Abstract
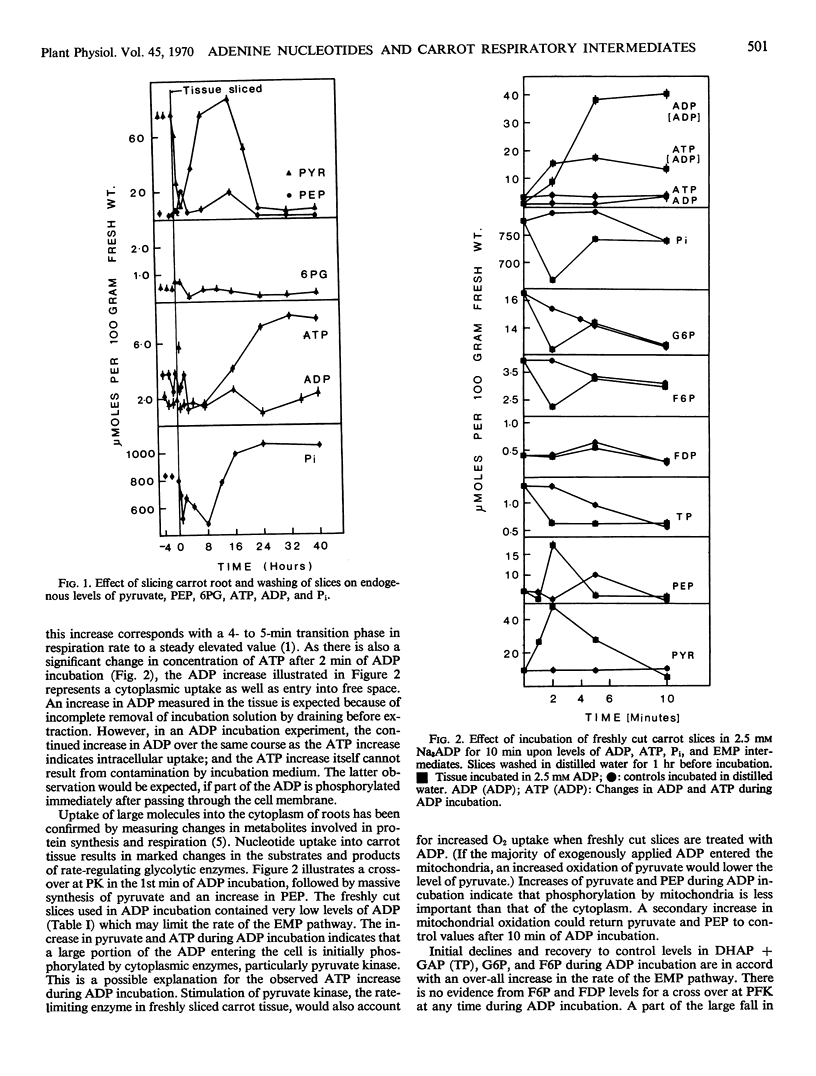
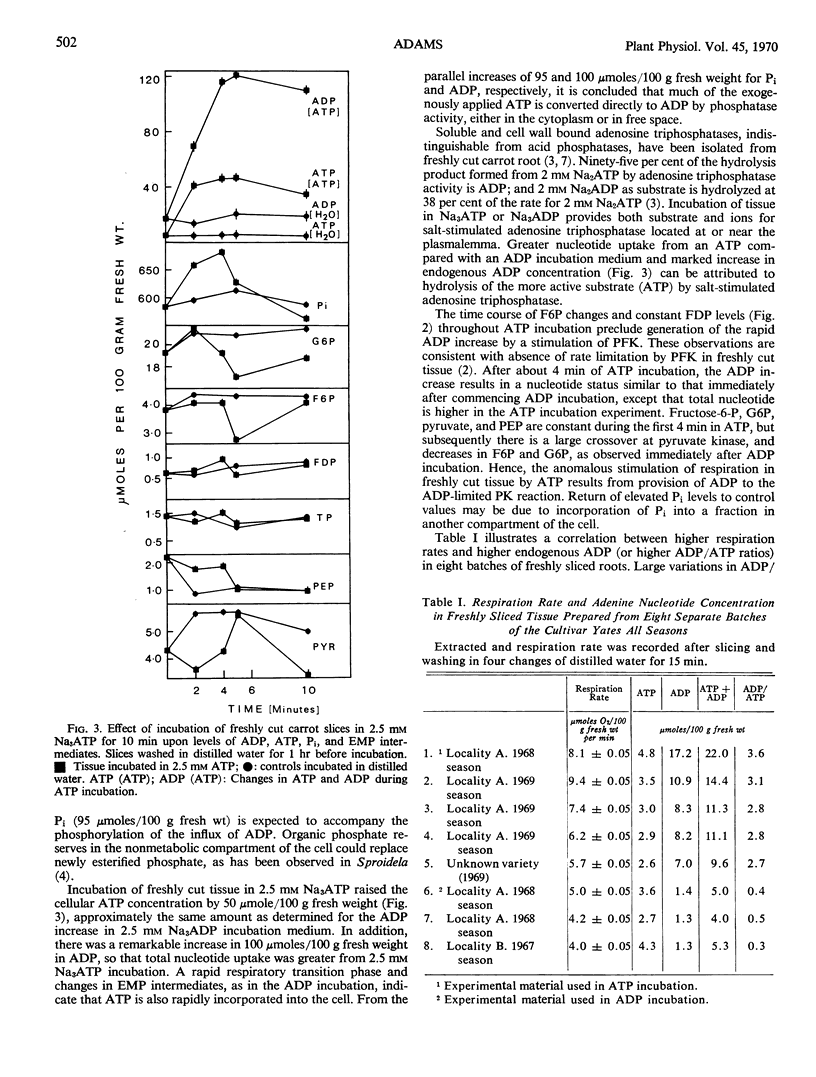
Incubation of freshly cut carrot tissue in Na3ADP and Na3ATP promotes a marked intracellular increase in both ADP and ATP. The rapid increase in ATP during an ADP incubation and in ADP during an ATP incubation results from the activity of cytoplasmic enzyme systems upon the nucleotide absorbed into the cell from the incubation medium. There is a crossover at the pyruvate kinase reaction, but not at phosphofructokinase, when either ADP or ATP is added to freshly cut tissue. In tissue slices washed in distilled water, pyruvate kinase exhibits a negative crossover in the first 2 hours and a positive crossover between 2 and 10 hours after cutting. Cutting induces large changes in levels of nucleotides and glycolytic intermediates. There is an immediate depletion of these compounds upon cutting, so that nucleotides are added to a system where respiration rate is limited by endogenous nucleotide level.
Variation in respiratory values for fresh cut tissue can be explained in terms of a range of endogenous ADP levels in different tissue batches. Nucleotide incubation experiments are discussed in relation to the provision of ADP to rate-limiting pyruvate kinase during the first phase in development of the induced respiration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. B. Effect of adenine nucleotides on the respiration of carrot root slices. Plant Physiol. 1970 Apr;45(4):495–499. doi: 10.1104/pp.45.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. B., Rowan K. S. Glycolytic control of respiration during aging of carrot root tissue. Plant Physiol. 1970 Apr;45(4):490–494. doi: 10.1104/pp.45.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRACHET J. Effects of ribonuclease on the metabolism of living root-tip cells. Nature. 1954 Nov 6;174(4436):876–877. doi: 10.1038/174876a0. [DOI] [PubMed] [Google Scholar]
- Bieleski R. L. Effect of phosphorus deficiency on levels of phosphorus compounds in spirodela. Plant Physiol. 1968 Aug;43(8):1309–1316. doi: 10.1104/pp.43.8.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALGARNO L., BIRT L. M. Free fatty acids in carrot-tissue preparations and their effect on isolated carrot mitochondria. Biochem J. 1963 Jun;87:586–596. doi: 10.1042/bj0870586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Key J. L. Polyribosome formation and RNA synthesis during aging of carrot-root tissue. Proc Natl Acad Sci U S A. 1967 May;57(5):1338–1344. doi: 10.1073/pnas.57.5.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward F. C., Preston G. METABOLIC PROCESSES OF POTATO DISCS UNDER CONDITIONS CONDUCIVE TO SALT ACCUMULATION. Plant Physiol. 1940 Jan;15(1):23–61. doi: 10.1104/pp.15.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan D., Macdonald I. R. Development of soluble and insoluble invertase activity in washed storage tissue slices. Plant Physiol. 1967 Mar;42(3):456–458. doi: 10.1104/pp.42.3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleur J. D., Uritani I. Respiratory Activity of the Mitochondrial Fractions Isolated from Healthy Potato Tubers and from Tuber Tissue Incubated after Cutting or Infection with Ceratocystis fimbriata. Plant Physiol. 1965 Nov;40(6):1008–1012. doi: 10.1104/pp.40.6.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]


