Abstract
Inflammatory bowel disease (IBD) patients have an increased risk of venous thromboembolism (VTE), which represents a significant cause of morbidity and mortality. The most common sites of VTE in IBD patients are the deep veins of the legs and pulmonary system, followed by the portal and mesenteric veins. However, other sites may also be involved, such as the cerebrovascular and retinal veins. The aetiology of VTE is multifactorial, including both inherited and acquired risk factors that, when simultaneously present, multiply the risk to the patient. VTE prevention involves correcting modifiable risk factors, such as disease activity, vitamin deficiency, dehydration and prolonged immobilisation. The role of mechanical and pharmacological prophylaxis against VTE using anticoagulants is also crucial. However, although guidelines recommend thromboprophylaxis for IBD patients, this method is still poorly implemented because of concerns about its safety and a lack of awareness of the magnitude of thrombotic risk in these patients. Further efforts are required to increase the rate of pharmacological prevention of VTE in IBD patients to avoid preventable morbidity and mortality.
Keywords: Inflammatory bowel disease, Venous thromboembolism, Thromboembolic prophylaxis, Anticoagulants, Unfractionated heparin, Low molecular weight heparin
Core tip: Inflammatory bowel diseases (IBD) patients have an increased risk of venous thromboembolism (VTE) that represents a significant cause of morbidity and mortality. The prevention of VTE involves the correction of modifiable risk factors, such as: disease activity, vitamin deficiency, dehydration and prolonged immobilization. Essential is also the role of mechanical and pharmacological prophylaxis. However, thromboprophylaxis in IBD patients, although guideline-recommended is still poorly implemented because of concerns about its safety and, over all, the lack of awareness of the magnitude of thrombotic risk in these patients. Further efforts are required to increase the rate of pharmacological prevention of VTE in IBD so to avoid some preventable morbidity and mortality.
INTRODUCTION
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic disorders that predominantly affect the bowel; however, IBD can also be associated with numerous extraintestinal complications. Among these complications, venous thromboembolism (VTE) is particularly important, due to both its high prevalence and its significant morbidity and mortality[1-9]. However, despite extensive evidence supporting the association between IBD and VTE, among physicians, there is still a lack of recognition of this risk, with dangerous consequences for patients[8,10]. Thus, the aim of this review is to summarise the most recent evidence regarding the prevention and treatment of VTE in IBD patients in light of the newest epidemiological data on this feared association.
EPIDEMIOLOGY AND CLINICAL FEATURES OF VTE IN IBD PATIENTS
Epidemiological data
IBD patients have a 2- to 3-fold increased risk of developing deep venous thrombosis (DVT) and pulmonary embolism (PE) compared with the general population[1,3-10]. In their population-based cohort study, Bernstein et al[7] found an incidence rate of DVT of 30.7 per 10000 person-years in IBD patients (30.0 for UC patients and 31.4 for CD patients) and 14.9 per 10000 person-years for PE in the entire IBD population (19.8 for UC and 10.3 for CD). The overall relative risk (RR) of VTE reported in this study was of 3.47 (95%CI: 2.94-4.09)[7]. These findings were confirmed by a recent, large population-based study from Denmark reporting an incidence rate of VTE of 24 per 10000 person-years among IBD patients (24.4 for UC patients and 23.3 for CD) compared with an incidence rate of 13.4 per 10000 person-years in a non-IBD cohort matched for age and gender[4]. Although the incidence of VTE increases with age, the highest RR for VTE was observed among patients younger than 40 years of age[3-7], whereas no significant differences linked to sex or the type of IBD were found[1-10]. VTE occurred more frequently during phases of active disease and in patients with extended disease (pancolitis in UC patients and extensive colonic involvement in CD)[3-6]. Recently, Grainge et al[5] conducted an epidemiological study that aimed to quantify the risk of VTE during different activity phases of IBD. The researchers confirmed that IBD patients had the highest risk of VTE at the time of a flare (hazard ratio of 8.4 compared with controls), although an increased risk still persisted during disease quiescence (hazard ratio of 2.1 compared with controls). These findings support the hypothesis of a procoagulant tendency in IBD patients[2,11]. Indeed, Miehsler et al[3] demonstrated that VTE is a specific feature of IBD because neither rheumatoid arthritis, another chronic inflammatory disease, nor celiac disease, another chronic bowel disease, was accompanied by an increased risk of VTE compared with controls. Furthermore, these data were confirmed by the finding that among 17 chronic illnesses that were evaluated using the United Kingdom primary care General Practice Research Database, only cancer and heart failure carried a greater risk of VTE than IBD did[12]. Interestingly, another study reported that the RR of VTE in pregnant females with IBD was even greater than in pregnant females without IBD with an OR of 8.44 (95%CI: 3.71-19.20) for UC and 6.12 (95%CI: 2.91-12.9) for CD[13]. Regarding the mortality rate in patients with IBD and VTE, the existing data indicate, significant 2.5-fold-increased odds of mortality associated with VTE-related hospitalisations compared with non-VTE-related hospitalisations[8]. In addition, a revisit of a series of 98 IBD patients with VTE evaluated at the Mayo Clinic over a decade (1990-2000) reported a 22% mortality rate[6], which is similar to the 18% mortality rate that was reported in a cohort of IBD patients with VTE two decades earlier at the same institution[9].
VTE location
VTE occurs primarily in the deep veins of the legs and in the pulmonary system and, less frequently, in the cerebrovascular system, portal vein, retinal vein, and mesenteric veins (Figure 1)[14-17]. Recently, a cohort study aimed to determine the location and clinical features of the first VTE in IBD patients and confirmed this finding[18]. Of 157 IBD patients with a history of VTE, 142 (90.4%) had DVT and/or PE, whereas 15 (9.6%) had cerebral, portal, mesenteric, splenic or internal jugular vein thrombosis[18].
Figure 1.
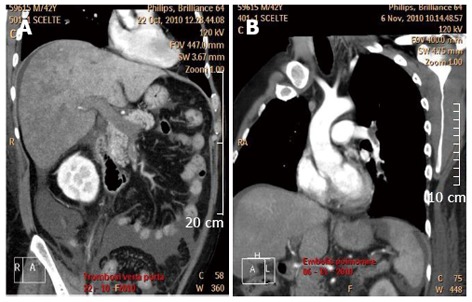
Computed tomography scan showing portal vein thrombosis (A) and a pulmonary embolism (B) in a patient with active ulcerative colitis.
Risk factors for VTE
Although a detailed exploration of risk factors is beyond the scope of this review, we must remember that VTE in patients with IBD is a multifactorial event that involves both hereditary and acquired factors that can coexist, thereby multiplying the individual prothrombotic risk[2,11,19]. The main modifiable acquired risk factors for VTE in IBD patients are reported in Table 1. Several are intuitively more frequent in IBD patients compared with the general population, such as dehydration, indwelling catheters, prolonged immobilisation, hyperhomocysteinaemia, surgical interventions, and active disease with an “inflammatory burden”. The mutual interactions between inflammation and coagulation have been extensively studied, and IBD represents a paradigmatic model for this complex interplay[20,21]. Indeed, in IBD, several mechanisms triggered by active inflammation are involved in moving the coagulative balance towards a prothrombotic state, including (1) increased plasmatic levels of recognised risk factors for thrombosis, several of which are also considered to be acute-phase reactants, and decreased levels of natural anticoagulants; (2) reduced fibrinolytic activity; (3) endothelial abnormalities that are mainly represented by the downregulation of the anticoagulant thrombomodulin and endothelial protein C receptor, which in turn affects the conversion of protein C into its activated form; and (4) abnormalities of platelets, such as thrombocytosis and increased activation and aggregation[11]. Concerning the inherited risk factors for VTE, the most common factors are: factor V Leiden mutation, G20210A mutation of the prothrombin gene, and homozygous C677T mutation in the methylenetetrahydrofolate reductase gene. However, no increased prevalence of these genetic prothrombotic factors has been found in IBD patients with or without VTE[19]. Therefore, patients with IBD face both the VTE risk factors (acquired and inherited) identified in the general population and those specific to IBD, and the prevention and control of those risk factors are of paramount importance for thromboprophylaxis in this patient population.
Table 1.
Acquired risk factors for venous thromboembolism in inflammatory bowel disease patients and modalities for their prevention and/or treatment
| Risk factor | Prevention/treatment modality |
| Active disease ("inflammatory burden") | Effective anti-inflammatory treatment |
| Smoking | Programmes for smoking cessation |
| Oral contraceptive use | Advise alternative methods of contraception |
| Hyperhomocysteinaemia | Assess the presence of vitamin deficiency (vitamins B6 and B12 and folic acid) and correct if necessary |
| Dehydration | Provide adequate hydration |
| Prolonged immobilisation | Early mobilisation, especially after surgery; graduated compression stockings or pneumatic devices |
| Infections | Timely diagnosis and treatment of infections |
| Indwelling catheters | Limit the use of venous catheters; when possible, administer oral and enteral nutrition |
| Obesity | Encourage weight loss (diet, exercise) |
| Long-distance travel | Frequent ambulation, exercise, hydration |
PROPHYLAXIS AGAINST VTE IN IBD PATIENTS
Non-pharmacological prophylaxis
As described above, VTE in IBD is a multifactorial process in which acquired risk factors seem to play the most important role; therefore, these factors’ prevention and/or treatment can lead to effective prophylaxis. Hydration, correction of deficiencies in vitamins (particularly in vitamins B6 and B12 and folate) that can reduce homocysteine levels[22], graduated compression stockings or pneumatic devices, and early mobilisation after surgery should be always considered, especially in hospitalised IBD patients (Table 1). Additionally, even in the absence of direct evidence, it might be expected that the control of disease activity could decrease the risk of VTE by reducing the already-mentioned procoagulant factors that are closely associated with active inflammation. Furthermore, many drugs used for IBD treatment have shown anti-inflammatory activity and an anticoagulant effects. Indeed, mesalamine can reduce platelet activation[23], azathioprine and 6-mercaptopurine inhibit platelet aggregation in vitro[24], and infliximab normalises haemostatic parameters and reduces the amount of circulating microparticles and the levels of prothrombotic sCD40L in CD patients[25,26].
Pharmacological prophylaxis
Prophylactic anticoagulation in IBD patients is recommended by several practice guidelines for conditions associated with a higher risk of VTE, particularly in hospitalised patients with active disease[27-31]. Low molecular-weight heparin (LMWH) and unfractionated heparin (UH) are recommended for thromboprophylaxis in IBD patients. The recommendations for VTE prophylaxis in IBD patients that are included in the current guidelines are summarised in Table 2. Although no randomised controlled trials (RCTs) have specifically assessed the efficacy of anticoagulation for VTE prophylaxis in IBD patients, several RCTs have demonstrated that in acutely ill medical patients pharmacological prophylaxis significantly reduces the incidence of VTE[32,33]. Only data from observational studies including IBD patients undergoing thromboprophylaxis with LMWH in the perioperative setting are available[34,35]. Scarpa et al[34] collected data on 755 colorectal surgical procedures, 383 of which were performed in IBD patients. All patients had received 4000 IU/d LMWH from the day of operation through to the discharge. Six postoperative thromboembolic events occurred in this population, all in IBD patients; of these events, two occurred in CD patients (clinical DVT rate of 1.2%) and four occurred in UC patients (clinical DVT rate of 2.6%)[34]. Similar data were reported by an Irish study in which the rates of postoperative VTE were evaluated both in 79 UC patients undergoing 180 major intra-abdominal surgeries and in 18 patients with familial adenomatous polyposis (FAP) undergoing 35 surgical operations of similar complexity[35]. All patients were treated with standard perioperative VTE prophylaxis. Only three UC patients (1.7%) developed VTE, compared with no patients with FAP[35]. Unfortunately, in both of these studies, a control group without prophylaxis was not included. Therefore, we can only hypothesise a benefit of LMWH prophylaxis because the VTE rates reported in a large cohort of hospitalised IBD patients in the United States were similar to those found in the above-mentioned studies. More specifically, Nguyen et al[8] extracting data from 73197 discharges for CD and 43645 discharges for UC and found that the crude rates of VTE were 21 per 1000 hospitalisations for UC patients and 13.9 per 1000 hospitalisations for CD patients. However, we should note that in the population analysed by Nguyen et al[8] only 18% and 11% of CD and UC patients, respectively, underwent bowel surgery during their hospitalisations and that abdominal surgery is a strong predictor of developing VTE. In conclusion, available evidence on the efficacy of thromboprophylaxis in IBD is still scarce, and RCTs that aim to ascertain this issue are warranted.
Table 2.
Published guidelines for the prevention of venous thromboembolism in inflammatory bowel disease patients
| Scientific society (reference) | Recommendations | Type of population at risk |
| European Crohn's and Colitis Organisation (ECCO)[29] | Mechanical thromboprophylaxis and/or heparin administration (UH or LMWH) | UC |
| European Crohn's and Colitis Organisation (ECCO)[28] | Consider VTE prophylaxis (UH, LMWH, or fondaparinux) in all hospitalised patients | CD |
| British Society of Gastroenterology (BSG)[31] | Pharmacological VTE prophylaxis for hospitalised patients with severe UC | UC |
| American College of Gastroenterology (ACG)[30] | VTE prophylaxis with heparin for hospitalised patients with severe UC | UC |
| American College of Chest Physicians (ACCP)[27] | Mechanical thromboprophylaxis with GCS or IPC; anticoagulant thromboprophylaxis with LMWH, UH or fondaparinux when bleeding risk decreases | Acutely ill hospitalised medical patients at increased risk of thrombosis who are bleeding or at high risk of bleeding |
GCS: Graduated compression stockings; IPC: Intermittent pneumatic compression; CD: Crohn’s disease; UC: Ulcerative colitis; VTE: Venous thromboembolism; LMWH: Low molecular-weight heparin; UH: Unfractionated heparin.
Issues associated to the adherence to the pharmacological prophylaxis
Another key topic is the low rate of VTE pharmacologic prophylaxis in hospitalised IBD patients, and, particularly in those admitted for medical services compared with those admitted for surgery, despite the recommendations provided by the guidelines[36,37]. The inadequate use of anticoagulants for VTE prophylaxis in IBD is mainly related to two factors: (1) gastroenterologists’ lack of awareness of both the increased risk of VTE in IBD patients and the guideline-recommended use of pharmacological prophylaxis in hospitalised IBD patients[38]; and (2) concerns about the safety of anticoagulant drugs in patients with active IBD[36,39]. However, a recent retrospective study of 974 IBD inpatients with a reported rate of pharmacological prophylaxis of 80% at admission showed that the rates of major and minor bleeding were similar for patients who received VTE prophylaxis and those who did not[36]. Moreover, VTE prophylaxis was not associated with major postoperative bleeding[36]. Indirect evidence of the safety of anticoagulation in IBD patients during an active flare also comes from certain clinical trials in which UH or LMWH was used to treat UC[40-42]. A meta-analysis of eight clinical trials showed that few serious adverse events were observed in patients treated with UH or LMWH compared with controls, with no significant difference in any trial[41]. In particular, only in one study, three patients with moderate-to-severe UC included in the heparin group were withdrawn from the study because of worsening of rectal bleeding[42]. One of these patients required urgent colectomy. Additionally, in the control group, one patient developed toxic megacolon and underwent urgent surgery. The remaining seven clinical trials showed no bleeding-related adverse events in their heparin groups[41]. All of this evidence confirms that the prophylactic use of anticoagulants in hospitalised IBD patients with acute disease is safe, despite the presence of bleeding at admission. Another unresolved issue is whether thromboprophylaxis should be extended to all ambulatory patients with disease exacerbation or only to a subgroup considered to be at a higher risk of VTE. As previously reported, the highest risk of VTE in IBD patients is during phases of active disease[3-5]; however, in the context of active disease, Grainge et al[5] found that the RR was higher during non-hospitalised periods than during hospitalised periods (hazard ratio of 18.8 vs 3.2). These data suggest that hospitalisation should not be considered as the only discriminant factor for thromboprophylaxis and that anticoagulation could also be extended to a subgroup of ambulatory patients with active disease and other significant risk factors for VTE. However, this finding should be interpreted with caution because the absolute risk of VTE is much more informative than the RR, although not always known for an individual patient. In fact, in the same study, the absolute risk of VTE of a patient hospitalised for an IBD flare was nearly six times higher than the absolute risk during an ambulatory flare (37.5 per 1000 person-years vs 6.4 per 1000 person-years)[5]. Thus, for each patient with active IBD, the absolute risk of VTE should be carefully assessed, including the personal and family histories of VTE, the presence of cardiovascular or respiratory diseases, obesity, information on the use of oral contraceptives and smoking status, the presence of genetic prothrombotic risk factors, reduced mobility, and the presence of venous catheters[43]. Additionally, as previously reported, also disease features could help in assessing the individual prothrombotic risk[3-10]. Lastly, it is well known that surgery represents a major risk factor for VTE, particularly in patients with IBD[8], and thromboprophylaxis is universally performed during the perioperative period. A recent retrospective review of patient data obtained from the American College of Surgeons National Surgical Quality Improvement Program aimed to identify modifiable risk factors for short-term (30-d) postoperative VTE[44]. The study reported that the following actions can potentially reduce the incidence of VTE in the surgical setting: correcting preoperative coagulopathy and/or anaemia, improving nutritional status, reducing steroid use, operating early to avoid emergency surgery, and limiting anaesthesia time[44].
TREATMENT OF VTE IN IBD PATIENTS
The treatment of VTE in patients affected by IBD is the same as the treatment for subjects without IBD[28,29]. If there is no haemodynamically significant bleeding or an indication of thrombolysis, LMWH is the ideal treatment. LMWH is usually switched to an oral vitamin K antagonist (i.e., warfarin). The duration of therapy with anticoagulants is not well established because the possibility of VTE recurrence in IBD patients should be balanced with the bleeding risk caused by anticoagulant use. In fact, a recent study showed that IBD patients who experience their first episode of unprovoked VTE have a 33% risk of a second episode of VTE within 5 years, with a risk of recurrence that is 2.5-fold higher than that of non-IBD patients after an initial episode of unprovoked VTE[45]. Nguyen and Bernstein[46] conducted a decision analysis study to compare the costs and effectiveness of time-limited anticoagulation (for 6 mo) and extended anticoagulation for the management of VTE in IBD. The study found that among IBD patients who have had unprovoked VTE, the benefits of long-term coagulation in reducing recurrent VTE outweigh the risks of the associated bleeding. In particular, extended anticoagulation may be more appropriate for patients who developed VTE in the absence of active disease or other transient provoking factors[46]. In the general population, local thrombolytic therapy is indicated for massive thrombosis and for life-threatening VTE, and several cases of successful catheter-directed thrombolytic treatment in IBD patients have been reported[47]. Additionally, the placement of inferior vena cava (IVC) filters is indicated in cases of floating thrombi in the deep veins of the legs and recurrent PE despite anticoagulant therapy and in cases with a high risk of bleeding (Figure 2)[48].
Figure 2.
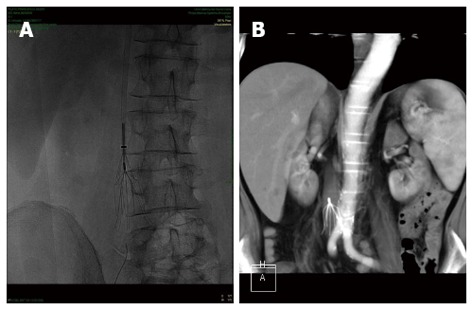
Plain abdominal Rx (A) and computed tomography scan (B) showing an inferior vena cava filter placed for the prevention of recurrent pulmonary embolism in a patient with Crohn’s disease and deep venous thrombosis.
CONCLUSION
IBD patients have a risk of VTE that is 2- to 3-fold greater than that of the general population. This risk is higher during disease flares, both for inpatients and outpatients. However, during hospitalisation, multiple prothrombotic risk factors other than active disease act synergistically, multiplying the absolute risk of VTE. Because VTE has significant morbidity and mortality, its prevention is mandatory. VTE prevention involves correcting modifiable risk factors and administering pharmacological prophylaxis. However, although guidelines recommend thromboprophylaxis for IBD patients, it is still poorly implemented because of concerns about its safety and a lack of awareness of the magnitude of thrombotic risk in these patients. Therefore, further efforts are required to increase the rate of pharmacological prevention of VTE in IBD patients to avoid preventable morbidity and mortality.
Footnotes
P- Reviewers: Femia AN, Feuerstein JD, Kisiel JB, Zhang L S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Yuhara H, Steinmaus C, Corley D, Koike J, Igarashi M, Suzuki T, Mine T. Meta-analysis: the risk of venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:953–962. doi: 10.1111/apt.12294. [DOI] [PubMed] [Google Scholar]
- 2.Papa A, Scaldaferri F, Danese S, Guglielmo S, Roberto I, Bonizzi M, Mocci G, Felice C, Ricci C, Andrisani G, et al. Vascular involvement in inflammatory bowel disease: pathogenesis and clinical aspects. Dig Dis. 2008;26:149–155. doi: 10.1159/000116773. [DOI] [PubMed] [Google Scholar]
- 3.Miehsler W, Reinisch W, Valic E, Osterode W, Tillinger W, Feichtenschlager T, Grisar J, Machold K, Scholz S, Vogelsang H, et al. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut. 2004;53:542–548. doi: 10.1136/gut.2003.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kappelman MD, Horvath-Puho E, Sandler RS, Rubin DT, Ullman TA, Pedersen L, Baron JA, Sørensen HT. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population-based nationwide study. Gut. 2011;60:937–943. doi: 10.1136/gut.2010.228585. [DOI] [PubMed] [Google Scholar]
- 5.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375:657–663. doi: 10.1016/S0140-6736(09)61963-2. [DOI] [PubMed] [Google Scholar]
- 6.Solem CA, Loftus EV, Tremaine WJ, Sandborn WJ. Venous thromboembolism in inflammatory bowel disease. Am J Gastroenterol. 2004;99:97–101. doi: 10.1046/j.1572-0241.2003.04026.x. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein CN, Blanchard JF, Houston DS, Wajda A. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: a population-based cohort study. Thromb Haemost. 2001;85:430–434. [PubMed] [Google Scholar]
- 8.Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:2272–2280. doi: 10.1111/j.1572-0241.2008.02052.x. [DOI] [PubMed] [Google Scholar]
- 9.Talbot RW, Heppell J, Dozois RR, Beart RW. Vascular complications of inflammatory bowel disease. Mayo Clin Proc. 1986;61:140–145. doi: 10.1016/s0025-6196(12)65200-8. [DOI] [PubMed] [Google Scholar]
- 10.Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: an epidemiological review. Am J Gastroenterol. 2011;106:713–718. doi: 10.1038/ajg.2011.53. [DOI] [PubMed] [Google Scholar]
- 11.Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol. 2007;102:174–186. doi: 10.1111/j.1572-0241.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 12.Huerta C, Johansson S, Wallander MA, García Rodríguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167:935–943. doi: 10.1001/archinte.167.9.935. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen GC, Boudreau H, Harris ML, Maxwell CV. Outcomes of obstetric hospitalizations among women with inflammatory bowel disease in the United States. Clin Gastroenterol Hepatol. 2009;7:329–334. doi: 10.1016/j.cgh.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Johns DR. Cerebrovascular complications of inflammatory bowel disease. Am J Gastroenterol. 1991;86:367–370. [PubMed] [Google Scholar]
- 15.Landman C, Nahon S, Cosnes J, Bouhnik Y, Brixi-Benmansour H, Bouguen G, Colombel JF, Savoye G, Coffin B, Abitbol V, et al. Portomesenteric vein thrombosis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:582–589. doi: 10.1097/MIB.0b013e31827eea5f. [DOI] [PubMed] [Google Scholar]
- 16.Schneiderman JH, Sharpe JA, Sutton DM. Cerebral and retinal vascular complications of inflammatory bowel disease. Ann Neurol. 1979;5:331–337. doi: 10.1002/ana.410050405. [DOI] [PubMed] [Google Scholar]
- 17.Hatoum OA, Spinelli KS, Abu-Hajir M, Attila T, Franco J, Otterson MF, Telford GL, Binion DG. Mesenteric venous thrombosis in inflammatory bowel disease. J Clin Gastroenterol. 2005;39:27–31. [PubMed] [Google Scholar]
- 18.Papay P, Miehsler W, Tilg H, Petritsch W, Reinisch W, Mayer A, Haas T, Kaser A, Feichtenschlager T, Fuchssteiner H, et al. Clinical presentation of venous thromboembolism in inflammatory bowel disease. J Crohns Colitis. 2013;7:723–729. doi: 10.1016/j.crohns.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Papa A, Danese S, Grillo A, Gasbarrini G, Gasbarrini A. Review article: inherited thrombophilia in inflammatory bowel disease. Am J Gastroenterol. 2003;98:1247–1251. doi: 10.1111/j.1572-0241.2003.07491.x. [DOI] [PubMed] [Google Scholar]
- 20.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131:417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 21.Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109:2698–2704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 22.Oldenburg B, Van Tuyl BA, van der Griend R, Fijnheer R, van Berge Henegouwen GP. Risk factors for thromboembolic complications in inflammatory bowel disease: the role of hyperhomocysteinaemia. Dig Dis Sci. 2005;50:235–240. doi: 10.1007/s10620-005-1588-y. [DOI] [PubMed] [Google Scholar]
- 23.Carty E, MacEy M, Rampton DS. Inhibition of platelet activation by 5-aminosalicylic acid in inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1169–1179. doi: 10.1046/j.1365-2036.2000.00824.x. [DOI] [PubMed] [Google Scholar]
- 24.Thomas G, Skrinska VA, Lucas FV. The influence of glutathione and other thiols on human platelet aggregation. Thromb Res. 1986;44:859–866. doi: 10.1016/0049-3848(86)90031-9. [DOI] [PubMed] [Google Scholar]
- 25.Hommes DW, van Dullemen HM, Levi M, van der Ende A, Woody J, Tytgat GN, van Deventer SJ. Beneficial effect of treatment with a monoclonal anti-tumor necrosis factor-alpha antibody on markers of coagulation and fibrinolysis in patients with active Crohn’s disease. Haemostasis. 1997;27:269–277. doi: 10.1159/000217467. [DOI] [PubMed] [Google Scholar]
- 26.Danese S, Sans M, Scaldaferri F, Sgambato A, Rutella S, Cittadini A, Piqué JM, Panes J, Katz JA, Gasbarrini A, et al. TNF-alpha blockade down-regulates the CD40/CD40L pathway in the mucosal microcirculation: a novel anti-inflammatory mechanism of infliximab in Crohn’s disease. J Immunol. 2006;176:2617–2624. doi: 10.4049/jimmunol.176.4.2617. [DOI] [PubMed] [Google Scholar]
- 27.Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, Le H, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e195S–e226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Assche G, Dignass A, Reinisch W, van der Woude CJ, Sturm A, De Vos M, Guslandi M, Oldenburg B, Dotan I, Marteau P, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Special situations. J Crohns Colitis. 2010;4:63–101. doi: 10.1016/j.crohns.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Van Assche G, Dignass A, Bokemeyer B, Danese S, Gionchetti P, Moser G, Beaugerie L, Gomollón F, Häuser W, Herrlinger K, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013;7:1–33. doi: 10.1016/j.crohns.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–23; quiz 524. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 31.Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 32.Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146:278–288. doi: 10.7326/0003-4819-146-4-200702200-00007. [DOI] [PubMed] [Google Scholar]
- 33.Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–879. doi: 10.1161/01.CIR.0000138928.83266.24. [DOI] [PubMed] [Google Scholar]
- 34.Scarpa M, Pilon F, Pengo V, Romanato G, Ruffolo C, Erroi F, Elisa B, Frego M, Ossi E, Manzato E, et al. Deep venous thrombosis after surgery for inflammatory bowel disease: is standard dose low molecular weight heparin prophylaxis enough? World J Surg. 2010;34:1629–1636. doi: 10.1007/s00268-010-0490-8. [DOI] [PubMed] [Google Scholar]
- 35.O’Connor OJ, Cahill RA, Kirwan WO, Redmond HP. The incidence of postoperative venous thrombosis among patients with ulcerative colitis. Ir J Med Sci. 2005;174:20–22. doi: 10.1007/BF03169142. [DOI] [PubMed] [Google Scholar]
- 36.Ra G, Thanabalan R, Ratneswaran S, Nguyen GC. Predictors and safety of venous thromboembolism prophylaxis among hospitalized inflammatory bowel disease patients. J Crohns Colitis. 2013;7:e479–e485. doi: 10.1016/j.crohns.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Tinsley A, Naymagon S, Enomoto LM, Hollenbeak CS, Sands BE, Ullman TA. Rates of pharmacologic venous thromboembolism prophylaxis in hospitalized patients with active ulcerative colitis: results from a tertiary care center. J Crohns Colitis. 2013;7:e635–e640. doi: 10.1016/j.crohns.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Sam JJ, Bernstein CN, Razik R, Thanabalan R, Nguyen GC. Physicians’ perceptions of risks and practices in venous thromboembolism prophylaxis in inflammatory bowel disease. Dig Dis Sci. 2013;58:46–52. doi: 10.1007/s10620-012-2435-6. [DOI] [PubMed] [Google Scholar]
- 39.Tinsley A, Naymagon S, Trindade AJ, Sachar DB, Sands BE, Ullman TA. A survey of current practice of venous thromboembolism prophylaxis in hospitalized inflammatory bowel disease patients in the United States. J Clin Gastroenterol. 2013;47:e1–e6. doi: 10.1097/MCG.0b013e31824c0dea. [DOI] [PubMed] [Google Scholar]
- 40.Papa A, Danese S, Gasbarrini A, Gasbarrini G. Review article: potential therapeutic applications and mechanisms of action of heparin in inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1403–1409. doi: 10.1046/j.1365-2036.2000.00860.x. [DOI] [PubMed] [Google Scholar]
- 41.Shen J, Ran ZH, Tong JL, Xiao SD. Meta-analysis: The utility and safety of heparin in the treatment of active ulcerative colitis. Aliment Pharmacol Ther. 2007;26:653–663. doi: 10.1111/j.1365-2036.2007.03418.x. [DOI] [PubMed] [Google Scholar]
- 42.Panés J, Esteve M, Cabré E, Hinojosa J, Andreu M, Sans M, Fernandez-Bañares F, Feu F, Gassull MA, Piqué JM. Comparison of heparin and steroids in the treatment of moderate and severe ulcerative colitis. Gastroenterology. 2000;119:903–908. doi: 10.1053/gast.2000.18159. [DOI] [PubMed] [Google Scholar]
- 43.Zitomersky NL, Verhave M, Trenor CC. Thrombosis and inflammatory bowel disease: a call for improved awareness and prevention. Inflamm Bowel Dis. 2011;17:458–470. doi: 10.1002/ibd.21334. [DOI] [PubMed] [Google Scholar]
- 44.Wallaert JB, De Martino RR, Marsicovetere PS, Goodney PP, Finlayson SR, Murray JJ, Holubar SD. Venous thromboembolism after surgery for inflammatory bowel disease: are there modifiable risk factors? Data from ACS NSQIP. Dis Colon Rectum. 2012;55:1138–1144. doi: 10.1097/DCR.0b013e3182698f60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novacek G, Weltermann A, Sobala A, Tilg H, Petritsch W, Reinisch W, Mayer A, Haas T, Kaser A, Feichtenschlager T, et al. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology. 2010;139:779–87, 787.e1. doi: 10.1053/j.gastro.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen GC, Bernstein CN. Duration of anticoagulation for the management of venous thromboembolism in inflammatory bowel disease: a decision analysis. Am J Gastroenterol. 2013;108:1486–1495. doi: 10.1038/ajg.2013.220. [DOI] [PubMed] [Google Scholar]
- 47.Tabibian JH, Streiff MB. Inflammatory bowel disease-associated thromboembolism: a systematic review of outcomes with anticoagulation versus catheter-directed thrombolysis. Inflamm Bowel Dis. 2012;18:161–171. doi: 10.1002/ibd.21307. [DOI] [PubMed] [Google Scholar]
- 48.Lopez PR, Stewart DW, Smalligan RD. Recurrent deep vein thrombosis despite warfarin therapy in a patient with Crohn’s disease. Postgrad Med. 2010;122:181–184. doi: 10.3810/pgm.2010.05.2155. [DOI] [PubMed] [Google Scholar]


