Abstract
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer death worldwide, with region specific etiologies. Despite improvements made in the diagnosis of HCC, the prognosis of HCC patients remains poor due to the high recurrence rate of HCC. There is an urgent need for development of prognostic biomarkers to predict the risk of recurrence in HCC patients after “curative” treatment. Such stratification may aid in patient management and development of personalized medicine for HCC treatment. Omics based studies facilitate the study of global changes in biomolecules in a disease in a high throughput manner, and hence are well poised to understand the complex changes which led to HCC recurrence. The quantitative nature of data obtained from omics based studies allow for development of prognostic biomarkers based on changes in gene, protein and metabolite expression. In this review, we surveyed the application of transcriptomics, proteomics and metabolomics in the study of HCC recurrence. We summarised the data in the literature from these three fields of studies that claimed to be prognostic for HCC recurrence. We critiqued on the limitations of each area of research and the challenges faced in translating the research results for clinical application in predicting HCC recurrence.
Keywords: Liver cancer, Biomarker, Transcriptomics, Proteomics, Metabolomics, Recurrence
Core tip: Hepatocellular carcinoma (HCC) patients have poor prognosis, largely due to the high incidence of recurrence. There is an urgent need for prognostic biomarkers to stratify patients with higher risk of recurrence to aid in clinical management. This review surveys the use of transcriptomics, proteomics and metabolomics in identifying prognostic biomarkers for HCC recurrence. Integration of data from various omics field allow for understanding of major pathways that were dysregulated in HCC recurrence, which could pave the way for development of personalized medicine for management of HCC recurrence.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer and third leading cause of cancer related deaths worldwide, with about 667000 new cases per annum[1]. HCC usually develops on patients with liver cirrhosis, and the causes are region specific. Etiological factors of HCC include chronic hepatitis B and C viral infection, chronic alcohol consumption and consumption of alfatoxin-B1 contaminated food. Diagnosis of HCC has improved significantly, from the study of histological features of the tissue to the use of radiography, such as magnetic resonance imaging (MRI) and computed tomography (CT)[2]. The improvements allow for earlier diagnosis of HCC, which opens up treatment options to the patients. Currently, liver transplantation and resection remains as the only curative treatment for HCC. However, about 60% of the patients suffered from HCC relapse even after curative resection, thus resulting in a poor prognosis for HCC[3]. Hence, it is imperative to look for prognostic markers that could predict the risk of recurrence in HCC patients so that these patients could benefit from increased surveillance which could increase overall survival rates. Understanding the molecular mechanism behind HCC recurrence could lead to the development of novel therapeutics that could potentially be applied as palliative treatments for recurrent cancers.
Differences in etiology of early and late recurrence
It is widely believed that there are two major types of HCC recurrence[4]. Dissemination of the remnant tumour cells after surgical resection results in recurrent tumours at other parts of the liver. Recurrence of this type usually peaks within two years after resection (early recurrence). Clinicopathological features associated with early recurrence include increased tumour size, presence of venous invasion, increased serum alpha-fetoprotein (AFP) protein and mRNA concentrations[3,5-8]. The cirrhotic liver may also act as a “field” for tumour development. The diseased liver undergoes further molecular changes under the influence of factors such as hepatitis virus infection to form dysplastic nodules, which would lead to formation of de novo tumours, resulting in recurrence. The recurrent tumours are usually independent from the primary tumours, and tumours of this type peak at three years after resection (late recurrence). The biological and clinical differences between these two types of recurrence models leads to differences in research approaches, with the primary tumour tissues commonly used for studies on early recurrence, and surrounding non-tumour tissues as the samples for late recurrence.
Prognostic values of known HCC oncogenes and tumour suppressors
The prognostic values of oncogenes and tumour suppressors implicated in HCC carcinogenesis had been mixed. High nuclear β-catenin[9] and elevated c-Myc expression[10] were correlated with vascular invasion and shorter recurrence free survival (RFS), suggesting the involvement of Wnt signaling activation in promoting HCC recurrence. The combination of three factors, namely phosphatase and tensin homolog downregulation, overexpression of p53 and increase in proliferating cell nuclear antigen labelling index, was associated with lower disease specific survival and early tumour recurrence[11]. Hotspot mutations of p53 (R249S and V157F) were associated with a stem cell phenotype, which is linked to a poor overall survival (OS) in HCC patients[12]. Interestingly, mutations in p53 and DNA mismatch repair protein Human mutS homolog 2 (hMSH2) had been linked to intrahepatic metastasis, as opposed to late recurrence[13]. Mutations in p53 and hMSH2 might confer a more aggressive phenotype which could account for early recurrence of HCC patients. Loss of Serine/Threonine kinase 11 (STK11) expression was also correlated with increased vascular invasion, shorter disease free survival (DFS) and OS in a Chinese cohort of HCC patients[14]. Although these oncogenes and tumour suppressors had been correlated with HCC prognosis, their prognostic value remains contradictory. Kondo et al[15] had shown that C-met overexpression has been linked to an increase in HCC recurrence, while this correlation was not observed in a recent publication by Lee et al[16]. Although tumour suppressor p16 has been found to be widely inactivated in HCC, p16 has not been shown to be a prognostic marker for HCC[17,18].
The heterogeneity of HCC suggests that various molecular players in related pathways are likely to be differentially regulated during the process of HCC recurrence. Omics based studies provide the opportunity to provide quantitative information on global expression profiles of various biomolecules in HCC progression in a high throughput and bias-free manner. The identification of dysregulated biomolecules can be used as prognostic biomarkers to predict HCC recurrence. In this review, we surveyed the application of “omics” for identification of prognostic biomarkers for predicting HCC recurrence. Emphasis will be placed on transcriptomics, proteomics and metabolomics, which studies the gene, protein and metabolite expression levels respectively.
TRANSCRIPTOMIC STUDIES AND GENE EXPRESSION PROFILING
Transcriptomics involves the study of expression of all transcribed genes in a genome wide manner. This analysis can be conducted in a high throughput form in the form of DNA microarrays, where cDNA is hybridized onto DNA probes that are present on a chip. Such analysis allows us to profile and identify genes that are differentially expressed with different disease states and clinical outcomes. Among all the global profiling technologies available, DNA microarray and related sequencing techniques are the most commonly used method to obtain molecular “signatures” for prediction of HCC recurrence. Transcriptomic studies that generate information for prediction of recurrence fall into two major groups. The first group of studies uses clinical information to segregate and develop a gene signature that could be used to predict the possible clinical outcome in a separate cohort. The second group of studies identifies distinct subgroup of patients that have the defined phenotype. In the following section, we will review some of the main discoveries that were obtained from the transcriptomic studies (Table 1) and the biological significance behind the gene signatures.
Table 1.
Gene expression studies of hepatocellular carcinoma for prediction of recurrence
| Ref. | Sample type | Screening platform | Virus type | Characteristics for distinguishing groups | Number of marker genes | Predictive accuracy for recurrence |
| Iizuka et al[19] (2003) | Tumour tissues | Oligonucleotide microarray | HBV < HCV | Early IHR within 1 yr after surgery | 12 | 25 in 27 independent patients (93%) |
| Kurokawa et al[20] (2004) | Tumour tissues | PCR based array | HBV < HCV | Early IHR within 2 yr after surgery | 20 | 29 in 40 independent patients (72.5%) |
| Budhu et al[35] (2006) | Non-tumour tissues | cDNA microarray | Almost all HBV | Venous invasion or extrahepatic metastasis | 17 | 87 of 95 independent patients (92%) |
| Ho et al[34] (2006) | Tumour tissues | cDNA microarray | HBV > HCV | Venous invasion | 14 | 26 of 35 independent samples (74.3%) |
| Lee et al[37] (2006) | Tumour tissues | Oligonucleotide microarray | HBV > HCV | Hepatoblast gene signature | 907 | P < 0.001 in 66 patients (Probability of recurrence) |
| Okamoto et al[21] (2006) | Non-tumour tissues | cDNA microarray | All HCV | Single nodular HCC vs multicentric HCC | 36 | 30 of 40 training samples (75%) |
| Wang et al[22] (2007) | Tumour tissues and non-tumour tissues | Oligonucleotide microarray | HBV > HCV | HCC recurrence | 57 | 84% in 25 independent samples, sensitivity 86%, specificity 82% |
| Hoshida et al[23] (2008) | FFPE non-tumour tissues | DASL assay | HBV < HCV | Late recurrence more than 2 yr after resection | 132 | P = 0.003 in 224 patients in validation set (Probability of late recurrence) |
| Somura et al[24] (2008) | Tumour tissues | Oligonucleotide microarray/qRT-PCR | HBV < HCV | Early IHR within 1 yr after surgery | 3 | 35 of 43 independent patients (81.4%) |
| Tanaka et al[25] (2008) | Tumour tissues | Oligonucleotide microarray | HBV > HCV | Aggressive recurrence exceeding Milan Criteria | 1 (AURKB) | 54 of 67 independent patients (80.5%) |
| Woo et al[26] (2008) | Tumour tissues | Oligonucleotide microarray | All HBV | Early IHR within 1 yr after surgery | 628 | P = 0.0018 in 139 independent patients (Probability of early recurrence) |
| Yoshioka et al[27] (2009) | Tumour tissues | Oligonucleotide microarray | HBV < HCV | Early IHR within 2 yr after surgery | 172 | P < 0.0001 in 97 independent patients (Probability of RFS) |
| Roessler et al[28] (2010) | Tumour tissues | Oligonucleotide microarray | HBV > HCV | Early IHR within 2 yr after surgery | 161 | P = 0.0057 for cohort 1, P = 0.017 for cohort 2 (Probability of RFS) |
| Tsuchiya et al[29] (2010) | Non-tumour tissues | Oligonucleotide microarray | All HCV | Late recurrence more than 1 yr after resection | 38 | P < 0.0001 in 44 training samples (Probability of RFS) |
| Woo et al[38] (2010) | Tumour tissues | Oligonucleotide microarray | HBV > HCV | Cholangiocarcinoma-like signature | 625 | P = 0.037 in cohort 1 of 61 patients, P = 0.004 for cohort 2 of 78 patients (Probability of RFS) |
| Weng et al[30] (2012) | Tumour tissues, PBMC | Oligonucleotide microarray | All HBV | Early IHR within 1 yr after surgery | 3 | P < 0.001 in 80 independent patients (Probability of RFS) |
| Xieraili et al[31] (2012) | Tumour tissues | Oligonucleotide microarray | HBV < HCV | Early IHR | 1 (VIL1) | P = 0.025 in 90 independent patients (Probability of RFS) |
| Tsunedomi et al[32] (2013) | Tumour tissues | Oligonucleotide microarray | All HCV | Early IHR within 1 yr after surgery | 1 (ABCB6) | 89% sensitivity, 55% specificity, 86% PPV, 62% NPV in 20 independent patients |
Under the heading “virus type”, HBV > HCV means that more patients in the study are HBV positive compared to HCV positive patients. HBV < HCV means there are more HCV positive patients in the study. DASL: Complementary cDNA-mediated annealing, selection, extension and ligation; IHR: Intrahepatic recurrence; RFS: Recurrence free survival; PBMC: Peripheral blood mononuclear cells; HBV: Hepatitis B virus; HCV: Hepatitis C virus; HCC: Hepatocellular carcinoma.
Predictive signatures obtained using recurrence as sample classifier
Most of the gene-profiling studies fall under this category, where early or late recurrence is used as the sample classifier for derivation of gene signatures that can predict recurrence[19-32]. Studies that are interested in predicting early recurrence commonly used HCC tumour tissues as molecular changes leading to intrahepatic metastasis seems to be programmed within the primary tumour. Most of the earlier studies had included a heterogenous study group in both the discovery and validation set to reflect the clinical setting where most patients would have vastly different clinicopathological features. In some studies, homogenous patient cohorts were chosen in the discovery phase to minimize confounding factors that might affect the molecular signatures of tumour samples. These include profiling patients with specific etiology [hepatitis B virus (HBV)-only[26,30] and hepatitis C virus (HCV)-only[21,29,32]] and patients with no clinicopathological factors that were correlated to early recurrence[27]. Gene profiling experiments had shown an overexpression of genes involved in proliferation[26,27,30], metastasis [26,31] and immune evasion[19,20]. In particular, SP1 and PPARα were identified by Woo et al[26] to be the common regulators of many genes overexpressed and downregulated in the high risk group respectively, which makes them attractive targets of drug design for treatment of HCC.
Late recurrence is often associated with the development of multicentric recurrence of HCC. Chronic infection by HBV and HCV results in the production of a cirrhotic liver which would promote the formation of de novo tumours, leading to late recurrence. Due to the difference in clonal origins of the recurrent and the primary tumour[33], non-tumour cirrhotic tissues were used for prediction of late recurrence. In an effort to identify gene signatures related to multicentric recurrence, Okamoto and colleagues profiled the gene expression of noncancerous liver tissue of 40 HCV positive patients with single nodular and multicentric HCC group[21]. A 36 gene signature was used to create a prediction score which could predict the risk of patients of multicentric recurrence, with an increase in prediction score conferring a higher risk of multicentric recurrence. However, no validation studies were performed to rate the performance of the prediction score in an independent data set.
Hoshida et al[23] studied the feasibility of gene-expression profiling in formalin-fixed, paraffin-embedded (FFPE) tissues, in which gene expression profiling of fixed tumour and adjacent non-tumour tissues from 105 patients were performed to obtain gene signatures that could predict overall survival and recurrence. Although they were unable to obtain a gene signature that could discriminate between patients with early recurrence from profiling of tumour tissues, they developed a 132 late-recurrence gene set (recurrence after 2 years of surgery). The gene set was validated using FFPE non-tumour tissues from two other countries with different etiology and clinicopathological features, thus suggesting the robustness of the late recurrence signature.
Lastly, to understand gene expression signatures in HCV patients, Tsuchiya and colleagues profiled the tumour and non-tumour tissues of HCV positive patients[29]. Similar to Hoshida et al[23], a 11 gene set was able to segregate patients with and without recurrence one year after resection. Bioinformatics analysis revealed that hepatic nuclear factor 4-alpha and interferon gamma centred interactomes as the top two significant networks, suggesting the role of chronic HCV infection in promoting late recurrence and disease progression.
Predictive signatures based on phenotype linked to recurrence
Transcriptomics studies in this group define the sample with specific phenotypes that are usually correlated with either recurrence or aggressive nature of tumours. These phenotypes act as surrogate markers for prediction of recurrence in patients. The desired phenotype can be related to pathological features that are correlated with the predictive parameters, such as vascular invasion and metastasis. Ho and colleagues reported a 14 gene set which could classify patients according to likelihood of vascular invasion[34]. They had demonstrated the utility of this gene set in predicting recurrence after surgical resection for HCC in an independent cohort of American Joint Committee of Cancer stage I patients without histological evidence of vascular invasion. Budhu et al[35] profiled the noncancerous hepatic tissues from patients with venous or extrahepatic metastasis and patients without metastasis to understand the role of the hepatic microenvironment in promoting metastasis. An immune response signature, corresponding to a global Th1/Th2-cytokine like shift was observed in patients with metastasis. A 17 gene list reflecting this shift in immune response was demonstrated to be able to segregate patients with and without metastasis, and this gene signature was able to predict both recurrence and survival of patients. Although both gene signatures have demonstrated the prognostic ability to predict recurrence, venous invasion might not be present in the tumours of all recurrent patients. Hence, the applicability of these gene sets in predicting recurrence might be limited.
Roessler et al[28] analysed the capability of a previously generated tumour-based metastatic signature in predicting recurrence of patients. The metastatic signature was able to predict early recurrence and overall survival of two independent cohorts with high sensitivity and good specificity. Importantly, the two cohorts have different etiological features, with one cohort consisting of HBV-positive Chinese patients, and the other cohort consisting of a mix of Chinese, European and American patients with different Hepatitis viral infection. This suggests the robustness of the classifier in predicting early recurrence and overall survival in a heterogenous group of patients. Combination of the gene signature with other clinicopathological data such as Barcelona-Clinic Liver Cancer staging and AFP levels improved prediction outcome.
Cancer stem cells have the ability to self-renew, differentiate and grow into a new tumour and are usually refractory to apoptosis. These cells do not die after conventional chemotherapy and often leads to the growth of tumour after curative treatment, resulting in recurrence. These cells are believed to arise from normal stem cells or progenitor cells[36]. HCC tumours bearing signatures of hepatic progenitor cells might possibly contribute to a poor prognosis, resulting in earlier recurrence and shorter overall survival. Two studies had linked the use of such signatures in predicting recurrence and survival of patients. Lee et al[37] and colleagues integrated data from rat fetal hepatoblasts and adult rat hepatocytes, together with human HCC to identify HCC tumours with similar gene expression as hepatoblasts[37]. The hepatoblast subgroup (Hb subtype) was independently associated with poorer survival and recurrence. Tumours in the Hb subtype exhibit higher expression of hepatic oval cells as well as genes suggesting the central role of JUN overactivation in recurrence and poor survival of Hb subtype patients.
Cholangiocarcinoma (CC) is one of major type of primary liver cancer with poorer prognosis compared to HCC. An intermediate form of combined hepatocellular-cholangiocarcinoma (CHC) was suggested to derive from bipotential liver stem cells. To identify cholangiocarcinoma-like traits in HCC which might identify patients with poorer prognosis, Woo et al[38] performed a genome wide profiling to identify differentially expressed genes between HCC and CC. The CC signature included well known biomarkers for CC and hepatic progenitor cells and HCC patients with CC signature (CLHCC) exhibited a shorter recurrence free survival and overall survival. The CC signature was validated on two independent cohorts of subjects, with tumours having CC signature showing shorter RFS and OS. A combination of CC and stem-cell (ES) signatures were able to further segregate patients with different prognosis, with CC and ES positive patients having the worst prognosis for RFS and OS.
Signatures based on defined phenotypes provide information with regards to the underlying biology behind recurrence through the identification of subgroups of patients with higher risk of recurrence based on specific phenotypes linked to recurrence and aggressive cancer progression. However, the predictive accuracy of such signatures may be lower than signatures based on clinical data since these signatures were used as surrogates for prediction of recurrence.
Gene signatures from HBV and HCV treatment
HBV and HCV infection are the most important etiological factors for HCC development, hence antiviral therapies after resection may be useful as adjuvant therapy to improve prognosis of the patients. The current approved treatment for hepatitis B infection are interferon-α (IFN-α) and nucleos(t)ide analogues (NA), while treatment for hepatitis C infection involves the combination of pegylated IFN-α and guanosine analog ribavirin (RBV). Several studies had compared the prognosis of HCC patients with and without antiviral treatments. In general, these studies had demonstrated the beneficial effects of antiviral treatments in preventing HCC recurrence, especially in patients with HCV infection. These results were summarized in a review article by Du et al[39].
Transcriptomic studies had been performed on two different types of treatment for HCV infection to understand the molecular mechanism and the possible side effects of treatment. Changes in gene expression of the blood mononuclear cells (PMBC) in patients with chronic HCV infection treated with IFN-α-RBV treatment were assessed using DNA microarray[40,41]. DNA sensing pathways and RIG-I like receptor signaling pathways were upregulated and ribosomal pathway was downregulated upon IFNα-RBV treatment. The downregulation of ribosomal pathway suggests a suppressive effect of IFN-α/RBV treatment in protein translation, which could explain the side effects of interferon treatment (such as fatigue) as well as its inhibitory effect on viral replication. The other study by Honda et al[42] involved the understanding of molecular effects of Peretinoin against recurrent HCC. Peretinoin is an acyclic retinoid which was previously reported to be efficacious in reducing the incidence of recurrent HCC. Gene expression profiling of liver tissues of HCV-positive HCC patients before and after peretinoin treatment showed that the expression levels of retinoid target genes, interferon target genes and tumour suppressor related genes were markedly elevated after peretinoin treatment. This was accompanied by a decreased expression of Wnt, Mammalian target of rapamycin and tumour progression related genes. This study highlighted the molecular basis for understanding the efficacy of peretinoin in preventing HCC recurrence, as well as the major molecular pathways that could be related to HCC recurrence.
Limitations of transcriptomics
Many gene profiling experiments had been performed in the search for a gene signature that could predict HCC recurrence with high accuracy. The predictive accuracy of these studies are promising, however these studies usually use a small sample size which might artificially inflate the predictive accuracy of the gene signature. There are few genes that overlap between these studies, which might be due to the use of different microarray platform, sample population, definition of the sample labels (e.g., different cut-off times used to define early recurrence) and the type of tissues profiled (frozen tissues vs FFPE). Villanueva et al[43] recently tested the predictive value of 22 published gene signatures that were reported to be prognostic for HCC on FFPE tissues corresponding to 201 patients. Only two signatures, namely G3 signature from tumour tissues[44] and poor survival signature from non-tumour tissues[23] were associated with shorter RFS and OS, suggesting the validity of the signature depends on the criteria set for the sample labels and the type of tissue profiled (frozen tissues vs FFPE tissues). In an effort to demonstrate the robustness of the gene signatures, cross-platform validation of signatures on patient cohorts with different etiology had been performed in several studies. However, there are still no gene signatures that had shown clinical significance in predicting recurrence on large sample sizes. A meta-analysis on the existing gene expression studies would provide the impetus to select and determine a gene signature that might best predict recurrence in patients, which could potentially be used clinically. In addition, identification of pathways that are commonly found to be dysregulated in different gene signatures might grant more insights to biological changes related to HCC recurrence. This is because gene profiling experiments are inherently noisy and some of the genes in the gene signature might not discriminate the recurrence and non-recurrence groups.
MIRNA AND HCC RECURRENCE
MicroRNAs (miRNA) are highly conserved, endogenous single-stranded non-coding RNA of 17-25 nucleotides. Long precursor RNAs with stem-loop structures are processed by Drosha and DICER to form mature miRNAs. Mature miRNAs post-transcriptionally regulate gene expression via base pairing with the 3’-UTR of target mRNAs, resulting in cleavage or translational repression. There are more than 2000 mature miRNAs found in human (miRBase, Release 20.0) (http://www.mirbase.org/)[45], and they are involved in several biological events at physiological and pathological conditions, such as embryonic development, cell growth, differentiation, apoptosis, and invasion[46].
In the following section, we discuss some of the recent studies on large scale profiling of miRNAs in the discovery of biomarkers for HCC recurrence (summarized in Table 2). The postoperative recurrence rate and OS in the HCC patients could be used to determine their values as prognostic marker(s), and as potential target in anti-HCC therapies. In some of these studies, the miRNA biomarkers were also found to be associated with hepatocarcinogenesis, orthotopic liver transplantation (OLT), or chemotherapy drug treatment for HCC.
Table 2.
miRNA for prediction of recurrence in hepatocellular carcinoma
| Ref. | Sample type | Screening platform | Virus type | Characteristics for distinguishing groups | Number of candidate miRNA | Predictive accuracy for recurrence |
| Fornari et al[53] (2009) | Tumour and non-tumour tissues | qRT-PCR | HBV < HCV | miR-122 levels | 1 (miR-122) | P = 0.05 for 45 independent patients (Recurrence rate) |
| Fornari et al[54] (2010) | Tumour and non-tumour tissues | qRT-PCR | HBV < HCV | Late recurrence beyond two years after surgery | 1 (miR-199a-3p) | P = 0.043 for 36 independent patients (Recurrence rate) |
| Augello et al[47] (2012) | FFPE tumour tissues | qRT-PCR | All HCV | Stages of HCC progression | 18 | P = 0.042 for 61 independent patients (Percent Recurrence) |
| Han et al[52] (2012) | FFPE tumour tissues | qRT-PCR | Mostly HBV | Recurrence after OLT | 1 (miR-155) | P < 0.001 for 100 training patients (RFS) |
| Huang et al[51] (2012) | Non-tumour tissues | qRT-PCR | HBV > HCV | Early IHR within 6 mo after surgery | 6 | P < 0.001 for 216 independent patients |
| Shih et al[48] (2012) | Tumour and non-tumour tissues | qRT-PCR | Not mentioned | HCC and non-tumour | 15 | P = 0.005 for 68 training samples and 13 independent samples (RFS) |
| Xia et al[50] (2012) | Tumour and non-tumour tissues | qRT-PCR | Mostly HBV | early IHR within 2 years after surgery | 1 (miR-214) | P = 0.009 for 50 independent patients (RFS) |
| Zhu et al[49] (2012) | FFPE tumour and non-tumour tissues | qRT-PCR | All HBV | Early IHR after surgery | 1 (miR-29a-5p) | AUC = 0.708 for 112 independent patients |
OLT: Orthotopic liver transplantation; IHR: Intrahepatic recurrence; RFS: Recurrence free survival; AUC: Area under curve; HCV: Hepatitis C virus; HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma; qRT-PCR: Quantitative reverse transcription polymerase chain reaction.
miRNA as molecular classifiers for tumour progression
A low-density array was used to profile the levels of 664 miRNAs in 60 hepatitis C-positive liver tissues from patients of different stages of hepatocarcinogenesis to identify miRNAs that are deregulated during HCV-related liver carcinogenesis[47]. A 18-miRNA signature was found to distinguish cirrhosis, liver dysplasia and HCC lesions. Among these miRNAs, miR-515-3p, miR-518a-3p, miR520f and miR-525-3p were members of the chromosome 19 miRNA cluster (C19MC), and were selectively over-expressed in HCC. miR-512-3p was significantly over-expressed in early HCC as compared with high-grade dysplasia, whereas miR-519d and 525-3p were over-expressed in tumour as compared with either low- or high-grade dysplastic nodules in the HBV-related samples. In another 61 HCC and matched cirrhosis cases, 11 miRNA members of C19MC were up-regulated in HCC compared to non-neoplastic counterparts. The increase in C19MC miRNA was also associated with poor clinico-pathological features, tumour recurrence and shorter OS time. This was the first study reporting the over-expression of chr19 miRNA in predicting HCC recurrence and OS, suggesting C19MC as potential target for therapies strategies against HCC.
Expression profiling of miRNAs in 68 HCC and 21 non-tumoural liver tissues has identified 15 miRNAs as potential classifier of HCC[48]. Among these miRNAs, miR-214 was found to be down-regulated in HCC, and this was associated with short RFS and OS. The deregulation of miR-214 was also associated with higher serum AFP, and advanced tumour stage in HCC patients. Furthermore, over-expression of miR-214 in HCC cells inhibited xenograft tumour cell proliferation, microvascularity of tumours and surrounding tissue, and suppressed orthotopic xenograft tumour formation in nude mice. miR-214 was thus proposed as a tumour suppressor of HCC associated with poor prognosis, and implicated in dedifferentiation, induction of stemness, and tumour transformation of hepatocytes. Lastly, the authors showed that the miR-214/HDGF paracrine pathway might be responsible for regulation of angiogenesis during HCC development, suggesting this pathway as potential target for anti-HCC therapy since angiogenesis plays a crucial role in HCC development.
miRNA as predictive signatures of recurrence
In a recent report, Zhu et al[49] analysed the miRNAs in microdissected HBV-related HCC tissues to detect miRNAs that can predict early recurrence after HCC resection. Increase in miR-29a-5p was found to be associated with early tumour recurrence and shorter OS, including patients with early stage HCC. The authors showed that miR-29a-5p was an independent prognostic indicator for early tumour recurrence after HCC resection. This association of high miR-29a-5p with metastasis-related early recurrence of HCC concurs with deregulation of miR-29 family members in cancers. However, further functional studies would be necessary to understand the mechanism of miR-29a-5p’s role in HCC recurrence to aid the development of cancer therapeutics.
In a study by Xia et al[50], down-regulation of miRNA-214 was also found to be associated with early recurrence of HCC, cancer cell invasion, and stem-like traits. The authors also demonstrated that the degree in the reduction of miR-214 correlated with the invasiveness and metastatic ability of liver cancer cell lines. Moreover, overexpression of miR-214 inhibits growth and invasion in vitro and tumourgenicity in vivo. This study also found that enhancer of zeste homologue 2 (EZH2) and β-catenin (CTNNB1) are down-stream targets of tumour suppressor miR-214. The increase in EZH2 and CTNNB1, and the decrease in miR-214 in HCC patients correlated with early recurrence and could predict poor survival. The results derived from this study could help in the development of therapeutics against HCC.
Huang et al[51] has compared the miRNA profiles of HCC liver tissues with that of non-tumour liver tissues to discover postoperative prognostic predictors for HCC. A panel of 20 miRNAs was identified from this study of 12 HCC patients (6 with better prognosis and 6 with poorer prognosis), of which high expression of miR-155, miR-15a, miR-432, miR-486-3p, miR-15b, and miR-30b were significantly associated with shorter RFS. miRNA-155 were also found higher in tumour tissues of patients with post-OLT HCC recurrence as compared to those of non-recurrence patients in an independent study[52]. Patients with higher miR-155 expression had poorer recurrence-free survival and shorter OS, independent of serum AFP level. Functional studies on HCC cell lines implicate the role of miR-155 in proliferation, survival and invasion[51,52]. Thus, miR-144 could be a good prognostic marker for recurrence risk stratification and target for anticancer therapy in HCC patients.
The reduction of miR-122 level, which is common in HCCs, affects HCC development and progression, and low miR-122 expression was shown to correlate with a shorter time to recurrence in patients resected for HCC[53]. miR-122 was found to promote the stability and transcriptional activity of p53, and lowers the invasiveness of HCC-derived cell lines, partially through regulating cyclin G1. Furthermore, miR-122, and silencing of cyclin G1 increases the sensitivity of the cancer cells to doxorubicin, one of the commonly used drugs for intermediate-advanced HCCs. In another study by Fornari et al[54], a shorter time to recurrence after HCC resection was found in patients with lower miR-199a-3p levels, and re-expression of miR-199a-3p in HCC-derived cell lines resulted in blockage of G1-S transition, lowered invasion capability, and increased response to doxorubicin treatment. These studies suggested that combined chemo- and miRNA-based therapy is a potential strategy for HCC treatment. Although miRNAs are potential clinically useful biologic metrics to guide patient selection and therapeutic interventions, it should be noted that there could be issues with the toxicity and off-target effects for miRNA as therapies in HCC.
PROTEOMICS STUDIES OF HCC
Proteomics is another key area of study which has been extensively used in cancer studies. The dynamic nature of proteins and its response to external and internal stimuli in the disease state might not be apparent from the transcripts, making proteomic studies an attractive method to understand disease progression. Quantitative expression proteomics allow for identification of proteins that are differentially regulated between two groups of patients, which could be used to identify prognostic biomarkers for HCC recurrence. Such studies involve the separation and identification of the proteins in a complex mixture such as tissue lysates. Separation of proteins is commonly performed using two-dimensional electrophoresis (2DE), which separates proteins according to their pI and molecular weight on a polyacrylamide gel, while separation of peptides is usually performed using liquid chromatography. This is coupled to mass spectrometry such as matrix assisted laser desorption/ionization or electrospray ionization for identification of proteins at a high level of automation and sensitivity. Limited proteomic studies have been performed to identify prognostic biomarkers that could predict recurrence in HCC patients after liver resection or liver transplantation. In the following section, we will discuss about the main findings from these papers (Table 3).
Table 3.
Proteomic studies of hepatocellular carcinoma for prediction of recurrence
| Ref. | Sample type | Screening platform | Characteristics for distinguishing groups | Candidate biomarkers | Validation method |
| Yokoo et al[55] (2007) | Tumour tissues | 2D-DIGE, MALDI-TOF MS | Early IHR within 6 mo after resection | 23 protein panel | 2D-DIGE |
| Orimo et al[59] (2008) | Tumour and non-tumour tissues | 2D-DIGE, LC-MS/MS | Histological differentiation of tumours | Adenomatous polyposis coli-end-binding protein 1 (EB1) | IHC |
| Yi et al[56] (2008) | Paired tumour and non-tumour tissues | 2DE, MALDI-TOF/TOF MS | Early IHR within 1 yr after resection | Mortalin (HSPA9) | qPCR, Immunoblotting, IHC |
| Bai et al[58] (2009) | Tumour tissues | cICAT, 2DLC-MS/MS | Recurrence after liver transplantation | Calpain small subunit 1 (CAPN4) | qRT-PCR, Immunoblotting, TMA |
| Cheng et al[57] (2011) | Tumour tissues | 2DE, MALDI-TOF/TOF MS | Recurrence after liver transplantation | N-myc downstream-regulated gene 1 (NDRG1) | Immunoblotting, IHC |
| Kanamori et al[60] (2011) | Tumour and non-tumour tissues | 2DLC-MS/MS | Tumour and non-tumour | Talin-1 (TLN1) | IHC |
IHR: Intrahepatic recurrence; IHC: Immunohistochemistry; TMA: Tissue microarray; qRT-PCR: Quantitative reverse transcription polymerase chain reaction; 2D-DIGE: Two-dimensional difference gel electrophoresis; MALDI: Matrix-assisted laser desorption/ionization; LC-MS: Liquid chromatography–mass spectrometry.
Proteomics identification of predictive biomarkers for recurrence
Yokoo et al[55] used a two-dimensional difference gel electrophoresis (2D-DIGE) approach to compare the proteome of 12 patients with early intrahepatic metastasis within 6 mo after surgery with 15 patients who did not have recurrence within 2 years after resection. A total of 23 differentially expressed proteins were identified and these proteins were involved in wide range of biological processes. This 23 protein list was validated on a set of 13 patients with a high predictive accuracy of 92%. However, the validation method was performed using 2D-DIGE, which required skilled personnel to perform and may not be easily translated.
In a similar study, Yi et al[56] used a differential proteomic approach with 2DE to profile the tumour and non-cancerous tissue of 103 Chinese HBV-positive patients. Mortalin was found to be overexpressed in the tumours of patients who recurred within 1 year post surgery, with high predictive accuracy and sensitivity. Elevated mortalin levels were correlated with the presence of venous invasion and advanced tumour stages, while increased mortalin levels were found in HCC cell lines with higher metastatic ability. The authors proposed that mortalin played a role in promoting the metastatic property of HCC and is a positive predictor for early HCC recurrence.
A similar approach was used by Cheng and colleagues to identify protein biomarkers that could discriminate patients with higher risk of recurrence after liver transplantation[57]. 2DE profiling of tumour tissues was performed on 143 patients with and without recurrence after liver transplantation. N-myc Downstream-regulated gene 1 (NDRG1) was significantly overexpressed in patients with recurrence compared to non-recurrence patients. Knockdown of NDRG1 in HepG2 cells reduced growth, invasion and migration ability of the cells, thus suggesting the role of NDRG1 in promoting proliferation and metastasis. Positive NDRG1 expression was also correlated with presence of venous invasion, advanced tumour staging, high serum AFP concentration and recurrence.
Likewise in the search of biomarkers for HCC recurrence after liver transplantation, Bai and colleagues utilized the cleavable isotope-coded affinity tag technology, and 2-Dimensional Liquid Chromatography coupled with tandem mass spectrometry (2D-LC MS/MS) to quantitate relative protein changes between patients with or without recurrence after liver transplantation[58]. A total of 52 differentially regulated proteins was identified, among which calpain small subunit 1 (Capn4) was demonstrated to be consistently overexpressed in both tumour tissues of recurrent patients and HCC cell lines with higher metastatic ability. Knockdown of Capn4 was shown to be an independent prognostic factor for recurrence and survival of HCC patients through multivariate analysis.
Proteomics identification of biomarkers related to HCC progression and its correlation with recurrence
Histological differentiation has been one of the pathological factors for poor prognosis. To identify molecular players in HCC differentiation, Orimo et al[59] profiled a total of 27 HCC tissues with different degrees of histological differentiation using 2D-DIGE. 26 unique proteins were identified to be differentially expressed, of which Adenomatous Polypopsis Coli-End Binding Protein 1 (EB1), which is controlled by factors linked to HCC malignancy such as c-Myc, RhoA and CDC42, was chosen for further validation. Increased EB1 expression was found to be associated with poorer histological differentiation, higher cumulative recurrence rate and shorter overall survival.
In another study to identify proteins related to HCC progression, Kanamori and colleagues used 2D-LC MS/MS to interrogate the proteome of 4 early HCC and 4 non-HCC tissues derived from two cases of liver transplant surgery[60]. Among the 83 proteins that were differentially expressed in the tumour and non-tumour tissues, Talin-1, a cytoskeletal protein was chosen for further validation. Immunohistochemical analysis revealed the direct correlation of Talin-1 expression with poor histological differentiation, and using 50% cells staining as a cutoff, patients with more than 50% cell staining were shown to have significantly shorter disease free survival rate.
Limitations of proteomics
In proteomics studies, validation of results obtained from the discovery phase would usually be performed using antibody based methods, such as immunoblotting or immunohistochemical analysis, which are costly. Hence, proteomic studies are often followed by validation of single potential prognostic markers that usually has functional implications related to HCC progression/metastasis or has the highest fold difference between the sample groups. However, due to the highly heterogeneous nature of HCC recurrence, a single prognostic marker might not be specific and sensitive enough to detect patients with high risk of recurrence. Multiple prognostic markers could possibly provide higher predictive accuracy as compared to single prognostic marker.
METABOLOMICS STUDIES OF HCC
Metabolomics is a large scale quantitative and qualitative study of small molecules (< 1 kDa), which commonly involves the use of high-throughput liquid chromatography (e.g., high performance liquid chromatography or ultra performance liquid chromatography) and mass spectrometry or nuclear magnetic resonance spectroscopy for an unbiased comparison of global metabolite profiles in biological samples, such as urine, serum and tissues. Importantly, alterations in the metabolites represent downstream events of transcriptome and proteome changes, and reflect dynamic responses to endogenous and exogenous factors, such as pathological, environmental or lifestyle effects. Metabolomics study of cancers was largely based on the hypothesis by Otto Warburg: tumour cells contain altered metabolic profiles mainly due to modified mitochondrial respiration and glycolytic pathways. Current findings from metabolomics have concurred with the Warburg phenomenon of altered tumour metabolism, with an increased glycolysis but reduced mitochondrial oxidative phosphorylation. Being one of the largest metabolic organs in the body, HCC progression would result in huge alterations in the metabolome of liver, making metabolomics an attractive field for identifying diagnostic and prognostic biomarkers for HCC. Metabolomics has been applied to look for diagnostic biomarkers[61-67]. To the best of our knowledge, there was only one study that applied metabolomics in search for predictive biomarkers for HCC recurrence, which would be discussed below.
In a pioneer urine metabolomics study to identify differential metabolic changes in patients with early HCC recurrence after resection, Ye et al[68] used GC-TOF/MS to profile urine samples of patients before and after surgery. Energy metabolism, amino acid metabolism, nucleoside metabolism, tricarboxylic acid cycle, and gut floral metabolism were found altered after surgical removal of tumour. This work identified metabolic signatures of HCC recurrence comprising of up-regulated lactate excretion, succinate production, purine and pyrimidine nucleosides turnover, glycine, serine and threonine metabolism, aromatic amino acid turnover, cysteine and methionine metabolism, and glyoxylate metabolism. These findings reflect the increase in proliferation, decrease in mitochondria oxidative phosphorylation and increased oxidative stress in recurrent patients that is correlated with the growth and increase in tumour load. In addition, 5 metabolites, namely ethanolamine, lactic acid, acotinic acid, phenylalanine and ribose were determined to be combinatorial biomarkers for discriminating early recurrent patients from non-recurrent patients post-surgery. Findings from metabolomics studies of urine would allow widespread non-invasive screening and surveillance instead of diagnostic tests using AFP and liver ultrasound that are prohibitively expensive. However, validation on a large cohort is needed to confirm the robustness and accuracy of this metabolomics signature in predicting HCC recurrence.
Limitations of metabolomics
Metabolomics is a powerful tool that can be used to quantitatively measure the differences in metabolite abundance between two different disease states. The ability to perform such studies in a large range of biological samples, including urine samples in which collection is easy and non-invasive, makes it an attractive platform for translation to clinical use. However, current studies lack validation of results in large sample cohorts. Moreover, the amount of biological information that could be extracted from metabolomics studies might be limited. This is because metabolic pathways are greatly interlinked and various proteins might be involved in the catabolism/anabolism of a particular metabolite. Hence, it might be difficult to attribute the changes in metabolite abundance to the expression/activity of a particular protein or a pathway.
PERSPECTIVES
HCC is a lethal disease with complex etiology. Despite improvements in detection methods, the prognosis of HCC remains poor, mainly due to the high recurrence rate after surgical interventions. Hence there is a pressing need for prognostic biomarkers that could stratify patients according to risk of cancer recurrence. Understanding the molecular mechanisms behind recurrence could allow for the development of personalized therapy to target recurrence with different biological characteristics. Through the use of omics based methods in studying HCC recurrence, we had generated huge amounts of data on the possible signatures of recurrence. However, such information has not been proven to be useful clinically for HCC prognosis. Systematic evaluation of these data on a large scale in a prospective study would be needed to verify the prognostic accuracy in a statistically convincing manner.
Currently, the molecular mechanisms underlying HCC recurrence remain largely enigmatic. There are no candidate genes that are commonly identified by the three omics methods. Different omics platforms could provide biological insights of HCC recurrence in a complementary fashion, despite their advantages and limitations (Table 4). Integration of data from different omics platform could reinforce the understanding and classification of HCC patients by highlighting common pathways that are dysregulated in subsets of patients (Figure 1). Such studies had already been applied in the metabolomics study, where different metabolomics signatures were associated with different subclasses of HCC patients classified via gene expression profiling[69]. Classification of patients would provide an avenue for personalised medicines which specifically targets the main players leading to recurrence, resulting in better clinical outcomes and improvements in quality of life. With advancements in technology and bioinformatics, the complete characterization of HCC recurrence should be achievable in the near future.
Table 4.
Advantages and limitations of the three different omics methodologies
| Method | Advantages | Limitations |
| Transcriptomics | Large dataset of dysregulated genes identified, provides insights to biological mechanisms of diseaseAffordable priceHas provided successful example of translation to clinical use (e.g., Oncotype Dx™ in predicting breast cancer recurrence) | Differences in platform contribute to lack of overlap in signatures between different studiesHigh possibility of noise present in the gene listPoor correlation between transcript and protein levels |
| Proteomics | Direct measurement of biological effectorsValidation method (IHC and TMA) routinely performed in pathology labs | Small number of validated targetsPrice, availability and quality of antibodies for validation work |
| Metabolomics | Amenable to different types of samples which can be obtained in a non-invasive manner | Lack of large-scale validation of metabolite signaturesLimited biological information obtained |
IHC: Immunohistochemistry; TMA: Tissue Microarray.
Figure 1.
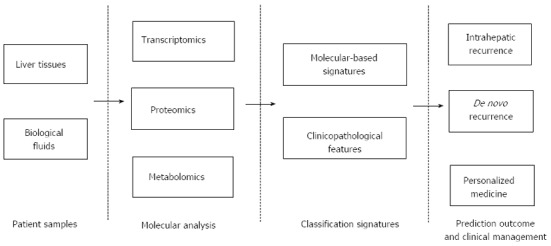
Predictive systems for outcome of hepatocellular carcinoma patients. Tissue samples (tumour and non-tumour) as well as biological fluids (blood, urine etc.) are used for molecular analysis via omics based methodologies to obtain molecular based signatures for HCC recurrence. The combination of these signatures and clinicopathological features (e.g., HBV or HCV infection, serum AFP levels, tumour staging) would be used to generate predictive systems for intrahepatic recurrence and de novo recurrence. Personalised treatment could be generated and administered based on the molecular basis of recurrence. HCC: Hepatocellular carcinoma; HBV: Hepatitis B virus; HCV: Hepatitis C virus; AFP: Alpha-fetoprotein.
Footnotes
Supported by The National Medical Research Council (NMRC), Singapore (NMRC grant 1259/2010), and a National University of Singapore President’s Graduate Fellowship to Lee SC
P- Reviewers: Julie NL, Wu JJ, Wu ZJ S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
References
- 1.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 2.Mancuso A. Management of hepatocellular carcinoma: Enlightening the gray zones. World J Hepatol. 2013;5:302–310. doi: 10.4254/wjh.v5.i6.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Med. 2008;359:2045–2047. doi: 10.1056/NEJMe0807581. [DOI] [PubMed] [Google Scholar]
- 5.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. [PubMed] [Google Scholar]
- 6.Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, Poovorawan Y. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000;31:302–308. doi: 10.1097/00004836-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Tsai TJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Hsia CY, Wu CW. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery. 2000;127:603–608. doi: 10.1067/msy.2000.105498. [DOI] [PubMed] [Google Scholar]
- 8.Morimoto O, Nagano H, Miyamoto A, Fujiwara Y, Kondo M, Yamamoto T, Ota H, Nakamura M, Wada H, Damdinsuren B, et al. Association between recurrence of hepatocellular carcinoma and alpha-fetoprotein messenger RNA levels in peripheral blood. Surg Today. 2005;35:1033–1041. doi: 10.1007/s00595-005-3077-5. [DOI] [PubMed] [Google Scholar]
- 9.Zulehner G, Mikula M, Schneller D, van Zijl F, Huber H, Sieghart W, Grasl-Kraupp B, Waldhör T, Peck-Radosavljevic M, Beug H, et al. Nuclear beta-catenin induces an early liver progenitor phenotype in hepatocellular carcinoma and promotes tumor recurrence. Am J Pathol. 2010;176:472–481. doi: 10.2353/ajpath.2010.090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin X, Li YW, Jin JJ, Zhou Y, Ren ZG, Qiu SJ, Zhang BH. The clinical and prognostic implications of pluripotent stem cell gene expression in hepatocellular carcinoma. Oncol Lett. 2013;5:1155–1162. doi: 10.3892/ol.2013.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu TH, Wang CC, Huang CC, Chen CL, Hung CH, Chen CH, Wang JH, Lu SN, Lee CM, Changchien CS, et al. Down-regulation of tumor suppressor gene PTEN, overexpression of p53, plus high proliferating cell nuclear antigen index predict poor patient outcome of hepatocellular carcinoma after resection. Oncol Rep. 2007;18:1417–1426. [PubMed] [Google Scholar]
- 12.Woo HG, Wang XW, Budhu A, Kim YH, Kwon SM, Tang ZY, Sun Z, Harris CC, Thorgeirsson SS. Association of TP53 mutations with stem cell-like gene expression and survival of patients with hepatocellular carcinoma. Gastroenterology. 2011;140:1063–1070. doi: 10.1053/j.gastro.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yano M, Hamatani K, Eguchi H, Hirai Y, MacPhee DG, Sugino K, Dohi K, Itamoto T, Asahara T. Prognosis in patients with hepatocellular carcinoma correlates to mutations of p53 and/or hMSH2 genes. Eur J Cancer. 2007;43:1092–1100. doi: 10.1016/j.ejca.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 14.Huang YH, Chen ZK, Huang KT, Li P, He B, Guo X, Zhong JQ, Zhang QY, Shi HQ, Song QT, et al. Decreased expression of LKB1 correlates with poor prognosis in hepatocellular carcinoma patients undergoing hepatectomy. Asian Pac J Cancer Prev. 2013;14:1985–1988. doi: 10.7314/apjcp.2013.14.3.1985. [DOI] [PubMed] [Google Scholar]
- 15.Kondo S, Ojima H, Tsuda H, Hashimoto J, Morizane C, Ikeda M, Ueno H, Tamura K, Shimada K, Kanai Y, et al. Clinical impact of c-Met expression and its gene amplification in hepatocellular carcinoma. Int J Clin Oncol. 2013;18:207–213. doi: 10.1007/s10147-011-0361-9. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Lee J, Sohn I, Mao M, Kai W, Park CK, Lim HY. A survey of c-MET expression and amplification in 287 patients with hepatocellular carcinoma. Anticancer Res. 2013;33:5179–5186. [PubMed] [Google Scholar]
- 17.Tannapfel A, Busse C, Weinans L, Benicke M, Katalinic A, Geissler F, Hauss J, Wittekind C. INK4a-ARF alterations and p53 mutations in hepatocellular carcinomas. Oncogene. 2001;20:7104–7109. doi: 10.1038/sj.onc.1204902. [DOI] [PubMed] [Google Scholar]
- 18.Anzola M, Cuevas N, Lopez-Martinez M, Martinez de Pancorbo M, Burgos JJ. p16INK4A gene alterations are not a prognostic indicator for survival in patients with hepatocellular carcinoma undergoing curative hepatectomy. J Gastroenterol Hepatol. 2004;19:397–405. doi: 10.1111/j.1440-1746.2003.03305.x. [DOI] [PubMed] [Google Scholar]
- 19.Iizuka N, Oka M, Yamada-Okabe H, Nishida M, Maeda Y, Mori N, Takao T, Tamesa T, Tangoku A, Tabuchi H, et al. Oligonucleotide microarray for prediction of early intrahepatic recurrence of hepatocellular carcinoma after curative resection. Lancet. 2003;361:923–929. doi: 10.1016/S0140-6736(03)12775-4. [DOI] [PubMed] [Google Scholar]
- 20.Kurokawa Y, Matoba R, Takemasa I, Nagano H, Dono K, Nakamori S, Umeshita K, Sakon M, Ueno N, Oba S, et al. Molecular-based prediction of early recurrence in hepatocellular carcinoma. J Hepatol. 2004;41:284–291. doi: 10.1016/j.jhep.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto M, Utsunomiya T, Wakiyama S, Hashimoto M, Fukuzawa K, Ezaki T, Hanai T, Inoue H, Mori M. Specific gene-expression profiles of noncancerous liver tissue predict the risk for multicentric occurrence of hepatocellular carcinoma in hepatitis C virus-positive patients. Ann Surg Oncol. 2006;13:947–954. doi: 10.1245/ASO.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Wang SM, Ooi LL, Hui KM. Identification and validation of a novel gene signature associated with the recurrence of human hepatocellular carcinoma. Clin Cancer Res. 2007;13:6275–6283. doi: 10.1158/1078-0432.CCR-06-2236. [DOI] [PubMed] [Google Scholar]
- 23.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somura H, Iizuka N, Tamesa T, Sakamoto K, Hamaguchi T, Tsunedomi R, Yamada-Okabe H, Sawamura M, Eramoto M, Miyamoto T, et al. A three-gene predictor for early intrahepatic recurrence of hepatocellular carcinoma after curative hepatectomy. Oncol Rep. 2008;19:489–495. [PubMed] [Google Scholar]
- 25.Tanaka S, Arii S, Yasen M, Mogushi K, Su NT, Zhao C, Imoto I, Eishi Y, Inazawa J, Miki Y, et al. Aurora kinase B is a predictive factor for the aggressive recurrence of hepatocellular carcinoma after curative hepatectomy. Br J Surg. 2008;95:611–619. doi: 10.1002/bjs.6011. [DOI] [PubMed] [Google Scholar]
- 26.Woo HG, Park ES, Cheon JH, Kim JH, Lee JS, Park BJ, Kim W, Park SC, Chung YJ, Kim BG, et al. Gene expression-based recurrence prediction of hepatitis B virus-related human hepatocellular carcinoma. Clin Cancer Res. 2008;14:2056–2064. doi: 10.1158/1078-0432.CCR-07-1473. [DOI] [PubMed] [Google Scholar]
- 27.Yoshioka S, Takemasa I, Nagano H, Kittaka N, Noda T, Wada H, Kobayashi S, Marubashi S, Takeda Y, Umeshita K, et al. Molecular prediction of early recurrence after resection of hepatocellular carcinoma. Eur J Cancer. 2009;45:881–889. doi: 10.1016/j.ejca.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Roessler S, Jia HL, Budhu A, Forgues M, Ye QH, Lee JS, Thorgeirsson SS, Sun Z, Tang ZY, Qin LX, et al. A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res. 2010;70:10202–10212. doi: 10.1158/0008-5472.CAN-10-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuchiya M, Parker JS, Kono H, Matsuda M, Fujii H, Rusyn I. Gene expression in nontumoral liver tissue and recurrence-free survival in hepatitis C virus-positive hepatocellular carcinoma. Mol Cancer. 2010;9:74. doi: 10.1186/1476-4598-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng L, Du J, Zhou Q, Cheng B, Li J, Zhang D, Ling C. Identification of cyclin B1 and Sec62 as biomarkers for recurrence in patients with HBV-related hepatocellular carcinoma after surgical resection. Mol Cancer. 2012;11:39. doi: 10.1186/1476-4598-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xieraili M, Yasen M, Mogushi K, Obulhasim G, Mayinuer A, Aihara A, Tanaka S, Mizushima H, Tanaka H, Arii S. Villin 1 is a predictive factor for the recurrence of high serum alpha-fetoprotein-associated hepatocellular carcinoma after hepatectomy. Cancer Sci. 2012;103:1493–1501. doi: 10.1111/j.1349-7006.2012.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsunedomi R, Iizuka N, Yoshimura K, Iida M, Tsutsui M, Hashimoto N, Kanekiyo S, Sakamoto K, Tamesa T, Oka M. ABCB6 mRNA and DNA methylation levels serve as useful biomarkers for prediction of early intrahepatic recurrence of hepatitis C virus-related hepatocellular carcinoma. Int J Oncol. 2013;42:1551–1559. doi: 10.3892/ijo.2013.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YJ, Yeh SH, Chen JT, Wu CC, Hsu MT, Tsai SF, Chen PJ, Lin CH. Chromosomal changes and clonality relationship between primary and recurrent hepatocellular carcinoma. Gastroenterology. 2000;119:431–440. doi: 10.1053/gast.2000.9373. [DOI] [PubMed] [Google Scholar]
- 34.Ho MC, Lin JJ, Chen CN, Chen CC, Lee H, Yang CY, Ni YH, Chang KJ, Hsu HC, Hsieh FJ, et al. A gene expression profile for vascular invasion can predict the recurrence after resection of hepatocellular carcinoma: a microarray approach. Ann Surg Oncol. 2006;13:1474–1484. doi: 10.1245/s10434-006-9057-1. [DOI] [PubMed] [Google Scholar]
- 35.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, Wang XW. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Costa FF, Le Blanc K, Brodin B. Concise review: cancer/testis antigens, stem cells, and cancer. Stem Cells. 2007;25:707–711. doi: 10.1634/stemcells.2006-0469. [DOI] [PubMed] [Google Scholar]
- 37.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 38.Woo HG, Lee JH, Yoon JH, Kim CY, Lee HS, Jang JJ, Yi NJ, Suh KS, Lee KU, Park ES, et al. Identification of a cholangiocarcinoma-like gene expression trait in hepatocellular carcinoma. Cancer Res. 2010;70:3034–3041. doi: 10.1158/0008-5472.CAN-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du Y, Su T, Ding Y, Cao G. Effects of antiviral therapy on the recurrence of hepatocellular carcinoma after curative resection or liver transplantation. Hepat Mon. 2012;12:e6031. doi: 10.5812/hepatmon.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor MW, Tsukahara T, Brodsky L, Schaley J, Sanda C, Stephens MJ, McClintick JN, Edenberg HJ, Li L, Tavis JE, et al. Changes in gene expression during pegylated interferon and ribavirin therapy of chronic hepatitis C virus distinguish responders from nonresponders to antiviral therapy. J Virol. 2007;81:3391–3401. doi: 10.1128/JVI.02640-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta R, Kim S, Taylor MW. Suppression of ribosomal protein synthesis and protein translation factors by Peg-interferon alpha/ribavirin in HCV patients blood mononuclear cells (PBMC) J Transl Med. 2012;10:54. doi: 10.1186/1479-5876-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honda M, Yamashita T, Yamashita T, Arai K, Sakai Y, Sakai A, Nakamura M, Mizukoshi E, Kaneko S. Peretinoin, an acyclic retinoid, improves the hepatic gene signature of chronic hepatitis C following curative therapy of hepatocellular carcinoma. BMC Cancer. 2013;13:191. doi: 10.1186/1471-2407-13-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villanueva A, Hoshida Y, Battiston C, Tovar V, Sia D, Alsinet C, Cornella H, Liberzon A, Kobayashi M, Kumada H, et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology. 2011;140:1501–1512.e2. doi: 10.1053/j.gastro.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyault S, Rickman DS, de Reyniès A, Balabaud C, Rebouissou S, Jeannot E, Hérault A, Saric J, Belghiti J, Franco D, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 45.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiemer EA. The role of microRNAs in cancer: no small matter. Eur J Cancer. 2007;43:1529–1544. doi: 10.1016/j.ejca.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Augello C, Vaira V, Caruso L, Destro A, Maggioni M, Park YN, Montorsi M, Santambrogio R, Roncalli M, Bosari S. MicroRNA profiling of hepatocarcinogenesis identifies C19MC cluster as a novel prognostic biomarker in hepatocellular carcinoma. Liver Int. 2012;32:772–782. doi: 10.1111/j.1478-3231.2012.02795.x. [DOI] [PubMed] [Google Scholar]
- 48.Shih TC, Tien YJ, Wen CJ, Yeh TS, Yu MC, Huang CH, Lee YS, Yen TC, Hsieh SY. MicroRNA-214 downregulation contributes to tumor angiogenesis by inducing secretion of the hepatoma-derived growth factor in human hepatoma. J Hepatol. 2012;57:584–591. doi: 10.1016/j.jhep.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 49.Zhu HT, Dong QZ, Sheng YY, Wei JW, Wang G, Zhou HJ, Ren N, Jia HL, Ye QH, Qin LX. MicroRNA-29a-5p is a novel predictor for early recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection. PLoS One. 2012;7:e52393. doi: 10.1371/journal.pone.0052393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia H, Ooi LL, Hui KM. MiR-214 targets β-catenin pathway to suppress invasion, stem-like traits and recurrence of human hepatocellular carcinoma. PLoS One. 2012;7:e44206. doi: 10.1371/journal.pone.0044206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang YH, Lin KH, Chen HC, Chang ML, Hsu CW, Lai MW, Chen TC, Lee WC, Tseng YH, Yeh CT. Identification of postoperative prognostic microRNA predictors in hepatocellular carcinoma. PLoS One. 2012;7:e37188. doi: 10.1371/journal.pone.0037188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han ZB, Chen HY, Fan JW, Wu JY, Tang HM, Peng ZH. Up-regulation of microRNA-155 promotes cancer cell invasion and predicts poor survival of hepatocellular carcinoma following liver transplantation. J Cancer Res Clin Oncol. 2012;138:153–161. doi: 10.1007/s00432-011-1076-z. [DOI] [PubMed] [Google Scholar]
- 53.Fornari F, Gramantieri L, Giovannini C, Veronese A, Ferracin M, Sabbioni S, Calin GA, Grazi GL, Croce CM, Tavolari S, et al. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009;69:5761–5767. doi: 10.1158/0008-5472.CAN-08-4797. [DOI] [PubMed] [Google Scholar]
- 54.Fornari F, Milazzo M, Chieco P, Negrini M, Calin GA, Grazi GL, Pollutri D, Croce CM, Bolondi L, Gramantieri L. MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2010;70:5184–5193. doi: 10.1158/0008-5472.CAN-10-0145. [DOI] [PubMed] [Google Scholar]
- 55.Yokoo H, Kondo T, Okano T, Nakanishi K, Sakamoto M, Kosuge T, Todo S, Hirohashi S. Protein expression associated with early intrahepatic recurrence of hepatocellular carcinoma after curative surgery. Cancer Sci. 2007;98:665–673. doi: 10.1111/j.1349-7006.2007.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yi X, Luk JM, Lee NP, Peng J, Leng X, Guan XY, Lau GK, Beretta L, Fan ST. Association of mortalin (HSPA9) with liver cancer metastasis and prediction for early tumor recurrence. Mol Cell Proteomics. 2008;7:315–325. doi: 10.1074/mcp.M700116-MCP200. [DOI] [PubMed] [Google Scholar]
- 57.Cheng J, Xie HY, Xu X, Wu J, Wei X, Su R, Zhang W, Lv Z, Zheng S, Zhou L. NDRG1 as a biomarker for metastasis, recurrence and of poor prognosis in hepatocellular carcinoma. Cancer Lett. 2011;310:35–45. doi: 10.1016/j.canlet.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Bai DS, Dai Z, Zhou J, Liu YK, Qiu SJ, Tan CJ, Shi YH, Huang C, Wang Z, He YF, et al. Capn4 overexpression underlies tumor invasion and metastasis after liver transplantation for hepatocellular carcinoma. Hepatology. 2009;49:460–470. doi: 10.1002/hep.22638. [DOI] [PubMed] [Google Scholar]
- 59.Orimo T, Ojima H, Hiraoka N, Saito S, Kosuge T, Kakisaka T, Yokoo H, Nakanishi K, Kamiyama T, Todo S, et al. Proteomic profiling reveals the prognostic value of adenomatous polyposis coli-end-binding protein 1 in hepatocellular carcinoma. Hepatology. 2008;48:1851–1863. doi: 10.1002/hep.22552. [DOI] [PubMed] [Google Scholar]
- 60.Kanamori H, Kawakami T, Effendi K, Yamazaki K, Mori T, Ebinuma H, Masugi Y, Du W, Nagasaka K, Ogiwara A, et al. Identification by differential tissue proteome analysis of talin-1 as a novel molecular marker of progression of hepatocellular carcinoma. Oncology. 2011;80:406–415. doi: 10.1159/000330734. [DOI] [PubMed] [Google Scholar]
- 61.Beyoğlu D, Idle JR. The metabolomic window into hepatobiliary disease. J Hepatol. 2013;59:842–858. doi: 10.1016/j.jhep.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soga T, Sugimoto M, Honma M, Mori M, Igarashi K, Kashikura K, Ikeda S, Hirayama A, Yamamoto T, Yoshida H, et al. Serum metabolomics reveals γ-glutamyl dipeptides as biomarkers for discrimination among different forms of liver disease. J Hepatol. 2011;55:896–905. doi: 10.1016/j.jhep.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 63.Nahon P, Amathieu R, Triba MN, Bouchemal N, Nault JC, Ziol M, Seror O, Dhonneur G, Trinchet JC, Beaugrand M, et al. Identification of serum proton NMR metabolomic fingerprints associated with hepatocellular carcinoma in patients with alcoholic cirrhosis. Clin Cancer Res. 2012;18:6714–6722. doi: 10.1158/1078-0432.CCR-12-1099. [DOI] [PubMed] [Google Scholar]
- 64.Wang B, Chen D, Chen Y, Hu Z, Cao M, Xie Q, Chen Y, Xu J, Zheng S, Li L. Metabonomic profiles discriminate hepatocellular carcinoma from liver cirrhosis by ultraperformance liquid chromatography-mass spectrometry. J Proteome Res. 2012;11:1217–1227. doi: 10.1021/pr2009252. [DOI] [PubMed] [Google Scholar]
- 65.Ressom HW, Xiao JF, Tuli L, Varghese RS, Zhou B, Tsai TH, Ranjbar MR, Zhao Y, Wang J, Di Poto C, et al. Utilization of metabolomics to identify serum biomarkers for hepatocellular carcinoma in patients with liver cirrhosis. Anal Chim Acta. 2012;743:90–100. doi: 10.1016/j.aca.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen T, Xie G, Wang X, Fan J, Qiu Y, Zheng X, Qi X, Cao Y, Su M, Wang X, et al. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Mol Cell Proteomics. 2011;10:M110.004945. doi: 10.1074/mcp.M110.004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan Y, Yin P, Tang L, Xing W, Huang Q, Cao D, Zhao X, Wang W, Lu X, Xu Z, et al. Metabolomics study of stepwise hepatocarcinogenesis from the model rats to patients: potential biomarkers effective for small hepatocellular carcinoma diagnosis. Mol Cell Proteomics. 2012;11:M111.010694. doi: 10.1074/mcp.M111.010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ye G, Zhu B, Yao Z, Yin P, Lu X, Kong H, Fan F, Jiao B, Xu G. Analysis of urinary metabolic signatures of early hepatocellular carcinoma recurrence after surgical removal using gas chromatography-mass spectrometry. J Proteome Res. 2012;11:4361–4372. doi: 10.1021/pr300502v. [DOI] [PubMed] [Google Scholar]
- 69.Beyoğlu D, Imbeaud S, Maurhofer O, Bioulac-Sage P, Zucman-Rossi J, Dufour JF, Idle JR. Tissue metabolomics of hepatocellular carcinoma: tumor energy metabolism and the role of transcriptomic classification. Hepatology. 2013;58:229–238. doi: 10.1002/hep.26350. [DOI] [PMC free article] [PubMed] [Google Scholar]


