Abstract
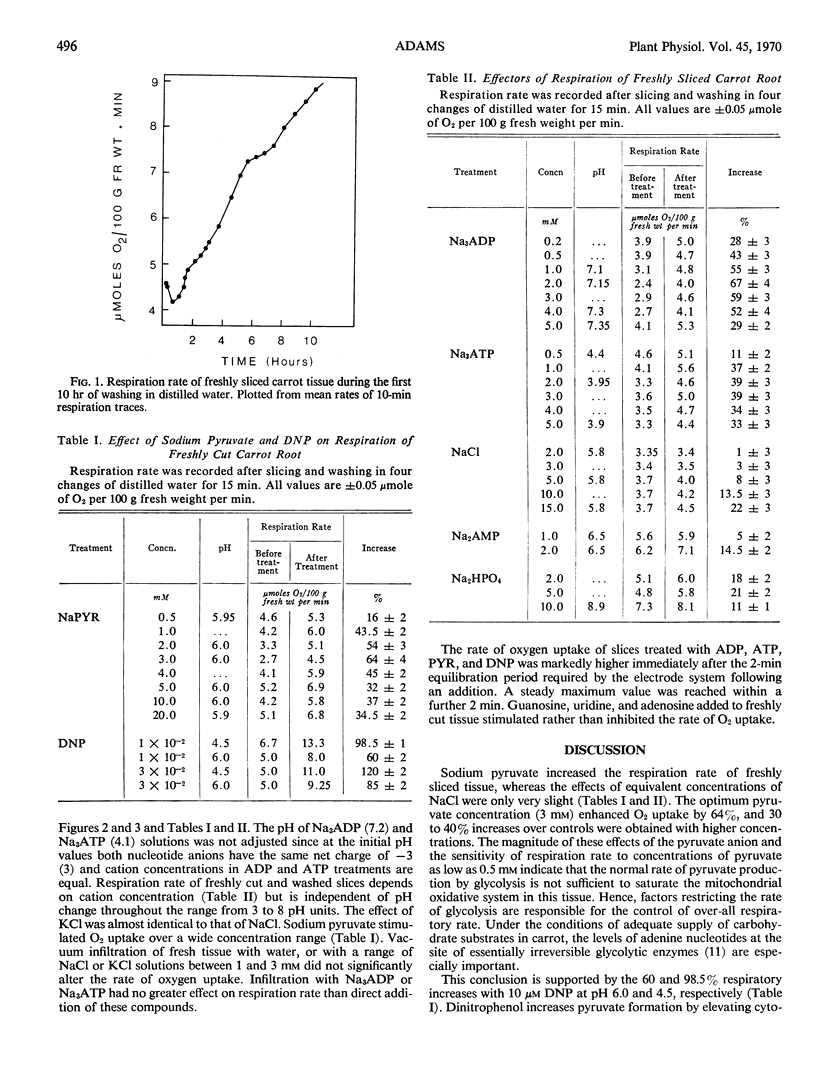
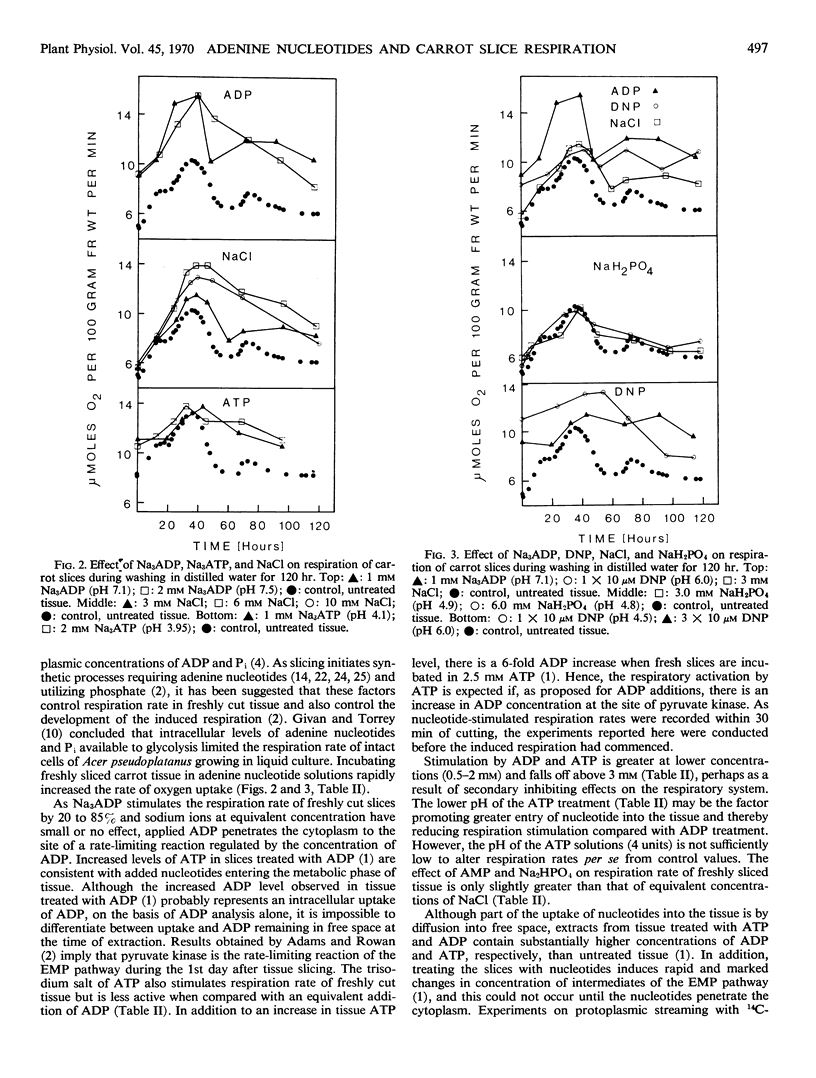
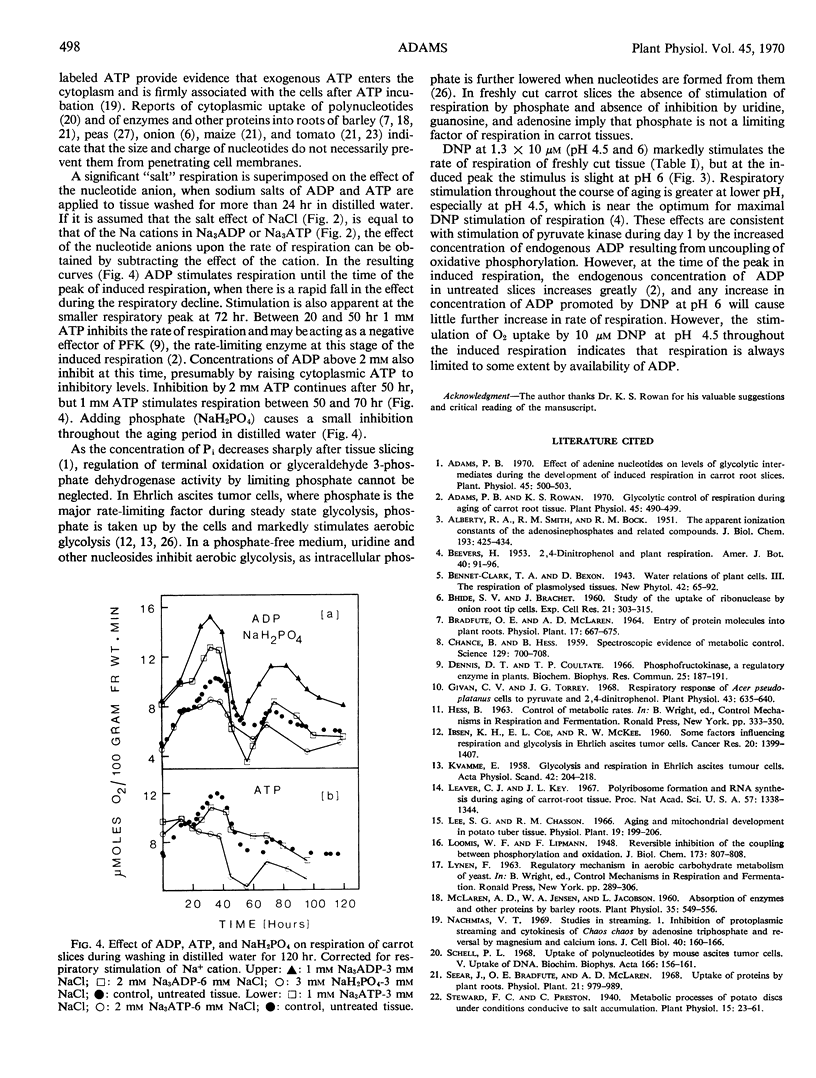
Sodium pyruvate and dinitrophenol stimulated O2 uptake of freshly cut phloem parenchyma from carrot roots by 63 and 120% at optimal concentrations, indicating that production of pyruvate by glycolysis regulates over-all respiratory rate. Adding 0.5 to 6.7 mm Na3ADP and Na3ATP to slices rapidly stimulates respiration rate by 20 to 85%. The effect is greater at the lower end of this concentration range and is not due to change in pH or active cation uptake. It is suggested that treating tissue with both nucleotides stimulates pyruvate kinase, the rate-limiting step in respiration of freshly cut slices, by increasing the concentration of endogenous ADP. Adenosine diphosphate continued to stimulate O2 uptake until the peak of induced respiration, but ATP inhibited respiration during development and decline of this peak. Absence of respiratory stimulation by NaH2PO4 and of respiratory inhibition by added nucleosides confirms that inorganic phosphate is not a limiting factor of respiration in freshly cut slices. The stimulation of respiration rate of these slices by dinitrophenol is consistent with results from experiments in which ADP and ATP were applied to the tissue.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERTY R. A., SMITH R. M., BOCK R. M. The apparent ionization constants of the adenosinephosphates and related compounds. J Biol Chem. 1951 Nov;193(1):425–434. [PubMed] [Google Scholar]
- Adams P. B. Effect of adenine nucleotides on levels of glycolytic intermediates during the development of induced respiration in carrot root slices. Plant Physiol. 1970 Apr;45(4):500–503. doi: 10.1104/pp.45.4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. B., Rowan K. S. Glycolytic control of respiration during aging of carrot root tissue. Plant Physiol. 1970 Apr;45(4):490–494. doi: 10.1104/pp.45.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., HESS B. Spectroscopic evidence of metabolic control. Science. 1959 Mar 13;129(3350):700–708. doi: 10.1126/science.129.3350.700. [DOI] [PubMed] [Google Scholar]
- Dennis D. T., Coultate T. P. Phosphofructokinase, a regulatory enzyme in plants. Biochem Biophys Res Commun. 1966 Oct 20;25(2):187–191. doi: 10.1016/0006-291x(66)90578-x. [DOI] [PubMed] [Google Scholar]
- Givan C. V., Torrey J. G. Respiratory Response of Acer pseudoplatanus Cells to Pyruvate and 2,4-Dinitrophenol. Plant Physiol. 1968 Apr;43(4):635–640. doi: 10.1104/pp.43.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBSEN K. H., COE E. L., McKEE R. W. Some factors influencing respiration and glycolysis in Ehrlich ascites tumor cells. Cancer Res. 1960 Oct;20:1399–1407. [PubMed] [Google Scholar]
- KVAMME E. Glycolysis and respiration in Ehrlich ascites tumour cells. I. Phosphate metabolism in relation to glycolysis and the Crabtree effect. Acta Physiol Scand. 1958 Jun 2;42(3-4):204–218. doi: 10.1111/j.1748-1716.1958.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Leaver C. J., Key J. L. Polyribosome formation and RNA synthesis during aging of carrot-root tissue. Proc Natl Acad Sci U S A. 1967 May;57(5):1338–1344. doi: 10.1073/pnas.57.5.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren A. D., Jensen W. A., Jacobson L. Absorption of Enzymes and Other Proteins by Barley Roots. Plant Physiol. 1960 Sep;35(5):549–556. doi: 10.1104/pp.35.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmias V. T. Studies on streaming. I. Inhibition of protoplasmic streaming and cytokinesis of Chaos chaos by adenosine triphosphate and reversal by magnesium and calcium ions. J Cell Biol. 1969 Jan;40(1):160–166. doi: 10.1083/jcb.40.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell P. L. Uptake of polynucleotides by mouse ascites tumor cells. V. Uptake of DNA. Biochim Biophys Acta. 1968 Aug 23;166(1):156–161. doi: 10.1016/0005-2787(68)90499-1. [DOI] [PubMed] [Google Scholar]
- Steward F. C., Preston G. METABOLIC PROCESSES OF POTATO DISCS UNDER CONDITIONS CONDUCIVE TO SALT ACCUMULATION. Plant Physiol. 1940 Jan;15(1):23–61. doi: 10.1104/pp.15.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ULRICH J. M., MCLAREN A. D. THE ABSORPTION AND TRANSLOCATION OF C14-LABELED PROTEINS IN YOUNG TOMATO PLANTS. Am J Bot. 1965 Feb;52:120–126. [PubMed] [Google Scholar]
- Vaughan D., Macdonald I. R. Development of soluble and insoluble invertase activity in washed storage tissue slices. Plant Physiol. 1967 Mar;42(3):456–458. doi: 10.1104/pp.42.3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]


