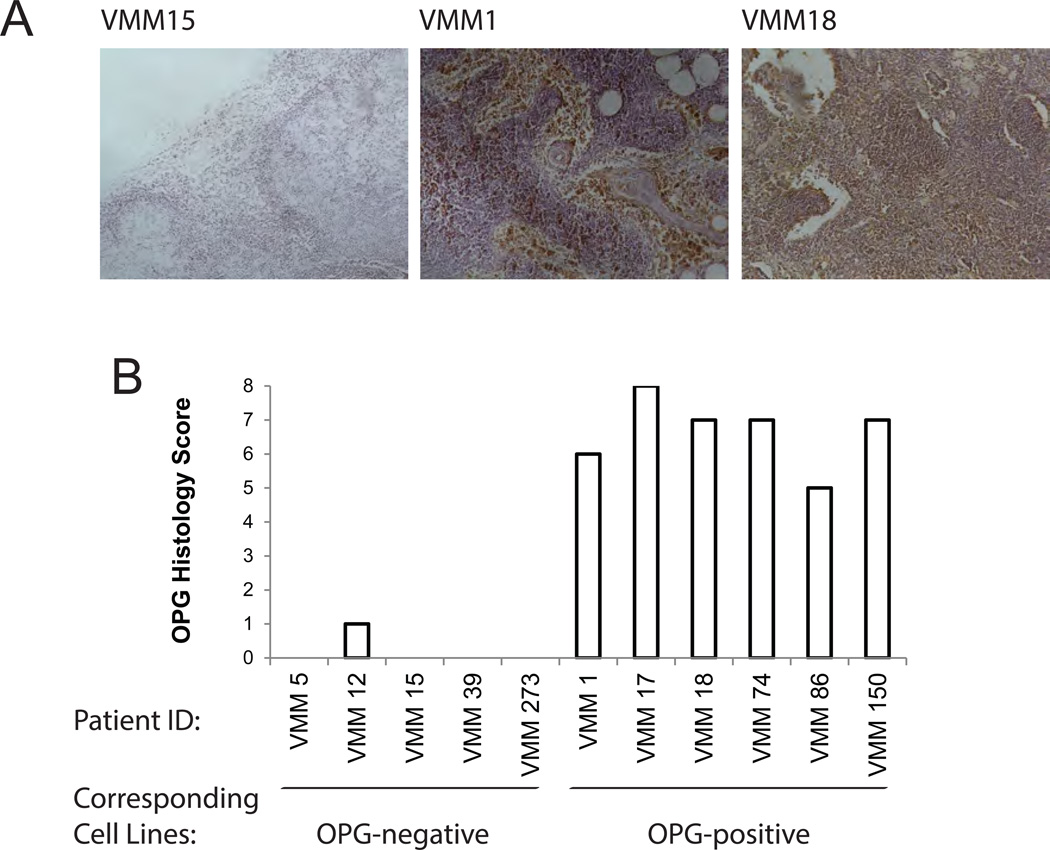
To exclude the possibility that OPG production by melanoma cell lines was an artifact of in vitro culture, OPG production was assessed directly ex vivo (Fig. 2A). OPG staining in melanoma-infiltrated LN biopsies (Fig. 2B) was concordant with protein production by the melanoma cell lines generated from the same LNs (correlation coefficient of soluble OPG and OPG immunostaining score = 0.848). We conclude that melanomas differentially produce the TNF receptor superfamily molecule OPG, in vitro and in situ, and the mechanism regulating OPG expression and secretion remains undefined.
Figure 2. Immunoreactivity of metastatic melanoma to anti-OPG mAb.
A) Representative OPG staining in melanoma-infiltrated lymph nodes biopsies. 10X magnification. B) Histology score of OPG staining for melanoma cell lines.
Melanoma OPG production in vitro is induced by the inflammatory cytokine TNF-α in cells expressing surface TNF receptor 1
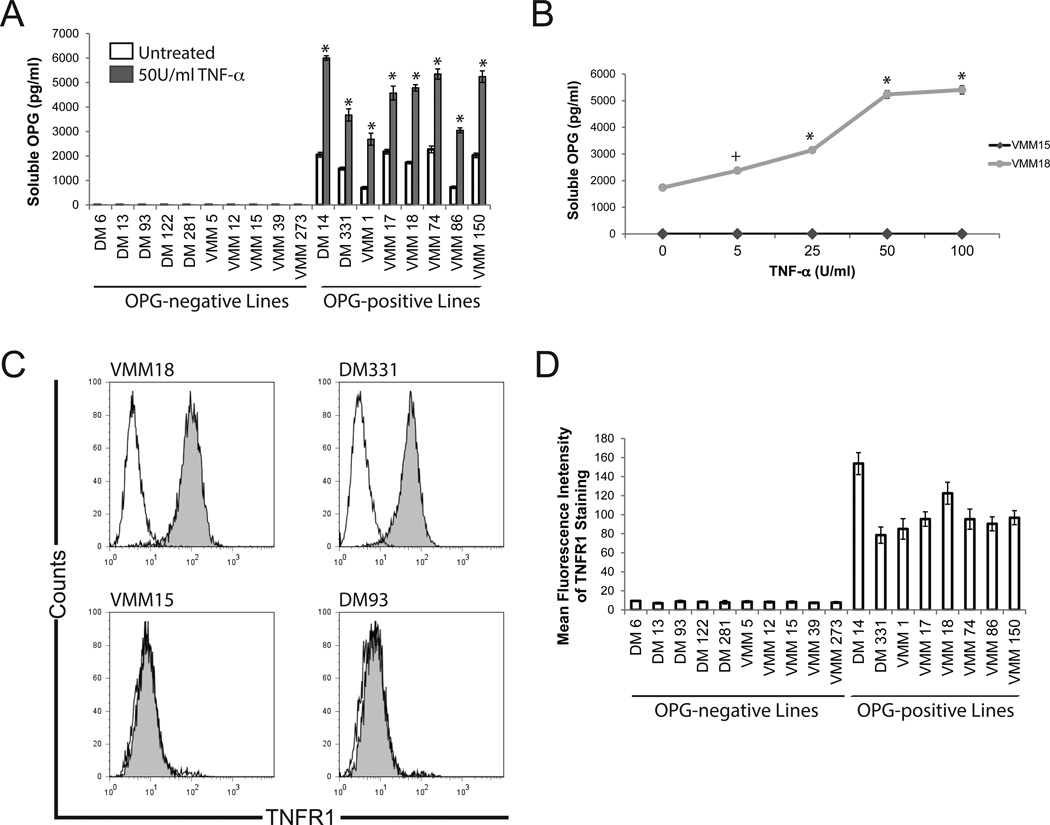
TNF-α has been demonstrated to promote the expression of OPG by synovial fibroblasts17, vascular endothelium22, and prostate cancer cell lines18. We hypothesized that TNF-α would induce or enhance melanoma OPG production. We confirmed that TNF-α induced human vascular endothelial cell (VEC) production of OPG in a dose-dependent manner (Supplemental Figure 1). We then treated melanoma cells with TNF-α (50 U/ml, optimal dose for induction of OPG in VEC). Interestingly, TNF-α significantly (p < 0.005) increased OPG production in cells previously demonstrated to constitutively secrete OPG, but TNF-α treatment failed to induce OPG in cells that do not constitutively express OPG (Fig. 3A), suggesting differential responsiveness to TNF-α signaling by various melanoma cell lines. Treatment with a range of TNF-α doses (0 – 100 U/ml) similarly enhanced, but did not induce, OPG (Fig. 3B), suggesting that the absence of OPG induction was not a consequence of TNF-α dose. We next evaluated melanoma cell lines for expression of surface TNFR1 and TNFR2. Significant staining for TNFR1 was observed exclusively on the melanoma lines previously described as constitutive producers of soluble OPG, whereas OPG-negative cell lines lacked significant surface TNFR1 staining (Fig. 3C–D). TNFR1 expression (MFI) and constitutive OPG production (lines producing >31pg/ml without treatment) were concordant (correlation coefficient = 0.831). There was no significant staining for TNFR2, regardless of the OPG production status of the melanoma cells (Supplemental Figure 2).
Figure 3. TNF-α-mediated OPG production by human melanoma cell lines.
A) TNF-α enhances but does not induce OPG production. Soluble OPG production by human melanoma cells was measured after 72 h culture by specific ELISA. Data are mean ±standard deviation of three replicates in a single experiment; one of 3 similar experiments shown. *, p<0.005 compared to untreated sample of the same cell line. B) TNF-α induces OPG production in a dose-dependent manner. Soluble OPG production was measured in response to a range of TNF-α-doses. Representative data from two cell lines; comparable dose responses were observed for the lines shown in panel A. Data are mean ±standard deviation of three replicates in a single experiment from one of three similar experiments. +, p < 0.05 compared to untreated sample. *, p<0.005 compared to untreated sample. C) OPG-producing melanoma cells express TNFR1. Representative data from one of three similar experiments. D) OPG-producing, but not OPG-negative, melanoma express surface TNFR1. Data are mean fluorescent intensity of staining on melanoma cells lines. Data are means ± standard deviation from three independent stainings in a single experiment. Data are representative of three similar experiments.
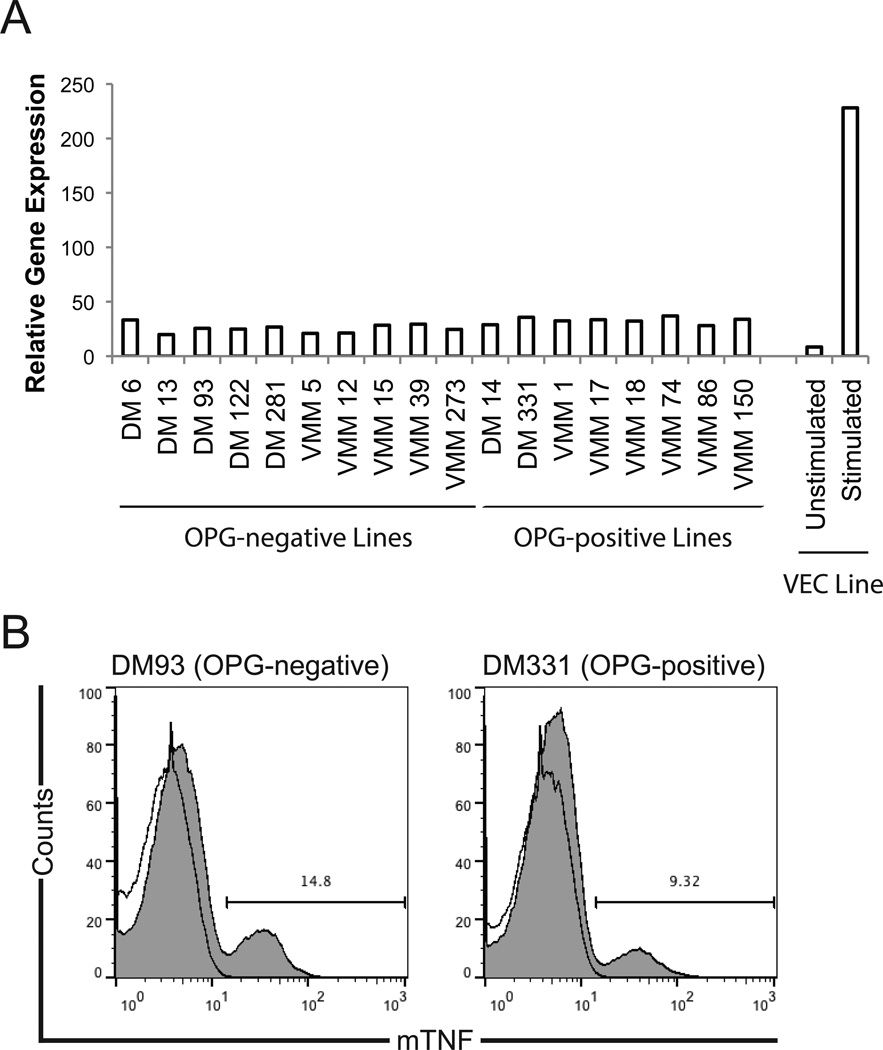
Based on these observations, we hypothesized that melanoma production of OPG may be regulated by autocrine TNF-α signaling through TNFR1. In cultured melanoma cells, soluble TNF-α was not detected in the supernatants of any melanoma cell line evaluated by specific ELISA (limit of detection ~15pg/ml; not shown); however, TNF-α message was detected in all melanoma lines tested (Fig. 4A) at levels greater than unstimulated VEC but significantly less than IFN-γ and LPS-stimulated VEC23. Therefore, we assessed melanomas expression of membrane-bound TNF-α (mTNF). Using specific mAb (clone 640124), we observed staining for mTNF on all melanoma cell lines tested (Fig. 4B), although mTNF was restricted to a subset (~9–15%) of the total cell population. Thus, melanoma cell lines constitutively express uncleaved mTNF but not soluble TNF-α protein.
Figure 4. Melanoma cells express membrane-bound TNF-α.
A) Melanoma cells express TNF-α gene message. Expression of TNF-α message was assessed by real time PCR, relative to the housekeeping gene GADPH. Representative data from one of three similar experiments are shown. B) Melanoma cells express mTNF. Open histograms, unstained; shaded histograms, stained with specific mAb. Staining with isotype control was not different than unstained (not shown). Representative data from one of three similar experiments; comparable data were obtained for all cell lines listed in Panel A.
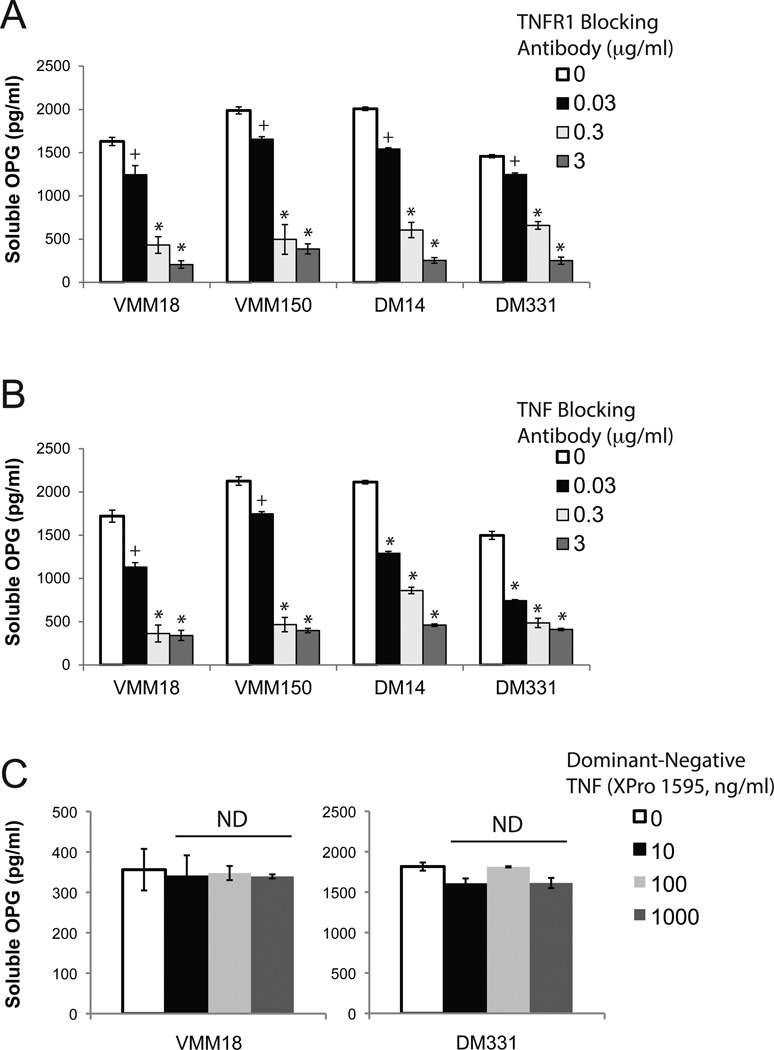
We next evaluated whether melanomas stimulate OPG production through autocrine signaling of mTNF via TNFR1. In four melanoma lines that constitutively express TNFR1 and produce OPG (VMM18, VMM150, DM14, and DM331), blockade of the TNFR1 engagement with specific mAb (clone 16805) modulated soluble OPG in a dose-dependent manner (Fig. 5A). TNF-α blocking mAb (clone 6401) also diminished OPG production in an Ab dose-dependent manner (Fig. 5B). Addition of isotype control Ab (clone 11711) did not affect OPG production (Supplemental Figure 3). To selectively inhibit soluble TNF-α, a dominant negative TNF that forms nonfunctional heterotrimers with nascent soluble TNF (XPro 1595, Xencor) was used25,26; absorption of soluble TNF-α with the dominant-negative reagent did not affect OPG production. Thus, we conclude that melanoma cells induce OPG production by autocrine signaling of mTNF through TNFR1.
Figure 5. TNF-α or TNFR1 blockade reduces OPG production by human melanoma cell lines.
A) Blocking TNFR1 reduces melanoma OPG production. Soluble OPG production by melanoma cells was assessed after 72 h culture in the presence of TNFR1-specific Ab. B) Blocking TNF-α in culture reduces melanoma OPG production. Soluble OPG production by melanoma cells was assessed after 72 h culture, in the presence of TNF-α-specific Ab. C) Absorption of soluble TNF-α using a dominant-negative construct (Xencor XPro 1595) does not alter OPG production by melanoma. In all panels, data are mean ± standard deviation of three replicates in a single experiment; one of 3 similar experiments shown. +, p < 0.05 compared to untreated sample. *, p<0.005 compared to untreated sample. ns, p > 0.05 compared to untreated sample.
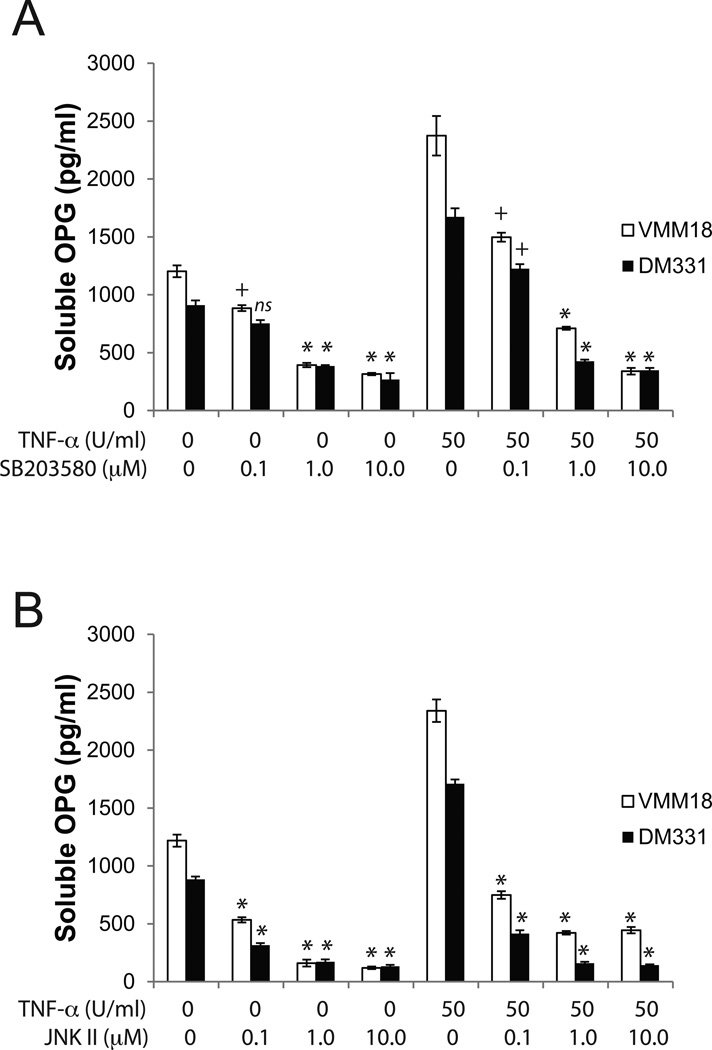
The specific signal pathways that mediate TNFR1 induction of OPG expression remain undefined. TNF-α is a strong inducer of MAPK-mediated signal transduction and p38 MAPK activation is preferentially required for maximal cytokine-induced OPG secretion in osteosarcoma cells27. Further, both p38 and c-Jun N-terminal kinase (JNK) are rapidly and potently activated in response to TNF-α28,29. Therefore, we investigated the role of MAPK pathways in the TNF-α-mediated production of OPG by melanoma cells. In a dose-dependent manner, pharmacologic inhibitors of p38 (SB203580) or JNK (JNK inhibitor II) decreased OPG production by TNFR1-expressing melanoma, even with exogenous TNF-α in the cultures (Fig. 6). At high concentrations of inhibitor, OPG production was largely ablated, although cell viability remained ~100% during the assay period (not shown). Collectively, these studies demonstrate that melanoma cell lines produce soluble OPG through engagement of TNFR1 by autocrine or exogenous TNF-α, leading to signaling through p38 and JNK and induction of OPG gene expression.
Figure 6. TNF-α-induced OPG production is mediated through the MAPK pathway.
A) Inhibition of p38-MAPK signaling with SB203580 diminishes melanoma production of OPG. B) Inhibition of JNK signaling using JNK inhibitor II suppressed melanoma production of OPG. For both panels, cells were pretreated for 1h with inhibitor then cultured for an additional 24h, and OPG assessed by ELISA. Data are mean ±standard deviation of three replicates in a single experiment; one of 3 representative experiments shown. +, p < 0.05; *, p<0.005, compared to the comparable TNF-treated sample without inhibitor.
Discussion
Although OPG expression by tumors is associated with poor clinical prognosis8–10 and a high propensity to metastasize to bone, no previous data have described the production or regulation of OPG in melanoma. We demonstrate that a subset of melanomas constitutively produce soluble OPG. OPG expression is regulated by the presence or absence of TNFR1 on melanomas, and melanomas drive OPG production in the absence of accessory cells through autocrine signaling via surface expression of mTNF. Our results demonstrate that human melanomas express mTNF, albeit in only a subset of the cell population. The mechanisms that regulate mTNF expression remain under investigation. In sum, these data demonstrate that the proinflammatory cytokine TNF-α induces other TNF superfamily molecules in melanomas and suggest pleotropic roles for TNF in the tumor microenvironment.
In the context of the inflamed tumor microenvironment, TNF-α may be produced by infiltrating immune cells30 as an anti-tumor effector molecule, and exogenous TNF-α has been used as a clinical therapy and induced partial responses in some patients31. Given that TNF-α has the capacity to induce tumor-derived OPG, which is associated with enhanced tumor survival13, these data suggest a duality in TNF-α-mediated activity in the tumor microenvironment, potentially inducing both anti- and pro-tumor effects. While TNF-α may promote immune cell activation and direct tumor lysis, our data raise the possibility that TNF-α may inadvertently lead to the induction of tumor metastasis into osseous compartments. The primary route of melanoma metastasis from the epidermis occurs via the afferent lymphatics to skin-draining lymph nodes (LN), with subsequent dissemination to other organs; this process is mediated by the chemoattraction of CXCR3-expressing melanoma into the draining lymphatics32 via the chemokine ligands CXCL9, CXCL10, and CXCL11. These molecules are upregulated in lymphatic endothelium in response inflammatory cytokines, including TNF-α43. Thus, exogenous TNF-α treatment may directly enhance metastatic capacity of melanoma and alter the microenvironment to favor tumor migration by enhancing OPG and chemokine production.
A primary physiologic function of OPG is to protect newly-forming bone from T cell mediated lysis. Through its capacity to competitively bind and sequester RANKL and TRAIL, OPG may impart regulatory activity on immune responses in non-osseous compartments. RANKL enhances DC longevity and adjuvant properties33, and OPG-mediated sequestrating of RANKL can impair T cell-mediated interactions with RANK-expressing dendritic cells (DC)34, thereby dampening immune activation. In tumors, OPG-mediated neutralization of TRAIL impairs immune-mediated clearance of neoplastic cells35,36. Thus, TNF-α may have a paradoxical effect of suppressing inflammation and immune activation in the tumor microenvironment. A two-arm clinical trial comparing combined TNF-α and IFN-γ vs. escalated TNF-α alone concluded that Stage III melanoma patients had a worse prognosis in the TNF-α only treatment arm37, and the possibility that TNF-α enhances metastatis or mediates immune suppression through OPG should be considered in the context of clinical application of TNF or TNF-inducing therapies, and recently-developed anti-TNF therapies (such as Remicade or Enbrel) should be considered to suppress OPG in the tumor microenvironment.
We also demonstrate that melanomas differentially express TNFR1. Although surface TNFR1 has been reported in melanoma19,20, no previous report has shown a differential expression of surface TNFR1 across a set of different melanoma lines. Consistent with the absence of TNFR1 expression, some melanomas have been shown to be insensitive to the presence of its ligand (TNF-α)19. The molecular mechanism that regulates melanoma TNFR1 expression or maintenance on the cell surface remains unknown and is the subject of ongoing investigation in our lab. The present data suggest that surface TNFR1 expression may be a discriminator of melanoma reactivity to immune effectors and exogenous cytokine-based therapy.
Materials and Methods
Melanoma cell lines and tissue samples
Human melanoma cell lines generated at Duke University (DM6, DM13, DM14, DM93, DM122, DM281, and DM331) and the University of Virginia (VMM1, VMM5, VMM12, VMM15, VMM17, VMM18, VMM39, VMM74, VMM86, VMM150, and VMM273) were provided by Dr. Craig L. Slingluff, Jr. (Department of Surgery, University of Virginia School of Medicine). These lines were established from melanoma-infiltrated LN, as described38–40. Although not authenticated by SNP analysis, all melanoma cell lines were verified for expression of melanocyte differentiation proteins (tyrosinase, gp100, and Mart-1) and monitored for HLA status to ensure consistency of the populations (not shown); these internal validations were most recently performed within the 3 months preceding experimentation. LNCaP prostate cell line (CRL-1740) and HEK-293 cell line (CRL-1573) were obtained from ATCC and passaged less than 6 times. Unless noted, tumor cells were cultured in RPMI-1640 (Cellgro/Mediatech) supplemented with FBS (HyClone), HEPES-buffered saline solution (Gibco), and L-glutamine (Gibco). Human VEC were obtained from Lonza Biologicals, maintained in endothelial cell growth media (Lonza), and passaged less than 6 times. All cell lines were maintained in 75cm2 flasks (Corning) in a humidified 5% CO2 environment at 37 °C, and medium was changed twice weekly. For assays, subconfluent cells were trypsinized (Gibco, 10% solution in HBSS), washed, and seeded at the indicated numbers into sterile flat-bottom tissue-culture plates (Corning).
Measurement of osteoprotegerin secretion in vitro
Melanoma cells were seeded in 12-well tissue culture plates at a density of 1.0 × 106 cells/well. In some cultures, medium was supplemented with TNF-α (0–100 U/ml, R&D Systems), as indicated. OPG concentration was evaluated in supernatant by specific ELISA (R&D Systems).
To evaluate the role of cytokines in stimulating melanoma OPG production, melanoma cells were plated in 24-well plates at a density of 100,000 cells/well and incubated overnight. Cell cultures were supplemented with a dose-range of blocking antibodies (0.03–3.0 µg/ml) against TNF-α (clone 6401, R&D Systems), TNFR1 (clone 16805, R&D Systems), or isotype controls (mouse IgG1, clone 11711, R&D Systems) at 24-hour (h) intervals. To selectively inhibit solTNF, melanomas were supplemented with a dominant negative TNF that forms nonfunctional heterotrimers with nascent solTNF (XPro 1595, generous gift from Xencor, Inc.)25,26. Supernatant for assay was collected at 72-h and OPG measured by specific ELISA, per manufacturer’s instructions.
To assess the impact of specific kinase inhibitors on melanoma OPG production, melanoma cells were plated as described. Media was replaced with serum-free RPMI-1640 for a minimum of 12-h, then cultures were supplemented with TNF-α for 24 h. Prior to cytokine exposure, some culture wells were pretreated for 1-h with serum-free RPMI-1640 supplemented with various concentrations of pharmacologic inhibitors of MAPK (SB203580, InvivoGen) or c-Jun N-terminal kinase (JNK; JNK inhibitor II, EMD/Millipore). After treatment, the viability of melanoma cells was assessed using alamarBlue (Invitrogen), as described41.
OPG expression in situ
Paraffin-embedded tissue sections of melanoma tumors were obtained from the Department of Surgery and Department of Pathology, University of Virginia School of Medicine, under Human Investigation Committee Protocol 10598. Sections were stained for OPG using mouse anti-human OPG mAb (R&D Systems clone 69146, 5µg/ml) and an HRP-DAB cell-staining system (R&D Systems), then counterstained with Mayer’s Hematoxylin solution (Sigma-Aldrich). The samples were mounted using Permount (Fisher Scientific) and photographed with a Zeiss AxioScope A2 and MRc5 image acquisition system. The stained tissues were scored based on intensity and proportion of staining. Intensity was scored on a scale of 0 (negative), 1 (weak), 2 (moderate), and 3 (strong), whereas proportion was scored on a scale of 0 (0%), 1 (0.1%–1%), 2 (2%–10%), 3 (11%–33%), 4 (34%–66%), and 5 (67%–100%). A cumulative staining score ranging from 0 to 8 was obtained by combining the intensity and proportion scores.
Intracellular Staining for OPG protein
Melanoma cells were permeabilized using eBioscience FoxP3 intracellular staining reagents, per the manufacturer’s recommendation. OPG was assessed using a monoclonal antibody (1µg/106 cells, R&D Systems clone 69127) and specific fluorochrome-conjugated secondary reagent (Jackson ImmunoResearch). Stained cells were analyzed using a Becton Dickenson FacsCalibur.
Real Time-PCR evaluation of OPG and TNF-α gene expression
Total RNA from human melanoma cell lines was isolated with a spin column-based system, per manufacturer’s instructions (Qiagen). Purified RNA was evaluated by agarose gel electrophoresis and quantified spectrophotometrically using a NanoDrop 1000. Total RNA was converted to first-strand cDNA by reverse transcriptase (SuperScript III, Invitrogen), and gene-specific cDNA content determined by ABI TaqMan chemistry with SYBR Green reporter dye on a ABI PRISM 7900HT instrument.
A 220 bp cDNA fragment of human OPG was amplified using primer pairs (Sigma Genosys) modified from Penno et al.18 and verified against NCBI GenBank: Pair 1: 5’-GAA CCC CAG AGC GAA ATA CAG-3’ and 5’-AAG AAT GCC TCC TCA CAC AGG-3’, Pair 2: 5’-GGC AAC ACA GCT CAC AAG AA-3’ and 5’-CTG GGT TTG CAT GCC TTT AT-3’. Primers for real-time analysis of human TNF-α (Hs01113624_g1) were obtained from Applied Biosystems.
The amplification reaction consisted of denaturation at 95°C for 30 seconds (s), annealing at 52°C for 30s, and elongation at 72°C for 90s, for 40 cycles. To verify that only a single PCR product was amplified per transcript, dissociation curve data was analyzed through the 7900HT Sequence Detection Software (SDS). To account for differences in starting material, quantitative PCR was also carried out for each cDNA sample using housekeeping gene-specific primers (human GAPDH and β-actin) synthesized at the University of Virginia Biomolecular Research Core Facility. The relative amount of target and housekeeping gene in each sample was determined using the comparative Ct method.
Membrane TNF and surface TNF receptor analysis
To evaluate the expression of mTNF and surface TNF receptors, subconfluent melanomas were stained with specific mAbs (α-TNF clone 6401 and α-TNFR1 clone 16803, R&D Systems; α-TNFR2 clone hTNFRm1, BD Biosciences) and evaluated using a Becton Dickenson FacsCalibur.
Statistics
Quantitative protein expression (ELISA) data are reported as the mean ± standard deviation of triplicate determinations in a single experiment, and representative data from multiple experiments are shown. For comparison of ELISA data, two-tailed Student’s t test or Mann-Whitney rank sum test was performed using MiniTab 16 Software.
Supplementary Material
Significance.
Production of the tumor necrosis factor receptor superfamily member osteoprotegerin (OPG) is a poor prognostic in several cancers. No study has evaluated the production or regulation of OPG in human metastatic melanoma. We demonstrate that OPG production may be differentially regulated in melanoma metastases, based on the presence or absence of surface TNF receptors. Further, we demonstrate that membrane-bound TNF-α drives OPG production, suggesting a potential therapeutic target.
Acknowledgments
Support for these studies was provided by: The David A. Harrison Undergraduate Research Fund (JLO); Sigma Xi Grants-in-Aid of Research (JLO); Virginia Academy of Science (JLO); the Melanoma Research Alliance (DMW); and USPHS R01 CA134799 (DWM). We thank Dr. Kenneth S.K. Tung (Department of Pathology, University of Virginia) for assistance with interpretation and scoring of histology specimens. We thank Dr. David E. Szymkowski (Xencor, Inc.) for guidance on the use of Xpro 1595. We appreciate the insightful comments of Drs. Constance Brinckerhoff and Molly Harris-Jenkins (Departments of Medicine and Biochemistry, Geisel School of Medicine at Dartmouth).
Footnotes
Conflicts of Interest: None
References
- 1.Theoleyre S, et al. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine & Growth Factor Reviews. 2004;15:457–475. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Simonet WS, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 3.Yasuda H, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emery JG, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. The Journal of Biological Chemistry. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 5.Whyte MP, et al. Osteoprotegerin deficiency and juvenile Paget’s disease. The New England Journal of Medicine. 2002;347:175–184. doi: 10.1056/NEJMoa013096. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa T, et al. Animal models for bone and joint disease. Bone disease of osteoprotegerin deficient mouse. Clinical Calcium. 2011;21:190–196. [PubMed] [Google Scholar]
- 7.Holen I, Shipman CM. Role of osteoprotegerin (OPG) in cancer. Clinical Science. 2006;110:279–291. doi: 10.1042/CS20050175. [DOI] [PubMed] [Google Scholar]
- 8.Ito R, et al. Expression of osteoprotegerin correlates with aggressiveness and poor prognosis of gastric carcinoma. Virchows Archiv: an International Journal of Pathology. 2003;443:146–151. doi: 10.1007/s00428-003-0845-8. [DOI] [PubMed] [Google Scholar]
- 9.Jung K, et al. Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. International Journal of Cancer. 2004;111:783–791. doi: 10.1002/ijc.20314. [DOI] [PubMed] [Google Scholar]
- 10.Mizutani Y, et al. Prognostic significance of serum osteoprotegerin levels in patients with bladder carcinoma. Cancer. 2004;101:1794–1802. doi: 10.1002/cncr.20550. [DOI] [PubMed] [Google Scholar]
- 11.Tsukamoto S, et al. Clinical significance of osteoprotegerin expression in human colorectal cancer. Clinical Cancer Research. 2011;17:2444–2450. doi: 10.1158/1078-0432.CCR-10-2884. [DOI] [PubMed] [Google Scholar]
- 12.Brand RE, et al. Serum biomarker panels for the detection of pancreatic cancer. Clinical Cancer Research. 2011;17:805–816. doi: 10.1158/1078-0432.CCR-10-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher JL, et al. Osteoprotegerin overexpression by breast cancer cells enhances orthotopic and osseous tumor growth and contrasts with that delivered therapeutically. Cancer Research. 2006;66:3620–3628. doi: 10.1158/0008-5472.CAN-05-3119. [DOI] [PubMed] [Google Scholar]
- 14.Fili S, Karalaki M, Schaller B. Mechanism of bone metastasis: the role of osteoprotegerin and of the host-tissue microenvironment-related survival factors. Cancer Letters. 2009;283:10–19. doi: 10.1016/j.canlet.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. American Journal of Surgery. 1978;135:807–810. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XD, et al. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Research. 1999;59:2747–2753. [PubMed] [Google Scholar]
- 17.Kubota A, Hasegawa K, Suguro T, Koshihara Y. Tumor necrosis factor-alpha promotes the expression of osteoprotegerin in rheumatoid synovial fibroblasts. The Journal of Rheumatology. 2004;31:426–435. [PubMed] [Google Scholar]
- 18.Penno H, et al. Osteoprotegerin secretion from prostate cancer is stimulated by cytokines, in vitro. Biochemical and Biophysical Research Communications. 2002;293:451–455. doi: 10.1016/S0006-291X(02)00242-5. [DOI] [PubMed] [Google Scholar]
- 19.Thomas WD, Hersey P. CD4 T cells kill melanoma cells by mechanisms that are independent of Fas (CD95) International Journal of Cancer. 1998;75:384–390. doi: 10.1002/(sici)1097-0215(19980130)75:3<384::aid-ijc10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Carrel S, et al. Expression of type A and B tumor necrosis factor (TNF) receptors on melanoma cells can be regulated by dbc-AMP and IFN gamma. International Journal of Cancer. 1995;62:76–83. doi: 10.1002/ijc.2910620115. [DOI] [PubMed] [Google Scholar]
- 21.Delgado-Calle J, et al. Role of DNA methylation in the regulation of the RANKL-OPG system in human bone. Epigenetics. 2012;7 doi: 10.4161/epi.7.1.18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olesen P, Ledet T, Rasmussen LM. Arterial osteoprotegerin: increased amounts in diabetes and modifiable synthesis from vascular smooth muscle cells by insulin and TNF-alpha. Diabetologia. 2005;48:561–568. doi: 10.1007/s00125-004-1652-8. [DOI] [PubMed] [Google Scholar]
- 23.Ranta V, et al. Human vascular endothelial cells produce tumor necrosis factor-alpha in response to proinflammatory cytokine stimulation. Critical Care Medicine. 1999;27:2184–2187. doi: 10.1097/00003246-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Liu D, et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. The Journal of Clinical Investigation. 2012;122:2221–2233. doi: 10.1172/JCI59535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steed PM, et al. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science. 2003;301:1895–1898. doi: 10.1126/science.1081297. [DOI] [PubMed] [Google Scholar]
- 26.Zalevsky J, et al. Dominant-negative inhibitors of soluble TNF attenuate experimental arthritis without suppressing innate immunity to infection. Journal of Immunology. 2007;179:1872–1883. doi: 10.4049/jimmunol.179.3.1872. [DOI] [PubMed] [Google Scholar]
- 27.Pantouli E, Boehm MM, Koka S. Inflammatory cytokines activate p38 MAPK to induce osteoprotegerin synthesis by MG-63 cells. Biochemical and Biophysical Research Communications. 2005;329:224–229. doi: 10.1016/j.bbrc.2005.01.122. [DOI] [PubMed] [Google Scholar]
- 28.Dérijard B, et al. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 29.Lin A, et al. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 30.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Reviews. 2006;25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 31.Lejeune FJ, Liénard D, Matter M, Rüegg C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immunity. 2006;6:6. [PubMed] [Google Scholar]
- 32.Kawada K, et al. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Research. 2004;64:4010–4017. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- 33.Josien R, et al. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. The Journal of Experimental Medicine. 2000;191:495–502. doi: 10.1084/jem.191.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong BR, Josien R, Choi Y. TRANCE is a TNF family member that regulates dendritic cell and osteoclast function. Journal of Leukocyte Biology. 1999;65:715–724. doi: 10.1002/jlb.65.6.715. [DOI] [PubMed] [Google Scholar]
- 35.Sheikh MS, Fornace AJ. Death and decoy receptors and p53-mediated apoptosis. Leukemia. 2000;14:1509–1513. doi: 10.1038/sj.leu.2401865. [DOI] [PubMed] [Google Scholar]
- 36.Zauli G, Melloni E, Capitani S, Secchiero P. Role of full-length osteoprotegerin in tumor cell biology. Cellular and Molecular Life Sciences. 2009;66:841–851. doi: 10.1007/s00018-008-8536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaglini M, et al. Treatment of in-transit metastases from cutaneous melanoma by isolation perfusion with tumour necrosis factor-alpha (TNF-alpha), melphalan and interferon-gamma (IFN-gamma). Dose-finding experience at the National Cancer Institute of Milan. Melanoma Research. 1994;4(Suppl 1):35–38. [PubMed] [Google Scholar]
- 38.Darrow TL, Slingluff CL, Seigler HF. The role of HLA class I antigens in recognition of melanoma cells by tumor-specific cytotoxic T lymphocytes. Evidence for shared tumor antigens. Journal of Immunology. 1989;142:3329–3335. [PubMed] [Google Scholar]
- 39.Hogan KT. Identification of Novel and Widely Expressed Cancer/Testis Gene Isoforms That Elicit Spontaneous Cytotoxic T-Lymphocyte Reactivity to Melanoma. Cancer Research. 2004;64:1157–1163. doi: 10.1158/0008-5472.can-03-2209. [DOI] [PubMed] [Google Scholar]
- 40.Yamshchikov GV, et al. Sequential immune escape and shifting of T cell responses in a long-term survivor of melanoma. Journal of Immunology. 2005;174:6863–6871. doi: 10.4049/jimmunol.174.11.6863. [DOI] [PubMed] [Google Scholar]
- 41.Mullins DW, Walker TM, Burger CJ, Elgert KD. Taxol-mediated changes in fibrosarcoma-induced immune cell function: modulation of antitumor activities. Cancer Immunology, Immunotherapy. 1997;45:20–28. doi: 10.1007/s002620050396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.