Abstract
Background
The publication of clinical prediction rules (CPRs) studies has risen significantly. It is unclear if this reflects increasing usage of these tools in clinical practice or how this may vary across clinical areas.
Aim
To review clinical guidelines in selected areas and survey GPs in order to explore CPR usefulness in the opinion of experts and use at the point of care.
Design and setting
A review of clinical guidelines and survey of UK GPs.
Method
Clinical guidelines in eight clinical domains with published CPRs were reviewed for recommendations to use CPRs including primary prevention of cardiovascular disease, transient ischaemic attack (TIA) and stroke, diabetes mellitus, fracture risk assessment in osteoporosis, lower limb fractures, breast cancer, depression, and acute infections in childhood. An online survey of 401 UK GPs was also conducted.
Results
Guideline review: Of 7637 records screened by title and/or abstract, 243 clinical guidelines met inclusion criteria. CPRs were most commonly recommended in guidelines regarding primary prevention of cardiovascular disease (67%) and depression (67%). There was little consensus across various clinical guidelines as to which CPR to use preferentially. Survey: Of 401 responders to the GP survey, most were aware of and applied named CPRs in the clinical areas of cardiovascular disease and depression. The commonest reasons for using CPRs were to guide management and conform to local policy requirements.
Conclusion
GPs use CPRs to guide management but also to comply with local policy requirements. Future research could focus on which clinical areas clinicians would most benefit from CPRs and promoting the use of robust, externally validated CPRs.
Keywords: clinical prediction rules, clinical guidelines, survey
INTRODUCTION
Clinical guidelines are developed systematically based on best available evidence to aid clinical decision making.1 The use of appropriately validated and tested clinical prediction rules (CPRs) is one way of implementing evidence-based medicine (EBM) for diagnosis and prognosis in clinical practice. CPRs are defined as tools that quantify the contributions of history, clinical examination, and diagnostic tests to stratify a patient in terms of the probability of having a target disorder (diagnostic CPR) or a future health outcome (prognostic CPR).1 An example is the Goldman CPR, which uses a combination of clinical and electrocardiograph findings to risk-stratify patients presenting with chest pain as low, moderate, or high risk of a cardiac cause.2 Smaller proportions of CPRs go further and recommend management decisions based on their algorithms, for instance, the modified Centor score for streptococcal throat infection stratifies patients based on symptoms and clinical signs and then uses this to direct the need for antibiotic prescription.3,4
However, there are well-recognised barriers to implementing CPRs at the point of care.5,6 One such barrier is a tendency to develop more CPRs for the same clinical situation, rather than validating existing models.7 The significant increase in the publication of CPRs in recent years suggests an increased interest on the part of researchers at least in such models.8,9 It is unclear if this reflects increasing usage of these tools in clinical practice or how this may vary across clinical areas.
This study investigated whether published CPRs have been considered useful by expert bodies and at the point of clinical care. To answer the first question, a review of international clinical guidelines produced on behalf of expert bodies was performed, and to answer the second, a well-defined group of UK clinicians, GPs were surveyed about their use of CPRs in selected clinical areas.
How this fits in
The use of appropriately validated and tested clinical prediction rules (CPRs) is one way of implementing evidence-based medicine for diagnosis and prognosis in clinical practice and publication of CPRs has risen significantly. This study showed that recommendation of CPRs by clinical guidelines varied according to clinical area. Surveyed GPs reported using CPRs most frequently in the clinical domains of cardiovascular disease and depression, primarily to guide management and adhere to local policy requirements. Future efforts could focus on determining in which areas of practice CPRs would be most beneficial for clinicians and patients, and promoting the use of robust, externally validated CPRs.
METHOD
Review of clinical guidelines
The aim was to identify clinical guidelines in eight selected areas in which the authors had prior knowledge that at least one CPR potentially relevant to primary care had been published:
primary prevention of cardiovascular disease (CVD);
TIA/stroke diagnosis and management;
diabetes mellitus screening, diagnosis or risk assessment;
fracture risk assessment in osteoporosis screening and management;
lower limb fractures diagnosis;
breast cancer diagnosis, screening, and risk assessment;
depression diagnosis and management; and
acute childhood infections, namely meningitis, influenza, urinary tract infection, gastroenteritis, otitis media, tonsillitis, pneumonia, and bronchiolitis.
Search strategy
A PubMed search used the ‘Practice Guideline’ publication type and was expanded to include documents with any of the words; ‘Guideline[s]’, ‘Framework’, ‘Standards’, ‘Recommendation[s]’, ‘Guidance’, ‘Consensus’, ‘Statement’ or ‘Practice Guideline’ in the title, producing a highly sensitive search (n = 106 088). To make the search more specific limits were applied: English language; exclusion of publication types News, Randomized Controlled Trial, Meta-Analysis, Clinical Trial, Letter and Comment; published between 1 June 2000 and 31 May 2010, resulting in 41 228 records. This guidelines search was then combined with subject-specific searches designed by researchers familiar with each of the clinical domains.
In addition, the websites of the National Guideline clearing house, the National Institute for Health and Clinical Excellence (NICE) and the Scottish Intercollegiate Guideline Network (SIGN) were accessed and searched (included as additional sources in Appendices 1a and 1b).
Study selection
Documents identified from these subject-specific searches were eligible for inclusion if they met the following criteria: (a) contains systematically developed statements that include recommendations, strategies, or information that assists clinicians and patients to make decisions about appropriate health care for specific clinical presentations; (b) produced by medical speciality associations — relevant professional societies, public or private organisations, government agencies, or healthcare providers at the state, national, or international level; (c) full text freely available in print or electronic format; and (d) current and most recent available version of the guideline available. Documents identified by the search and meeting the above four criteria were considered to be ‘clinical guidelines’ for the purposes of this review.
The difficulty of formally defining CPRs has been discussed previously.8 For the purposes of this review, a pre-existing definition10 was adapted to define a clinical prediction rule as ‘a predefined combination of (two or more) questions, symptoms, signs and tests that provides information on risk, diagnosis, or prognosis’. Formal diagnostic criteria were not considered to be CPRs. For the purposes of this review, a CPR was considered to be ‘predefined’ if the guideline cites an article on the CPR in a peer-reviewed journal.
Data extraction
In each clinical area, one researcher searched through titles and abstracts for their specific search. Each potentially relevant full-text article was independently reviewed in duplicate and relevant data extracted. To be considered to be ‘recommended’ by the guideline, use of language that recommends, encourages, or promotes the use of the CPR was required. Discrepancies were resolved by consensus or by a third adjudicating reviewer. For each clinical area the total number of guidelines retrieved, the number and proportion recommending use of at least one CPR, and the most commonly recommended CPRs are reported. For the acute infections in children domain, guidelines were included if children were mentioned specifically in the title or if the guideline could be applied to both adults and children.
Survey of GPs
Participants
Participants were GPs in the UK, registered with the General Medical Council, recruited from Doctors.net.uk. To estimate the percentage using each CPR with a standard error of approximately 2.5%, a sample size of 400 GPs stratified by NHS Strategic Health Authority and seniority/position was requested. Doctors.net.uk sent invitations to members followed by reminders until 400 GPs had completed the questionnaire. Participants were asked for their year of qualification, and role in the practice.
Survey
In consultation with academic GP colleagues, 25 CPRs potentially relevant to UK general practice were selected. Modifications made after the in-house pilot included the addition of four CPRs: one of these, the NICE traffic light algorithm for childhood infection, did not meet the criteria for a CPR in the review of guidelines, but was considered a CPR by participants in the pilot survey. The resulting 29 included CPRs were grouped under six clinical areas, for presentation to survey responders, as shown in Table 1. Pragmatic considerations regarding survey length precluded the inclusion of all clinical areas studied in the first part of this study. Responders were asked which CPRs they had heard of and how often they used them. They were asked for reasons why they did or did not use each CPR, using the following options: (a) aid diagnosis; (b) assessing severity; (c) to guide therapy; (d) to guide referral; (e) comply with clinical guidelines/Quality Outcomes Framework (QOF); (f) automatically generated by practice software; and (g) inform or educate patients. A free text field was provided for other reasons for not using CPRs or to indicate any additional CPRs not included in the survey.
Table 1.
List of clinical prediction rules (CPRs) included in the survey
| Clinical area | Clinical prediction rule | Description | Reference |
|---|---|---|---|
| Cardiovascular disease | QRISK or QRISK2 | 10-year risk of heart attack or stroke, based on QRESEARCH database | 21 |
| Joint British Societies (JBS) charts | Based on Framingham risk equation with adjustments | 22 | |
| New Zealand (NZ) Tables | Cardiovascular Risk Calculator from the New Zealand Guidelines Group | 23 | |
| Sheffield Tables | Based on Framingham risk function | 24 | |
| Any Framingham Risk Score | Cardiovascular risk assessment, based on Framingham study | 25 | |
| Systematic Coronary Risk Evaluation (SCORE) risk charts | The European cardiovascular disease risk assessment model | 26 | |
| PROCAM risk score | Cardiovascular risk assessment, based on Prospective Cardiovascular Münster (PROCAM) Study | 27 | |
|
| |||
| Anxiety and depression | Patient Health Questionnaire (PHQ)-2 | 2-item version of PHQ-9 | 28 |
| Patient Health Questionnaire (PHQ)-9 | 9-item depression module of the PHQ | 29 | |
| Generalised Anxiety Scale (GAD)-2 | 2-item subscale of GAD-7 | 30 | |
| Generalised Anxiety Scale (GAD)-7 | 7-item anxiety measure | 30 | |
| Hospital anxiety and depression scale (HADs) | Self-reported rating instrument for anxiety and depression | 31 | |
| Beck Depression Inventory (BDI) | Self-reported items, each correlating to a symptom of major depressive disorder experienced over the preceding 2 weeks | 32 | |
|
| |||
| Fracture | Ottawa ankle rule | Decision rule for the selective use of radiography in acute ankle injuries | 33 |
| Ottawa knee rule | Decision rule for the selective use of radiography in acute knee injuries | 34 | |
| Ottawa foot rule | (Also known as the Ottawa ankle rule) | 33 | |
| Pittsburgh knee rule | Decision rule for the selective use of radiography in acute knee injuries | 35 | |
|
| |||
| Cancer | Gail risk score | Breast cancer risk assessment tool | 36 |
| Risk Assessment in Genetics (RAGs) | Evaluation of genetic risk of cancer | 37 | |
|
| |||
| Infection | Yale | Observation scales to identify serious illness in febrile children | 38 |
| NICE traffic light system | From the NICE guideline on feverish illness in children | 39 | |
| CRB65 | Grades severity of community-acquired pneumonia in terms of 30-day mortality | 17 | |
| STREP score | Modified Centor Score for Streptococcal Pharyngitis in children and adults | 40 | |
| CENTOR score | Diagnosis of Streptococcal Pharyngitis in adults | 3 | |
|
| |||
| General medical | ABCD or ABCD2 score | Prediction of very early stroke risk after transient ischaemic attack at 7 days | 41 |
| California score | Risk of stroke at 90 days | 42 | |
| CHADS or CHADS2 | Atrial fibrillation stroke risk | 43 | |
| Wells score (DVT) | Risk of deep vein thrombosis | 44 | |
| Wells score (PE) | Risk of pulmonary embolism | 45 | |
RESULTS
Review of clinical guidelines
An overview of the search strategy is presented in Appendix 1a and 1b. A total of 7637 records were screened by title and/or abstract and 243 eligible clinical guidelines in eight clinical areas were identified and included in the review.
A summary of clinical guideline numbers retrieved and named CPRs recommended according to clinical domain is presented in Table 2. Overall, CPRs were most commonly recommended in the clinical domains of primary prevention of cardiovascular disease (67%), depression (67%), TIA/stroke (63%), and breast cancer (51%). For lower limb fractures and fracture risk assessment in osteoporosis, CPRs were recommended in 40% and 38% of reviewed guidelines respectively. CPRs were least often recommended in the clinical domains of diabetes mellitus (20%) and acute childhood infections (16%). Overall, there was little consensus across reviewed guidelines as to which, if any, CPR to use preferentially.
Table 2.
Clinical guidelines review of clinical prediction rules (CPRs) recommendations
| Clinical area | Total number of guidelines included | CPR recommended (%) | Named CPRs recommended (number of guidelines) | Other CPRs recommended not in clinical domain |
|---|---|---|---|---|
| Cardiovascular disease (primary prevention) | 45 | 30 (67) | Risk scores derived from the Framingham Heart Study (21);25 Systematic Coronary Risk Evaluation (SCORE) tool (5) ;26 UK Prospective Diabetes Study (UKPDS) Risk Engine (4);46 Risk stratification system of the 2003 European Society of Cardiology (3);47 CHADS or CHADS2 score (2);43 Cardiovascular Life Expectancy model (2);48 Heartscore (1);26 Reynolds Risk Score (1);49 ASSIGN (1);50 Risk stratification system 1999 World Health Organization guidelines (1).51 | None |
| Transient ischaemic attack (TIA) or stroke (diagnosis and management) | 16 | 10 (63) | ABCD or ABCD2 (5);41,52 Framingham risk score (1);53 FAST (Facial weakness, Arm weakness, Speech difficulty, Time to act) (6);54 Rule Out Stroke in the Emergency Room (ROSIER) (5); 55 Melbourne acute stroke scale (MASS) (1).56 | For depression (GHQ12, PHQ9 and SAD-Q), for malnutrition (MUST) and the Glasgow Coma Scale 19,29,57–59 |
| Diabetes mellitus (Screening, risk assessment or diagnosis) | 20 | 4 (20) | Finnish Diabetes Risk Score (FINDRISC) (3); 60 Diabetes Risk Calculator (1).61 | For cardiovascular risk assessment (Framingham risk score) 25 |
| Osteoporosis (Fracture risk assessment) | 25 | 9 (38) | WHO/FRAX algorithm (5);62 Osteoporosis Risk Assessment Index (2); 13 Simple Calculated Osteoporosis Risk Estimation questionnaire (1); 63 Two ultrasound indices; the stiffness index and the quantitative ultrasound index (1). | None |
| Lower limb fractures (Diagnosis and management) | 20 | 8 (40) | Ottawa Ankle Rule (2); 33 Ottawa Knee rule (4);34 Meniscal Pathology Composite score (1);64 Function Score of De Bie (1) 4. 65 | None |
| Acute childhood infections a (Diagnosis and management) | 73 | 12 (16) | Tonsillitis: Centor score (3);3 Pneumonia: CURB-65 (5);17 Pneumonia Severity Index/Patient Outcome Research Team (PORT) (5); 66 World Health Organization (WHO) criteria (1); 67 Meningitis: Glasgow meningococcal septicaemia prognostic score (2); 68 Bacterial meningitis score (2); 69 Hoen’s software (1); 70 Meningitest (1).71 | Glasgow Coma Scale 19 |
| Breast cancer (Risk assessment, screening and referral) | 32 | 16 (51) | Original or modified Gail model (5); 72 Claus model (4); 73 BRCAPRO (4); 74 Mammaprint tool (3); 75 Oncotype Diagnosis Recurrence score (3); 76 Adjuvant Risk score (3); 77 Nottingham Prognostic Index (1); 78 Manchester Score (1); 79 Risk Assessment in Genetics (RAGS) Tool (1); 37 Family History Risk Assessment Tool (1); 80 Tyrer Tool (1). 81 | None |
| Depression (Diagnosis and management) | 12 | 8 (67) | Patient Health Questionnaire (PHQ)-9 (4); 29 Two-item screener (4); 28 Hamilton Rating Scale for Depression (3); 82 Beck Depression Inventory (BDI) (2); 32 Montgomery Asberg Depression Rating Scale (MADRS) (2); 83 Edinburgh Postnatal Depression Inventory (EPDI) (1); 11 Quick Inventory of Depressive Symptomatology (QID-SR) (1); 84 Cornell Scales for Depression in Dementia (CSDD) (1); 85 Bech-Rafaelsen Melancholia Scale (BRMS) (1); 86 Hospital Anxiety and Depression Scale (HADS) (1); 31 Clinical Global Impressions (CGI) scale (1); 87 Geriatric Depression Scale (GDS) (1); 88 Centre for Epidemiological Studies Depression Scale (CES-D) (1).89 | CAGE-AID 90 |
For the acute childhood infections of otitis media, bronchiolitis, gastroenteritis, urinary tract infection, and influenza, no CPR recommendations were found in reviewed guidelines.
Survey of UK GPs
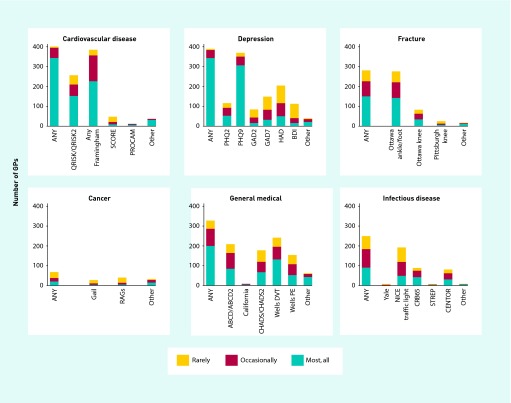
A total of 401 responses were collected from Scotland (12%), Northern Ireland (3%), Wales (4.5%), and England (80.5%), spread among each of the 10 English Strategic Health Authority regions. Participants qualified between 1969 and 2005 (median 1995) and most (65%) were GP principals or partners. Figure 1 shows the reported frequency of use of any CPRs in each clinical domain, of specific CPRs indicated by name, and of other CPRs not included in the questionnaire design but named by responders in the free text field. In CVD these other CPRs included ASSIGN (n = 23 GPs, all based in Scotland) and the UKPDS Risk Engine (n = 1). In depression, other CPRs included the Edinburgh Postnatal Depression Scale (24 GPs) and Mini-Mental State Examination for dementia (n = 3).11,12 In addition to the fracture CPRs listed in the questionnaire, seven used the FRAX tool for osteoporosis.13 In cancer, additional CPRs mentioned were the Gleason score for prostate cancer staging (n = 13 GPs),14 Dukes staging for colorectal cancer (n = 5),15 and the Tumour Nodes Metastases cancer staging system (n = 4).16 In addition to infection CPRs listed in the survey, five (1%) reported using the CURB65 score17 and two (0.5%) reported having used the APACHE II score for ICU mortality.18 In the section entitled general medical, additional CPRs mentioned were the Glasgow Coma Scale (n = 11 GPs), Epworth sleepiness scale (n = 5), and Rockall score for risk of upper gastrointestinal bleeding (n = 3).19 Responders additionally used the free text to report using the International Prostate Symptom Score for benign prostatic hyperplasia (n = 7),20 various alcohol use questionnaires (n = 6), and others (all n≤2). Reported reasons for CPR use are presented in Table 3.
Figure 1.
Self-reported use of clinical prediction rules by 401 UK GPs in selected clinical areas.
Table 3.
Number (%) of GPs reporting respective reasons for using clinical prediction rules
| Cardiovascular disease, n (%) | Anxiety and depression n (%) | Fracture n (%) | Cancer n (%) | Infection n (%) | General medical n (%) | |
|---|---|---|---|---|---|---|
| Aid diagnosis | 143 (36) | 217 (54) | 225 (56) | 48 (12) | 92 (23) | 219 (55) |
| Assessing severity | 191 (48) | 279 (70) | 66 (16) | 39 (10) | 168 (42) | 142 (35) |
| To guide therapy | 336 (84) | 207 (52) | 64 (16) | 30 (7) | 118 (29) | 188 (47) |
| To guide referral | 89 (22) | 134 (33) | 146 (36) | 59 (15) | 106 (26) | 218 (54) |
| Comply with clinical guidelines/QOF | 267 (67) | 313 (78) | 17 (4) | 21 (5) | 37 (9) | 106 (26) |
| Automatically generated by practice software | 106 (26) | 31 (8) | 4 (1) | 4 (1) | 1 (0.002) | 19 (5) |
| Inform or educate patients | 220 (55) | 123 (31) | 50 (12) | 30 (7) | 44 (11) | 75 (19) |
| Other | 1 (0.002) | 8 (2) | 8 (2) | 2 (0.005) | 1 (0.002) | 3 (1) |
QOF = Quality and Outcomes Framework.
The main reason for not using named CPRs related to lack of familiarity (Table 4). Other reasons, reported in free text fields, included preference for own clinical judgement (for CPRs listed under depression, infection, and general medical), greater relevance to secondary care settings (fracture and cancer), and perceived lack of utility (depression and cancer).
Table 4.
Number (%) of GPs who do not use the respective clinical prediction rules and of these, the number (%) who had never heard of them
| Clinical area | CPR | Do not use n (%) | Never heard of it, n (%) |
|---|---|---|---|
| Cardiovascular disease | QRISK or QRISK2 | 140 (35) | 88 (22) |
| Joint British Societies (JBS) risk charts | 78 (19) | 33 (8) | |
| JBS risk calculator | 152 (38) | 96 (24) | |
| New Zealand tables | 355 (89) | 313 (78) | |
| Framingham risk score | 58 (14) | 6 (1) | |
| Systematic Coronary Risk Evaluation (SCORE) | 364 (91) | 332 (83) | |
| PROCAM | 98 (393) | 94 (378) | |
|
| |||
| Anxiety and depression | Patient Health Questionnaire (PHQ)-2 | 292 (73) | 251 (63) |
| PHQ9 | 33 (8) | 17 (4) | |
| Generalised Anxiety Scale (GAD)-2 | 318 (79) | 246 (61) | |
| GAD7 | 247 (62) | 152 (38) | |
| Hospital and Anxiety Depression scale (HADs) | 198 (49) | 54 (13) | |
| Beck Depression Inventory (BDI) | 287 (72) | 108 (27) | |
|
| |||
| Fracture | Ottawa ankle | 125 (31) | 83 (21) |
| Ottawa knee | 315 (79) | 284 (71) | |
| Ottawa foot | 312 (78) | 289 (72) | |
| Pittsburgh knee | 390 (97) | 378 (94) | |
|
| |||
| Cancer | Gail risk score | 377 (94) | 363 (91) |
| Risk Assessment in Genetics (RAGs) | 365 (91) | 346 (86) | |
|
| |||
| Infection | Yale | 391 (98) | 387 (97) |
| NICE traffic light | 213 (53) | 172 (43) | |
| CRB65 | 320 (80) | 313 (78) | |
| STREP score | 390 (97) | 386 (96) | |
| Centor score | 323 (81) | 305 (76) | |
|
| |||
| General medical | ABCD or ABCD2 | 194 (48) | 173 (43) |
| California score | 394 (98) | 386 (96) | |
| CHADS or CHADS2 | 230 (57) | 208 (52) | |
| Wells score for DVT | 163 (41) | 139 (35) | |
| Wells score for PE | 247 (62) | 228 (57) | |
DISCUSSION
Summary
Of the eight clinical domains studied, guidelines most commonly recommended CPRs for primary prevention of CVD and depression. For CVD, a total of 10 different cardiovascular risk assessment models were recommended, most commonly those derived from the Framingham Heart Study. Surveyed GPs reported using these tools in practice also, with most using Framingham derived scores, the Joint British Societies risk score, or QRISK, primarily to guide therapy. Other reported reasons for use of these CPRs were to inform or educate patients, comply with guidelines/Quality and Outcomes Framework (QOF), and to assess disease severity. For depression, a total of 13 different models were recommended in eight reviewed guidelines, most commonly the PHQ-9. This was also utilised by most of the surveyed GPs who indicated that guideline or QOF conformance was the most common reason for use, followed by assessing severity and as a diagnostic aid. The Ottawa ankle rule for ankle fracture assessment, although infrequently recommended by reviewed guidelines, was used by most of the surveyed GPs, primarily to aid diagnosis. For breast cancer, although about half of reviewed guidelines recommended the use of a CPR model for risk assessment, these tools were very infrequently utilised by surveyed GPs. Most were either unaware of these tools or preferred to use UK referral guidelines, which dictate that suspected cancer cases need specialist review within 2 weeks.
Both the survey and review suggest that there are varying influences regarding use of CPRs in clinical practice. Use of these tools may vary geographically as illustrated by the guidelines review, where the QRISK2 score was recommended by UK guidelines only, and within the UK by the survey, with the ASSIGN algorithm being used exclusively by Scottish GPs. As already mentioned, national policy requirements in UK general practice also have an impact on CPR uptake. Overall, a lack of familiarity, preference for their own clinical judgement, or considering the CPR to be unnecessary were highlighted by surveyed GPs as the main impediments for use of these tools at the point of care. Examining the level of evidence for all CPRs included in this review was beyond the scope of this study. However, it is interesting to note that the Centor score for streptococcal throat infection, which has been broadly validated for use in general practice, was not used by most GPs (81%), with 76% of these reporting they had never heard of it, whereas the NICE traffic light system for the assessing childhood fever, which was developed for the purposes of the guideline, was used by almost half of the surveyed GPs. CPR use also varied according to clinical area, for example the CHADS and CHADS2 score for the prediction stroke risk in patients with atrial fibrillation and the Well’s score for DVT were not utilised by most surveyed GPs, although most were familiar with these CPRs.
Clinical guidelines offer scope to critically appraise published CPRs, which could help clinicians in making an informed decision regarding their use. However, in this review there was little such evaluation of CPRs evident and little consensus between guidelines as to which, if any, of these tools should be used preferentially.
Strengths and limitations
This study reviews wide-ranging international literature supplemented by a detailed survey of actual clinical practice in UK general practice. Each review was designed by a researcher with experience in the relevant area. The pre-selection of clinical domains in which CPRs exist allows comparison in terms of guideline recommendations and use in practice.
There are several limitations. First, for the purpose of rigorous review it was necessary to adopt a single, objective definition of a CPR. As there is no internationally agreed definition of a CPR, the definition used should be considered a working definition for a specific project rather than definitive for all purposes. Second, the literature review, of international scale and across eight clinical areas, is supplemented by a survey of primary care in a single national setting. The authors did not have the resources to conduct an international survey of primary, secondary, and tertiary care, and this led to a UK survey that looked at fewer CPRs than the literature review. Finally, the electronic survey mechanism used for this study gives no known denominator and represents a partially self-selecting population.
Comparison with existing literature
Previous studies of clinical prediction rules across multiple domains have assessed the properties of the rules, such as validity and impact, rather than their uptake by practicing clinicians7,9 Surveys of the uptake of CPRs have usually been restricted to single clinical domains.91–94 To the author’s knowledge this is the first large survey to compare the uptake of CPRs across multiple clinical domains and to relate this to a systematic evaluation of guideline recommendations.
Implications for research and practice
From a clinical perspective, CPRs were applied by surveyed GPs most frequently in the clinical domains of CVD and depression mainly to guide management and adhere to local policy requirements. Lack of awareness was cited as one of the reasons for not using CPRs in practice. Future efforts could focus on determining in which areas of practice CPRs would be most beneficial to clinicians and patients, for example, referral guidance at the primary–secondary care interface for high stakes diagnoses such as myocardial infarction and cancer. In addition, the implementation of poorly validated CPRs should be resisted. Research should instead be directed to developing robust, externally validated CPRs that have been shown to have a positive impact on the process and outcome of clinical care. It is these CPRs that should be promoted in clinical guidelines.
Acknowledgments
The authors are grateful to Dr Grainne Cousins and Dr Daniel Brandt for assistance with the literature searches.
Appendices 1a and 1b.
Literature search for clinical practice guidelines.
Funding
The authors wrote this paper for the International Diagnosis and Prognosis Prediction (IDAPP) working group. The survey of GPs represents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research programme (RP-PG-0407–10347). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. The guidelines review is supported by the Health Research Board (HRB) of Ireland through the HRB Centre for Primary Care Research under Grant HRC/2007/1.
Ethical approval
Not applicable.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
Richard Stevens is an author of the UKPDS Risk Engine, one of the clinical prediction rules studied in this manuscript. He has no financial stake in the Risk Engine.
Discuss this article
Contribute and read comments about this article: www.bjgp.org/letters
REFERENCES
- 1.McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA. 2000;284(1):79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Goldman L, Cook EF, Brand DA, et al. A computer protocol to predict myocardial infarction in emergency department patients with chest pain. N Engl J Med. 1988;318(13):797–803. doi: 10.1056/NEJM198803313181301. [DOI] [PubMed] [Google Scholar]
- 3.Centor RM, Witherspoon JM, Dalton HP, et al. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1(3):239–246. doi: 10.1177/0272989X8100100304. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Dolan JG, Centor RM. Optimal management of adults with pharyngitis – a multi-criteria decision analysis. BMC Med Inform Decis Mak. 2006;6:14. doi: 10.1186/1472-6947-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang ES, Wyer PC, Haynes RB. Knowledge translation: closing the evidence-to-practice gap. Ann Emerg Med. 2007;49(3):355–363. doi: 10.1016/j.annemergmed.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Reilly BM, Evans AT. Translating clinical research into clinical practice: impact of using prediction rules to make decisions. Ann Intern Med. 2006;144(3):201–209. doi: 10.7326/0003-4819-144-3-200602070-00009. [DOI] [PubMed] [Google Scholar]
- 7.Keogh C, Fahey T. Clinical Prediction Rules in Primary Care: what can be done to maximise their implementation? Clinical Evidence. 2010 http://clinicalevidence.bmj.com/x/mce/file/05-10-10.pdf (accessed 26 Feb 2014). [Google Scholar]
- 8.Keogh C, Wallace E, O’Brien KK, et al. Optimized retrieval of primary care clinical prediction rules from MEDLINE to establish a Web-based register. J Clin Epidemiol. 64(8):848–860. doi: 10.1016/j.jclinepi.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Toll DB, Janssen KJ, Vergouwe Y, Moons KG. Validation, updating and impact of clinical prediction rules: a review. J Clin Epidemiol. 2008;61(11):1085–1094. doi: 10.1016/j.jclinepi.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA. 1997;277(6):488–494. [PubMed] [Google Scholar]
- 11.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Kanis JA, Johnell O, Oden A, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gleason DF. The Veteran’s Administration Cooperative Urologic Research Group: histologic grading and clinical staging of prostatic carcinoma. In: Tannenbaum M, editor. Urologic pathology: the prostate. Phildelphia, PA: Lea and Febiger; 1977. pp. 171–198. [Google Scholar]
- 15.Dukes C. The classification of cancer of the rectum. J Pathol Bacteriol. 1932;35:323–332. [Google Scholar]
- 16.Denoix PF. Enquete permanent dans les centres anticancereaux. [Continuous survey in cancer treatment centres] Bull Inst Nat Hyg. 1946;1:70–75. [PubMed] [Google Scholar]
- 17.Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 19.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 20.Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 21.Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ. 2007;335(7611):136. doi: 10.1136/bmj.39261.471806.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular disease in clinical practice. Heart. 2005;91(Suppl 5):v1–52. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson R. Updated New Zealand cardiovascular disease risk-benefit prediction guide. BMJ. 2000;320(7236):709–710. doi: 10.1136/bmj.320.7236.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haq IU, Jackson PR, Yeo WW, Ramsay LE. Sheffield risk and treatment table for cholesterol lowering for primary prevention of coronary heart disease. Lancet. 1995;346(8988):1467–1471. doi: 10.1016/s0140-6736(95)92477-9. [DOI] [PubMed] [Google Scholar]
- 25.Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83(1):356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 26.Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 27.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105(3):310–315. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 31.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 33.Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269(9):1127–1132. doi: 10.1001/jama.269.9.1127. [DOI] [PubMed] [Google Scholar]
- 34.Stiell IG, Greenberg GH, Wells GA, et al. Derivation of a decision rule for the use of radiography in acute knee injuries. Ann Emerg Med. 1995;26(4):405–413. doi: 10.1016/s0196-0644(95)70106-0. [DOI] [PubMed] [Google Scholar]
- 35.Seaberg DC, Jackson R. Clinical decision rule for knee radiographs. Am J Emerg Med. 1994;12(5):541–543. doi: 10.1016/0735-6757(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 36.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 37.Coulson AS, Glasspool DW, Fox J, Emery J. RAGs: a novel approach to computerized genetic risk assessment and decision support from pedigrees. Methods Inf Med. 2001;40(4):315–322. [PubMed] [Google Scholar]
- 38.McCarthy PL, Sharpe MR, Spiesel SZ, et al. Observation scales to identify serious illness in febrile children. Pediatrics. 1982;70(5):802–809. [PubMed] [Google Scholar]
- 39.NICE. Feverish illness in children (CG47) (replaced by CG160) Feverish illness in children — Assessment and initial management in children younger than 5 years. http://guidance.nice.org.uk/CG47 (accessed 26 Feb 2014).
- 40.McIsaac WJ, Kellner JD, Aufricht P, et al. Empirical validation of guidelines for the management of pharyngitis in children and adults. JAMA. 2004;291(13):1587–1595. doi: 10.1001/jama.291.13.1587. [DOI] [PubMed] [Google Scholar]
- 41.Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005;366(9479):29–36. doi: 10.1016/S0140-6736(05)66702-5. [DOI] [PubMed] [Google Scholar]
- 42.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2001;284(22):2901–2906. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]
- 43.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 44.Wells PS, Hirsh J, Anderson DR, et al. A simple clinical model for the diagnosis of deep-vein thrombosis combined with impedance plethysmography: potential for an improvement in the diagnostic process. J Intern Med. 1998;243(1):15–23. doi: 10.1046/j.1365-2796.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- 45.Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416–420. [PubMed] [Google Scholar]
- 46.Kothari V, Stevens RJ, Adler AI, et al. UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke. 2002;33(7):1776–1781. doi: 10.1161/01.str.0000020091.07144.c7. [DOI] [PubMed] [Google Scholar]
- 47.2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21(6):1011–1053. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Grover SA, Abrahamowicz M, Joseph L, et al. The benefits of treating hyperlipidemia to prevent coronary heart disease. Estimating changes in life expectancy and morbidity. JAMA. 1992;267(6):816–822. [PubMed] [Google Scholar]
- 49.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 50.Woodward M, Brindle P, TunstallĐPedoe H. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC) Heart. 2007;93(2):172–176. doi: 10.1136/hrt.2006.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chalmers J, MacMahon S, Mancia G, et al. 1999 World Health Organization - International Society of Hypertension Guidelines for the management of hypertension. Guidelines sub-committee of the WHO. Clin Exp Hypertens. 1999;21(5–6):1009–1060. doi: 10.3109/10641969909061028. [DOI] [PubMed] [Google Scholar]
- 52.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- 53.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373(9665):739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harbison J, Hossain O, Jenkinson D, et al. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke. 2003;34(1):71–76. doi: 10.1161/01.str.0000044170.46643.5e. [DOI] [PubMed] [Google Scholar]
- 55.Nor AM, Davis J, Sen B, et al. The Recognition of Stroke in the Emergency Room (ROSIER) scale: development and validation of a stroke recognition instrument. Lancet Neurol. 4(11):727–734. doi: 10.1016/S1474-4422(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 56.Bray JE, Martin J, Cooper G, et al. Paramedic identification of stroke: community validation of the Melbourne ambulance stroke screen. Cerebrovasc Dis. 2005;20(1):28–33. doi: 10.1159/000086201. [DOI] [PubMed] [Google Scholar]
- 57.Goldberg D, Williams P. A user’s guide to the General Health Questionnaire. Basingstoke: NFER-Nelson; 1988. [Google Scholar]
- 58.Sutcliffe LM, Lincoln NB. The assessment of depression in aphasic stroke patients: the development of the Stroke Aphasic Depression Questionnaire. Clin Rehabil. 1998;12(6):506–513. doi: 10.1191/026921598672167702. [DOI] [PubMed] [Google Scholar]
- 59.Malnutrition Advisory Group . MAG guidelines for detection and management of malnutrition. Maidenhead: British Association for Parenteral and Enteral Nutrition (BAPEN); 2000. [Google Scholar]
- 60.Heikes KE, Eddy DM, Arondekar B, Schlessinger L. Diabetes Risk Calculator: a simple tool for detecting undiagnosed diabetes and pre-diabetes. Diabetes Care. 2008;31(5):1040–1045. doi: 10.2337/dc07-1150. [DOI] [PubMed] [Google Scholar]
- 61.Lindstrom J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26(3):725–731. doi: 10.2337/diacare.26.3.725. [DOI] [PubMed] [Google Scholar]
- 62.Cadarette SM, Jaglal SB, Kreiger N, et al. Development and validation of the Osteoporosis Risk Assessment Instrument to facilitate selection of women for bone densitometry. CMAJ. 2000;162(9):1289–1294. [PMC free article] [PubMed] [Google Scholar]
- 63.Lydick E, Cook K, Turpin J, et al. Development and validation of a simple questionnaire to facilitate identification of women likely to have low bone density. Am J Manag Care. 1998;4(1):37–48. [PubMed] [Google Scholar]
- 64.Lowery DJ, Farley TD, Wing DW, et al. A clinical composite score accurately detects meniscal pathology. Arthroscopy. 2006;22(11):1174–1179. doi: 10.1016/j.arthro.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 65.de Bie RA, de Vet HC, van den Wildenberg FA, et al. The prognosis of ankle sprains. Int J Sports Med. 1997;18(4):285–289. doi: 10.1055/s-2007-972635. [DOI] [PubMed] [Google Scholar]
- 66.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 67.WHO guidelines on detecting pneumonia in children. Lancet. 1991;338(8780):1453–1454. [PubMed] [Google Scholar]
- 68.Dubos F, Moulin F, Raymond J, et al. [Distinction between bacterial and aseptic meningitis in children: refinement of a clinical decision rule] Arch Pediatr. 2007;14(5):434–438. doi: 10.1016/j.arcped.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 69.Nigrovic LE, Kuppermann N, Malley R. Development and validation of a multivariable predictive model to distinguish bacterial from aseptic meningitis in children in the post-Haemophilus influenzae era. Pediatrics. 2002;110(4):712–719. doi: 10.1542/peds.110.4.712. [DOI] [PubMed] [Google Scholar]
- 70.Hoen B, Viel JF, Paquot C, et al. Multivariate approach to differential diagnosis of acute meningitis. Eur J Clin Microbiol Infect Dis. 1995;14(4):267–274. doi: 10.1007/BF02116518. [DOI] [PubMed] [Google Scholar]
- 71.Sinclair JF, Skeoch CH, Hallworth D. Prognosis of meningococcal septicaemia. Lancet. 1987;2(8549):38. doi: 10.1016/s0140-6736(87)93067-4. [DOI] [PubMed] [Google Scholar]
- 72.Chen J, Pee D, Ayyagari R, et al. Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J Natl Cancer Inst. 2006;98(17):1215–1226. doi: 10.1093/jnci/djj332. [DOI] [PubMed] [Google Scholar]
- 73.Claus EB, Risch N, Thompson WD. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet. 1991;48(2):232–242. [PMC free article] [PubMed] [Google Scholar]
- 74.Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62(1):145–158. doi: 10.1086/301670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Glas AM, Floore A, Delahaye LJ, et al. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics. 2006;7:278. doi: 10.1186/1471-2164-7-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 77.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19(4):980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 78.Blamey RW. The design and clinical use of the Nottingham Prognostic Index in breast cancer. Breast. 1996;5:156–157. [Google Scholar]
- 79.Evans DG, Eccles DM, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41(6):474–480. doi: 10.1136/jmg.2003.017996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58(4):299–308. doi: 10.1034/j.1399-0004.2000.580408.x. [DOI] [PubMed] [Google Scholar]
- 81.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 82.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 84.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 85.Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23(3):271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 86.Bech P, Rafaelsen OJ. The use of rating scales exemplified by a comparison of the Hamilton and the Bech-Rafaelsen Melancholia Scale. Acta Psychiatr Scand. 1980;62(Suppl 285):128–132. [Google Scholar]
- 87.Guy W. ECDEU Assessment Manual for Psychopharmacology — Revised. Rockville, MD: Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. (DHEW Publ No ADM 76–338). [Google Scholar]
- 88.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 89.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psycholog Measure. 1977;1:385–401. [Google Scholar]
- 90.Brown RL. Identification and office management of alcohol and drug disorders. In: Fleming M, Barry K, editors. Addictive disorders. St. Louis, MO: Mosby; 1992. pp. 25–43. [Google Scholar]
- 91.Beswick AD, Brindle P, Fahey T, Ebrahim S. NICE Clinical Guidelines, No. 67S. London: Royal College of General Practitioners (UK); 2008. May, A Systematic Review of Risk Scoring Methods and Clinical Decision Aids Used in the Primary Prevention of Coronary Heart Disease (Supplement) [PubMed] [Google Scholar]
- 92.Dallongeville J, Banegas JR, Tubach F, et al. Survey of physicians’ practices in the control of cardiovascular risk factors: the EURIKA study. Eur J Prev Cardiol. 2012;19(3):541–550. doi: 10.1177/1741826711407705. [DOI] [PubMed] [Google Scholar]
- 93.Porche K, Reymond L, Callaghan JO, Charles M. Depression in palliative care patients: a survey of assessment and treatment practices of Australian and New Zealand palliative care specialists. Aust Health Rev. 2014;38(1):44–50. doi: 10.1071/AH13041. [DOI] [PubMed] [Google Scholar]
- 94.Edwards QT, Maradiegue A, Seibert D, et al. Breast cancer risk elements and nurse practitioners’ knowledge, use, and perceived comfort level of breast cancer risk assessment. J Am Acad Nurse Pract. 2009;21(5):270–277. doi: 10.1111/j.1745-7599.2009.00405.x. [DOI] [PubMed] [Google Scholar]