Abstract
Objective
A novel form of food allergy has been described that initially became apparent from IgE reactivity with the drug cetuximab. Ongoing work regarding the etiology, distribution, clinical management, and cellular mechanisms of the IgE response to the oligosaccharide galactose-α-1,3-galactose (α-gal) is reviewed.
Data Sources
Brief review of the relevant literature in peer-reviewed journals.
Study Selection
Studies on the clinical and immunologic features, pathogenesis, epidemiology, laboratory evaluation, and management of IgE to α-gal are included in this review.
Results
Recent work has identified a novel IgE antibody response to the mammalian oligosaccharide epitope, α-gal, that has been associated with 2 distinct forms of anaphylaxis: (1) immediate-onset anaphylaxis during first exposure to intravenous cetuximab and (2) delayed-onset anaphylaxis 3 to 6 hours after ingestion of mammalian food products (eg, beef and pork). Study results have suggested that tick bites are a cause of IgE antibody responses to α-gal in the United States. Patients with IgE antibody to α-gal continue to emerge, and, increasingly, these cases involve children. Nevertheless, this IgE antibody response does not appear to pose a risk for asthma but may impair diagnostic testing in some situations.
Conclusion
The practicing physician should understand the symptoms, evaluation, and management when diagnosing delayed allergic reactions to mammalian meat from IgE to α-gal or when initiating treatment with cetuximab in patients who have developed an IgE antibody response to α-gal.
Introduction
The identification of food allergens typically begins, naturally enough, in the consumption of an offending food. However, in some instances, there are cryptic allergens that avoid obvious detection in food; alternatively, an allergic reaction to a medication can be the initial recognition of an allergy to an antigen also contained in food. For example, allergic reactions to bovine- and porcine-derived gelatin are not commonly described.1–3 However, patients allergic to gelatin must be aware that gelatin is an ingredient of some processed foods, gelatin colloids, and stabilizing agents in some vaccines and thus is a potentially cryptic allergen.1–9 Eluted when meat is cooked and cooled, gelatin also is present in some confectioneries (eg, marshmallows), food thickeners, dips, glazes, and icing and acts as a fat substitute in yogurt, mayonnaise, and ice cream.2,5,6,8 Gelatin can be found in sausage coatings and salami and is used to clarify fruit juice and wine.2,5,6 Thus, gelatin can be considered a potential occult food allergen because exposure is ubiquitous. That reactions to topical or oral gelatin can occur is supported by rare case reports of allergic reactions to “hydrolyzed protein” (gelatin) in shampoo, collagen implants, and “catgut” sutures and to gelatin present as a binding agent in tablets, capsules, suppositories, or confectioneries.2,5,6
Another example of a drug allergen that affects patients as a food allergy is the monoclonal antibody (Ab) cetuximab, which is the focus of the remainder of this review. Marketed under the trade name Erbitux (ImClone LLC/, a wholly-owned subsidiary of Eli Lilly and Company, New York, New York, and Bristol-Myers Squibb Company, Princeton, New Jersey), cetuximab is a chimeric epidermal growth factor receptor inhibitor used for the treatment of metastatic colorectal cancer and head and neck cancer that went into clinical use in March 2006. Shortly afterward, it became obvious that hypersensitivity reactions during the first infusion were more common than expected in an area of the southern United States.10 In some centers, these reactions were occurring in as many as 20% of patients treated.10 These reactions were generally so severe as to preclude subsequent use of the drug and occasionally proved fatal.11 Using an assay for cetuximab with the monoclonal Ab bound to an ImmunoCAP, investigations established that the reactions to cetuximab occurred in patients who had preexisting IgE antibodies to the oligosaccharide on the Fab portion of this molecule.11,12 Galactose-α-1,3-galactose (α-gal) is present at amino acids 88 and 299 on the Fab portion of the heavy chain.13 In fact, of the 21 distinct oligosaccharide structures identified in cetuximab, approximately 30% have at least 1 α-1,3–linked galactosyl residue as measured by peak area at time-of-flight mass spectrometry.13 Thus, cetuximab is in many ways uniquely suited to present α-gal as an antigen and to be used in assays to detect Ab binding to α-gal.
The α-gal oligosaccharide is a major blood group substance of nonprimate mammals and is well recognized as a target of IgG antibodies, which are present in the serum of all immunocompetent individuals.14 In contrast, the presence of IgE class Ab to α-gal in a defined area of the United States was initially an enigma. There did not appear to be any consistent relation with pollen, dust mite, fungal, or insect sensitivity. During this period, however, screening sera from an allergy clinic in Virginia identified a group of patients who had IgE to α-gal and a history of anaphylaxis or severe episodes of hives.15 In several cases, these patients were convinced that their episodes occurred 4 to 5 hours after eating beef or pork. Despite the inherent unlikelihood of an “immediate hypersensitivity” reaction occurring 4 to 5 hours after oral exposure, the rationale for testing was that this oligosaccharide (α-gal) is a blood group substance of nonprimate mammals.16 In keeping with that, there were strong correlations with IgE Ab to cow's milk, cat, and dog.12,15 Moreover, the patients were consistent in saying that they had no problems with chicken, turkey, or fish. Thus, 2 important pieces of data led to the identification of a “new” food allergy: (1) identification of the IgE Ab responsible for reactions to a drug allergen and (2) elucidation of the glycosylation pattern of the drug.
Increasing Clinical Identification
The authors initially published the description of 24 cases of reactions to red meat and subsequently documented the serum IgE Ab levels in 208 cases.15,17 Currently they are aware of at least 1,000 cases in Virginia and have estimated that there must be several thousand more affected patients in the United States, because cases of meat-induced, delayed anaphylaxis have been reported in South Carolina, Texas, Georgia, Louisiana, Alabama, Mississippi, Kentucky, Oklahoma, Illinois, West Virginia, and Nebraska. In most instances, the cases that come to the authors’ attention are those in which the history has been confirmed by a serum assay for IgE Ab to α-gal. However, these cases include a mixture of de-identified case reports from other physicians, cases enrolled in the authors’ own clinics, and series of cases enrolled by other physicians. In addition, the authors have knowledge of cases in locations where the landscape is unique or different. In her practice in the East Hampton portion of Long Island, Dr Erin McGintee has identified more than 60 patients with a history of delayed reactions to mammalian meat and a documented positive IgE to α-gal (unpublished data). Furthermore, cases of this kind have been reported from France, Sweden, Spain, Germany, Japan, Australia, and the United States.4,18–24
Development of IgE to α-Gal Response
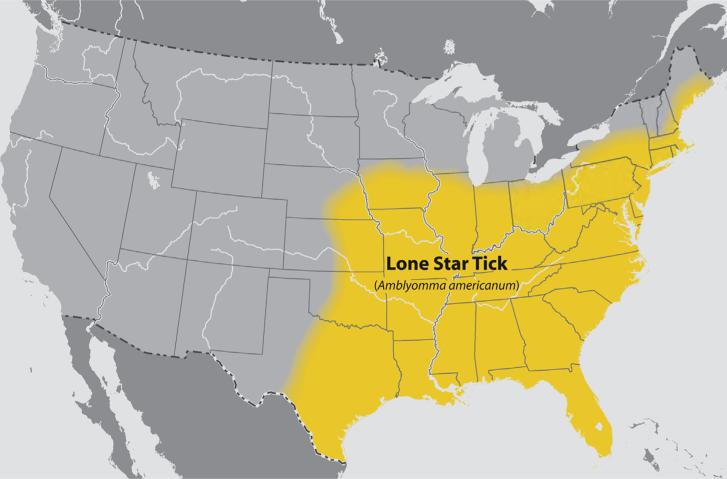
The first clue to what causes or leads to the development of the IgE response to α-gal came from the observation that the area of the initial reactions to cetuximab coincided with the maximum prevalence of Rocky Mountain spotted fever.25 That observation led to questioning patients about tick bites, serial observations after tick bites, and serologic studies using extracts of ticks to investigate IgE antibodies. Taken together, the results provided strong evidence that bites from the lone star tick, Amblyomma americanum, were a cause of IgE Ab responses to α-gal in the United States.25 Recent data have suggested an expanding range of A americanum, which is likely to increase the affected population (Fig 1). Interestingly, bites from the lone star tick are often severely pruritic and may persist for weeks.25 In addition, the authors became aware that Van Nunen et al22 had reported to the Sydney Allergy Society that some people who were bitten by ticks in southern Australia had developed an allergy to meat. More recently, Hamsten et al19 in Stockholm reported clear evidence that the α-gal epitope is present in the gut of Ixodes ricinus. However, the results did not provide answers about why this tick was such a potent cause of IgE responses to this oligosaccharide.
Figure 1.
The known distribution of the tick Amblyomma americanum is shown. Data are from the Centers for Disease Control and Prevention Web site (http://www.cdc.gov/ticks/geographic_distribution.html#lone-star), revised August 2011.
Studies on the Ab response to A americanum tick bites have examined the specificity of the antibodies and the isotypes involved. Using nuclear magnetic resonance, it has been confirmed that the antibodies are primarily directed at the 2 terminal galactoses.26 The ongoing IgG Ab response to α-gal, thought to be induced by continuous antigenic stimulation in the gut, is predominantly IgG2.27 After tick bites, the IgG antibodies increase in parallel with IgE antibodies, but they are predominantly IgG1.28 It remains unclear at this time whether the “natural” IgG2 Ab response influences the immune response induced by tick bites. In addition, the resulting Ab response (IgG2 vs IgE) likely reflects the route of sensitization (intestinal vs skin) and predicts clinical outcome (tolerance vs allergy). The dichotomous responses may allow the establishment of a model for understanding the mechanisms controlling immune responses leading to oral tolerance, on the one hand, or food allergy, on the other.
Insight into the Control of IgE Responses
The IgE Ab responses are generally considered to be T-cell dependent; however, there is evidence that the switch to IgE production can take place outside organized germinal centers.29,30 It is clear that IgE production to α-gal does not occur in humans by merely being exposed to this epitope from gut bacteria or by eating mammalian meat. Thus, it may be that after an “appropriate” tick bite, there is a local switch of B cells currently making antibodies to α-gal over to IgE production. Such a shift could occur in the skin or in the lymph nodes nearby the bite site. The authors recently analyzed the evidence about IgE production and argued that extensive recombination of IgE B cells in the germinal center is not an essential part of the human IgE response.30 Data using a murine model, however, showed that the production of IgE antibodies requires extensive rearrangement to achieve an affinity relevant to allergic disease.31 Evidence from human studies stands in contrast to the mouse data. In fact, it has been shown that medium- and low-affinity IgE at high IgE clonality can induce ample complex formation in vitro.32 A weak T-helper cell type 2 response (eg, low-dose exposure to pollen or mites) fails to induce a mature germinal center. In this situation, activated B cells undergo some somatic mutation and an isotype switch but do not develop into memory cells.29 The authors argue that the likely route for the switch from IgM to IgE in this allergy is in keeping with a fading response and occurs outside fully formed germinal centers.
Clinical Diagnosis and Management
Skin testing for IgE to α-gal using beef, pork, or lamb extracts in adult and pediatric patients has been challenging. Many patients have only small reactions (2–5 mm) to these allergens by skin prick testing, and intradermal tests have been used in adults to clarify the intermediate results.15 The authors have, on occasion, also performed intradermal testing in older teens and these results mirrored those seen in adults.33 Overall, the authors are more likely to use the in vitro assays and typically reassess IgE to α-gal levels every 8 to 12 months. Certainly one reason to monitor blood levels is that, based on the authors’ experience, if patients can avoid subsequent tick bites, the level of α-gal–specific IgE tends to decrease over time. In fact, some adult and pediatric patients with this form of allergy have been able to tolerate mammalian meat again after avoiding additional tick bites for 1 to 2 years (Commins and Platts-Mills, unpublished data).
Although the authors have performed mammalian meat challenges in adult patients to document the delayed appearance of clinical symptoms, these food challenges have produced significant symptoms beyond what the patient had reported after natural exposure (Figs 2 and 3). Because of the time course to symptoms, incremental dosing is not possible in the case of delayed reactions to mammalian meat and the entire dose must be given at the start of the challenge. Certainly, there were patients who reported only mild stomach upset or itching, whereas others developed anaphylaxis, complete with an increase in serum tryptase. Variability of the response to antigen during these challenges appears to be in keeping with protein-based food allergies (eg, egg or peanut), in which past reactions are not thought to be fully predictive of future allergic symptoms. Although all positive reactions during the meat challenges have been delayed, there is variation in the time of symptom onset. Some patients note symptoms beginning just within 3 hours after eating mammalian meat, whereas others develop symptoms closer to 6 hours. Interestingly, many patients with IgE to α-gal reported itching as their initial symptom of a food allergic response. Although the authors were aware of this through patient reports in the past, they were not aware of the prominent palmar and plantar pruritus with erythema and often urticaria. Variation also has been noted in the progression of symptoms, with some patients experiencing a much more rapid course once symptoms appeared. Interestingly, the variability of the response, including time of symptom onset, speed of symptom progression, and severity, does not appear to be related to the titer of soluble IgE to α-gal.
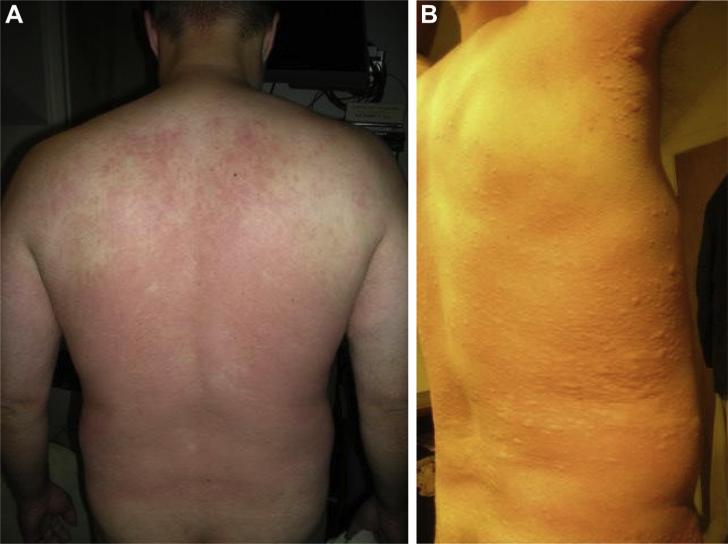
Figure 2.
(A) Patient with initial flushing and erythema of the back 345 minutes (5 hours 45 minutes) after a 150-g mammalian meat challenge. (B) His symptoms progressed to systemic urticaria before resolving with antihistamine.
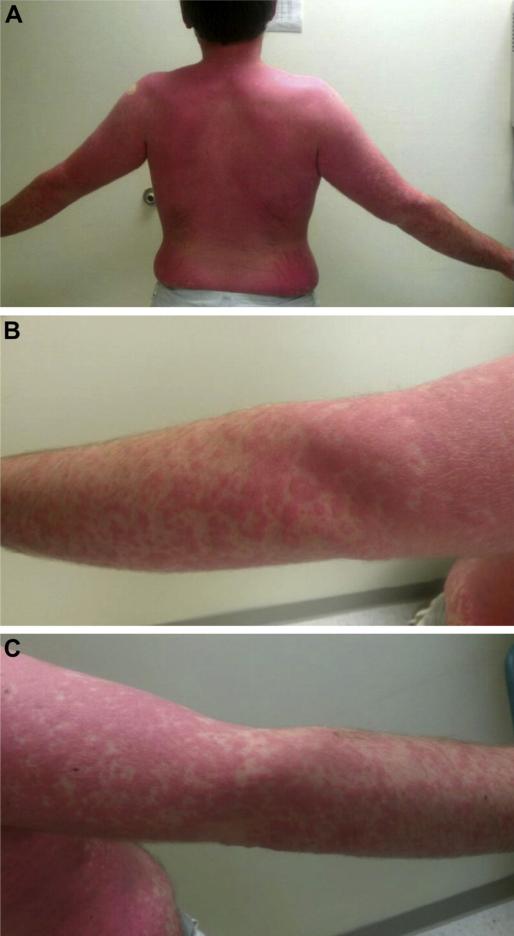
Figure 3.
(A) Patient with systemic urticaria that appeared 225 minutes (3 hours 45 minutes) after consuming 150 g of pork sausage. Enlarged photographs of the (B) right and (C) left arms show the coalesced discrete urticaria.
Despite the delayed food allergy being related to a soluble IgE response that can be a very high titer and account for 10% to 50% of the total IgE, IgE Ab to α-gal is not associated with rhinitis or asthma.17,25 To this end, the authors investigated a large group of patients who presented with delayed symptoms after eating red meat for a history of symptoms, lung function, and evidence of lung inflammation. The results provide compelling evidence that IgE Ab to α-gal does not create a risk for asthma.17 Thus, although in vitro assays for IgE Ab to cat extract have been consistently positive in patients with IgE to α-gal, this should not be taken as independent concern for worsening allergic rhinitis symptoms or asthma risk.15,34
Conclusion
The development of IgE Ab to α-gal not only has given rise to a novel form of anaphylaxis but also has provided a model for understanding the ways in which parasites induce IgE responses without creating a risk for inhalant allergic disease.17 Bites from the lone star tick can cause high-titer IgE Ab to α-gal and major increases in total IgE.25 Further, these responses often occur in adults older than 60 years and who have no allergic history. This is in keeping with the known ability of parasites that use a cutaneous route of entry or attachment to induce high levels of IgE, including IgE specific for oligosaccharides.35,36 The specific factors that govern the IgE response to α-gal are currently unknown. Future efforts are being directed at understanding the role of T cells in this response and the mechanisms of isotype switch. The authors are intrigued by the notion that it is possible the “natural” Ab response to α-gal is not influenced by the IgE response to the same epitope. Thus, it could be that allergic patients have 2 different responses against the same epitope that are not related, a mechanism the authors look forward to exploring.
Acknowledgments
Funding: This work was supported by National Institutes of Health grants K08 AI085190 (to S.P.C.) and R01 AI-20565 (to T.A.E.P.-M.).
Footnotes
Disclosures: Dr Platts-Mills has received research supplies from Phadia and Thermo Fisher.
References
- 1.Kelso JM, Jones RT, Yunginger JW. Anaphylaxis to measles, mumps, and rubella vaccine mediated by IgE to gelatin. J Allergy Clin Immunol. 1993;91:867–872. doi: 10.1016/0091-6749(93)90344-f. [DOI] [PubMed] [Google Scholar]
- 2.Kelso JM. The gelatin story. J Allergy Clin Immunol. 1999;103:200–202. doi: 10.1016/s0091-6749(99)70490-2. [DOI] [PubMed] [Google Scholar]
- 3.Mullins RJ, Richards C, Walker T. Allergic reactions to oral, surgical and topical bovine collagen. Anaphylactic risk for surgeons. Aust N Z J Ophthalmol. 1996;24:257–260. doi: 10.1111/j.1442-9071.1996.tb01589.x. [DOI] [PubMed] [Google Scholar]
- 4.Mullins RJ, James H, Platts-Mills TA, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-α-1,3-galactose. J Allergy Clin Immunol. 2012;129:1334–1342. e1. doi: 10.1016/j.jaci.2012.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakayama T, Kumagai T. Gelatin allergy. Pediatrics. 2004;113:170–171. doi: 10.1542/peds.113.1.170. author reply 171. [DOI] [PubMed] [Google Scholar]
- 6.Purello-D'Ambrosio F, Gangemi S, La Rosa G, Merendino RA, Tomasello F. Allergy to gelatin. Allergy. 2000;55:414–415. doi: 10.1034/j.1398-9995.2000.00618.x. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi M, Nakayama T, Inouye S. Food allergy to gelatin in children with systemic immediate-type reactions, including anaphylaxis, to vaccines. J Allergy Clin Immunol. 1996;98:1058–1061. doi: 10.1016/s0091-6749(96)80191-6. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Sicherer SH. Anaphylaxis following ingestion of candy fruit chews. Ann Allergy Asthma Immunol. 2005;94:530–533. doi: 10.1016/S1081-1206(10)61128-3. [DOI] [PubMed] [Google Scholar]
- 9.Worm M, Sterry W, Zuberbier T. Gelatin-induced urticaria and anaphylaxis after tick-borne encephalitis vaccine. Acta Derm Venereol. 2000;80:232. doi: 10.1080/000155500750043186. [DOI] [PubMed] [Google Scholar]
- 10.O'Neil BH, Allen R, Spigel DR, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25:3644–3648. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- 11.Pointreau Y, Commins SP, Calais G, Watier H, Platts-Mills TA. Fatal infusion reactions to cetuximab: role of immunoglobulin e-mediated anaphylaxis. J Clin Oncol. 2012;30:334. doi: 10.1200/JCO.2011.38.4701. author reply 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007;364:8–18. doi: 10.1016/j.ab.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti–alpha-galactosyl specificity. J Exp Med. 1984;160:1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Commins SP, Satinover SM, Hosen J, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123:426–433. doi: 10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008;1780:75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Commins SP, Kelly LA, Rönmark E, et al. Galactose-α-1,3-galactose-specific IgE is associated with anaphylaxis but not asthma. Am J Respir Crit Care Med. 2012;185:723–730. doi: 10.1164/rccm.201111-2017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caponelto P, Fischer J, Biedermann T. Gelatin-containing sweets can elicit anaphylaxis in a patient with sensitization to galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2013;1:302–303. doi: 10.1016/j.jaip.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Hamsten C, Starkhammar M, Tran TA, et al. Identification of galactose-α-1,3-galactose in the gastrointestinal tract of the tick Ixodes ricinus; possible relationship with red meat allergy. Allergy. 2013;68:549–552. doi: 10.1111/all.12128. [DOI] [PubMed] [Google Scholar]
- 20.Jappe U. [Update on meat allergy: α-Gal: a new epitope, a new entity?]. Hautarzt. 2012;63:299–306. doi: 10.1007/s00105-011-2266-y. [DOI] [PubMed] [Google Scholar]
- 21.Morisset M, Richard C, Astier C, et al. Anaphylaxis to pork kidney is related to IgE antibodies specific for galactose-alpha-1,3-galactose. Allergy. 2012;67:699–704. doi: 10.1111/j.1398-9995.2012.02799.x. [DOI] [PubMed] [Google Scholar]
- 22.Van Nunen SA, O'Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009;190:510–511. doi: 10.5694/j.1326-5377.2009.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 23.Sekiya K, Fukutomi Y, Nakazawa T, Taniguchi M, Akiyama K. Delayed anaphylactic reaction to mammalian meat. J Investig Allergol Clin Immunol. 2012;22:446–447. [PubMed] [Google Scholar]
- 24.Nuñez R, Carballada F, Gonzalez-Quintela A, Gomez-Rial J, Boquete M, Vidal C. Delayed mammalian meat-induced anaphylaxis due to galactose-α-1,3-galactose in 5 European patients. J Allergy Clin Immunol. 2011;128:1122–1124. doi: 10.1016/j.jaci.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Commins SP, James HR, Kelly LA, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol. 2011;127:1286–1293. e6. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plum M, Michel Y, Wallach K, et al. Close-up of the immunogenic α1,3-galactose epitope as defined by a monoclonal chimeric immunoglobulin E and human serum using saturation transfer difference (STD) NMR. J Biol Chem. 2011;286:43103–43111. doi: 10.1074/jbc.M111.291823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM. Interaction between human natural antiealpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988;56:1730–1737. doi: 10.1128/iai.56.7.1730-1737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rispens T, Derksen NI, Commins SP, Platts-Mills TA, Aalberse RC. IgE Production to α-Gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS One. 2013;8:e55566. doi: 10.1371/journal.pone.0055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aalberse RC, Platts-Mills TA. How do we avoid developing allergy: modifications of the TH2 response from a B-cell perspective. J Allergy Clin Immunol. 2004;113:983–986. doi: 10.1016/j.jaci.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 30.Davies JM, Platts-Mills TA, Aalberse RC. The enigma of IgE+ B-cell memory in human subjects. J Allergy Clin Immunol. 2013;131:972–976. doi: 10.1016/j.jaci.2012.12.1569. [DOI] [PubMed] [Google Scholar]
- 31.Xiong H, Dolpady J, Wabl M, Curotto de Lafaille MA, Lafaille JJ. Sequential class switching is required for the generation of high affinity IgE antibodies. J Exp Med. 2012;209:353–364. doi: 10.1084/jem.20111941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holm J, Willumsen N, Würtzen PA, Christensen LH, Lund K. Facilitated antigen presentation and its inhibition by blocking IgG antibodies depends on IgE repertoire complexity. J Allergy Clin Immunol. 2011;127:1029–1037. doi: 10.1016/j.jaci.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy J, Stallings A, Platts-Mills T, et al. Galactose-alpha-1,3-galactose and delayed anaphylaxis, angioedema, and urticaria in children. Pediatrics. 2013;131:1–8. doi: 10.1542/peds.2012-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gronlund H, Adedoyin J, Commins SP, Platts-Mills TA, van Hage M. The carbohydrate galactose-alpha-1,3-galactose is a major IgE-binding epitope on cat IgA. J Allergy Clin Immunol. 2009;123:1189–1191. doi: 10.1016/j.jaci.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arkestål K, Sibanda E, Thors C, et al. Impaired allergy diagnostics among parasite-infected patients caused by IgE antibodies to the carbohydrate epitope galactose-α 1,3-galactose. J Allergy Clin Immunol. 2011;127:1024–1028. doi: 10.1016/j.jaci.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Amoah A, Obeng B, Larbi I, et al. Peanut-specific IgE antibodies in asymptomatic Ghanaian children possibly caused by carbohydrate determinant cross-reactivity. J Allergy Clin Immunol. 2013;132:639–647. doi: 10.1016/j.jaci.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]