Abstract
Background
The British National Formulary for Children (BNFC) recommends dosing oral penicillins according to age-bands, weight-bands, or weight-based calculations. Because of the rising prevalence of childhood obesity, age-band-based prescribing could lead to subtherapeutic dosing.
Aim
To investigate actual oral penicillin prescribing by GPs in the UK with reference to the current BNFC age-band recommendations.
Design and setting
Descriptive analysis of UK prescriptions in the 2010 IMS Disease-Analyzer database (IMS-DA).
Method
A detailed database analysis was undertaken of oral penicillin prescriptions for 0–18 year olds from the 2010 IMS-DA. The prescription analysis included all available data on formulation, strength (mg), prescription quantity unit, package size, prescribed quantity, and volume.
Results
Considering amoxicillin alone, no infants (aged <1 year) were prescribed the BNFC 2011 edition recommended unit dose (62.5 mg), while the majority received double the dose (125 mg); among children aged 1–5 years, 96% were prescribed the recommended unit dose (125 mg), but 40% of 6–12 year olds and 70% of 12–18 year olds were prescribed unit doses below the BNFC recommendations. For otitis media, only those children aged <1 year received the recommended dose of amoxicillin (40–90 mg/kg/day). Similar variations in dosing across age-bands were observed for phenoxymethylpenicillin and flucloxacillin.
Conclusion
There is wide variation in the dosing of penicillins for children in UK primary care, with very few children being prescribed the current national recommended doses. There is an urgent need to review dosing guidelines, in relation to the weights of children today.
Keywords: antibiotic, dose, paediatric, penicillin, prescription, primary health care
INTRODUCTION
Oral penicillins are the most commonly prescribed antibiotics for children worldwide.1 In the UK the majority of paediatric antibiotic prescribing occurs in primary health care. In recent years, antibiotic prescribing has come under increasing scrutiny because of the threats posed by antimicrobial resistance. There have therefore been extensive efforts to rationalise physicians’ antibiotic prescribing practices in primary and secondary care to reduce the prevalence of inappropriate antimicrobial prescriptions for both children and adults.
Electronic healthcare databases, such as the IMS Disease-Analyzer (IMS-DA) and the former General Practice Research Database (now known as the Clinical Practice Research Datalink), present a useful means to study the prescribing habits of GPs in the UK. The IMS-DA contains anonymous records for approximately 2 million patients from about 125 general practices and 570 GPs. Information collated includes patient demographics, diagnoses, prescription details, laboratory data, and referrals. These databases have been recognised and validated as tools to conduct paediatric pharmacoepidemiological research and drug utilisation studies.2–4 Such studies provide population-level data representative of GP paediatric prescribing habits.
In the UK, paediatric prescribing usually follows the dosing recommendations of the British National Formulary for Children (BNFC).5 The BNFC oral penicillin paediatric dosing recommendations have remained largely unchanged for many decades; this is despite increases in the recommended adult doses of oral penicillins over this period, as well as the changing prevalence of childhood obesity and antimicrobial resistance.6,7 The BNFC recommends dosing oral penicillins in children according to age-bands, weight-bands, or weight-based calculations, and to double the dose in severe illness. This format — with multiple methods presented, each providing different ways to identify an ‘appropriate’ dose — gives the paediatric dosing guidelines potential ambiguity, which may confuse prescribers. However, many UK GPs routinely use the age-band dosing, first introduced in 1963,6,8 which allows rapid selection of the appropriate dose as recommended for the age of the child. This study aimed to analyse oral penicillin prescribing practices by GPs in the UK against the current BNFC age-band recommendations.
How this fits in
Even though oral penicillins have been widely used for many decades to treat infections in children, the paediatric dosing guidelines have remained complicated, with the potential to confuse prescribers. This research gives the first detailed assessment of current prescriber practice by UK GPs using data from the IMS Disease-Analyzer database. The findings show that age-band dosing is standard practice, but prescribers do not always adhere to the recommended prescribing guidance for the age-bands. In general, current dosing by UK GPs appears to be worryingly low, suggesting that some children may not be receiving a clinically effective dose.
METHOD
Amoxicillin, phenoxymethylpenicillin (penicillin V), and flucloxacillin are the most commonly prescribed oral penicillins in children in the UK.4 Detailed prescriptions for these penicillins for children aged 0–18 years in 2010 were analysed from the IMS-DA. The 2011–2012 edition of the BNFC was used as the standard source of nationally accepted dosing recommendations used in primary care. The 2011–2012 BNFC age-band dosing recommendations for each penicillin were as follows: each single dose halves between the age-bands from children aged 12–18 years (500 mg), to 6–12 years (250 mg), to 1–5 years (125 mg), to infants aged <1 year (62.5 mg). The IMS-DA includes data on formulation, strength, prescription quantity unit, package size, prescribed quantity, and volume, which together with these BNFC age-bands were used to calculate the daily unit dosing for each prescription. The amoxicillin prescriptions for acute otitis media and related inner ear infections (the commonest prescribing indication for amoxicillin) were analysed separately. The dose was calculated in mg/kg/day using average weights from the Health Survey for England 2010,9 because patient weight was not available in the IMS-DA.
RESULTS
In 2010, 388 926 children aged 0–18 years were registered on the database (51% male), representing 376 292 person-years. There were 65 737 prescriptions for oral penicillins written for 45 940 children: amoxicillin (63%), penicillin V (17%), and flucloxacillin (20%). Figure 1 displays the detailed breakdown of the unit doses of amoxicillin prescribed for each age-band in 2010 and includes the percentage of correct doses prescribed for each year group according to BNFC guidance. Detailed prescription data for amoxicillin showed:
In the age-band <1 year, no infant was prescribed the recommended unit dose (62.5 mg); the majority received double the unit dose (125 mg).
In the age-band 1–5 years, 96% were prescribed the recommended unit dose (125 mg).
40% of 6–12 year-olds and 70% of 12–18 year-olds were prescribed unit doses below the BNFC recommendations.
Figure 1.
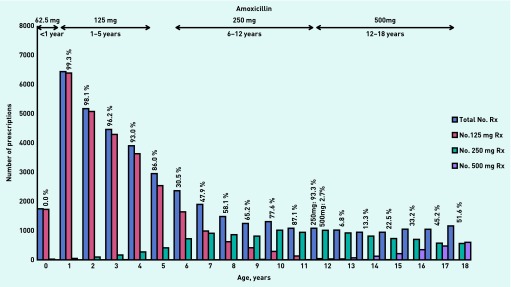
Unit doses of amoxicillin prescribed by age-bands in 2010. Percentages are calculated as the number of correct doses prescribed within each age-band according to BNFC 2011–2012 guidance.
Similar patterns of age-band prescribing were also found for penicillin V (Figure 2) and flucloxacillin (Figure 3):
In the age-band <1 year, no infant was prescribed the recommended unit dose (62.5 mg) for either penicillin V or flucloxacillin.
The majority of children in the age-band 1–5 years were prescribed the recommended unit dose (125 mg) of penicillin V and flucloxacillin.
Of the 6–12 year olds, children aged < 9 years were most often prescribed penicillin V and flucloxacillin at the unit dose recommended for the 1–5 year age band (125 mg), while children aged 9–12 years were prescribed the recommended unit dose (250 mg).
In the 12–18 years age-band, no child prescribed penicillin V and less than half of the children prescribed flucloxacillin received the recommended unit dose (500 mg).
Figure 2.
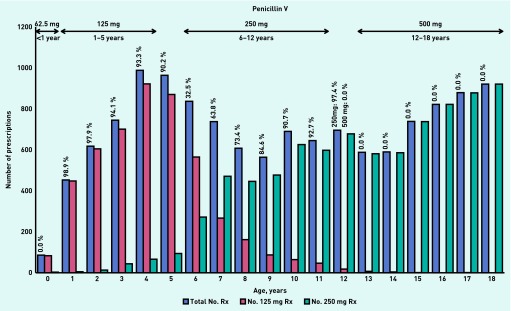
Unit doses of penicillin V prescribed by age-bands in 2010. Percentages are calculated as the number of correct doses prescribed within each age-band according to BNFC 2011–2012 guidance.
Figure 3.
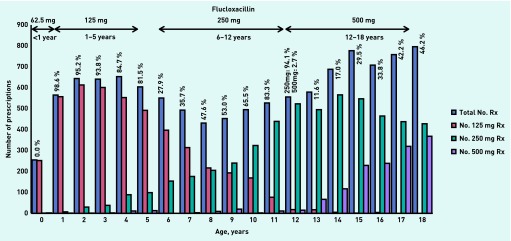
Unit doses of flucloxacillin prescribed by age-bands in 2010. Percentages are calculated as the number of correct doses prescribed within each age-band according to BNFC 2011–2012 guidance.
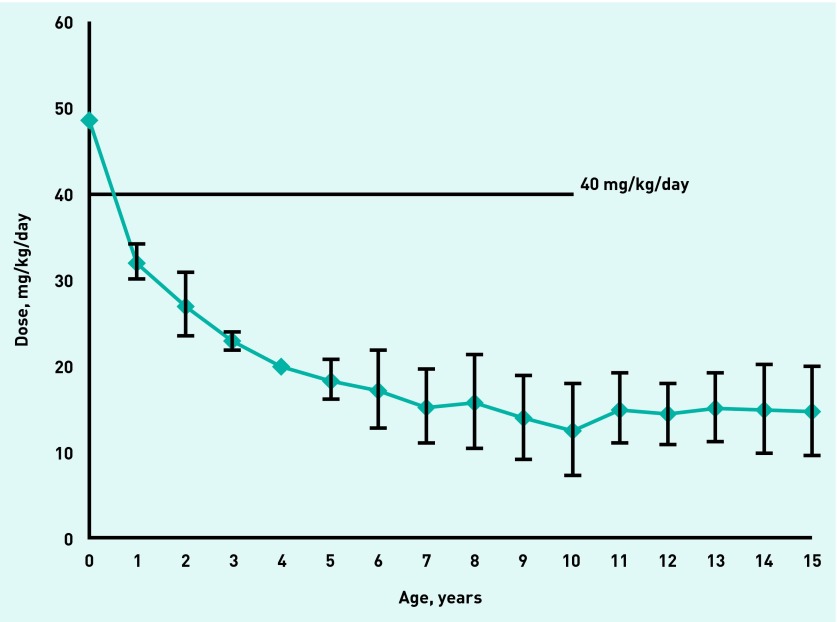
For otitis media and related inner ear infections, infants (aged <1 year) were the only group who received the recommended amoxicillin dose (40–90 mg/kg/day). For children aged 4–15 years, the prescriptions equated to 10–20 mg/kg/day, approximately 33% of the recommended dose (Figure 4).
Figure 4.
Average amoxicillin dose for prescriptions to treat ‘otitis media and related inner ear infections’ (error bars represent 95% confidence intervals).
DISCUSSION
Summary
GP prescribing practice in the UK varies widely in the dosing of oral penicillins for children. A significant proportion of community penicillin prescriptions for children (excepting infants) are at potentially subtherapeutic doses. This is exemplified in the treatment of acute otitis media and related inner ear infections, which were analysed separately because these are the most common conditions in childhood that can lead to the prescription of an oral penicillin in primary care.10 The BNFC guidance is clear that amoxicillin doses for acute otitis media should be at least 40 mg/kg/day. In addition, the doses described in the BNFC and the 2011 Summary of Product Characteristics for amoxicillin (Amoxil® Paediatric Suspension [GlaxoSmithKline UK]) for all indications in children weighing <40 kg (that is, aged <10 years) are 40–90 mg/kg/day. However, this study reveals that for children aged 4–15 years the IMS-DA prescriptions for otitis media and related inner ear infections were equivalent to only 10–20 mg/kg/day on average. The 2013 guidance from the American Academy of Pediatrics is that if antibiotics are to be used to treat acute otitis media, then the antibiotic of choice is amoxicillin at a dose of 80–90 mg/kg/day.11
Strengths and limitations
The IMS-DA database includes information representative of the UK population. The database has been validated for pharmacoepidemiological studies in the past, and identified as appropriate for paediatric medications research.2 However, such prescription database analyses are recognised to have some unavoidable limitations: the database captures information from ‘routine practice’ and is therefore subject to diagnostic, coding, and prescribing errors that potentially decrease validity but nevertheless happen in reality and could be seen as increasing their applicability and relevance to routine ‘real-life’ health care. The analysis cannot identify situations where GPs have either chosen to double the dose because they have judged the infection to be ‘severe’ (which would still be in keeping with BNFC guidance) or in situations of delayed prescribing, but these details were not available in this analysis. The weight of individual patients is not available in the IMS-DA, so the mg/kg/day dose calculations had to rely on average weights obtained from the Health Survey for England 2010.9 A simplified standard age-band single dose was used for the purposes of simultaneous analysis of all three oral penicillins; however, it is acknowledged that this does not exactly reflect the range of single doses that are included in some of the dose recommendations and the exact age-band limits used in the current (September 2013) BNFC. The exact age-band limits used in the current (September 2013) BNFC are now different to the range of single doses that were used in this study based on the 2011–2012 BNFC. For example, amoxicillin: the age-bands 1–5 years (previous single dose 125 mg), 6–12 years (previous single dose 250 mg), and 12–18 years (previous single dose 500 mg) have been merged so that the recommended single dose of children aged 5–18 years old is now 250 mg. However, this potentially further exacerbates under-dosing in those aged 12–18 years.
Comparison with existing literature
To the authors’ knowledge, there are no previous published healthcare database analyses of paediatric oral penicillin prescribing and dosing in primary care. However, this study’s findings are consistent with a smaller US study that reported under-dosing of amoxicillin in older and heavier children with otitis media, and marked departure by family physicians from US national guidance.12,13
Implications for research and practice
When following the current BNFC recommendations, GPs will still need to choose between age-bands, weight-bands, and weight-based calculations when prescribing oral penicillins for children. Age-bands and weight-bands are very well established and are simple for GPs and parents to understand. Currently, however, there is no standardised or evidence-based guideline to clarify which method of dose selection is optimum. For pragmatic purposes, many GPs in the UK will continue to use age-band dosing routinely. It is important that GPs are aware that these age-bands were devised many years ago and therefore the relative mg/kg dosing has changed and reduced over time in relation to the average weights of children today.6 This impact is compounded by the fact that when the adult doses of these penicillins were increased, these dose increases were never reflected in the paediatric dosing recommendations.6 A detailed qualitative assessment of current prescriber practice is required to understand reasons for the use of age-bands and barriers to medical implementation of weight-based dosing regimens. For example, it may be that apparently subtherapeutic doses are issued in the recognition that most infections are self-limiting; or that GPs are concerned about the risk of side effects with higher doses.14
Although many children do not require antibiotics, when the decision is made to write a prescription for immediate or delayed treatment, it should be at a dose sufficient to avoid therapeutic failure, as per the BNFC guidance. Importantly, previous pharmacokinetic studies have shown that using substantially lower oral doses can result in subtherapeutic concentrations of antibiotic penetrating the middle ear fluid.15 Therefore affected patients remain at risk of adverse drug reactions, such as antibiotic-associated diarrhoea, without the intended clinical benefit. Prescribing at a lower dose may increase the likelihood of antimicrobial resistance.7,16
The study recommends that UK GPs should ask local prescribing advisers to conduct practice audits to examine the accuracy of prescribed antibiotic doses in children and to aim for the target of 40 mg/kg/day when antibiotics are prescribed for acute otitis media, as recommended by the BNFC. Given the recent call to apply the evidence that interactive electronic tools to improve GP antibiotic prescribing among children are most effective when both parents and GPs are in consultation, it would be a relatively simple step to incorporate computer-based alerts to incorrectly prescribed antibiotic doses calculated on a child’s weight-band or age-band into electronic prescribing formularies.17
The study also recommends that the evidence base for the paediatric dosing of oral penicillins, which is historical,6 is strengthened through pharmacokinetic and/or pharmacodynamic studies to identify optimum dosing of these off-patent but crucially important antimicrobials for the treatment of common infectious diseases in children of different ages. While the majority of these studies to date are undertaken in secondary care settings, the opportunities to use novel pharmacokinetic methods, including population-based modelling, will facilitate the conduct of such investigations in primary care in the future.18 Conditions that present a high burden of disease at the population level and which result in the most antibiotic prescriptions will remain high on this priority list including, for example, community-acquired pneumonia and acute otitis media.15,19–22 Overall, there is an urgent need to review and update paediatric penicillin dosing guidelines, in relation to the weights of UK children today, and with consideration of current antimicrobial resistance patterns.
Funding
Sonia Saxena is funded by a National Institute for Health Research NIHR postdoctoral fellowship. This paper presents independent research funded by the NIHR. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Charlotte Barker is funded as a clinical research fellow by the Global Research in Paediatrics Network of Excellence, part of the European Union Seventh Framework Programme for Research (FP7/2007-2013, Grant Agreement number 261060).
Ethical approval
Ethical approval was given by the Independent Scientific and Ethical Advisory committee of IMS for the study (2009/ISEAC/001).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests. All authors declare no financial support for the submitted work from anyone other than their employer. Ian CK Wong, Mike Sharland, and Paul F Long have specific relationships with Wyeth Vaccines of John Wyeth & Brother Ltd, likewise Macey L Murray and Mike Sharland also have specific relationships with Pfizer UK that might have an interest in the submitted work.
Discuss this article
Contribute and read comments about this article: www.bjgp.org/letters
REFERENCES
- 1.Rossignoli A, Clavenna A, Bonati M. Antibiotic prescription and prevalence rate in the outpatient paediatric population: analysis of surveys published during 2000–2005. Eur J Clin Pharmacol. 2007;63(12):1099–1106. doi: 10.1007/s00228-007-0376-3. [DOI] [PubMed] [Google Scholar]
- 2.Neubert A, Sturkenboom MC, Murray ML, et al. Databases for pediatric medicine research in Europe: assessment and critical appraisal. Pharmacoepidemiol Drug Saf. 2008;17(12):1155–1167. doi: 10.1002/pds.1661. [DOI] [PubMed] [Google Scholar]
- 3.Sturkenboom MC, Verhamme KM, Nicolosi A, et al. Drug use in children: cohort study in three European countries. BMJ. 2008;337:a2245. doi: 10.1136/bmj.a2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson PL, Spyridis N, Sharland M, et al. Changes in clinical indications for community antibiotic prescribing for children in the UK from 1996 to 2006: will the new NICE prescribing guidance on upper respiratory tract infections just be ignored? Arch Dis Child. 2009;94(5):337–340. doi: 10.1136/adc.2008.147579. [DOI] [PubMed] [Google Scholar]
- 5.Paediatric Formulary Committee . BNF for children. London: British Medical Association, Royal Pharmaceutical Society, Royal College of Paediatrics and Child Health, and Neonatal and Paediatric Pharmacists Group; http://BNFc.org/BNFc/ (accessed 29 Jan 2014). [Google Scholar]
- 6.Ahmed U, Spyridis N, Wong IC, et al. Dosing of oral penicillins in children: is big child = half an adult, small child = half a big child, baby = half a small child still the best we can do? BMJ. 2011;343:d7803. doi: 10.1136/bmj.d7803. [DOI] [PubMed] [Google Scholar]
- 7.Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance: the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 8.Cole FM. Today’s drugs: paediatric prescribing (contd.). Treatment of infections in childhood. BMJ. 1963;2:1577–1578. [PMC free article] [PubMed] [Google Scholar]
- 9.Health & Social Care Information Centre Health Survey for England — 2010, Trend tables [NS] http://www.hscic.gov.uk/pubs/hse10trends (accessed 25 Feb 2014).
- 10.Thompson PL, Gilbert RE, Long PF, et al. Effect of antibiotics for otitis media on mastoiditis in children: a retrospective cohort study using the United Kingdom general practice research database. Pediatrics. 2009;123(2):424–430. doi: 10.1542/peds.2007-3349. [DOI] [PubMed] [Google Scholar]
- 11.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–e999. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 12.Christian-Kopp S, Sinha M, Rosenberg DI, et al. Antibiotic dosing for acute otitis media in children: a weighty issue. Pediatr Emerg Care. 2010;26(1):19–25. doi: 10.1097/PEC.0b013e3181cbeb00. [DOI] [PubMed] [Google Scholar]
- 13.Vernacchio L, Vezina RM, Mitchell AA. Management of acute otitis media by primary care physicians: trends since the release of the 2004 American Academy of Pediatrics/American Academy of Family Physicians clinical practice guideline. Pediatrics. 2007;120(2):281–287. doi: 10.1542/peds.2006-3601. [DOI] [PubMed] [Google Scholar]
- 14.Slight SP, Howard R, Ghaleb M, et al. The causes of prescribing errors in English general practices: a qualitative study. Br J Gen Pract. 2013 doi: 10.3399/bjgp13X673739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pichichero ME, Reed MD. Variations in amoxicillin pharmacokinetic/pharmacodynamic parameters may explain treatment failures in acute otitis media. Paediatr Drugs. 2009;11(4):243–249. doi: 10.2165/00148581-200911040-00003. [DOI] [PubMed] [Google Scholar]
- 16.Chung A, Perera R, Brueggemann AB, et al. Effect of antibiotic prescribing on antibiotic resistance in individual children in primary care: prospective cohort study. BMJ. 2007;335:429. doi: 10.1136/bmj.39274.647465.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore M. Editorial. Antibiotics: time to act. Br J Gen Pract. 2013;63(612):340–341. doi: 10.3399/bjgp13X668447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker CI, Standing JF, Turner MA, et al. Antibiotic dosing in children in Europe: can we grade the evidence from pharmacokinetic/pharmacodynamic studies — and when is enough data enough? Curr Opin Infect Dis. 2012;25(3):235–242. doi: 10.1097/QCO.0b013e328353105c. [DOI] [PubMed] [Google Scholar]
- 19.Esposito S, Cohen R, Domingo JD, et al. Antibiotic therapy for pediatric community-acquired pneumonia: do we know when, what and for how long to treat? Pediatr Infect Dis J. 2012;31(6):e78–e85. doi: 10.1097/INF.0b013e318255dc5b. [DOI] [PubMed] [Google Scholar]
- 20.Dagan R. The use of pharmacokinetic/pharmacodynamic principles to predict clinical outcome in paediatric acute otitis media. Int J Antimicrob Agents. 2007;30(Suppl 2):S127–S130. doi: 10.1016/j.ijantimicag.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Dagan R. Appropriate treatment of acute otitis media in the era of antibiotic resistance. Paediatr Drugs. 2010;12(Suppl 1):3–9. doi: 10.2165/11538720-S0-000000000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Isla A, Trocóniz IF, Canut A, et al. Pharmacokinetic/pharmacodynamic evaluation of amoxicillin, amoxicillin/clavulanate and ceftriaxone in the treatment of paediatric acute otitis media in Spain. Enferm Infecc Microbiol Clin. 2011;29(3):167–173. doi: 10.1016/j.eimc.2010.05.008. [DOI] [PubMed] [Google Scholar]