Abstract
AIM: To study the effects of cortactin on the tumor biology of SGC-7901 cells and identify the mechanism involved in the process.
METHODS: Cell lines in which cortactin was stably overexpressed or knocked down as well as the respective control cell lines were established by standard molecular methods. The effects of cortactin on the proliferation, migration and invasion capacity of SGC-7901 cells were assessed by the MTT assay, colony formation, flow cytometry, transwell migration and matrigel invasion. Nude mouse models were also used to assess the role of cortactin in the growth and metastasis of SGC-7901 cells in vivo. Western blotting analysis was performed to detect the expression of epidermal growth factor receptor (EGFR) and downstream molecules.
RESULTS: Cell lines in which cortactin was stably overexpressed or knocked down as well as control cell lines were successfully established and designated as LV5-cortactin-SGC, LV5-SGC, LV3-shRNA-SGC and LV3-SGC. Cortactin overexpression promoted SGC-7901 cell migration (340.7 ±12.6 vs 229.1 ± 23.2, P < 0.01) and invasion (71.6 ± 5.2 vs 48.4 ± 3.6, P < 0.01). Cortactin downregulation impaired SGC-7901 cell migration (136.2 ± 19.8 vs 225 ± 17) and invasion (29.2 ± 5.2 vs 49.6 ± 3.8, P < 0.01). The results from the MTT and colony formation assays results indicated increased LV5-cortactin-SGC cell proliferation and decreased LV3-shRNA-SGC cell proliferation compared to the control cells. Flow cytometry analysis demonstrated that cortactin overexpression promoted the proliferation index of SGC-7901 cells, and the results were reversed when cortactin was downregulated. Mouse tumor models confirmed that cortactin expression increased SGC-7901 cell proliferation and metastasis in vivo. Western blotting analysis revealed that cortactin elevated EGFR expression and activated the downstream molecules.
CONCLUSION: Cortactin expression promoted the migration, invasion and proliferation of SGC-7901 cells both in vivo and in vitro. The EGFR signaling pathway is mechanistically involved.
Keywords: Gastric cancer, Cortactin, Epidermal growth factor receptor, Invasion, Metastasis, Proliferation
Core tip: Cortactin is an actin-related protein 2/3 complex-activating and filamentous (F)-actin-binding protein that is implicated in tumor cell motility and metastasis. Clinical studies have shown that cortactin overexpression is often associated with the clinicopathological parameters and poor prognosis in a variety of cancers, including gastric cancer. In this study, the effects of cortactin on gastric cancer progression were investigated. The results showed that cortactin expression promoted SGC-7901 cell migration, invasion and proliferation both in vitro and in vivo. The epidermal growth factor receptor signaling pathway is mechanistically involved. Cortactin may serve as a novel therapeutic target of gastric cancer.
INTRODUCTION
Gastric carcinoma is one of the most common cancers in the world and the second leading cause of cancer death worldwide[1-3]. Over 70% of new cases and deaths occur in developing countries and the highest incidence rate is in China and other eastern Asian countries[3]. One recent study indicated that the 5-year overall survival of the disease was 53%[4]. In contrast, the 5-year survival rates for all tumor stages are only 20% to 25% in the western world, and the median survival time approximately 24 mo[5]. Surgery and adjuvant chemo-radiotherapy are traditional therapies that have a poor prognosis, therefore molecular targeted therapy has been studied extensively. Targeted therapy is based on a comprehensive understanding of the mechanisms governing gastric cancer progression, but an exact mechanism has yet to be elucidated.
Cortactin protein was first identified in chicken cells transformed by the src oncogene[6]. The gene encoding cortactin maps to chromosome 11q13, which is often amplified in many carcinomas, such as head and neck squamous cell carcinoma (HNSCC)[7]. Cortactin is an actin-related protein 2/3 (Arp2/3) complex-activating and filamentous (F)-actin-binding protein that is implicated in tumor cell motility and metastasis[8]. The protein is enriched in cortical structures such as membrane ruffles and lamellipodia[9]. The properties of cortactin indicate that it may be important for microfilament-membrane interactions as well as the transduction of signals from the cell surface to the cytoskeleton[9]. In carcinoma cells that constitutively overexpress cortactin, this protein accumulates in the cytoplasm as well as protruding leading lamellae or podosome-like structures, thereby contributing to the invasive potential of these tumor cells[10]. Cortactin overexpression often occurs in malignant tumors regardless of the chromosome 11q13 amplification and is associated with poor prognosis[11-15]. Cortactin has also been studied in gastric carcinoma tissues. The results indicated that cortactin overexpression directly correlates with more advanced cancer and lymph node stages as well as degree of differentiation; thus, cortactin overexpression is associated with unfavorable survival[16,17]. However, the exact role of cortactin in gastric cancer progression remains unknown.
In this study, molecular methods were used to overexpress and knockdown cortactin in SGC-7901 gastric carcinoma cells and the resulting phenotypes were analyzed. The overexpression of cortactin promoted the migration, invasion and proliferation of SGC-7901 cells in vitro. Cortactin overexpression enhanced tumor growth and metastatic capacity in vivo. The Western blotting results indicated that epidermal growth factor receptor (EGFR) expression was upregulated in the cortactin overexpression cells and that the downstream molecules were activated. Cortactin silencing resulted in the opposite effects. These observations are consistent with a previous report indicating that cortactin overexpression in a cervical cancer cell line markedly inhibits the ligand-induced down-regulation of EGFR[18]. In the same study, the RNAi-mediated reduction of cortactin expression in an 11q13-amplified HNSCC cell line accelerated EGFR degradation[18]. Therefore, cortactin and EGFR may synergistically contribute to the progression of gastric carcinoma. Cortactin may serve as a promising therapeutic target in gastric cancer.
MATERIALS AND METHODS
Cell culture
SGC-7901 cells (obtained from Professor Liang, School of Medicine, Tongji University) were cultured in RPMI 1640 (Gibco, Grand Island, NY, United States) supplemented with 10% fetal calf serum (FCS) and maintained at 37 °C in 5% CO2. HEK293T cells were cultured in DMEM with high glucose supplemented with 10% FCS and maintained at 37 °C in 5% CO2.
Antibodies and plasmids
Rabbit polyclonal anti-cortactin, anti-phospho-cortactin, anti-EGFR, anti-AKT, anti-phospho-AKT, anti-signal transducer and activator of transcription 3 (STAT3), anti-phospho-STAT3, anti-glyceraldehyde 3-phosphate dehydro- genase (GAPDH) and the secondary mouse anti-rabbit antibody were purchased from Epitomics (Burlingame, CA, United States). For overexpression and knockdown of cortactin, the pGLV5/H1/GFP+Puro and pGLV3/H1/GFP+Puro lentiviral plasmid were used, respectively. The PG-P1-VSVG, PG-P2-REV and PG-P3-RRE helper plasmids were employed to package the lentiviral vector (Genepharma, Shanghai, China).
The design of shRNA to silence the expression of cortactin
Two following validated cortactin shRNA sequences were used, (forward): 5’-GATCCGAAAGACTACTCCAGTGGTTTCAAGAGAACCACTGGAGTAGTCTTTCTTTTTTG-3’, and (reverse): 5’-AATTCAAAAAAGAAAGACTAC TCCAGTGGTTCTCTTGAAACCACTGGAGTAGTCTTTCG-3’. The non-specific control sequences were as follows: (forward) 5’-GATCCGTTCTC CGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAACT TTTT TG-3’, and (reverse) 5’-AATTCAAAAACCTAAGGTTAAGTCGCCCTCGCT CGAG CGAGGGCGACTTAACCTTAGG-3’. The two pairs were annealed and inserted into the BamHI and EcoRI sites of the pGLV3/H1/GFP+Puro lentiviral plasmid. The product was then transformed into Trans5α (TransGen Biotech, Beijing, China). Positive clones were selected and verified by restriction enzyme and sequence analysis.
Construction of cortactin overexpression plasmid
The cortactin coding sequence (CDS) was obtained by polymerase chain reaction (PCR) using the TransTaq High Fidelity PCR SuperMix I (TransGen Biotech, Beijing, China) in accordance with the manufacturer’s protocol. The following primers were used: (forward) 5’-ATAAGAATGCGGCCGCATG TGGAAAGCTTCAGCAGGCCACG-3’, and (reverse) 5’-CGGGATCCCTACT GCCGCAGCTCCACATAGTTG-3’. The cortactin CDS was inserted into to the NotI and BamHI sites of the pGLV5/H1/GFP+Puro lentiviral plasmid, and the product was transformed into Trans5α (TransGen Biotech, Beijing, China). Positive clones were selected and verified by PCR and sequence analysis.
Packaging of lentiviral vectors and the establishment of stable transfectants
The successfully constructed pGLV3-cortactin shRNA and the pGLV5-cortactin vector were transfected into HEK293T cells with PG-P1-VSVG, PG-P2-REV and PG-P3-RRE plasmids. All transfection reactions were performed using the GeneExpresso Max Transfection Reagent (Excellgen; Rockville, MD, United States) in accordance with the manufacturer’s instructions. Eight to twelve hours after transfection, the culture medium of the HEK293T cells was changed to fresh culture medium. The supernatant was then harvested at 72 h and titered using the HEK293T cell line. The pGLV3-control shRNA and pGLV5-control plasmids were also packaged simultaneously. The lentiviral stock titer was 1 × 108 TU/mL. SGC-7901 cells were cultured in six-well tissue culture plates and infected with the four lentiviral vectors at a multiplicity of infection of 40 for 24 h. The medium was replaced with fresh complete medium. After 3 d, cells were observed by fluorescence microscopy to confirm that greater than 90% of the cells were GFP-positive (Figure 1). Subsequently, the GFP-positive cells were screened by addition of 5 μg/mL puromycin. The resultant stable cell lines were designated as LV5-cortactin-SGC, LV5-SGC, LV3-shRNA-SGC, and LV3-SGC.
Figure 1.
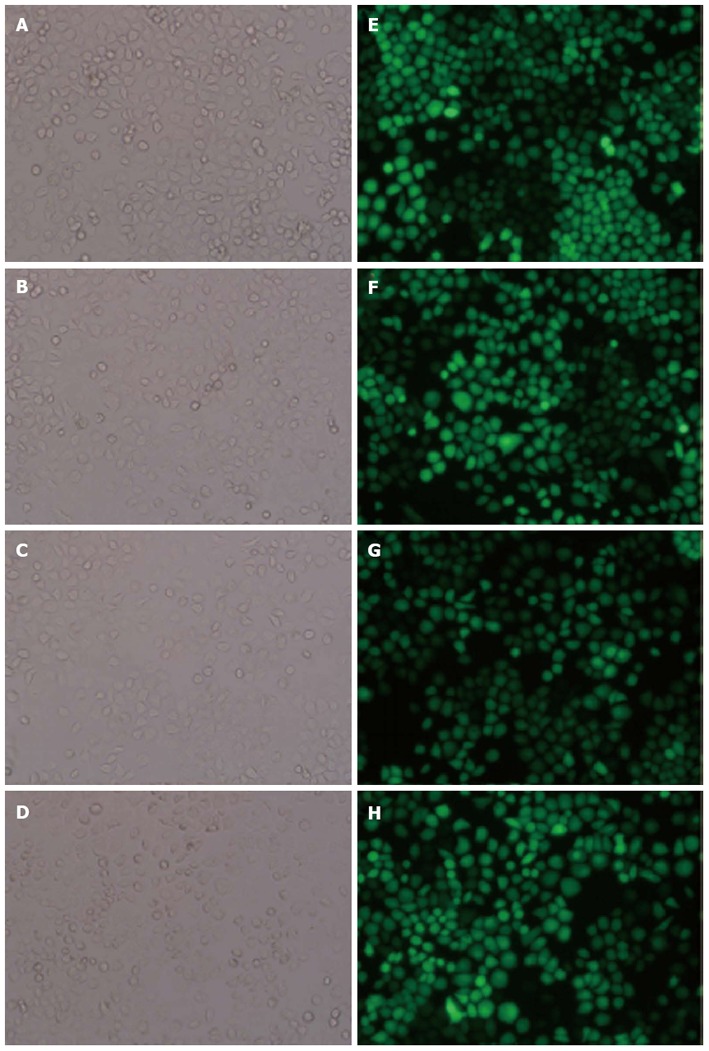
Establishment of the LV5-cortactin-SGC, LV5-SGC, LV3- shRNA-SGC, and LV3-SGC stable cell lines. A, E: LV5-cortactin-SGC; B, F: LV5-SGC; C, G: LV3-shRNA-SGC; D, H: LV3-SGC. The stable transfectants expressed green fluorescent protein. The left half of the figure was obtained under light field, and the right half was obtained under fluorescence. There is no morphological difference found between the stable cell lines.
Real-time quantitative reverse transcription PCR
Real time reverse transcription (RT) polymerase chain reactions were performed as previously described using GAPDH as an internal control[19]. The primer sequences used for q-RT-PCR are as follows: (cortactin forward primer) 5’-TGAGTGTGTGTTCTTCCCCAAG-3’, (cortactin reverse primer) 5’-CACGTGACCTTCTGGAAAGACA-3’. (GAPDH forward primer) was 5’-G GTGGTCTCCTCTGACTTCAACA-3’, (GAPDH reverse primer): 5’-GTTGCTG TAGCCAAATTCGTTGT-3’. All the experiments were performed in triplicate with 3 replicates.
Western blotting assay
Western blotting was performed as previously described[19] with antibodies against cortactin, phosphor-cortactin, GAPDH, EGFR, STAT3, phosphor-STAT3, AKT and phosphor-AKT at 1:1000 dilutions. The band intensity was detected using Image Quant LAS 4000 (GE Healthcare, Buckinghamshire, England) and analyzed using ImageJ software.
Cell migration and invasion assays
Cell migration and invasion assays were performed using 24-well Transwell chambers with a pore size of 8 μm (Costar, New York, NY, United States) planted with 5 × 104 cells in serum-free RPMI 1640. The lower chambers were filled with medium that contained 10% fetal bovine serum as the chemoattractant. For the invasion assays, the inserts were coated with 50 μL Matrigel (1:3 dilution; BD Bioscience, Franklin Lakes, NJ, United States). After culture for 24 h, the cells on the upper membrane surface were removed by scraping with a cotton swab, and the cells that passed through the filter were fixed and stained using the hematoxylin-eosin reagent. The invading cells were counted in five randomly selected high power fields using a microscope. All the experiments were performed in triplicate with 3 replicates and the mean was calculated.
Cell proliferation assay
In general, 1 × 103 cells were seeded per well in a 96-well culture plate. Fifty microliters of 5 mg/mL 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-diphenyltetraz oliumb-romide (Kengen, Nanjing, China) in 1 × phosphate-buffered saline was added to growth medium 48 and 72 h after plating. The cells were incubated for 4 h at 37 °C and then solubilized with 150 μL DMSO for 30 min. A 96-well plate reader (Bio-Rad, Philadelphia, United States) was used to measure the absorbance at 490 nm. All the experiments were performed in triplicate with 3 replicates and the mean was calculated.
Colony formation assay
After achieving 70% to 80% confluence, the cells were trypsinized, washed three times with phosphate-buffered saline (PBS), and then plated in triplicate at 1 × 103 cells/6 cm well. After 14 d of culture, the colonies were fixed with 4% paraformaldehyde and stained with crystal violet. The number of colonies per well was counted using a microscope and a cluster of 50 cells was designated as a colony. Three independent experiments were performed.
Flow cytometry analysis
Cells were plated at 2 × 105 cells per well in a 6-well culture plate and cultured for 48 h. The cells were then harvested, fixed with 70% ethanol, and washed with ice-cold PBS. The cells were stained with 50 μg/mL propidium iodide and 250 μg/mL RNase and incubated for 30 min in the dark at room temperature. The cells were subsequently analyzed by flow cytometry using a FACS Calibur (Becton Dickinson, Bedford, MA, United States).
In vivo assays of tumor growth and metastasis
Four- to six-week-old female BALB/c nude mice were purchased from the Slac Laboratory Animal Company (Shanghai, China) and fed in the Experimental Animal Center of Tongji University. All mice were maintained in a germ-free environment with free access to food and water. To examine the effects of cortactin on tumor cell proliferation and metastasis in vivo, LV5-cortactin-SGC, LV5-SGC, LV3-shRNA-SGC and LV3-SGC cells were used. For the in vivo proliferation assay, four nude mice from each group were injected subcutaneously with 5.0 × 106 cells. The tumor volumes were measured at the indicated times according to the following formula[20]: 0.5 × length × width2. After 8 wk, the mice that were injected subcutaneously were sacrificed and the tumors were dissected and measured to calculate the volume. For the spontaneous metastasis assay, the cells were injected into the inferior portion of the gastric serosa of 8 nude mice in each group. The mice were monitored every 3 d and sacrificed 12 wk after injection. The livers of the mice were dissected, and the liver metastases were evaluated. Paraffin-embedded tumors and livers were sectioned, and stained with hematoxylin and eosin. The slides were microscopically observed to confirm the presence of the tumor formation and metastasis in the mice. The average values were expressed as the mean ± SE. This animal experiment was approved by the ethic commission of the experimental center of Tongji University. The study was in accordance with the recommendations of the regional and country animal ethics committee.
Statistical analysis
The results are expressed as the mean ± SD. For the comparison of the means between two groups, a two-tailed t-test was used. For the statistical analysis of the in vivo metastases, the Mann Whitney U test was used. Statistical analysis was performed using SPSS software version 17.0. A P-value < 0.05 was considered statistically significant.
RESULTS
The establishment and identification of stable transfectants
Four stable recombinant cell lines were generated using the pGLV5-cortactin, pGLV5-control, pGLV3-cortactin shRNA and pGLV3-control lentiviruses, which were designated as LV5-cortactin-SGC, LV5-SGC, LV3-shRNA-SGC and LV3-SGC cells, respectively. As shown in Figure 1, the stable transfectants expressed green fluorescent protein, and no morphological differences were observed between the stable cell lines. Quantitative real-time PCR was performed to identify the levels of cortactin gene transcription in the cell lines. Cortactin mRNA transcription was significantly increased in LV5-cortactin-SGC cells compared with the LV5-SGC cells (P < 0.01, Figure 2A) and significantly reduced in the LV3-shRNA-SGC cells compared with the LV3-SGC cells (P < 0.01, Figure 2B). Western blotting analysis showed that the cortactin protein expression was increased by 2.6-fold in the LV5-cortactin-SGC cells compared with the LV5-SGC cells (Figure 2C). Cortactin protein expression was decreased by 70% in the LV3-shRNA-SGC cells compared with the LV3-SGC cells (Figure 2D). These results suggested that the inserted genes were highly efficient in the stable transfectants.
Figure 2.
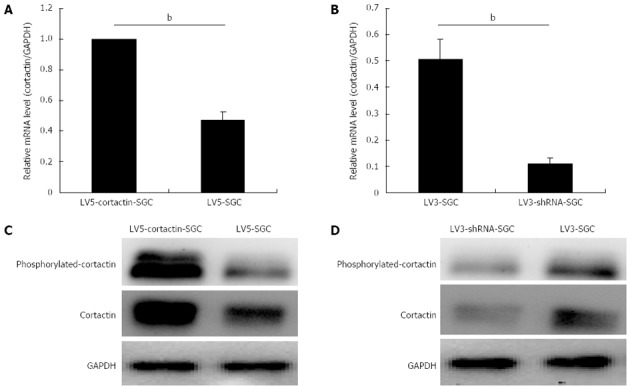
Cortactin expression at the mRNA and protein level in the recombinant cell lines. A, B: Bar chart represents quantitative real-time polymerase chain reaction assessment of the cortactin mRNA levels in the LV5-cortactin-SGC, LV5-SGC, LV3-SGC and LV3-shRNA-SGC cells (bP < 0.01 between groups, Student’s t-test); C: Western blotting analysis of cortactin and phosphorylated cortactin in the LV5-cortactin-SGC and LV5-SGC control cells. The cortactin and phosphorylated cortactin increased greatly in the LV5-cortactin-SGC cells compared with the LV5-SGC cells; D: Western blotting analysis of cortactin and phosphorylated cortactin in the LV3-shRNA-SGC and LV3-SGC control cells. The levels of cortactin and phosphorylated cortactin were decreased significantly in the LV3-shRNA-SGC cells compared with LV3-SGC cells. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Cortactin promote the SGC-7901cell migration and invasion in vitro
To determine the role of cortactin expression in gastric cancer cell migration and invasion, Transwell migration and invasion assays were performed. For the migration assay, Transwell chambers were used in absence of Matrigel. LV5-cortactin-SGC cell migration was significantly enhanced compared with that of LV5-SGC cells. The number of LV5-cortactin-SGC cells that migrated to the lower chamber was 340.7 ± 12.6 per high-power field (HPF) compared with 229.1 ± 23.2 per HPF for the LV5-SGC cells (P < 0.01; Figure 3A, B and E). Simultaneously, LV3-shRNA-SGC cell migration greatly decreased compared with that of LV3-SGC cells. The mean number of LV3-shRNA-SGC cells that migrated to the lower chamber was 136.2 ± 19.8 per HPF compared with 225 ± 17 per HPF for the LV3-SGC cells (P < 0.01; Figure 3C, D and F). For the invasion assay, Matrigel, an artificial extracellular matrix, was used. Similarly, LV5-cortactin-SGC cell invasion was significantly enhanced compared with that of the LV5-SGC cells (71.6 ± 5.2 vs 48.4 ± 3.6, P < 0.01; Figure 4A, B and E). Compared with LV3-SGC cells, the LV3-shRNA-SGC cells displayed reduced invasion (49.6 ± 3.8 vs 29.2 ± 5.2, P < 0.01; Figure 4C, D and F). These results indicated that cortactin expression promotes the migration and invasion potential of SGC-7901 gastric cancer cells.
Figure 3.
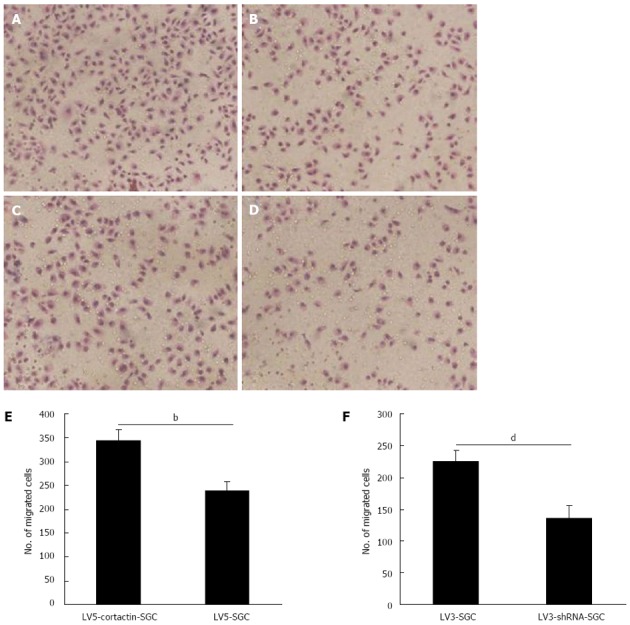
Cortactin expression promotes SGC-7901 cell migration. A-D: Number of LV5-cortactin-SGC cells (A) that migrated through the chamber was greatly increased compared with that for the LV5-SGC cells (B). The number of LV3-shRNA-SGC cells (D) that migrated through the chamber was greatly decreased compared with the number of migrating LV3-SGC cells (C); E: Statistical analysis of invasion by LV5-cortactin-SGC and control LV5-SGC cells (340.7 ± 12.6 vs 229.1 ± 23.2, bP < 0.01); F: Statistical analysis of invasion by LV3-shRNA-SGC and control LV3-SGC cells (136.2 ± 19.8 vs 225 ± 17, dP < 0.01). Student’s t-test was used, and P < 0.05 was considered statistically significant.
Figure 4.
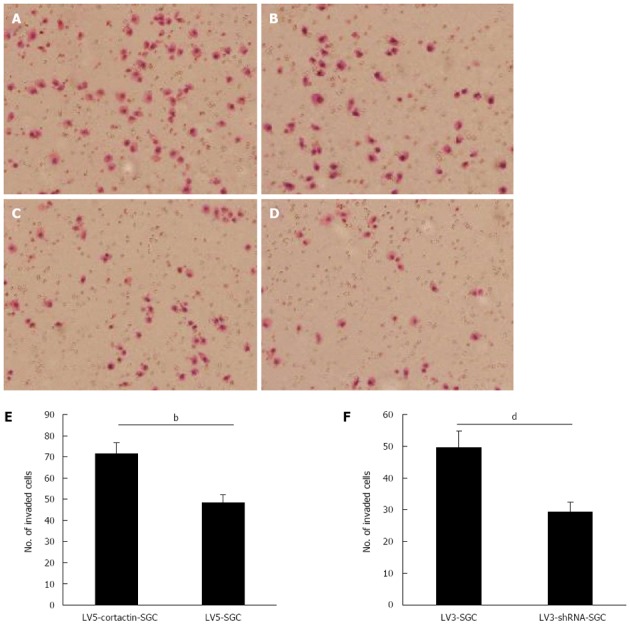
Cortactin expression promotes SGC-7901 cell invasions. A-D: Number of LV5-cortactin-SGC cells (A) that invaded the chamber was greatly increased compared with the number of invading LV5-SGC cells (B). The number of LV3-shRNA-SGC cells (D) that invaded the chamber was greatly decreased compared with the number of invading LV3-SGC cells (C); E: Statistical analysis of invasion by LV5-cortactin-SGC and control LV5-SGC cells (71.6 ± 5.2 vs 48.4 ± 3.6, bP < 0.01); F: Statistical analysis of invasion by LV3-shRNA-SGC and control LV3-SGC cells (49.6 ± 3.8 vs 29.2 ± 5.2, dP < 0.01). Student’s t-test was used, and P < 0.05 was considered statistically significant.
Cortactin promotes SGC-7901 gastric cancer cell proliferation
To investigate the effect of cortactin expression on gastric cancer cell growth, the MTT and colony formation assays were performed. The MTT assay results indicated an obvious increase in LV5-cortactin-SGC cell proliferation compared with the LV5-SGC cells on the second and the third days (P < 0.05; Figure 5A). The proliferation capacity of the LV3-shRNA-SGC cells significantly decreased compared with the LV3-SGC cells on the second (P < 0.05; Figure 5B) and the third days (P < 0.01; Figure 5B). The effect of cortactin on SGC-7901 cell growth was also confirmed by the colony formation assay. These results indicated that cortactin overexpression clearly contributes to an increased ability to form colonies (P < 0.01; Figure 5C, D and G). Moreover, cortactin downregulation significantly reduced of the colony formation capacity of the SGC-7901 cells (P < 0.05; Figure 5E, F and H). These results suggest that cortactin expression influences SGC-7901 cell growth and proliferation.
Figure 5.
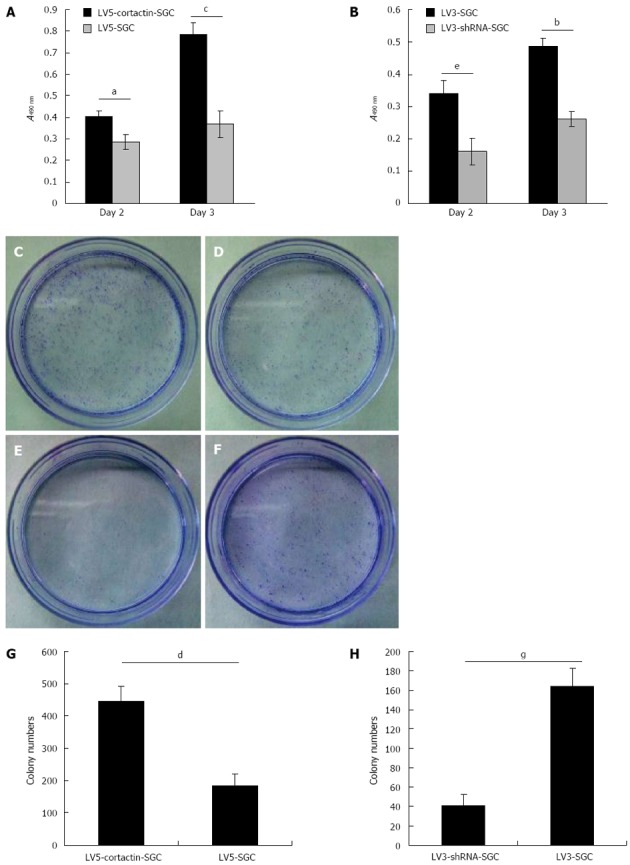
Cortactin expression promotes SGC-7901 cell proliferation. A: MTT assay results showed that the LV5-cortactin-SGC cell proliferation was increased on the second (aP < 0.05 vs LV5-SGC cells) and the third (cP < 0.05 vs LV5-SGC cells) days after plating; B: LV3-shRNA-SGC cell proliferation was significantly lower on the second (eP < 0.05 vs LV3-SGC cells) and third (bP < 0.01 vs LV3-SGC cells) days after plating. The colony formation assay confirmed the role of cortactin in SGC-7901 cell proliferation. The LV5-cortactin-SGC, LV5-SGC, LV3-shRNA-SGC and LV3-SGC cell colonies were stained with crystal violet (C-F, respectively); G: The number of LV5-cortactin-SGC cell colonies was greater than LV3-SGC cell colonies (dP < 0.01 vs the number of LV5-SGC cell colonies); H: The number of LV3-shRNA-SGC cell colonies was significantly less than of LV3-SGC cell colonies (gP < 0.05 vs the number of LV5-SGC cell colonies). Student’s t-test was used, and P < 0.05 was considered significantly significant.
Cortactin increases the proliferation index of SGC-7901 cells
To investigate the effects of cortactin expression on the cell cycle in SGC-7901 cells, the flow cytometry assay was performed. The proliferation index was calculated as (S + G2M)/(G0G1+S+G2M) according to a previous study[21]. The LV5-cortactin-SGC cell proliferation index was significantly higher than that of LV5-SGC cells (P < 0.05; Figure 6A, B and E). The proliferation index of the LV3-shRNA-SGC cells was significantly reduced compared with that of LV3-SGC cells (P < 0.01; Figure 6C, D and F). These results suggest that cortactin increases the proliferation index of SGC-7901 cells.
Figure 6.
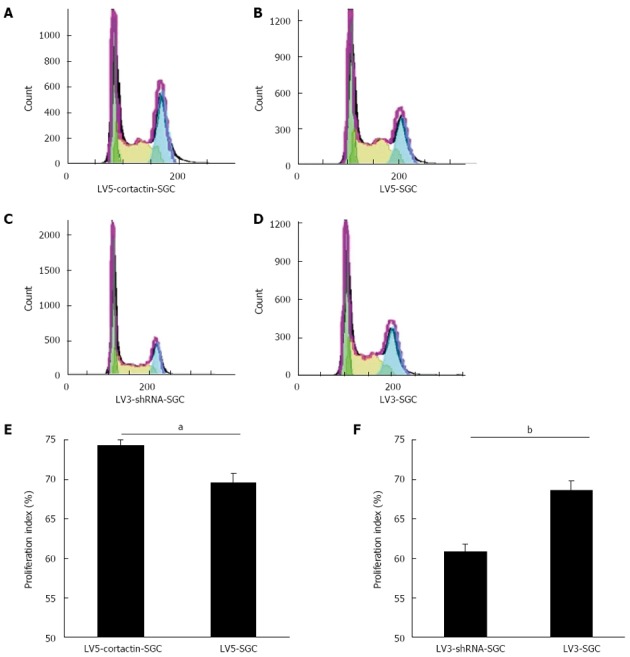
Effect of cortactin on the cell cycle as analyzed by flow cytometry. A-D: Cell-cycle stages of LV5-cortactin-SGC, LV5-SGC, LV3-shRNA-SGC and LV3-SGC cells were detected by flow cytometry; E: Proliferation indexes of LV5-cortactin-SGC and LV5-SGC cells, aP < 0.05 between groups was assessed by Student’s t-test; F: Proliferation indexes of LV3-shRNA-SGC and LV3-SGC cells, bP < 0.01 between groups was assessed by Student’s t-test. P < 0.05 was considered to be significantly significant.
Cortactin overexpression accelerates tumor growth and metastasis in vivo
To investigate the role of cortactin overexpression in the tumor growth and metastasis in vivo, the tumor formation and metastatic abilities of the LV5-cortactin-SGC, LV5-SGC, LV3-shRNA-SGC and LV3-SGC cells were compared in a mouse tumor model. For the tumor growth assay, the cells were inoculated subcutaneously in the nude mice and the tumor growth was measured weekly for 2 mo post inoculation. The tumors of the mice inoculated with the LV5-cortactin-SGC cells were obviously larger than the tumors of the mice inoculated with the LV5-SGC cells (608.2 ± 153.69 mm3 vs 274.5 ± 41.6 mm3, P < 0.05; Figure 7A). The tumors of the mice inoculated with the LV3-shRNA-SGC cells were smaller than that the tumors of the nude mice inoculated with the LV3-SGC cells (212.5 ± 77.2 vs 87.76 ± 33.6, P < 0.05; Figure 7B). The results suggest that cortactin increases tumor growth in vivo. For the spontaneous metastasis assay, the cells were injected into the inferior portion of the gastric serosa of the nude mice. The mice were sacrificed three months after inoculation, and the livers were dissected and analyzed. Six out of 8 mice injected with the LV5-cortactin-SGC cells developed liver metastasis compared with 2 out of 8 mice in the LV5-SGC cell group. In the LV5-cortactin-SGC group, the six mouse livers with metastases harbored 4, 3, 3, 2, 2 and 1 lesions. With regard to the mice injected with the LV5-SGC cells, the two livers of the mice with metastases harbored 1 and 2 lesions (P < 0.05, Mann Whitney U test; Figure 7C). Two mice developed metastases with 1 secondary tumor in the LV3-SGC group, and no metastasis was noted in the LV3-shRNA-SGC group (P > 0.05, Mann Whitney U test; Figure 7C). These results indicate that cortactin overexpression induces metastasis in vivo. Moreover, cortactin knockdown reduces the metastatic potential of the SGC-7901 cells; however, the results were not statistically significant. The primary and secondary tumors were also confirmed by microscopy (data not shown).
Figure 7.
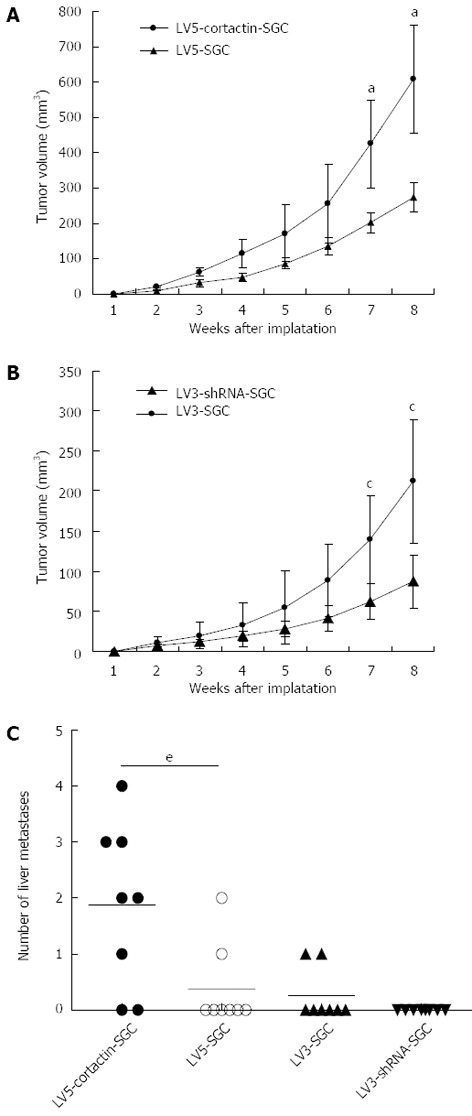
Cortactin expression promotes SGC-7901 cell growth and metastasis in vivo. A: Growth curves of the primary tumor volumes (mm3) of the LV5-cortactin-SGC and LV5-SGC cells (aP < 0.05 vs LV5-SGC cells, Student’s t-test); B: Growth curves of primary tumor volumes (mm3) of the LV3-shRNA-SGC and LV3-SGC cells (cP < 0.05 vs LV5-SGC cells, Student’s t-test); C: Number of liver metastases in the four cell lines (eP < 0.05 vs LV5-SGC cells, Mann Whitney U test).
Cortactin promotes the EGFR expression and activates the EGFR signaling pathway in SGC-7901 cells
To study the mechanism by which cortactin enhances the growth of SGC-7901cells in vitro and in vivo, the EGFR, STAT3, phosphorylated-STAT3, AKT, and phosphorylated-AKT protein levels were detected by Western- blotting. As shown in Figure 8, the levels of EGFR, phosphorylated-STAT3 and phosphorylated-AKT were increased in the LV5-cortactin-SGC cells compared with the LV5-SGC cells, and no difference in the total STAT3 and total AKT protein levels were observed between the two cell groups. The levels of EGFR, phosphorylated-STAT3 and phosphorylated-AKT were decreased in the LV3-shRNA-SGC cells compared with the LV3-SGC cells. Similarly, the expression of total STAT3 and total AKT was not different between the two cell groups. These results indicate that cortactin over- expression enhances the EGFR expression in the SGC-7901 cells and in turn constitutively activates the EGFR signaling pathway.
Figure 8.
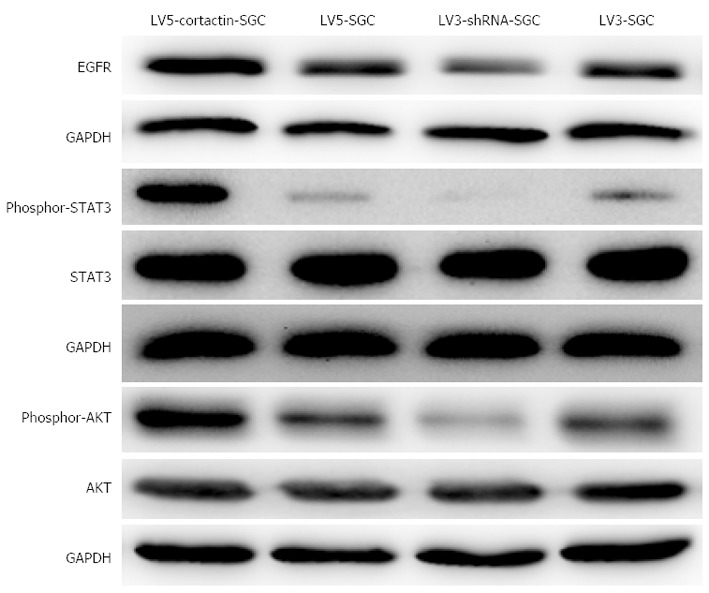
Cortactin expression promotes epidermal growth factor receptor expression and activates the downstream molecules AKT and signal transducer and activator of transcription 3. Western blotting results show that epidermal growth factor receptor (EGFR), phosphor-signal transducer and activator of transcription 3 (STAT3) and phosphor-AKT were increased in LV5-cortactin-SGC cells and decreased in LV3-shRNA-SGC cells compared with their control cells. No alteration in the expression of total STAT3 and total AKT was observed in the four cell lines.
DISCUSSION
Metastasis remains to be the major cause of treatment failure and poor prognosis in patients with malignant tumors, and it is a multistage process that involves the motility and migration of cells and proliferation in a new site[22]. Cortactin is a filamentous actin cross-linking protein and a substrate of Src protein tyrosine kinase. The overexpression of wild-type cortactin results in a significant increase in endothelial cell migration[23]. Cell migration is a coordinated process that involves dynamic changes in the actin cytoskeleton and its interplay with focal adhesions[24]. The cortactin N-terminus associates with F-actin, whereas the C-terminus interacts with focal adhesions[24]. The phosphorylation of cortactin tyrosine residues by the FAK-Src complex modulates cortactin’s interaction with FAK and increases its turnover at focal adhesions to promote cell motility[24]. Yamada et al[25] showed that cortactin knockdown results in the downregulation of adhesion molecules, such as E-cadherin, β-catenin, and epithelial cell adhesion molecule, and leads to the decreased invasion of oral squamous cell carcinoma cells. Nakane et al[26] showed that cortactin knockdown significantly diminished DU145 cell migration and invasion ability and no apparent morphological changes in the cells were observed by microscopy. Patel et al[27] showed that NIH3T3 fibroblasts overexpressing cortactin displayed increased motility and invasion in modified Boyden chamber assays without any striking morphological changes. These results are consistent with our observations. As shown in Figures 3 and 4, LV5-cortactin-SGC cell migration and invasion was significantly enhanced compared with the migration and invasion of the LV5-SGC cells, whereas LV3-shRNA-SGC cell migration and invasion was greatly reduced compared with the migration and invasion of the LV3-SGC cells. In addition, no morphological changes were observed in the four cell types (Figure 1). The mechanism that regulates cortactin-mediated effects on the migration and invasion of cancerous or non-cancerous cells was intensely investigated. One study indicates that cortactin is implicated in the cell-cell adhesion and cell spreading[28]. Cortactin overexpression results in enhanced cell migration and invasion via reduced cell spreading and intercellular adhesive strength[28]. Hill et al[29] reported that the cortactin gene was a transcriptional target of the hyaluronan/CD44 standard form signaling, and the mechanistic underpinning of CD44-mediated EMS1/cortactin transcription is dependent on a nuclear factor kappa-light-chain-enhancer of activated B cells in breast cancer cells. Cortactin is functionally important in CD44-mediated cell motility and adhesion in endothelial cells[29]. Clark et al[30] demonstrated that cortactin expression levels in HNSCC cells correlate with the formation of invadopodia and associated matrix degradation. Moreover, the secretion of the matrix metalloproteinases (MMP) MMP-2 and MMP-9 as well as the surface expression of MT1-MMP is dependent on the level of cortactin expression[30]. In the cortactin knockdown cells, the number of invadopodia was reduced and these cells were not able to degrade the extracellular matrix (ECM). In the reverse experiment, cortactin overexpression enhanced ECM degradation and invasiveness[30]. Bryce et al[31] found that cortactin promotes persistent of lamellipodial protrusion and enhances the rate of new adhesion formation in the lamellipodia through simultaneous interaction with the Arp2/3 complex and actin filaments. These functions are important in cell motility. Rothschild et al[8] showed that cortactin tyrosine phosphorylation is an essential requirement for effective HNSCC motility. Another study indicated that tyrosine phosphorylation of cortactin might act as a unique and naive switch in the regulation of cell motility[32]. The effects of cortactin on cell motility and invasion are becoming increasingly clear and more complex. The present study shows that cortactin phosphorylation was increased in the LV5-cortactin-SGC cells and decreased in the LV3-shRNA-SGC cells compared with the control cells (Figure 2C, D). These results may partly explain the alteration in migration and invasion in the four cell lines, but the specific mechanism requires further elucidation. Clinical studies gave demonstrations that cortactin overexpression is often associated with clinicopathological parameters and poor prognosis in a variety of cancers, such as HNSCC, breast cancer, pancreatic and ampulla of Vater adenocarcinomas, colorectal carcinoma, gastric cancer, non-small cell lung cancer and hepatocellular carcinoma[11-13,17,33-37]. This association may underscore the important role of cortactin in cell migration and invasion.
To evaluate the effect of cortactin expression on cell proliferation, the MTT assay, colony formation assays and flow cytometry were performed. The MTT assay (Figure 5A) and colony formation assay (Figure 5C, D and G) results showed that cortactin overexpression increased the proliferation of the LV5-cortactin-SGC cells compared with the LV5-SGC cells. LV3-shRNA-SGC cell proliferation significantly reduced compared with that of the LV3-SGC cells as assessed by the MTT assay (Figure 5B) and the colony formation assay (Figure 5E, F and H). Flow cytometry analysis demonstrated that cortactin overexpression increased the proliferation index of the LV5-cortactin-SGC cells compared with that of the LV5-SGC cells (Figure 6A, B and E). Cortactin downregulation impaired the proliferation index of LV3-shRNA-SGC cells compared with that of LV3-SGC cells (Figure 6C, D and F). The Western blotting results showed that cortactin overexpression promoted EGFR expression, and cortactin downregulation impaired EGFR expression. These results are consistent with a previous study showing that cortactin overexpression is associated with attenuated ligand-induced EGFR down-regulation in HeLa cells[18]. Moreover, RNAi-mediated reduction of cortactin expression accelerates EGFR degradation in HNSCC cell lines[18]. The molecules downstream of EGFR were also detected by Western blotting, and the levels of phosphorylated-STAT3 and phosphorylated-AKT were increased in the LV5-cortactin-SGC cells and decreased in the LV3-shRNA- SGC cells compared with their control cells. The levels of total STAT3 and total AKT were similar among the four cell types. These results directly support the notion that cortactin overexpression is implicated in EGFR upregulation and constitutive downstream signaling, thereby conferring a proliferation advantage to SGC-7901 cells. EGFR is frequently implicated in cancer cell proliferation, the inhibition of apoptosis and tumor-induced neovascularization via the activation of downstream signaling pathways, including the PI3K/Akt, the MAPK and the Jak2/STAT3 pathways. EGFR is often overexpressed in various types of tumors, such as salivary gland carcinomas, colorectal cancer (CRC), non-small-cell lung cancer (NSCLC), biliary tract cancer, and gastric cancer[38-43]. Galizia et al[43] showed that EGFR overexpression is correlated to poor prognosis in gastric cancer. However, Kim et al[44] showed opposite effects. Though the role of EGFR expression in gastric cancer prognosis is not clear, the present study demonstrated that EGFR is involved in the progression of gastric cancer. In addition to the ability to migration to secondary sites, the potential to grow from micrometastasis to a macrometastsis is an important factor in the metastatic process. Our mouse tumor growth and metastasis assay also confirmed that cortactin overexpression promotes the growth and metastasis of SGC-7901 cells in vivo. These more malignant phenotypes may be attributed to the overexpressions of cortactin and EGFR, which confer migration, invasion and proliferation advantages to SGC-7901 cells both in vivo and in vitro.
EGFR targeted therapy has been applied in various cancers, including NSCLC, HNSCC and CRC[41,45,46]. This treatment strategy is currently being explored in clinical trials for gastric cancer patients[47]. Though current EGFR targeting agents may produce dramatic responses, these drugs are effective in only a fraction of patients. Moreover, resistance to these agents frequently develops[40]. Therefore, effective treatments may involve a combination of different targeted agents or chemotherapeutic compounds. Based on the present study, cortactin may be a promising coupled target for gastric cancer in the future.
In conclusion, cortactin expression confers a more malignant phenotype to SGC-7901 cells both in vitro and in vivo. Cortactin expression upregulates EGFR expression, and cortactin and EGFR synergistically contribute to the progression of gastric cancer. Cortactin may serve as a novel therapeutic target for gastric cancer. The role of cortactin in the progression of gastric cancer warrants further studies.
COMMENTS
Background
Gastric carcinoma is one of the most common cancers in the world and the second leading cause of cancer death worldwide. Studies have shown that cortactin overexpression directly correlates with more advanced cancer and lymph node stages, the degree of differentiation and poor prognosis in gastric cancer. However, the mechanism by which cortactin affects gastric cancer progression remains largely unknown.
Research frontiers
Cortactin was first identified in chicken cells transformed by the src oncogene. Cortactin is involved in the progression of a variety of cancers, such as head and neck squamous cell carcinoma, breast cancer, pancreatic and ampulla of Vater adenocarcinomas, colorectal carcinoma, hepatocellular carcinoma, non-small cell lung cancer and gastric cancer. This research aims to clarify the effects of cortactin carcinogenesis.
Innovations and breakthroughs
Previous studies indicated that cortactin is associated with clinicopathological parameters and poor survival in gastric cancer. In this study, we investigated the role of cortactin in the development of the disease. The authors found that cortactin expression promotes the migration, invasion and proliferation of SGC-7901 cells both in vivo and in vitro. Additionally, cortactin upregulates epidermal growth factor receptor (EGFR) expression in gastric cancer cells. Cortactin and EGFR contribute synergistically to gastric cancer progression.
Applications
The study indicates that cortactin may serve as a novel therapeutic target for gastric cancer in the future.
Terminology
Cortactin protein was first identified in chicken cells transformed by the src oncogene. Cortactin is an actin-related protein 2/3 complex-activating and filamentous (F)-actin-binding protein that is implicated in tumor cell motility and metastasis. In carcinoma cells that constitutively overexpress cortactin, this protein accumulates in the cytoplasm as well as protruding leading lamellae or podosome-like structures, thereby contributing to the invasive potential of these tumor cells.
Peer review
The manuscript is conducted according to the highest standards of biomedical research. Furthermore, it addresses the topic of molecular biology of gastric cancer and clearly demonstrates the effects of cortactin on its progression, invasion and metastasis that matter on national and international scale. Authors represent a comprehensive study on the effects of cortactin on tumor biology of SGC-7901 cells and identify the mechanism involved in the process. He has no question with this paper and he highly recommends publication.
Footnotes
Supported by Grants from the Project of Shanghai Science and Technology Commission of China, No. 10411968400
P- Reviewers: Alshehabi Z, Bilir C, Guo JM, Komatsu K, Zheng XF S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson IG, Gotley DC, Barbour AP, Martin I, Jayasuria N, Thomas J, Smithers BM. Treatment results of curative gastric resection from a specialist Australian unit: low volume with satisfactory outcomes. Gastric Cancer. 2014;17:152–160. doi: 10.1007/s10120-013-0240-3. [DOI] [PubMed] [Google Scholar]
- 5.Meyer HJ, Wilke H. Treatment strategies in gastric cancer. Dtsch Arztebl Int. 2011;108:698–705; quiz 706. doi: 10.3238/arztebl.2011.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu H, Reynolds AB, Kanner SB, Vines RR, Parsons JT. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol Cell Biol. 1991;11:5113–5124. doi: 10.1128/mcb.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigo JP, García-Carracedo D, García LA, Menéndez S, Allonca E, González MV, Fresno MF, Suárez C, García-Pedrero JM. Distinctive clinicopathological associations of amplification of the cortactin gene at 11q13 in head and neck squamous cell carcinomas. J Pathol. 2009;217:516–523. doi: 10.1002/path.2462. [DOI] [PubMed] [Google Scholar]
- 8.Rothschild BL, Shim AH, Ammer AG, Kelley LC, Irby KB, Head JA, Chen L, Varella-Garcia M, Sacks PG, Frederick B, et al. Cortactin overexpression regulates actin-related protein 2/3 complex activity, motility, and invasion in carcinomas with chromosome 11q13 amplification. Cancer Res. 2006;66:8017–8025. doi: 10.1158/0008-5472.CAN-05-4490. [DOI] [PubMed] [Google Scholar]
- 9.Wu H, Parsons JT. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993;120:1417–1426. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuuring E, Verhoeven E, Litvinov S, Michalides RJ. The product of the EMS1 gene, amplified and overexpressed in human carcinomas, is homologous to a v-src substrate and is located in cell-substratum contact sites. Mol Cell Biol. 1993;13:2891–2898. doi: 10.1128/mcb.13.5.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YN, Choi JE, Bae JS, Jang KY, Chung MJ, Moon WS, Kang MJ, Lee DG, Park HS. Expression of cortactin and focal adhesion kinase in colorectal adenocarcinoma: correlation with clinicopathologic parameters and their prognostic implication. Korean J Pathol. 2012;46:454–462. doi: 10.4132/KoreanJPathol.2012.46.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.You TK, Kim KM, Noh SJ, Bae JS, Jang KY, Chung MJ, Moon WS, Kang MJ, Lee DG, Park HS. Expressions of E-cadherin, Cortactin and MMP-9 in Pseudoepitheliomatous Hyperplasia and Squamous Cell Carcinoma of the Head and Neck: Their Relationships with Clinicopathologic Factors and Prognostic Implication. Korean J Pathol. 2012;46:331–340. doi: 10.4132/KoreanJPathol.2012.46.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai WC, Lin CK, Lee HS, Gao HW, Nieh S, Chan DC, Jin JS. The correlation of cortactin and fascin-1 expression with clinicopathological parameters in pancreatic and ampulla of Vater adenocarcinoma. APMIS. 2013;121:171–181. doi: 10.1111/j.1600-0463.2012.02952.x. [DOI] [PubMed] [Google Scholar]
- 14.Yuan BZ, Zhou X, Zimonjic DB, Durkin ME, Popescu NC. Amplification and overexpression of the EMS 1 oncogene, a possible prognostic marker, in human hepatocellular carcinoma. J Mol Diagn. 2003;5:48–53. doi: 10.1016/S1525-1578(10)60451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo ML, Shen XM, Zhang Y, Wei F, Xu X, Cai Y, Zhang X, Sun YT, Zhan QM, Wu M, et al. Amplification and overexpression of CTTN (EMS1) contribute to the metastasis of esophageal squamous cell carcinoma by promoting cell migration and anoikis resistance. Cancer Res. 2006;66:11690–11699. doi: 10.1158/0008-5472.CAN-06-1484. [DOI] [PubMed] [Google Scholar]
- 16.Tsai WC, Jin JS, Chang WK, Chan DC, Yeh MK, Cherng SC, Lin LF, Sheu LF, Chao YC. Association of cortactin and fascin-1 expression in gastric adenocarcinoma: correlation with clinicopathological parameters. J Histochem Cytochem. 2007;55:955–962. doi: 10.1369/jhc.7A7235.2007. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Cao W, Mo M, Wang W, Wu H, Wang J. VEGF and cortactin expression are independent predictors of tumor recurrence following curative resection of gastric cancer. J Surg Oncol. 2010;102:325–330. doi: 10.1002/jso.21644. [DOI] [PubMed] [Google Scholar]
- 18.Timpson P, Lynch DK, Schramek D, Walker F, Daly RJ. Cortactin overexpression inhibits ligand-induced down-regulation of the epidermal growth factor receptor. Cancer Res. 2005;65:3273–3280. doi: 10.1158/0008-5472.CAN-04-2118. [DOI] [PubMed] [Google Scholar]
- 19.Song J, Peng XL, Ji MY, Ai MH, Zhang JX, Dong WG. Hugl-1 induces apoptosis in esophageal carcinoma cells both in vitro and in vivo. World J Gastroenterol. 2013;19:4127–4136. doi: 10.3748/wjg.v19.i26.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusano H, Akiba J, Ogasawara S, Sanada S, Yasumoto M, Nakayama M, Ueda K, Ueda K, Kurita T, Todoroki K, et al. Pegylated Interferon-α2a Inhibits Proliferation of Human Liver Cancer Cells In Vitro and In Vivo. PLoS One. 2013;8:e83195. doi: 10.1371/journal.pone.0083195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Shi R, Wang Y, Niu X. TAGLN suppresses proliferation and invasion, and induces apoptosis of colorectal carcinoma cells. Tumour Biol. 2013;34:505–513. doi: 10.1007/s13277-012-0575-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhang YY, Chen B, Ding YQ. Metastasis-associated factors facilitating the progression of colorectal cancer. Asian Pac J Cancer Prev. 2012;13:2437–2444. doi: 10.7314/apjcp.2012.13.6.2437. [DOI] [PubMed] [Google Scholar]
- 23.Huang C, Liu J, Haudenschild CC, Zhan X. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J Biol Chem. 1998;273:25770–25776. doi: 10.1074/jbc.273.40.25770. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Liu Y, Liao K. Tyrosine phosphorylation of cortactin by the FAK-Src complex at focal adhesions regulates cell motility. BMC Cell Biol. 2011;12:49. doi: 10.1186/1471-2121-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada S, Yanamoto S, Kawasaki G, Mizuno A, Nemoto TK. Overexpression of cortactin increases invasion potential in oral squamous cell carcinoma. Pathol Oncol Res. 2010;16:523–531. doi: 10.1007/s12253-009-9245-y. [DOI] [PubMed] [Google Scholar]
- 26.Nakane K, Fujita Y, Terazawa R, Atsumi Y, Kato T, Nozawa Y, Deguchi T, Ito M. Inhibition of cortactin and SIRT1 expression attenuates migration and invasion of prostate cancer DU145 cells. Int J Urol. 2012;19:71–79. doi: 10.1111/j.1442-2042.2011.02888.x. [DOI] [PubMed] [Google Scholar]
- 27.Patel AS, Schechter GL, Wasilenko WJ, Somers KD. Overexpression of EMS1/cortactin in NIH3T3 fibroblasts causes increased cell motility and invasion in vitro. Oncogene. 1998;16:3227–3232. doi: 10.1038/sj.onc.1201850. [DOI] [PubMed] [Google Scholar]
- 28.van Rossum AG, Moolenaar WH, Schuuring E. Cortactin affects cell migration by regulating intercellular adhesion and cell spreading. Exp Cell Res. 2006;312:1658–1670. doi: 10.1016/j.yexcr.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Hill A, McFarlane S, Mulligan K, Gillespie H, Draffin JE, Trimble A, Ouhtit A, Johnston PG, Harkin DP, McCormick D, et al. Cortactin underpins CD44-promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells. Oncogene. 2006;25:6079–6091. doi: 10.1038/sj.onc.1209628. [DOI] [PubMed] [Google Scholar]
- 30.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 31.Bryce NS, Clark ES, Leysath JL, Currie JD, Webb DJ, Weaver AM. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr Biol. 2005;15:1276–1285. doi: 10.1016/j.cub.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 32.Jia L, Uekita T, Sakai R. Hyperphosphorylated cortactin in cancer cells plays an inhibitory role in cell motility. Mol Cancer Res. 2008;6:654–662. doi: 10.1158/1541-7786.MCR-07-0220. [DOI] [PubMed] [Google Scholar]
- 33.Lee YY, Yu CP, Lin CK, Nieh S, Hsu KF, Chiang H, Jin JS. Expression of survivin and cortactin in colorectal adenocarcinoma: association with clinicopathological parameters. Dis Markers. 2009;26:9–18. doi: 10.3233/DMA-2009-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noh SJ, Baek HA, Park HS, Jang KY, Moon WS, Kang MJ, Lee DG, Kim MH, Lee JH, Chung MJ. Expression of SIRT1 and cortactin is associated with progression of non-small cell lung cancer. Pathol Res Pract. 2013;209:365–370. doi: 10.1016/j.prp.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Hirakawa H, Shibata K, Nakayama T. Localization of cortactin is associated with colorectal cancer development. Int J Oncol. 2009;35:1271–1276. doi: 10.3892/ijo_00000444. [DOI] [PubMed] [Google Scholar]
- 36.Zhao G, Huang ZM, Kong YL, Wen DQ, Li Y, Ren L, Zhang HY. Cortactin is a sensitive biomarker relative to the poor prognosis of human hepatocellular carcinoma. World J Surg Oncol. 2013;11:74. doi: 10.1186/1477-7819-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Zheng H, Hara T, Takahashi H, Masuda S, Wang Z, Yang X, Guan Y, Takano Y. Aberrant expression of cortactin and fascin are effective markers for pathogenesis, invasion, metastasis and prognosis of gastric carcinomas. Int J Oncol. 2008;33:69–79. [PubMed] [Google Scholar]
- 38.Clauditz TS, Gontarewicz A, Lebok P, Tsourlakis MC, Grob TJ, Münscher A, Sauter G, Bokemeyer C, Knecht R, Wilczak W. Epidermal growth factor receptor (EGFR) in salivary gland carcinomas: potentials as therapeutic target. Oral Oncol. 2012;48:991–996. doi: 10.1016/j.oraloncology.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Rokita M, Stec R, Bodnar L, Charkiewicz R, Korniluk J, Smoter M, Cichowicz M, Chyczewski L, Nikliński J, Kozłowski W, et al. Overexpression of epidermal growth factor receptor as a prognostic factor in colorectal cancer on the basis of the Allred scoring system. Onco Targets Ther. 2013;6:967–976. doi: 10.2147/OTT.S42446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gazdar AF. Epidermal growth factor receptor inhibition in lung cancer: the evolving role of individualized therapy. Cancer Metastasis Rev. 2010;29:37–48. doi: 10.1007/s10555-010-9201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pirker R, Pereira JR, von Pawel J, Krzakowski M, Ramlau R, Park K, de Marinis F, Eberhardt WE, Paz-Ares L, Störkel S, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol. 2012;13:33–42. doi: 10.1016/S1470-2045(11)70318-7. [DOI] [PubMed] [Google Scholar]
- 42.Harder J, Waiz O, Otto F, Geissler M, Olschewski M, Weinhold B, Blum HE, Schmitt-Graeff A, Opitz OG. EGFR and HER2 expression in advanced biliary tract cancer. World J Gastroenterol. 2009;15:4511–4517. doi: 10.3748/wjg.15.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galizia G, Lieto E, Orditura M, Castellano P, Mura AL, Imperatore V, Pinto M, Zamboli A, De Vita F, Ferraraccio F. Epidermal growth factor receptor (EGFR) expression is associated with a worse prognosis in gastric cancer patients undergoing curative surgery. World J Surg. 2007;31:1458–1468. doi: 10.1007/s00268-007-9016-4. [DOI] [PubMed] [Google Scholar]
- 44.Kim JS, Kim MA, Kim TM, Lee SH, Kim DW, Im SA, Kim TY, Kim WH, Yang HK, Heo DS, et al. Biomarker analysis in stage III-IV (M0) gastric cancer patients who received curative surgery followed by adjuvant 5-fluorouracil and cisplatin chemotherapy: epidermal growth factor receptor (EGFR) associated with favourable survival. Br J Cancer. 2009;100:732–738. doi: 10.1038/sj.bjc.6604936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein JD, Christopoulos A, Ahn SM, Gooding WE, Grandis JR, Kim S. Antitumor effect of vandetanib through EGFR inhibition in head and neck squamous cell carcinoma. Head Neck. 2012;34:1269–1276. doi: 10.1002/hed.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hubbard JM, Alberts SR. Alternate dosing of cetuximab for patients with metastatic colorectal cancer. Gastrointest Cancer Res. 2013;6:47–55. [PMC free article] [PubMed] [Google Scholar]
- 47.Liu L, Wu N, Li J. Novel targeted agents for gastric cancer. J Hematol Oncol. 2012;5:31. doi: 10.1186/1756-8722-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]


