Abstract
Background
Recent initiatives have focused on primary prevention to delay time to first myocardial infarction (MI). The aim of this study was to evaluate the change in risk factor profile over time in patients without known cardiovascular disease presenting with first MI.
Methods
In the American Heart Association's Get With The Guidelines–Coronary Artery Disease national registry, 100,884 patients without known cardiovascular disease presenting with acute MI from 408 hospitals were evaluated between 2002 and 2008. The time trends of the proportion of patients with cardiovascular risk factors (nonmodifiable: age >45 years for men or >55 years for women, male sex, modifiable: diabetes mellitus, hypertension, hyperlipidemia, tobacco use) were analyzed. Analyses were stratified by non–ST-segment elevation MI (NSTEMI) versus ST-segment elevation MI (STEMI).
Results
The proportion of patients with ≥3 of 6 traditional risk factors slightly decreased over time in the NSTEMI (69.5%-66.8%, P < .0001) and STEMI (68.9%-66.4%, P < .0001) cohorts. The proportion of patients with ≥2 of 4 modifiable risk factors increased from 52% to 59% and then declined to 52.1% (P < .0001) in the NSTEMI cohort but declined slightly in the STEMI cohort (50.9%-47.3%, P < .0001). After adjusting for age and gender, the time trend of proportion with diabetes mellitus, hypertension, and tobacco use declined in both cohorts. However, the proportion of patients with hyperlipidemia remained similar.
Conclusions
Although risk factor profiles in patients presenting with first MI have shown improvements over time, the changes are modest.
Over recent years, greater focus has been placed on primary prevention of cardiovascular disease. Large epidemiological studies have identified cardiovascular risk factors and development of global risk scores designed to predict the risk of coronary artery disease (CAD) in primary care offices.1-4 Identification of these cardiovascular risk factors has led to national guidelines aimed at improving the modifiable risk factors with lifestyle changes and pharmacologic therapy in patients at high risk for developing a cardiovascular event.5,6 The American Heart Association (AHA) took on a global approach to primary prevention with their publication of guidelines addressing how best to evaluate, manage, and follow-up people at risk for developing cardiovascular disease.7 The addition of community-based initiatives, such as the AHA's Life's Simple 7 program and increased public policy efforts to decrease exposure to first- and second-hand smoking, further aimed to delay a first cardiovascular event in individuals without known disease.8
Herein, we aim to use the AHA's Get With The Guidelines (GWTG)–CAD registry to provide some insight into the change in risk factor profile over time of patients without known cardiovascular disease presenting with their first episode of myocardial infarction (MI).
Methods
Study cohort
Data were collected from a total of 276,540 patients in 435 hospitals in the United States participating in the AHA's GWTG-CAD national registry between January 1, 2002, and December 31, 2008. The details of the registry have been described previously.9,10 Participation in this registry is voluntary and requires hospitals to submit clinical information on consecutive patients hospitalized for CAD using an online, interactive case report form and Patient Management Tool (Outcome Sciences, Inc, Cambridge, MA). Participating hospitals represent all regions of the country, including rural and urban settings and academic and community-based centers. Outcome Sciences, Inc, serves as the data collection and coordination center for GWTG. The Duke Clinical Research Institute (DCRI) serves as the data analysis center and has institutional review board approval to analyze the aggregate deidentified data for research purposes.
In the current study, patients with known CAD, MI, percutaneous coronary intervention, coronary artery bypass graft surgery, stroke, transient ischemic attack, or peripheral artery disease (n = 106,051) and those not presenting with an acute MI (n = 68,880) or with inconsistent data on diagnosis of non–ST-segment elevation MI (NSTEMI) versus ST-segment elevation MI (STEMI) (n = 725) were excluded. The final study cohort consisted of 100,884 patients without known cardiovascular disease presenting with first episode of MI at 408 hospitals (204 sites 2002-2003, 329 sites 2004-2006, 195 sites 2007-2008).
Measures
The primary measure of interest was a high-risk factor profile, defined as the presence of ≥3 of 6 traditional cardiovascular risk factors (age >45 years for men or >55 years for women, male sex, diabetes mellitus [DM], hypertension, hyperlipidemia, and tobacco use). Reported medical history defined the presence of DM, hypertension, and hyperlipidemia, whereas use of tobacco within the past 12 months defined tobacco use. Secondary measures of interest included a high modifiable risk factor profile, defined as ≥2 of 4 modifiable risk factors (DM, hypertension, hyperlipidemia, and tobacco use), and each individual risk factor. Primary and secondary measures were stratified by presentation of NSTEMI versus STEMI. All risk factors were collected since 2002, with the exception of hyperlipidemia, which was collected starting in 2003.
Statistical analyses
Baseline characteristics were compared across the 3 periods, 2002 to 2003, 2004 to 2006, and 2007 to 2008, in the NSTEMI and STEMI cohorts. Median (25th-75th percentiles) and percentages were reported to describe the distribution of continuous and categorical variables. Cochran-Mantel-Haenszel test with nonzero correlation statistic was used for continuous variables, and Cochran-Mantel-Haenszel test with row mean score statistic, for categorical variables. Logistic regression analyses were performed for binary variables, and regression analyses, for continuous variables. Generalized estimating equations were used to account for within-hospital clustering, and variables in the model included calendar year, patient groups (NSTEMI vs STEMI), and the interaction between calendar year and patient groups. For the modifiable risk factors, multivariable logistic regression analyses were performed with adjustment variables, age and gender, included in the regression model. The interaction term was tested to evaluate whether the time trend on risk factor differs between NSTEMI and STEMI cohorts.
All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). All P values were 2 sided, with P < .05 considered statistically significant.
Dr Shah received the AHA Young Investigator Database Seed Grant in support of the data analysis presented and was partially funded by an NIH/NHLBI grant (T32HL098129 in 2012; UL1 TR000038 in 2013). Data analysis was performed by the GWTG Data Coordinating Center at the DCRI. The authors are solely responsible for design and conduct of this study, all study analyses, and drafting and editing of the manuscript, and its final contents.
Results
Study population
Over time, 20.3% of the cohort (n = 20,465) presented between 2002 and 2003; 52.3% (n = 52,763), between 2004 and 2006; and 27.41% (n = 27,656), between 2007 and 2008. Of the 100,884 patients in the total cohort, 36.9% (n = 37,265) presented with an STEMI, and 63.1% (n = 63,619), as an NSTEMI.
Baseline characteristics
Baseline characteristics, stratified by type of MI and time of presentation (2002-2003, 2004-2006, 2007-2008), are shown in Table I. In the NSTEMI cohort, patients with a first manifestation of cardiovascular disease were of similar median age from 2002 to 2008 (median 67, 66, and 66 years, P = .11). However, when categorized by gender-stratified age thresholds traditionally used in the risk factor definition, the proportion of men >45 years old and women >55 years old decreasedslightly over time (87.5%-86.1%, P = .0003). In the STEMI cohort, patients with a first manifestation of cardiovascular disease were younger from 2002 to 2008 (median age 61 years, 60 years, 59 years, P < .0001) and demonstrated a similar trend when categorized by gender-stratified age thresholds (83.9%-81.7%, P = .0001).
Table I.
Baseline characteristics
| NSTEMI |
STEMI |
|||||||
|---|---|---|---|---|---|---|---|---|
| 2002-2003 (n = 12,945) | 2004-2006 (n = 33,671) | 2007-2008 (n = 17,003) | P * | 2002-2003 (n = 7520) | 2004-2006 (n = 19,092) | 2007-2008 (n = 10,653) | P * | |
| Age (y) | 67 (56-78) | 66 (55-78) | 66 (55-78) | .11 | 61 (52-73) | 60 (51-72) | 59 (51-70) | <.0001 |
| Age >45 y (men) or >55 y (women) (%) | 87.5 | 87.0 | 86.1 | .0003 | 83.9 | 83.0 | 81.7 | .0001 |
| Male sex (%) | 60.1 | 58.9 | 55.2 | <.0001 | 67.1 | 67.5 | 67.4 | .0001 |
| Body mass index (kg/m2) | 27 (24-32) | 28 (24-32) | 28 (24-32) | <.0001 | 27 (25-31) | 28 (25-31) | 28 (25-32) | <.0001 |
| Obesity (%) | 33.0 | 34.6 | 37.4 | <.0001 | 32.8 | 33.5 | 36.5 | <.0001 |
| Race (%) | <.0001 | <.0001 | ||||||
| White | 75.5 | 72.4 | 74.6 | 76.8 | 74.3 | 76.4 | ||
| Black | 6.2 | 7.2 | 7.8 | 5.4 | 5.9 | 5.8 | ||
| Hispanic | 7.9 | 8.2 | 5.8 | 6.9 | 7.9 | 6.2 | ||
| Asian | 3.1 | 3.7 | 4.2 | 3.1 | 3.4 | 3.0 | ||
| Insurance payors (%) | <.0001 | <.0001 | ||||||
| Medicare | 44.5 | 32.3 | 35.8 | 34.2 | 23.9 | 24.3 | ||
| Medicaid | 3.9 | 6.9 | 5.7 | 3.2 | 5.4 | 4.7 | ||
| Other | 28.4 | 50.0 | 44.5 | 32.9 | 53.2 | 49.0 | ||
| None | 4.3 | 9.3 | 6.6 | 7.6 | 15.6 | 11.9 | ||
| Medical history (%) | ||||||||
| Hypertension | 68.2 | 66.3 | 63.1 | <.0001 | 62.2 | 57.0 | 52.1 | <.0001 |
| Hyperlipidemia | 22.9 | 46.0 | 41.1 | <.0001 | 23.3 | 44.1 | 37.1 | <.0001 |
| DM | 33.1 | 31.0 | 26.6 | <.0001 | 27.3 | 24.0 | 19.4 | <.0001 |
| Heart failure | 13.2 | 13.4 | 8.5 | <.0001 | 8.1 | 6.6 | 3.1 | <.0001 |
| Renal insufficiency | 9.8 | 8.6 | 6.0 | <.0001 | 5.5 | 4.2 | 2.0 | <.0001 |
| None | 10.8 | 11.7 | 16.9 | <.0001 | 16.3 | 19.4 | 27.5 | <.0001 |
| Tobacco use (%) | 30.4 | 29.9 | 29.8 | .292 | 40.2 | 41.9 | 42.5 | . 003 |
| LDL cholesterol (mg/dL) | 106 (83-133) | 103 (80-130) | 102 (78-129) | <.0001 | 108 (85-135) | 106 (83-133) | 106 (82-131) | .0004 |
| HDL cholesterol (mg/dL) | 39 (32-47) | 37 (30-46) | 37 (31-46) | <.0001 | 39 (32-47) | 36 (30-44) | 36 (30-44) | <.0001 |
| Triglycerides (mg/dL) | 130 (89-193) | 125 (86-186) | 120 (83-179) | <.0001 | 128 (90-190) | 126 (87-186) | 126 (85-183) | .0100 |
| Ejection fraction <40% (%) | 16.9 | 17.5 | 17.1 | <.0001 | 21.0 | 21.3 | 21.4 | <.0001 |
Continuous data are presented as median (interquartile range).
Trend was tested using Cochran-Mantel-Haenszel with nonzero correlation statistic and with row mean score statistic for continuous and categorical variables, respectively.
Evaluation of metabolic profile showed that the prevalence of obesity (BMI ≥30 kg/m2) increased over time in both the NSTEMI (33.0%-37.4%, P < .0001) and STEMI (32.8%-36.5%, P < .0001) cohorts. In the current study, data on lipid profile was missing in almost one-third and one-fourth of the NSTEMI and STEMI cohorts, respectively, in each period. However, of the data available, the median low-density lipoprotein (LDL) cholesterol level decreased 4 mg/dL over the period of the study, from 106 (83-133) mg/dL to 102 (78-129) mg/dL (P < .0001) in the NSTEMI cohort, and only 2 mg/dL, from 108 (85-135) to 106 (82-131) mg/dL (P = .0004) in the STEMI cohort. Similarly, the median high-density lipoprotein (HDL) cholesterol level decreased 2 mg/dL (39 [32-47] mg/dL to 37 [31-46] mg/dL, P < .0001) and 3 mg/dL (39 [32-47] to 36 [30-44] mg/dL, P < .0001) in the NSTEMI and STEMI cohorts, respectively.
Traditional cardiovascular risk factors
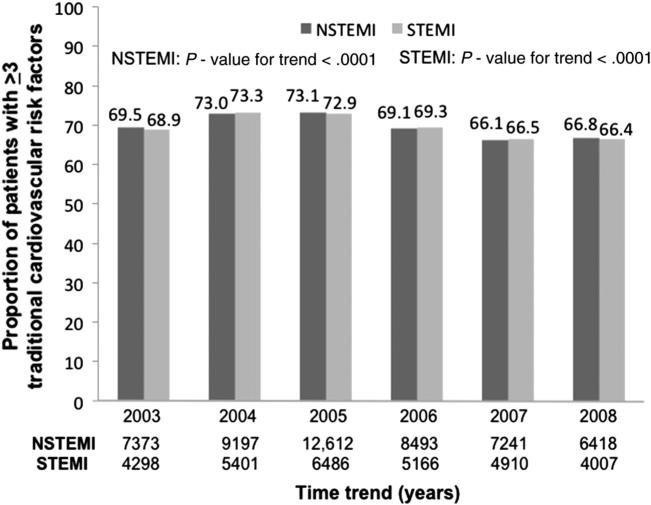
The proportion of patients with ≥3 traditional cardiovascular risk factors decreased over time in both the NSTEMI and STEMI cohorts (Figure 1). The number of traditional cardiovascular risk factors present is also evaluated by year (online Appendix Supplementary Table).
Figure 1.
Time trend of proportion of patients with ≥3 traditional cardiovascular risk factors in the NSTEMI and STEMI cohorts (significance presented as P value for trend).
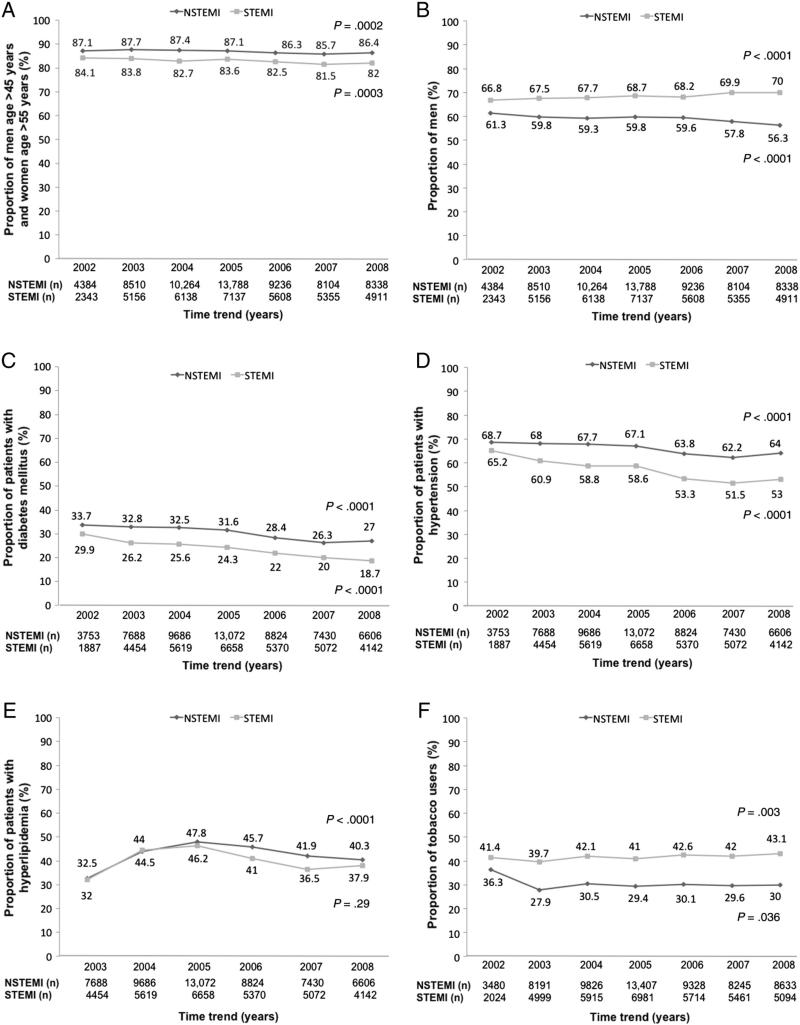
The traditional cardiovascular risk factors evaluated individually by year are presented in Figure 2. In the NSTEMI cohort, with the exception of hyperlipidemia, the proportions of patients with the individual risk factors have decreased since 2002. In the STEMI cohort, the proportions of patients with the risk factors of age, DM, and hypertension also decreased since 2002, but the proportions of men and tobacco users increased over time.
Figure 2.
Time trend of the proportion of men age >45 years or women age >55 years (A), men (B), patients with DM (C), patients with hypertension (D), patients with hyperlipidemia (E), and smokers (F) in the NSTEMI and STEMI cohorts (significance presented as P value for trend).
Modifiable cardiovascular risk factors
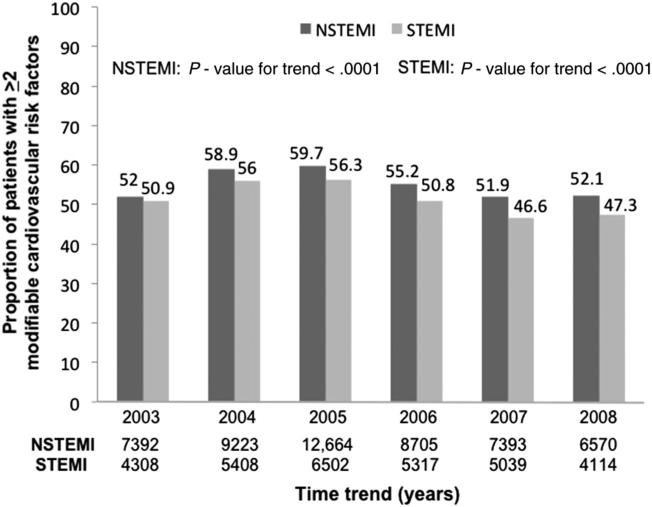
The proportion of patients with ≥2 modifiable risk factors also decreased over time in both the NSTEMI and STEMI cohorts (Figure 3). The time trend of modifiable risk factors adjusted for age and gender is presented in Table II. Similar to the unadjusted trends, the proportion of patients with a history of DM and hypertension decreased over time in both cohorts, with the magnitude of decrease greater in the STEMI cohort. However, after adjusting for age and gender, the proportion of patients with a history of hyperlipidemia remained the same over time in the NSTEMI cohort and marginally decreased in the STEMI cohort. The proportion of tobacco users decreased over time in both cohorts.
Figure 3.
Time trend of proportion of patients with ≥2 modifiable cardiovascular risk factors in the NSTEMI and STEMI cohorts (significance presented as P value for trend).
Table II.
Time trend (per 1 year) for modifiable cardiovascular risk factors after adjusting for age and gender
| Cohort | Odds ratio | 95% CI | P | Interaction P value | |
|---|---|---|---|---|---|
| DM* | NSTEMI | 0.93 | 0.92-0.95 | <.0001 | .02 |
| STEMI | 0.91 | 0.89-0.93 | <.0001 | ||
| Hypertension* | NSTEMI | 0.96 | 0.94-0.98 | <.0001 | .002 |
| STEMI | 0.93 | 0.91-0.95 | <.0001 | ||
| Hyperlipidemia† | NSTEMI | 1.00 | 0.98-1.03 | .73 | .001 |
| STEMI | 0.96 | 0.93-1.00 | .04 | ||
| Tobacco use* | NSTEMI | 0.97 | 0.96-0.98 | <.0001 | .23 |
| STEMI | 0.98 | 0.96-0.99 | .01 |
Examined from 2002 to 2008.
Examined from 2003 to 2008.
Discussion
In this large analysis of patients without known cardiovascular disease presenting with an MI, we demonstrated that the proportion of patients with multiple cardiovascular risk factors decreased over time. After adjustment for age and gender, we found a significant but small decrease over time in patients with a history of DM, hypertension, and tobacco use.
Over time patients without a known history of cardiovascular disease presenting with an MI demonstrate a progressively younger profile. This is counterintuitive as the prevalence of DM and hypertension has been reported to be lower in younger patients presenting with MI. An analysis of 122,458 patients from 14 randomized clinical trials similarly showed a decreasing prevalence of DM and hypertension as age groups decreased from 66 to 75 years to ≤45 years of age in patients presenting with NSTEMI and STEMI.11 However, 1 study did note an inverse association between age and number of CAD risk factors in patients with first MI.12 In our study, the decreasing trend of DM and hypertension over time remained present even after adjustment for age and gender. Whether this accurately reflects a low prevalence or is secondary to undiagnosed disease remains uncertain. The prevalence of metabolic risk in the form of DM and prediabetes is rapidly rising in the United States.13,14 While DM affects >24 million Americans, another 57 million are classified as having prediabetes.15
In the present study, prevalence of obesity increased over time, consistent with other reports. An analysis of the National Health and Nutrition Examination Survey (NHANES) demonstrates obesity in more than one-third of the adult population, and a subsequent analysis of the same database showed no increase in physical activity levels over the past 10 years.16,17 Together, these NHANES findings may explain the decrease in HDL cholesterol observed in the current report. With a rise in obesity and persistent trend for a sedentary lifestyle, it further seems unlikely that the overall prevalence of DM has truly decreased over time.18 Given the younger age at time of presentation, it is more likely that these patients have some degree of glucose intolerance and fit the criteria for prediabetes rather than DM.
Alternatively, the Health, Aging, and Body Composition Study lends support to unawareness of a diagnosis of DM with the condition being undiagnosed in almost one-third of participants.19 A review of the literature demonstrates hyperglycemia in up to 20% of patients without a diagnosis of DM presenting with MI.20 Furthermore, a prospective study of patients without a history of DM presenting with MI and an admission glucose level of <200 mg/dL found 35% with impaired glucose tolerance and 31% with DM after formal testing.21
Similarly, data from the NHANES showed only 23% of people with elevated blood pressure were treated with antihypertensive medications.22 Although an elevated blood pressure in 1 sitting does not constitute a diagnosis of hypertension, the proportion of people with true hypertension unaware of their diagnosis may not be insignificant. Certainly, primary care physicians have a higher threshold to diagnose and treat hypertension than recommended guidelines supporting the possibility of a higher prevalence of unawareness of the condition in younger patients.23
The diagnosis of hyperlipidemia did not decrease over time. As the body of evidence linking lower LDL cholesterol levels to decreased long-term mortality grows, more stringent cut-offs have been used to define hyperlipidemia.24,25 Although the third report from the National Cholesterol Education Program recommended optimal LDL cholesterol levels of ≤100 mg/dL in patients with CAD, there have been increasing data to support a lower threshold of ≤70 mg/dL.26 This trend has also extended to healthy men and women who demonstrate a higher risk of developing CAD as more recent data suggest improvement in rate of first long-term major adverse cardiovascular event on long-term follow-up with lipid-lowering therapy.27
In the current report, levels of LDL cholesterol decreased, consistent with data from the NHANES between 1988 and 2010.28 This is likely due to the relatively increasing diagnosis of hyperlipidemia and initiation of lipid-lowering therapy aimed at decreasing LDL cholesterol levels before presentation. Although there are insufficient data on medication use before admission in the current report, the same analysis of NHANES shows a significant increase in the proportion of age-adjusted adults on lipid-lowering therapy and, therefore, the proportion of adults with a diagnosis of hyperlipidemia from 1988 to 2010.28 The increase in proportion of adults on lipid-lowering therapy increased even more over time in those aged ≥50 years.28 The NHANES data between 1999 and 2006 further demonstrate significant increases in adults who had their lipid profile evaluated and reported being told that they had hypercholesterolemia.29
Of all the risk factors, tobacco use is one of the major preventable causes of cardiovascular disease. Tobacco use increases the risk of first MI by nearly 3-fold due to associated impairment of endothelial function, prothrombotic effects, and increased oxidative stress.30-35 Since California first implemented a state-wide smoking ban in 1998, 26 more states have implemented similar bans, and 12 other states have implemented partial smoking bans with the hope that not only will risks associated with second-hand smoke decrease, but also the number of smokers will decrease over time.36 In our study, after adjustment for age and gender, we observed a decrease in the proportion of tobacco users presenting with NSTEMI and STEMI. A large analysis of patients with CAD demonstrates a nearly 10-year decrease in age associated with tobacco use highlighting the strong association between age, tobacco use, and presentation of CAD.11
Few studies have evaluated the clinical risk factor profile of patients with a first episode of MI. One 30-year perspective of 8,898 patients hospitalized with an initial MI in a metropolitan area demonstrated increasing unadjusted prevalence of DM and hypertension and decreasing unadjusted rate of tobacco use over time from 1975 to 2005.37 Although this population was older over time, a subgroup analysis demonstrated an increase in the proportion of patients diagnosed with STEMI in the 1,703 patients <55 years old.37,38 This younger subgroup, however, also demonstrated increasing unadjusted prevalence of obesity, DM, and hypertension over time. Another registry from Denmark of 92,164 patients presenting with a first MI demonstrated similar increases in the unadjusted prevalence of DM but no real change in the age profile between 1997 and 2009.39 In contrast to the current report, prior cohorts are from 1 geographic location and have evaluated trends over longer periods dating back to when primary prevention guidelines were almost nonexistent. Other large national reports have not specifically evaluated first MI populations.40
Limitations
There are several limitations to this study. First, this is a retrospective analysis of a prospective registry. Data were collected by chart review and dependent on the quality and accuracy of that collection. As such, data on the risk factors presented are based on a provided history. Medication use before admission was not collected until late 2006 and, therefore, not available in almost 80% of the cohort. Similarly, blood pressure and hemoglobin A1c data are not available in >70% of the cohort. Fasting blood glucose levels and information on whether patients had regular visits to a physician in the years before presentation were not collected. Second, there are many potential sources of selection bias. Participation in the registry is voluntary, and the patients in the GWTGCAD registry may not be representative of all patients with first MI in the United States. The findings may have been influenced by hospitals joining or leaving the registry over the study period. There is also a survival time bias as the observations noted in the current study only pertain to survivors who arrive to a hospital. Third, family history of premature CAD, a traditional cardiovascular risk factor, was not collected. Finally, given the lack of blood pressure data and missing lipid data in approximately 25% of the cohort, a more specific scoring system, such as the Framingham risk score, could not be examined.
Conclusions
The prevalence of a concurrent history of risk factors such as DM, hypertension, and tobacco use has slightly decreased over time in an age- and gender-adjusted population, whereas the prevalence of hypercholesterolemia has overall remained the same. This discrepancy may reflect a different degree of evaluation, diagnosis, and treatment of these cardiovascular risk factors. The current analysis suggests small temporal trends in risk factors; however, more comprehensive data regarding implementation of primary prevention measures are needed in the MI population to gain a better understanding of where the disparities in the modification of cardiovascular risk factors and prevention of cardiovascular disease lie.
Supplementary Material
Footnotes
Disclosures
Dr Binita Shah: Research Grants: Guerbet, Takeda. Dr W. Frank Peacock: research grants: Abbott, Alere, Brahms, Novartis, Roche, The Medicine's Company; consultant: Abbott, Alere, BG, Cardiorentis, GE, Jannsen, Lily, The Medicine's Company, Singulex, Verathon; speaker's bureau: Abbott, Alere, Astra-Zeneca, Daichi-Sankyo; ownership interest: Comprehensive Research Associates LLC, Vital Sensors, Emergencies in Medicine LLC. Dr Gregg Fonarow: consultant: Novartis. Dr Adrian Hernandez: research grants: Bristol Myers Squibb, Janssen, Portola Pharmaceuticals. Dr Deepak L. Bhatt: advisory board: Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; board of directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; chair: AHA GWTG Steering Committee; honoraria: American College of Cardiology (Editor, Clinical Trials, Cardiosource), Belvoir Publications (Editor in Chief, Harvard Heart Letter), DCRI (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology); Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), WebMD (CME steering committees); data monitoring committees: DCRI; Harvard Clinical Research Institute; Mayo Clinic; Population Health Research Institute; research grants: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Roche, Sanofi Aventis, The Medicines Company; unfunded research: FlowCo, PLx Pharma, Takeda.
References
- 1.Kannel WB. Contributions of the Framingham Study to the conquest of coronary artery disease. Am J Cardiol. 1988;62:1109–12. doi: 10.1016/0002-9149(88)90558-9. [DOI] [PubMed] [Google Scholar]
- 2.Keys A. Coronary heart disease in seven countries. Circulation. 1970;41(suppl I):I–1-I-211. [PubMed] [Google Scholar]
- 3.Marmot MG, Syme SL, Kagan A, et al. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii, and California: prevalence of coronary and hypertensive heart disease and associated risk factors. Am J Epidemiol. 1975;102:514–25. doi: 10.1093/oxfordjournals.aje.a112189. [DOI] [PubMed] [Google Scholar]
- 4.Epstein FH, Ostrander LD, Jr, Johnson BC, et al. Epidemiological studies of cardiovascular disease in a total community—Tecumseh Michigan. Ann Intern Med. 1965;62:1170–87. doi: 10.7326/0003-4819-62-6-1170. [DOI] [PubMed] [Google Scholar]
- 5.Circulation. US Department of Health and Human Services; Public Health Service; National Institutes of Health; National Heart, Lung, and Blood Institute.; 2002. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Final Report. pp. 3143–420. [PubMed] [Google Scholar]
- 6.The Seventh Report of the Joint National Committee on Prevention. Detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 7.Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388–91. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 8. [May 1, 2013]; http://mylifecheck.heart.org.
- 9.Hong Y, LaBresh KA. Overview of the American Heart Association “Get with the Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5:179–86. doi: 10.1097/01.hpc.0000243588.00012.79. [DOI] [PubMed] [Google Scholar]
- 10.Smaha LA. American Heart Association. The American Heart Association Get With The Guidelines program. Am Heart J. 2004;148:S46–8. doi: 10.1016/j.ahj.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290:898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 12.Canto JG, Kiefe CI, Rogers WJ, et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. 2011;306:2120–7. doi: 10.1001/jama.2011.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1c criteria in the U.S. population in 1988-2006. Diabetes Care. 2010;33:562–8. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention . National diabetes fact sheet: general information and national estimates on diabetes in the United States. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2007. [January 19, 2013]. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. 2008. [Google Scholar]
- 16.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 17.Carlson SA, Fulton JE, Schoenborn CA, et al. Trend and prevalence estimates based on the 2008 Physical Activity Guidelines for Americans. Am J Prev Med. 2010;39:305–13. doi: 10.1016/j.amepre.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Ovbiagele B, Markovic D, Fonarow GC. Recent US patterns and predictors of prevalent diabetes among acute myocardial infarction patients. Cardiol Res Pract. 2011;2011:145615. doi: 10.4061/2011/145615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franse LV, Di Bari M, Shorr RI, et al. Type 2 diabetes in older well-functioning people: who is undiagnosed? Data from the Health, Aging, and Body Composition study. Diabetes Care. 2001;24:2065–70. doi: 10.2337/diacare.24.12.2065. [DOI] [PubMed] [Google Scholar]
- 20.Shah B, Amoroso NS, Sedlis SP. Hyperglycemia in nondiabetic patients presenting with acute myocardial infarction. Am J Med Sci. 2012;343:321–6. doi: 10.1097/MAJ.0b013e31822fb423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norhammar A, Tenerz A, Nilsson G, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet. 2002;359:2140–4. doi: 10.1016/S0140-6736(02)09089-X. [DOI] [PubMed] [Google Scholar]
- 22.Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med. 2001;345:479–86. doi: 10.1056/NEJMoa010273. [DOI] [PubMed] [Google Scholar]
- 23.Hyman DJ, Pavlik VN. Self-reported hypertension treatment practices among primary care physicians: blood pressure thresholds, drug choices, and the role of guidelines and evidence-based medicine. Arch Intern Med. 2000;160:2281–6. doi: 10.1001/archinte.160.15.2281. [DOI] [PubMed] [Google Scholar]
- 24.LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 25.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 26.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 27.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 28.Carroll MD, Kit BK, Lacher DA, et al. Trends in lipids and lipoproteins in US adults, 1988-2010. JAMA. 2012;308:1545–54. doi: 10.1001/jama.2012.13260. [DOI] [PubMed] [Google Scholar]
- 29.Ford ES, Li C, Pearson WS, et al. Trends in hypercholesterolemia, treatment and control among United States adults. Int J Cardiol. 2010;140:226–35. doi: 10.1016/j.ijcard.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 30.Prescott E, Hippe M, Schnohr P, et al. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ. 1998;316:1043–7. doi: 10.1136/bmj.316.7137.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–55. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 32.Pitilo RM, Clarke JM, Harris D, et al. Cigarette smoking and platelet adhesion. Br J Haematol. 1984;58:627–32. doi: 10.1111/j.1365-2141.1984.tb06109.x. [DOI] [PubMed] [Google Scholar]
- 33.Davis JW, Shelton L, Eigenberg DA, et al. Effects of tobacco and non-tobacco cigarette smoking on endothelium and platelets. Clin Pharmacol Ther. 1985;37:529–33. doi: 10.1038/clpt.1985.83. [DOI] [PubMed] [Google Scholar]
- 34.Galea G, Davidson RJL. Haematological and haemorheological changes associated with cigarette smoking. J Clin Pathol. 1985;38:978–84. doi: 10.1136/jcp.38.9.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Njølstad I, Arnesen E, Lund-Larsen PG. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction. A 12-year follow-up of the Finnmark Study. Circulation. 1996;93:450–6. doi: 10.1161/01.cir.93.3.450. [DOI] [PubMed] [Google Scholar]
- 36.Bruintjes G, Bartelson BB, Hurst P, et al. Reduction in acute myocardial infarction hospitalization after implementation of a smoking ordinance. Am J Med. 2011;124:647–54. doi: 10.1016/j.amjmed.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Floyd KC, Yarzebski J, Spencer FA, et al. A 30-year perspective (1975-2005) into the changing landscape of patients hospitalized with initial acute myocardial infarction: Worcester Heart Attack Study. Circ Cardiovasc Qual Outcomes. 2009;2:88–95. doi: 10.1161/CIRCOUTCOMES.108.811828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McManus DD, Piacentine SM, Lessard D, et al. Thirty-year (1975 to 2005) trends in the incidence rates, clinical features, treatment practices, and short-term outcomes of patients b55 years of age hospitalized with an initial acute myocardial infarction. Am J Cardiol. 2011;108:477–82. doi: 10.1016/j.amjcard.2011.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen KW, Hvelplund A, Abildstrøm SZ, et al. Prognosis and treatment in patients admitted with acute myocardial infarction on weekends and weekdays from 1997 to 2009. Int J Cardiol. 2013;168:1167–73. doi: 10.1016/j.ijcard.2012.11.071. [DOI] [PubMed] [Google Scholar]
- 40.Rogers WJ, Canto JG, Lambrew CT, et al. Temporal trends in the treatment of over 1.5 million patients with myocardial infarction in the U.S. from 1990 through 1999. J Am Coll Cardiol. 2000;36:2056–63. doi: 10.1016/s0735-1097(00)00996-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.