Abstract
Modulation of the phosphorylation status of proteins by both kinases and phosphatases plays an important role in cellular signal transduction. Challenge of host cells by Legionella pneumophila manipulates the phosphorylation state of multiple host factors. These changes play roles in bacterial uptake, vacuole modification, cellular survival, and the immune response. In addition to modification by host cell kinases in response to the bacterium, L. pneumophila translocates bacterial kinases into the host cell that may contribute to further signaling modifications. Proper regulation of host cell signaling by L. pneumophila is necessary for its ability to replicate intracellulary, while avoiding host defenses.
1 Introduction
Cellular signaling pathways allow cells to sense changing environmental conditions by responding to both extracellular and intracellular stimuli. One mechanism of signal transduction involves the reversible phosphorylation of protein substrates. Phosphorylation and dephosphorylation reactions are performed by cellular kinases and phosphatases, respectively. Signaling through phosphorylation allows for rapid cellular responses to varying stimuli at distinct cellular localizations, allowing for signal specificity.
Signaling through phosphorylation is an evolutionarily conserved mechanism found in all domains of life (Manning et al. 2002). In eukaryotes, the common sites of phosphorylation by kinases on target proteins are either serine or threonine residues, or less commonly, tyrosines (Blom et al. 1999). The kinases that target these residues can either be specific serine/threonine or tyrosine kinases, or may be more promiscuous dual-specificity kinases which can target multiple residues (Ubersax and Ferrell 2007).
Signal transduction through phosphorylation plays an important role in many cellular processes. Responses such as innate immune signaling, cell cycle control, metabolism, cytoskeletal modification, response to cellular stress, and recognition of extracellular ligands are all controlled by phosphorylation-mediated signaling cascades (Manning et al. 2002). Substrate phosphorylation induces changes important for signaling and function including modulation of enzymatic activity, substrate stability, and interactions with other factors (Zhang et al. 2002). Defects in kinase-mediated signaling are implicated in multiple disease states including cancer, diabetes, severe combined immunodeficiency, and rheumatoid arthritis (Cohen 2001).
Because of the importance of kinase-mediated signaling pathways in cellular physiology and the immune response, pathogens have developed mechanisms to subvert these pathways for their own benefit. One of the earliest identified examples of this was a Yersinia effector protein, YopH, which is translocated into the host cytosol where it dephosphorylates tyrosine residues on multiple substrates (Guan and Dixon 1990). CagA is a Helicobacter pylori virulence factor which, once translocated into the host, is phosphorylated by Abl and Src family kinases, resulting in its binding to host cell proteins through their Src homology 2 domains, leading to host cell cytoskeletal modifications (Backert et al. 2010). Direct inactivation of host kinases is a mechanism of action of the lethal factor (LF) component of the Bacillus anthracis multi-subunit anthrax toxin. LF is a metalloprotease that cleaves host kinases, inhibiting their activity by limiting their ability to interact with substrates (Duesbery et al. 1998; Vitale et al. 1998). Lastly, Shigella encodes a phosphothreonine lyase, OspF, which irreversibly removes a phosphate by cleavage of the carbon–oxygen bond of target phosphothreonine residues (Li et al. 2007).
The intracellular pathogen Legionella pneumophila regulates host cell function in order to develop a niche permissive for replication. Much of the ability of L. pneumophila to accomplish this is dependent on its type IV secretion system (T4SS), termed Icm/Dot (intracellular multiplication/defect in organelle trafficking) (Marra et al. 1992; Berger and Isberg 1993). This system translocates ~300 proteins into the host cell after contact with the bacterium (Burstein et al. 2009; Huang et al. 2011; Zhu et al. 2011). These Icm/Dot translocated substrates (IDTS) have been shown to play roles in modulating host cell processes such as translation, cell survival, membrane trafficking, ubiquitination, and cytoskeletal dynamics (Nagai et al. 2002; Laguna et al. 2006; Kubori et al. 2008; Fontana et al. 2011; Franco et al. 2012). Although the absence of a single IDTS rarely results in an intracellular growth defect, likely due to functional redundancy among these substrates, the host cell factors and processes that they target are often required for high levels of replication (Dorer et al. 2006; O’Connor et al. 2012).
Host cell signaling through modulation of the phosphorylation states of proteins plays an important role in L. pneumophila intracellular replication. These signaling pathways are activated in response to L. pneumophila challenge and are further altered by the pathogen for its own benefit. During Legionella host cell binding and uptake, the phosphorylation status of multiple proteins is modulated (Venkataraman et al. 1997; Coxon et al. 1998; Tachado et al. 2008). Mitogen-activated protein kinase (MAPK) pathways are also activated during challenge (Welsh et al. 2004; Shin et al. 2008; Fontana et al. 2012). These pathways are altered by Legionella in an Icm/Dot dependent and independent manner to regulate the host response. Also activated is the transcription factor NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) which is important for maintaining host cell viability during intracellular replication (Losick and Isberg 2006; Abu-Zant et al. 2007; Shin et al. 2008; Bartfeld et al. 2009; Fontana et al. 2011). Both MAPK and NF-κB signaling are mediated by Protein kinase C (PKC) which has also been shown to activate innate immune pathways in response to L. pneumophila challenge (N’Guessan et al. 2007; Vardarova et al. 2009). Lastly, Legionella translocates protein kinases into the host which may further modulate these pathways (de Felipe et al. 2008; Ge et al. 2009; Hervet et al. 2011).
2 Modulation of Protein Phosphorylation During Binding and Uptake by Host Cells
Initial studies of signal transduction following the interaction of host cells with L. pneumophila showed the global modification of the phosphorylation status of multiple proteins. Large-scale changes in protein phosphorylation have been linked to bacterial binding, uptake, and intracellular replication (Venkataraman et al. 1997; Coxon et al. 1998; Susa and Marre 1999). The change in the phosphorylation status of these proteins is mediated by both host kinases and phosphatases and there appears to be cell type specificity to these modifications (Venkataraman et al. 1997; Coxon et al. 1998; Tachado et al. 2008; Charpentier et al. 2009).
2.1 Protein Phosphorylation During Uptake by Mammalian Cells
Opsonization of L. pneumophila with complement C3b and C3bi induces binding to the host cell complement receptor 3 (CR3). This results in one of the most efficient mechanisms of L. pneumophila uptake by monocytes. CR3-mediated uptake of L. pneumophila is inhibited by the tyrosine protein kinase (TPK) inhibitors genistein and tyrphostin (Coxon et al. 1998). The inhibition of uptake by TPK inhibitors correlates with a decrease in actin polymerization in response to L. pneumophila as cells treated with these inhibitors did not show the marked increase in actin polymerization that was seen in untreated infected cells (Coxon et al. 1998).
Due to the role of tyrosine kinases in complement-mediated uptake of L. pneumophila, the global change in protein phosphorylation in response to L. pneumophila challenge was analyzed. CR3-mediated uptake of L. pneumophila by monocytes induces the tyrosine phosphorylation of multiple protein targets (Coxon et al. 1998). This phosphorylation is not dependent on the ability of L. pneumophila to replicate intracellularly, as an avirulent mutant, as well as Escherichia coli, also induce similar patterns of tyrosine phosphorylation (Coxon et al. 1998).
Challenge of MRC-5 lung epithelial cells also induces changes in the phosphorylation state of multiple proteins (Susa and Marre 1999). Both bacterial and eukaryotic proteins are believed to be phosphorylated as pretreatment with cycloheximide did not inhibit synthesis of all proteins that are phosphorylated during infection. Interestingly, little to no change in serine/threonine phosphorylation was observed while tyrosine phosphorylation was seen for many proteins (Susa and Marre 1999). Tyrosine phosphorylation is believed to be required for later stages of replication as an intracellular growth defect was observed for genistein treated cells at 24, but not four, hours post-infection (Susa and Marre 1999).
Studies utilizing chemical inhibitors have further revealed the importance of tyrosine phosphorylation for L. pneumophila uptake. A screen designed to identify host factors important for translocation of IDTS showed that the compound RWJ-60475, which has been shown to target the receptor protein tyrosine phosphate phosphatase, CD45, inhibited translocation, as well as bacterial uptake (Charpentier et al. 2009). Further studies revealed that bone marrow-derived macrophages (BMDMs) from CD45/CD148 CD148 double knockout mice were inhibited for uptake, while BMDMs from CD45 knockout mice were not. CD148 is also a receptor protein tyrosine phosphate phosphatase which is believed to function redundantly to (Zhu et al. 2008) and it, as well as other tyrosine phosphatases, may be an off target substrate of RWJ-60475. While the CD45/CD148-deficient BMDMs where defective in L. pneumophila uptake, they were able to efficiently phagocytose E. coli (Charpentier et al. 2009). This may point to a L. pneumophila specific mechanism of uptake by which it is taken up into a replication-competent niche within the host.
Though large-scale global changes in serine/threonine phosphorylation are not seen during host cell challenge with L. pneumophila, there is evidence that specific serine/threonine kinases may play a role in the early stages of L. pneumophila infection. Protein kinase B (PKB/Akt) Protein kinase B (PKB/Akt) is a serine/threonine kinase which functions in signaling pathways downstream phosphoinositide 3-kinase (PI3K) of phosphoinositide 3-kinase (PI3K) (Franke et al. 1995). Though there appears to be cell type differences in the requirement of PI3K activation for Legionella uptake (Khelef et al. 2001), J774A.1 macrophages are inhibited for L. pneumophila uptake when treated with chemical PI3K inhibitors (Tachado et al. 2008; Charpentier et al. 2009). Consistent with these results, Akt is phosphorylated within 15 min of J774A.1 challenge, but this activation is not seen when PI3K is inhibited (Tachado et al. 2008). Akt signaling may also be involved in L. pneumophila induced apoptosis of T-cells, when challenged at a high MOI, as it is dephosphorylated under these conditions (Takamatsu et al. 2010).
2.2 Modulation of Amoebal Protein Tyrosine Phosphorylation
Hartmannella vermiformis is a protozoan host within which L. pneumophila is found in the environment (Fields et al. 1990; Fields 1996). Invasion of L. pneumophila into this host is mediated by the host cell Gal/GalNAc lectin receptor, a homolog of the mammalian β2 transmembrane receptors (Adams et al. 1993; Venkataraman et al. 1997). Uptake of L. pneumophila can be blocked by the addition of Gal or GalNAc, as well as by monoclonal antibodies targeting this receptor (Venkataraman et al. 1997). Challenge of H. vermiformis by L. pneumophila induces the tyrosine dephosphorylation of the Gal/GalNAc lectin receptor and this dephosphorylation is inhibited by the addition of Gal or GalNac, consistent with a requirement for L. pneumophila receptor binding (Venkataraman et al. 1997).
In addition to the dephosphorylation of the Gal/GalNAc lectin receptor, L. pneumophila induces the dephosphorylation of multiple other tyrosine phosphorylated proteins, including those associated with this lectin (Venkataraman et al. 1998; Venkataraman and Kwaik 2000). Protein dephosphorylation is mediated by the activation of protein tyrosine phosphatases, rather than inactivation of a kinase, and this activation appears to be unique to L. pneumophila as E. coli does not induce this dephosphorylation (Venkataraman et al. 1997; Venkataraman et al. 1998). Host protein dephosphorylation is associated with L. pneumophila binding, but not uptake, as it is seen in H. vermiformis pretreated with methylamine, which inhibits uptake, or infection with invasion-defective L. pneumophila mutants (Venkataraman et al. 1998). The dephosphorylation is also reversible as washing away extracellular bacteria results in the tyrosine phosphorylation of these protein substrates (Venkataraman et al. 1998).
The proteins which are dephosphorylated in response to Legionella challenge include cytoskeletal proteins involved in actin rearrangement such as paxillin, vinculin, and pp125FAK. Dephosphorylation of these actin-associated proteins may result in cytoskeletal disassembly which could be responsible for the unique mechanisms of uptake of L. pneumophila into amoebal hosts (Venkataraman et al. 1998). While the tyrosine phosphorylation status of host proteins appears to be important for L. pneumophila uptake by a variety of cell types, it appears that dephosphorylation of protein targets, rather than phosphorylation seen in mammalian systems, mediates uptake into H. vermiformis.
3 MAPK Signaling Pathways
Mitogen-activated protein kinases are serine/threonine kinases that are activated by an evolutionally conserved signal transduction pathway, allowing eukaryotic cells to respond to environmental conditions through a kinase-mediated signaling cascade (Caffrey et al. 1999). MAPK signaling has been shown to play roles in cell cycle progression, metabolism, cytoskeletal dynamics, apoptosis, and the inflammatory response (Johnson and Lapadat 2002).
MAPK signaling proceeds through a phosphorelay system beginning with MAPK kinase kinase (MAPKKK) activation in response to a stimulus. Activation of MAPKKKs occurs by the phosphorylation of specific tyrosine and threonine residues. As the signaling cascade continues, MAPKKKs transfer a phosphate to a MAPK kinase (MAPKK), activating it and allowing it to phosphorylate a specific MAPK (Ray and Sturgill 1988; Johnson and Lapadat 2002). MAPK activation, by phosphorylation of a Thr-X-Tyr motif, induces cellular changes through the phosphorylation of transcription factors, kinases, and cytoskeletal proteins (Cargnello and Roux 2011).
There are four well-characterized conventional MAPK families found in multicellular eukaryotes: ERK1/2, SAPK/JNK, p38, and ERK5. These families are activated by cellular stresses, growth factors, protein synthesis inhibition, and cytokines (Cargnello and Roux 2011). Detection of pathogens, by pattern recognition receptors (PRRs), is also an important activator of MAPK signaling as Tolllike receptor (TLR) and nucleotide-binding oligomerization domain like receptor (NLR) detection of pathogen-associated molecular patterns (PAMPs) is linked to the MAPK response (Weinstein et al. 1992; Swantek et al. 2000; Girardin et al. 2001).
Modulation of MAPK pathways appears to be a common theme of host cell subversion by pathogens. SAPK/JNK and p38 pathways are activated following pathogen detection by TLRs or NLRs, leading to an enhanced immune response (Kobayashi et al. 2005; Huang et al. 2009). Inhibition of MAPK signaling has been shown for multiple pathogens including B. anthracis, by the activity of its LF toxin, and Vibrio parahaemolyticus, through the action of a type III secretion system effector protein, VopA (Duesbery et al. 1998; Trosky et al. 2004).
3.1 Pathogen-Associated Molecular Pattern and Icm/Dot Dependent Induction of MAPK Activation
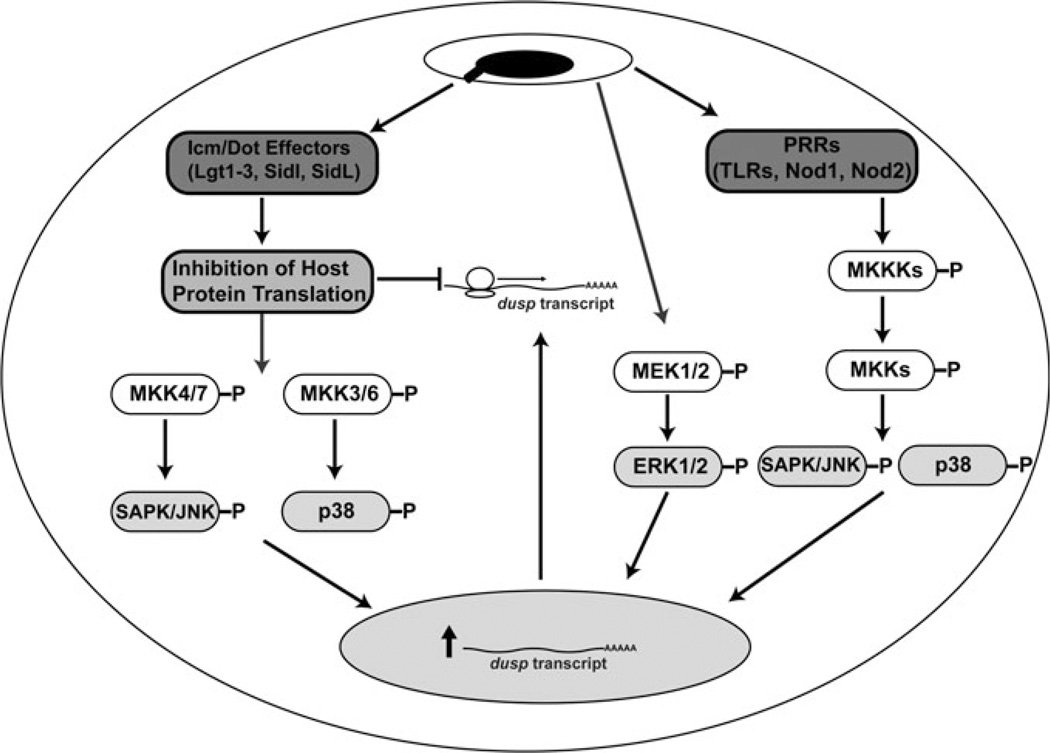
MAPK activation in response to L. pneumophila challenge has been detected in numerous mammalian cell types, including primary macrophages and epithelial cells (Fig. 1) (Welsh et al. 2004; N’Guessan et al. 2007; Shin et al. 2008). In monocyte-derived macrophages, phosphorylation of SAPK/JNK, ERK1/2, and p38 is seen within 15 min of bacterial challenge (Welsh et al. 2004). This early MAPK response is independent of the translocation of substrates by the T4SS as an icm/dot− mutant (GL10) also induces this host cell response. Interestingly, when later time points are observed, p38 and JNK phosphorylation has returned to basal levels in the icm/dot− mutant-challenged cells while activation is maintained in cells infected with wild-type L. pneumophila (Welsh et al. 2004). Activation at late time points is also dependent on the translocation of specific effectors as a ΔicmS mutant, which retains T4SS dependent pore formation but is defective in the translocation of many effectors, did not induce SAPK/JNK or p38 phosphorylation (Zuckman et al. 1999; Coers et al. 2000; Bardill et al. 2005; Ninio et al. 2005; Cambronne and Roy 2007; Shin et al. 2008). ERK phosphorylation does not show this pattern of activation as its activation is not maintained at 1 h post-challenge with either an icm/dot− mutant or wild-type strain (Welsh et al. 2004; Shin et al. 2008).
Fig. 1.
MAPK signaling during L. pneumophila infection. L. pneumophila induced MAPK activation occurs by both Icm/Dot dependent and independent pathways. ERK1/2, SAPK/JNK, and p38 pathways are activated in an Icm/Dot independent manner. Activation of SAPK/JNK and p38 occurs by pathogen recognition receptor (PRR) signaling while induction of ERK1/2 occurs by an unknown mechanism (Shin et al. 2008). Icm/Dot dependent MAPK activation is induced by the inhibition of protein synthesis by five translocated effectors (Lgt1-3, SidI, and SidL) which, through an unknown mechanism, activate MAPKKs that phosphorylate p38 and SAPK/JNK (Fontana et al. 2012). Signaling through MAPKs results in enhanced transcription of target genes, including those encoding dual-specificity protein phosphatases (Dusps), which dephosphorylate MAPKs in a feedback response (Losick and Isberg 2006; Shin et al. 2008; Li et al. 2009). Dusp translation is inhibited by the action of protein synthesis inhibitors; preventing this response (Isberg Lab, unpublished results)
Signaling both upstream and downstream of MAPK activation is seen during L. pneumophila challenge. MAPKKs in the p38, SAPK/JNK, and ERK pathway are activated with kinetics consistent with their ability to induce MAPK phosphorylation (Fig. 1) (Welsh et al. 2004; Shin et al. 2008). MAPK activation during challenge is also productive as c-Jun, a transcription factor that is modulated through phosphorylation by SAPK/JNK, is activated (Shin et al. 2008; Scharf et al. 2010). Lysates from challenged cells have also been shown to have activity toward ELK and ATF, ERK, and p38 substrates, respectively (Welsh et al. 2004).
Maximal induction of MAPK signaling by Legionella is dependent on extracellular and cytosolic sensing of bacterial PAMPs. Myd88−/−Trif−/− BMDMs, which are defective in TLR dependent pathogen recognition, challenged with L. pneumophila, do not show p38 or SAPK/JNK phosphorylation until 1 h post-challenge. This pattern of activation is also seen in Myd88−/−Rip2−/− BMDMs, which are defective in TLR, as well as Nod1 and Nod2 signaling. In these cells, no activation, at any time point, of either SAPK/JNK or p38 was detected when challenged with an icm/dot− L. pneumophila mutant (Shin et al. 2008). In contrast to SAPK/JNK and p38, ERK activation is independent of both TLR and Nod signaling (Fig. 1) (Shin et al. 2008).
The differential responses to wild-type and Icm/Dot deficient strains pointed to an early MAPK response that is Myd88/Nod dependent, Icm/Dot independent, and a later response that is Myd88/Nod independent, Icm/Dot dependent. This Icm/Dot dependent response appears to be upstream of MAPKK activation as MKK4 and MKK3/6 are both phosphorylated in Myd88−/−Rip2−/− BMDMs when challenged with wild type, but not icm/dot−, L. pneumophila (Fig. 1) (Shin et al. 2008).
Five IDTS, which inhibit host protein synthesis, have been shown to be the T4SS factors which result in the late activation of SAPK/JNK and p38 (Fontana et al. 2011, 2012). A strain lacking these five effectors (Lgt1, Lgt2, Lgt3, SidI, and SidL) is incapable of eliciting a SAPK/JNK or p38 response in Myd88−/−- Nod1−/−Nod2−/− BMDMs (Fig. 1). Complementation of this strain with a single effector (Lgt3), but not one with an inactivating point mutation, restores MAPK signaling, indicating that the cellular response to inhibition of protein biosynthesis, rather than a single effector, elicits this phenotype (Fontana et al. 2012).
3.2 Effects of MAPK Activation During Infection
The implications of MAPK signaling in response to L. pneumophila challenge have been analyzed using chemical inhibitors of MAPK pathways. Macrophages challenged with L. pneumophila, and treated with p38 or JNK inhibitors, are defective in the transcriptional induction of IL-1α and IL-1β (Shin et al. 2008; Fontana et al. 2012). Challenge of epithelial cells with L. pneumophila induces the expression of human β-defensin-2 (hBD-2), an antimicrobial peptide, MUC5AC, a major mucin protein, and prostaglandin E2 (PGE2), which regulates lung surfactant secretion as well as the immune response (N’Guessan et al. 2007; Scharf et al. 2010; Morinaga et al. 2012). hBD-2 production by cells challenged with L. pneumophila is decreased in cells preincubated with either JNK or p38 inhibitors (Scharf et al. 2010). In epithelial cells pretreated with ERK or JNK inhibitors, MUC5AC expression is inhibited (Morinaga et al. 2012). PGE2 induction in response to L. pneumophila is also inhibited by both ERK and p38 inhibitors (N’Guessan et al. 2007).
The effect of pharmacokinetic inhibition of MAPKs on L. pneumophila intracellular replication is less clear. In monocyte-derived macrophages, inhibition of p38 or JNK, but not ERK, significantly inhibited intracellular growth observed at 48 h post-infection (Welsh et al. 2004). In epithelial cells, the JNK inhibitor SB600125 inhibited replication over a 24 h time period, consistent with treatment of monocyte-derived macrophages with this inhibitor (Morinaga et al. 2012). Another JNK inhibitor, JNK II, did not inhibit L. pneumophila replication in epithelial cells (Scharf et al. 2010). Inhibition of ERK or p38 had no effect on replication in epithelial cells (Scharf et al. 2010; Morinaga et al. 2012). Although in some cases, there appears to be reduction of intracellular growth in the presence of pharmacological inhibitors of MAPK, it should be noted that L. pneumophila mutants that fail to activate MAPK are still able to grow intracellularly. Therefore, MAPK activation in response to L. pneumophila does not appear to be required for intracellular growth.
3.3 Amoebal MAPK Activation
Activation of MAPK signaling is also observed during challenge of Dictyostelium discoideum, a natural amoebal host of L. pneumophila (Li et al. 2009). D. discoideum encodes two MAPKs, ERK1, and ERK2, which are analogous to the mammalian ERK family (Gaskins et al. 1994; Segall et al. 1995). During challenge with L. pneumophila, ERK1 is phosphorylated to a maximal level 1 h post-infection and activation continues for at least 4 h (Li et al. 2009). As with mammalian cells, ERK1 activation was observed with similar kinetics when D. discoideum was challenged with an icm/dot− mutant (Li et al. 2009).
3.4 Role of Dusps in the MAPK Response
Negative regulation of MAPK signaling in D. discoideum is necessary for efficient L. pneumophila replication. One mechanism of MAPK modulation is through Dual-specificity protein phosphatases (Dusps) which are upregulated following MAPK signaling. Dusp activation provides a feedback loop whereby MAPKs are dephosphorylated to attenuate the initial response (Patterson et al. 2009). A screen to identify D. discoideum mutants defective for L. pneumophila intracellular replication identified DupA, a protein which encodes an N-terminal eukaryotic protein kinase domain, as well as a dual-specificity protein phosphatase domain. dupA transcript expression is enhanced in L. pneumophila infected cells, consistent with a MAPK feedback response. A dupA mutant showed constitutive ERK1 activity which may explain the L. pneumophila growth defect in these cells and points to the importance of the phosphatase activity of this protein (Li et al. 2009).
Enhanced Dusp transcription has also been shown to occur in mammalian cells following L. pneumophila challenge (Fig. 1) (Losick and Isberg 2006; Shin et al. 2008). This expression is dependent on maximal MAPK signaling as the L. pneumophila strain, lacking five effectors shown to inhibit protein synthesis, does not induce Dusp expression (Haenssler and Isberg 2011). However, the importance of this enhanced expression in mammalian cells is unclear, because even though the transcript is increased, Dusp protein levels do not increase. It appears that the inhibition of protein synthesis by L. pneumophila, while enhancing Dusp transcript expression through MAPK activation, may be preventing the Dusp feedback response by inhibiting Dusp translation (unpublished results, Asrat and Isberg).
4 The Role of Protein Kinase C in the Response to L. pneumophila
The PKC family of serine/threonine kinases responds to varying environmental stimuli allowing for a broad range of responses including cell cycle progression, apoptosis, motility, and gene transcription (Ghayur et al. 1996; Black 2000; Ventura and Maioli 2001; Xiao and Liu 2012). PKCs can be categorized into three subfamilies based on their structural features and the requirement of different upstream signals for activation. Conventional PKCs (α, βI-II, and γ) require diacylglycerol (DAG) and Ca2+ to stimulate kinase activity. Novel PKCs (δI-III, ε, η, and θI-II) are activated downstream of DAG but do not require Ca2+. Lastly, atypical PKCs (ζ and ι/λ) require neither DAG or Ca2+ (Tan and Parker 2003).
Members of each family of PKCs play important roles in both adaptive and innate immunity in response to bacterial pathogens. PKCs localize to the phagosome during phagocytosis and their pharmacological inhibition has been shown to limit uptake in some systems (Garcia-Garcia and Rosales 2002). Signaling downstream of both cytokine receptors and TLRs is mediated by varying isoforms of PKC. Inhibition, or the absence, of specific isoforms has been shown to block NF-κB and MAPK activation, as well as subsequent cytokine expression (Tan and Parker 2003; Loegering and Lennartz 2011).
4.1 Involvement of PKCs During Uptake
Studies of complement-mediated uptake of L. pneumophila have pointed to an important role of PKC in this process. Treatment of human monocytes with either staurosporine or calphostin C, both PKC inhibitors, decreased the ability of these cells to take up C3b/C3bi coated L. pneumophila. This was correlated with a decrease in actin polymerization when these cells were challenged, relative to untreated cells. Treatment with these chemicals 2 h post-infection resulted in only a minor reduction in bacterial growth observed at 48 h, implicating that while PKCs are important for CR3 mediated uptake, they may not play a role in intracellular replication (Coxon et al. 1998).
The importance of a conventional PKC, PKC-α, during L. pneumophila uptake and intracellular growth has also been studied. RAW 264.7 murine macrophages expressing a dominant negative PKC-α showed no defect in L. pneumophila uptake (St-Denis et al. 1999). Interestingly, expression of the dominant negative PKC-α resulted in RAW 264.7 cells which were permissive for L. pneumophila intracellular replication. These data are consistent with a model in which PKC-α is not required for bacterial uptake but is necessary for the restriction of bacterial growth exhibited by RAW 264.7 macrophages.
4.2 Innate Immune Signaling Mediated by PKC
Protein kinase C activation has been shown to occur in multiple systems in response to L. pneumophila. Early studies revealed that the addition of a L. pneumophila heat shock protein, Hsp60, to mouse peritoneal macrophages induced PKC activation (Retzlaff et al. 1996). Challenge of epithelial cells by L. pneumophila has also been shown to activate multiple PKC isotypes (N’Guessan et al. 2007; Vardarova et al. 2009). This activation was decreased in cells challenged with strains deficient in flagellin or treated with heat-killed Legionella, indicating that multiple signals from the pathogen are required for high-level activation (Vardarova et al. 2009).
Activation of PKC by L. pneumophila results in the initiation of innate immune signaling pathways. Enhanced IL-1β transcription, seen during treatment of macrophages with L. pneumophila Hsp60, is blocked by chemical inhibition of PKC (Retzlaff et al. 1996). Release of granulocyte macrophage colony-stimulating factor (GM-CSF) and PGE2 is also limited by PKC inhibition (N’Guessan et al. 2007; Vardarova et al. 2009). Studies using chemical inhibitors of specific PKC isoforms have shown that PKC-α is involved in the activation of NF-κB, while PKC-ε is relevant to c-Jun signaling, in response to L. pneumophila challenge (Vardarova et al. 2009).
5 The NF-κB Response to L. pneumophila Challenge
Signaling through the regulated transcription factor NF-κB results in changes in the expression of hundreds of genes, including proinflammatory cytokines and regulators of cell survival and differentiation (Natoli 2009). In an unstimulated cell, NF-κB hetero- and homodimers are inhibited by interaction with the inhibitor of κB (IκB) family proteins. NF-κB signaling is initiated when IκB kinases (IKKs) are activated, leading to the phosphorylation of IκB which results in its ubiquitination and subsequent proteasomal degradation. Released NF-κB translocates to, and is maintained in the nucleus where it binds to κB sequences located in the promoter and enhancer regions of target genes (Li and Verma 2002).
NF-κB signaling is an innate immune response to pathogens initiated by both PRRs and cytokine receptors. The TNF-α and IL-1 receptors signal through IKK to activate NF-κB during the response to these extracellular ligands (Kelliher et al. 1998; Verstrepen et al. 2008). Detection of PAMPs by TLR-, NOD-, and NOD-like receptors leads directly to IKK activation and downstream NF-κB induction (Ogura et al. 2001; Fritz et al. 2006; Kawai and Akira 2007).
5.1 The Biphasic Activation of NF-κB
The activation of NF-κB and its regulated genes is strongly induced following L. pneumophila host cell challenge (Fig. 2). Activation, as measured by nuclear localization of p65 (RelA), a subunit of the canonical form of NF-κB, is seen within 3 h post-challenge of replication permissive BMDMs from A/J mice, U937 cells, or human monocyte-derived macrophages (Losick and Isberg 2006; Abu-Zant et al. 2007). Inactivation of the Icm/Dot T4SS, through a dotA mutation, severely limits this response, as a 10-fold higher MOI is required to elicit a strong NF-κB response. Activation in response to icm/dot− mutants is also transient and maintained for only 2 h post-infection, whereas challenge with a wild-type strain continues to elicit a response up to 14 h post-infection (Losick and Isberg 2006; Abu-Zant et al. 2007; Shin et al. 2008; Bartfeld et al. 2009). Induction is also limited in a ΔicmS L. pneumophila host cell challenge, indicating that the translocation of specific effectors, rather than a cellular response to the T4SS apparatus, is responsible (Losick and Isberg 2006).
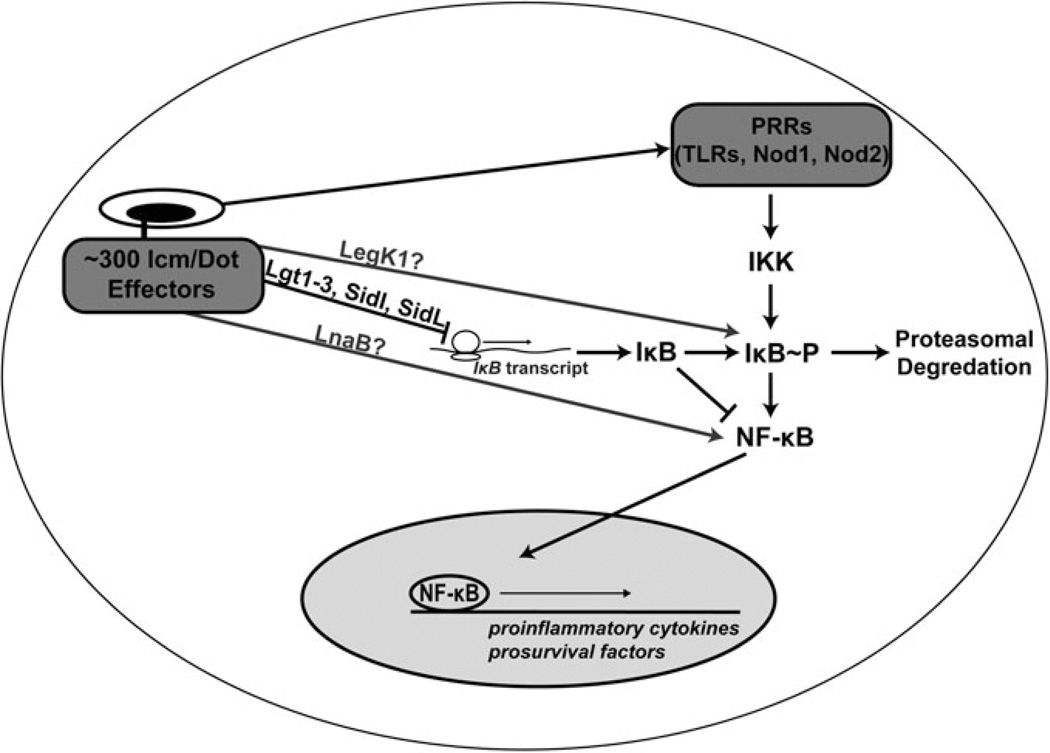
Fig. 2.
NF-κB activation in response to Legionella. NF-κB activation occurs when the inhibitor of κB (IκB) is phosphorylated by the IκB kinase (IKK), leading to its degradation and the translocation of NF-κB to the nucleus. IKK is activated by L. pneumophila in an Icm/Dot independent manner through PRRs, leading to IκB degradation (Shin et al. 2008). High levels of sustained NF-κB activation, in response to L. pneumophila challenge, are due to the Icm/Dot mediated translocation of five effectors (Lgt1-3, SidI, and SidL) which inhibit translation of host cell proteins, including IκB (Fontana et al. 2011). LegK1, an Icm/Dot translocated Ser/Thr kinase, is able to phosphorylate IκB in vitro, but its activity in vivo is unclear (Ge et al. 2009). LnaB is another translocated substrate which activates NF-κB by an unknown mechanism (Losick et al. 2010). NF-κB activation leads to enhanced transcription of prosurvival factors, as well as inflammatory cytokines (Losick and Isberg 2006; Abu-Zant et al. 2007)
Studies of TLR signaling responsible for the NF-κB response to L. pneumophila have shown that, similar to MAPK activation, this response is biphasic in nature. In epithelial cells depleted for the TLR adaptor protein Myd88, or in Myd88−/− BMDMs, there is a dramatic inhibition of the early (1 hpi) NF-κB response (Shin et al. 2008; Bartfeld et al. 2009). At later time points (8 hpi), NF-κB activation is detectable in these hosts when challenged with the wild type, but not with an Icm/Dot deficient strain (Bartfeld et al. 2009). These data pointed to an early Icm/Dot independent response and a later Icm/Dot dependent response.
Recognition of intracellular pathogens by NLRs results in signaling through the adaptor protein Rip2 to activate NF-κB (Chin et al. 2002; Kobayashi et al. 2002; Hayden and Ghosh 2012). Therefore, the role of Rip2 in NF-κB activation in response to L. pneumophila was assayed. Unlike Myd88-dependent signaling, Rip2-dependent signaling is dispensable for NF-κB activation by both wild-type and icm/dot− L. pneumophila (Shin et al. 2008). Interestingly, BMDMs deficient in both Myd88 and Rip2 are defective for NF-κB activation, even at late time points during wild-type L. pneumophila challenge (Shin et al. 2008). This indicates that though the late NF-κB response is Icm/Dot dependent, it also requires an additional pattern recognition signal mediated by either TLR or NOD pathways. This is in contrast to MAPK signaling which only requires a single signal for activation.
5.2 Translocated Substrates Implicated in NF-κB Activity
Multiple groups have attempted to identify the translocated substrates of the Icm/Dot T4SS required for the late activation of NF-κB. Ectopic expression of multiple IDTS in HEK293T cells showed moderate (>3-fold) enhancement in NF-κB activity, as measured by a NF-κB-luciferase reporter (Losick et al. 2010). Two substrates, LnaB and LegK1, exhibited greater than 100-fold induction of NF-κB activity (Ge et al. 2009; Losick et al. 2010). The role of these effectors in the induction of NF-κB during L. pneumophila challenge is currently unclear as a ΔlnaB strain only modestly reduced NF-κB activity while the absence of LegK1 had no effect (Fig. 2) (Losick et al. 2010).
In addition to their role in modulating the host MAPK pathways, the five L. pneumophila translocated substrates that inhibit host cell translation also play a role in the NF-κB response (Fig. 2). These proteins inhibit the translation of IκB, which is degraded in response to IKK activation by pattern recognition. In BMDMs challenged with a ΔdotA strain, IκB protein levels are decreased shortly after infection, but then return to close to uninfected levels, while in a wild-type infection IκB levels remain low (Shin et al. 2008; Fontana et al. 2011). In cells challenged with a strain lacking the five translation inhibitors, results are similar to a ΔdotA infection in which IκB levels return following an early depletion (Fontana et al. 2011). Therefore, in the mutant strain lacking the translation inhibitors, there is a lack of induction of NF-κB activity, as this strain shows lower levels of translocation of the p65 NF-κB subunit into the nucleus, relative to that observed during a wild-type infection (Fontana et al. 2011).
5.3 NF-κB and Host Cell Survival
The outcome of NF-κB activation by L. pneumophila appears to be twofold. The first is the enhanced transcription of NF-κB target genes, including cytokines, which play a role in the host response to limit the pathogen (Losick and Isberg 2006; Abu-Zant et al. 2007). The second, which is essential for the ability of L. pneumophila to replicate intracellularly, is the activation of prosurvival factors. A/J BMDMs, expressing either a dominant negative IκB, which is inhibitory for NF-κB activation, or treated with caffeic acid phenethyl ester (CAPE), which prevents the nuclear translocation of NF-κB, undergo enhanced cell death in response to L. pneumophila (Losick and Isberg 2006). CAPE treatment of human monocyte-derived macrophages, challenged with the AA100 strain, does not induce enhanced cell death, indicating that there may be cell type and strain specificity (Abu-Zant et al. 2007). Regardless of differences in cellular survival, CAPE treatment limits L. pneumophila replication in both cell types (Losick and Isberg 2006; Abu-Zant et al. 2007).
6 L. pneumophila T4SS Translocated Kinases
L. pneumophila encodes five proteins (LegK1-5) with homology to serine/threonine kinases (de Felipe et al. 2005; Hervet et al. 2011). LegK1-4 are present in all sequenced L. pneumophila strains, while LegK5 is found only in the Lens isolate (Hervet et al. 2011). Of these, LegK1-4 have been shown to be translocated by the Icm/Dot T4SS (de Felipe et al. 2008; Shin et al. 2008; Ge et al. 2009; Hervet et al. 2011). Modulation of host signaling through Ser/Thr kinases appears to be a unique mechanism as none of the other identified IDTS have homology to either tyrosine kinases or protein phosphatases (Haenssler and Isberg 2011).
The best studied of these translocated substrates is the previously mentioned LegK1. When expressed in eukaryotic cells, LegK1, but not LegK2 or LegK3, activated NF-κB (Ge et al. 2009). This activation required the kinase activity of LegK1 as a point mutation in its ATP binding site inhibited its ability to activate NF-κB. Recombinant LegK1 is able to phosphorylate the NF-κB inhibitor, IκB, showing that, in vitro, it is able to trigger the canonical NF-κB activation pathway. p100, which is processed into p52 after phosphorylation in the noncanonical pathway of NF-κB activation, was also shown to be phosphorylated by recombinant LegK1 (Ge et al. 2009). The importance of the phosphorylation during intracellular growth is unclear, as a strain lacking legK1 is able to replicate in both BMDMs and the natural amoebal host Acanthamoeba castellanii (Ge et al. 2009; Losick et al. 2010). Furthermore, strains lacking the five translation inhibitors are unable to activate NF-κB above the levels seen in a dotA− strain, calling into question the relevance of the in vitro-demonstrated IκB phosphorylation.
Though specific host targets for LegK2-5 have not been identified, each has begun to be characterized in vitro as well as in vivo. Recombinant LegK2-5 proteins were shown to have autokinase activity, as well as the ability to transfer a phosphate group to the eukaryotic myelin basic protein in vitro (Hervet et al. 2011). In contrast, recombinant LegK1 did not exhibit either of these activities, showing that its ability to phosphorylate NF-κB pathway factors may be specific (Hervet et al. 2011). When A. castellanii was challenged with L. pneumophila strain Lens lacking each of the LegK proteins, a Δlegk2 strain exhibited delayed replication and decreased host cell cytotoxicity relative to the wild-type, and other legK deletion strains. This may be due to an inability of this strain to form a replication-competent vacuole as Legionella-containing vacuoles (LCVs) showed a defect in recruitment of the ER chaperone calnexin (Hervet et al. 2011).
7 Conclusions
The ability of L. pneumophila to replicate within a host is dependent on host cell signaling through changes in the phosphorylation state of protein substrates. Modulation of these signaling pathways by Legionella is just one example of how this pathogen is able to subvert normal cellular processes for its own benefit. During initial host cell contact, multiple proteins are phosphorylated and may play a role in bacterial uptake. Legionella activates both MAPK and NF-κB signaling pathways, when PRRs are engaged, early during infection. These pathways are further activated by the translocation of five IDTS which inhibit host protein synthesis. PKC activation is also involved in these, as well as other, innate immune signaling pathways. Finally, although their targets are currently unknown, L. pneumophila translocates at least four effector proteins shown to have protein kinase activity. Further research will elucidate the targets of these proteins and what role they may play in the modulation of the important signaling pathways regulated by protein phosphorylation.
Abbreviations
- BMDMs
Bone marrow-derived macrophages
- CAPE
Caffeic acid phenethyl ester
- CR3
Complement receptor 3
- DAG
Diacylglycerol
- Dusp
Dual-specificity protein phosphatase
- hBD-2
Human β-defensin-2
- IDTS
Icm/Dot translocated substrates
- IκB
Inhibitor of κB
- Icm/Dot
Intracellular multiplication/defect in organelle trafficking
- IKK
IκB kinase
- LCV
Legionella-containing vacuole
- LF
Lethal factor
- MAPKK
MAPK kinase
- MAPKKK
MAPK kinase kinase
- MAPK
Mitogen-activated protein kinase
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NLR
Nucleotide-binding oligomerization domain like receptor
- PAMP
Pathogen-associated molecular pattern
- PRR
Pattern recognition receptor
- PI3K
Phosphoinositide 3-kinase
- PGE2
Prostaglandin E2
- PKB/Akt
Protein kinase B
- PKC
Protein kinase C
- TLR
Toll-like receptor
- T4SS
Type IV secretion system
- TPK
Tyrosine protein kinase
Contributor Information
Andrew D. Hempstead, Department of Molecular Biology and Microbiology, Tufts University School of Medicine, Boston, MA 02111, USA Graduate Program in Molecular Microbiology, Sackler School of Graduate Biomedical Science, Tufts University School of Medicine, Boston, MA 02111, USA.
Ralph R. Isberg, Email: ralph.isberg@tufts.edu, Howard Hughes Medical Institute, Tufts University School of Medicine, Boston, MA 02111, USA; Department of Molecular Biology and Microbiology, Tufts University School of Medicine, Boston, MA 02111, USA.
References
- Adams SA, Robson SC, Gathiram V, Jackson TF, Pillay TS, et al. Immunological similarity between the 170 kD amoebic adherence glycoprotein and human beta 2 integrins. Lancet. 1993;341:17–19. doi: 10.1016/0140-6736(93)92483-a. [DOI] [PubMed] [Google Scholar]
- Abu-Zant A, Jones S, Asare R, Suttles J, Price C, et al. Anti-apoptotic signaling by the Dot/Icm secretion system of L. pneumophila. Cell Microbiol. 2007;9:246–264. doi: 10.1111/j.1462-5822.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- Backert S, Tegtmeyer N, Selbach M. The versatility of Helicobacter pylori CagA effector protein functions: the master key hypothesis. Helicobacter. 2010;15:163–176. doi: 10.1111/j.1523-5378.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- Bardill JP, Miller JL, Vogel JP. IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol Microbiol. 2005;56:90–103. doi: 10.1111/j.1365-2958.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Bartfeld S, Engels C, Bauer B, Aurass P, Flieger A, et al. Temporal resolution of two-tracked NF-kappaB activation by Legionella pneumophila. Cell Microbiol. 2009;11:1638–1651. doi: 10.1111/j.1462-5822.2009.01354.x. [DOI] [PubMed] [Google Scholar]
- Berger KH, Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Black JD. Protein kinase C-mediated regulation of the cell cycle. Front Biosci. 2000;5:D406–D423. doi: 10.2741/black. [DOI] [PubMed] [Google Scholar]
- Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- Burstein D, Zusman T, Degtyar E, Viner R, Segal G, et al. Genome-scale identification of Legionella pneumophila effectors using a machine learning approach. PLoS Pathog. 2009;5:e1000508. doi: 10.1371/journal.ppat.1000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey DR, O’Neill LA, Shields DC. The evolution of the MAP kinase pathways: coduplication of interacting proteins leads to new signaling cascades. J Mol Evol. 1999;49:567–582. doi: 10.1007/pl00006578. [DOI] [PubMed] [Google Scholar]
- Cambronne ED, Roy CR. The Legionella pneumophila IcmSW complex interacts with multiple Dot/Icm effectors to facilitate type IV translocation. PLoS Pathog. 2007;3:e188. doi: 10.1371/journal.ppat.0030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier X, Gabay JE, Reyes M, Zhu JW, Weiss A, et al. Chemical genetics reveals bacterial and host cell functions critical for type IV effector translocation by Legionella pneumophila. PLoS Pathog. 2009;5:e1000501. doi: 10.1371/journal.ppat.1000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin AI, Dempsey PW, Bruhn K, Miller JF, Xu Y, et al. Involvement of receptorinteracting protein 2 in innate and adaptive immune responses. Nature. 2002;416:190–194. doi: 10.1038/416190a. [DOI] [PubMed] [Google Scholar]
- Coers J, Kagan JC, Matthews M, Nagai H, Zuckman DM, et al. Identification of Icm protein complexes that play distinct roles in the biogenesis of an organelle permissive for Legionella pneumophila intracellular growth. Mol Microbiol. 2000;38:719–736. doi: 10.1046/j.1365-2958.2000.02176.x. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in human health and disease. The Sir Hans Krebs Medal Lecture. Eur J Biochem. 2001;268:5001–5010. doi: 10.1046/j.0014-2956.2001.02473.x. [DOI] [PubMed] [Google Scholar]
- Coxon PY, Summersgill JT, Ramirez JA, Miller RD. Signal transduction during Legionella pneumophila entry into human monocytes. Infect Immun. 1998;66:2905–2913. doi: 10.1128/iai.66.6.2905-2913.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felipe KS, Glover RT, Charpentier X, Anderson OR, Reyes M, et al. Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog. 2008;4:e10000117. doi: 10.1371/journal.ppat.1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felipe KS, Pampou S, Jovanovic OS, Pericone CD, Ye SF, et al. Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J Bacteriol. 2005;187:7716–7726. doi: 10.1128/JB.187.22.7716-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorer MS, Kirton D, Bader JS, Isberg RR. RNA interference analysis of Legionella in Drosophila cells: exploitation of early secretory apparatus dynamics. PLoS Pathog. 2006;2:e34. doi: 10.1371/journal.ppat.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesbery NS, Webb CP, Leppla SH, Gordon VM, Klimpel KR, et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- Fields BS. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- Fields BS, Nerad TA, Sawyer TK, King CH, Barbaree JM, et al. Characterization of an axenic strain of Hartmannella vermiformis obtained from an investigation of nosocomial legionellosis. J Protozool. 1990;37:581–583. doi: 10.1111/j.1550-7408.1990.tb01269.x. [DOI] [PubMed] [Google Scholar]
- Fontana MF, Banga S, Barry KC, Shen X, Tan Y, et al. Secreted bacterial effectors that inhibit host protein synthesis are critical for induction of the innate immune response to virulent Legionella pneumophila. PLoS Pathog. 2011;7:e1001289. doi: 10.1371/journal.ppat.1001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana MF, Shin S, Vance RE. Activation of host mitogen-activated protein kinases by secreted Legionella pneumophila effectors that inhibit host protein translation. Infect Immun. 2012;80:3570–3575. doi: 10.1128/IAI.00557-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco IS, Shohdy N, Shuman HA. The Legionella pneumophila effector VipA is an actin nucleator that alters host cell organelle trafficking. PLoS Pathog. 2012;8:e1002546. doi: 10.1371/journal.ppat.1002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia E, Rosales C. Signal transduction during Fc receptor-mediated phagocytosis. J Leukoc Biol. 2002;72:1092–1108. [PubMed] [Google Scholar]
- Gaskins C, Maeda M, Firtel RA. Identification and functional analysis of a developmentally regulated extracellular signal-regulated kinase gene in Dictyostelium discoideum. Mol Cell Biol. 1994;14:6996–7012. doi: 10.1128/mcb.14.10.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Xu H, Li T, Zhou Y, Zhang Z, et al. A Legionella type IV effector activates the NFkappaB pathway by phosphorylating the IkappaB family of inhibitors. Proc Natl Acad Sci U S A. 2009;106:13725–13730. doi: 10.1073/pnas.0907200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghayur T, Hugunin M, Talanian RV, Ratnofsky S, Quinlan C, et al. Proteolytic activation of protein kinase C delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin SE, Tournebize R, Mavris M, Page AL, Li X, et al. CARD4/Nod1 mediates NFkappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan KL, Dixon JE. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990;249:553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- Haenssler E, Isberg RR. Control of host cell phosphorylation by Legionella pneumophila. Front Microbiol. 2011;2:64. doi: 10.3389/fmicb.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervet E, Charpentier X, Vianney A, Lazzaroni JC, Gilbert C, et al. Protein kinase LegK2 is a type IV secretion system effector involved in endoplasmic reticulum recruitment and intracellular replication of Legionella pneumophila. Infect Immun. 2011;79:1936–1950. doi: 10.1128/IAI.00805-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Shi LZ, Chi H. Regulation of JNK and p38 MAPK in the immune system: signal integration, propagation and termination. Cytokine. 2009;48:161–169. doi: 10.1016/j.cyto.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Boyd D, Amyot WM, Hempstead AD, Luo ZQ, et al. The E Block motif is associated with Legionella pneumophila translocated substrates. Cell Microbiol. 2011;13:227–245. doi: 10.1111/j.1462-5822.2010.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, et al. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- Khelef N, Shuman HA, Maxfield FR. Phagocytosis of wild-type Legionella pneumophila occurs through a wortmannin-insensitive pathway. Infect Immun. 2001;69:5157–5161. doi: 10.1128/IAI.69.8.5157-5161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- Kubori T, Hyakutake A, Nagai H. Legionella translocates an E3 ubiquitin ligase that has multiple U-boxes with distinct functions. Mol Microbiol. 2008;67:1307–1319. doi: 10.1111/j.1365-2958.2008.06124.x. [DOI] [PubMed] [Google Scholar]
- Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR. A Legionella pneumophila translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci U S A. 2006;103:18745–18750. doi: 10.1073/pnas.0609012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xu H, Zhou Y, Zhang J, Long C, et al. The phosphothreonine lyase activity of a bacterial type III effector family. Science. 2007;315:1000–1003. doi: 10.1126/science.1138960. [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Li Z, Dugan AS, Bloomfield G, Skelton J, Ivens A, et al. The amoebal MAP kinase response to Legionella pneumophila is regulated by DupA. Cell Host Microbe. 2009;6:253–267. doi: 10.1016/j.chom.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loegering DJ, Lennartz MR. Protein kinase C and toll-like receptor signaling. Enzyme Res. 2011;2011:537821. doi: 10.4061/2011/537821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick VP, Haenssler E, Moy MY, Isberg RR. LnaB: a Legionella pneumophila activator of NF-kappaB. Cell Microbiol. 2010;12:1083–1097. doi: 10.1111/j.1462-5822.2010.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick VP, Isberg RR. NF-kappaB translocation prevents host cell death after low-dose challenge by Legionella pneumophila. J Exp Med. 2006;203:2177–2189. doi: 10.1084/jem.20060766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Plowman GD, Hunter T, Sudarsanam S. Evolution of protein kinase signaling from yeast to man. Trends Biochem Sci. 2002;27:514–520. doi: 10.1016/s0968-0004(02)02179-5. [DOI] [PubMed] [Google Scholar]
- Marra A, Blander SJ, Horwitz MA, Shuman HA. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc Natl Acad Sci U S A. 1992;89:9607–9611. doi: 10.1073/pnas.89.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga Y, Yanagihara K, Araki N, Migiyama Y, Nagaoka K, et al. Live Legionella pneumophila induces MUC5AC production by airway epithelial cells independently of intracellular invasion. Can J Microbiol. 2012;58:151–157. doi: 10.1139/w11-123. [DOI] [PubMed] [Google Scholar]
- N’Guessan PD, Etouem MO, Schmeck B, Hocke AC, Scharf S, et al. Legionella pneumophila -induced PKCalpha-, MAPK-, and NF-kappaB-dependent COX-2 expression in human lung epithelium. Am J Physiol Lung Cell Mol Physiol. 2007;292:L267–L277. doi: 10.1152/ajplung.00100.2006. [DOI] [PubMed] [Google Scholar]
- Nagai H, Kagan JC, Zhu X, Kahn RA, Roy CR. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science. 2002;295:679–682. doi: 10.1126/science.1067025. [DOI] [PubMed] [Google Scholar]
- Natoli G. Control of NF-kappaB-dependent transcriptional responses by chromatin organization. Cold Spring Harb Perspect Biol. 2009;1:a000224. doi: 10.1101/cshperspect.a000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninio S, Zuckman-Cholon DM, Cambronne ED, Roy CR. The Legionella IcmS-IcmW protein complex is important for Dot/Icm-mediated protein translocation. Mol Microbiol. 2005;55:912–926. doi: 10.1111/j.1365-2958.2004.04435.x. [DOI] [PubMed] [Google Scholar]
- O’Connor TJ, Boyd D, Dorer MS, Isberg RR. Aggravating genetic interactions allow a solution to redundancy in a bacterial pathogen. Science. 2012;338:1440–1444. doi: 10.1126/science.1229556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, et al. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- Patterson KI, Brummer T, O’Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- Ray LB, Sturgill TW. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci U S A. 1988;85:3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retzlaff C, Yamamoto Y, Okubo S, Hoffman PS, Friedman H, et al. Legionella pneumophila heat-shock protein-induced increase of interleukin-1 beta mRNA involves protein kinase C signalling in macrophages. Immunology. 1996;89:281–288. doi: 10.1046/j.1365-2567.1996.d01-735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf S, Hippenstiel S, Flieger A, Suttorp N, N’Guessan PD. Induction of human beta-defensin-2 in pulmonary epithelial cells by Legionella pneumophila : involvement of TLR2 and TLR5, p38 MAPK, JNK, NF-kappaB, and AP-1. Am J Physiol Lung Cell Mol Physiol. 2010;298:L687–L695. doi: 10.1152/ajplung.00365.2009. [DOI] [PubMed] [Google Scholar]
- Segall JE, Kuspa A, Shaulsky G, Ecke M, Maeda M, et al. A MAP kinase necessary for receptor-mediated activation of adenylyl cyclase in Dictyostelium. J Cell Biol. 1995;128:405–413. doi: 10.1083/jcb.128.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Case CL, Archer KA, Nogueira CV, Kobayashi KS, et al. Type IV secretiondependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila. PLoS Pathog. 2008;4:e1000220. doi: 10.1371/journal.ppat.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Denis A, Caouras V, Gervais F, Descoteaux A. Role of protein kinase C-alpha in the control of infection by intracellular pathogens in macrophages. J Immunol. 1999;163:5505–5511. [PubMed] [Google Scholar]
- Susa M, Marre R. Legionella pneumophila invasion of MRC-5 cells induces tyrosine protein phosphorylation. Infect Immun. 1999;67:4490–4498. doi: 10.1128/iai.67.9.4490-4498.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swantek JL, Tsen MF, Cobb MH, Thomas JA. IL-1 receptor-associated kinase modulates host responsiveness to endotoxin. J Immunol. 2000;164:4301–4306. doi: 10.4049/jimmunol.164.8.4301. [DOI] [PubMed] [Google Scholar]
- Tachado SD, Samrakandi MM, Cirillo JD. Non-opsonic phagocytosis of Legionella pneumophila by macrophages is mediated by phosphatidylinositol 3-kinase. PLoS ONE. 2008;3:e3324. doi: 10.1371/journal.pone.0003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu R, Takeshima E, Ishikawa C, Yamamoto K, Teruya H, et al. Inhibition of Akt/GSK3beta signalling pathway by Legionella pneumophila is involved in induction of T-cell apoptosis. Biochem J. 2010;427:57–67. doi: 10.1042/BJ20091768. [DOI] [PubMed] [Google Scholar]
- Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem J. 2003;376:545–552. doi: 10.1042/BJ20031406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trosky JE, Mukherjee S, Burdette DL, Roberts M, McCarter L, et al. Inhibition of MAPK signaling pathways by VopA from Vibrio parahaemolyticus. J Biol Chem. 2004;279:51953–51957. doi: 10.1074/jbc.M407001200. [DOI] [PubMed] [Google Scholar]
- Ubersax JA, Ferrell JE., Jr Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- Vardarova K, Scharf S, Lang F, Schmeck B, Opitz B, et al. PKC(alpha) and PKC(epsilon) differentially regulate Legionella pneumophila -induced GM-CSF. Eur Respir J. 2009;34:1171–1179. doi: 10.1183/09031936.00171908. [DOI] [PubMed] [Google Scholar]
- Venkataraman C, Gao LY, Bondada S, Kwaik YA. Identification of putative cytoskeletal protein homologues in the protozoan host Hartmannella vermiformis as substrates for induced tyrosine phosphatase activity upon attachment to the Legionnaires’ disease bacterium, Legionella pneumophila. J Exp Med. 1998;188:505–514. doi: 10.1084/jem.188.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman C, Haack BJ, Bondada S, Abu Kwaik Y. Identification of a Gal/GalNAc lectin in the protozoan Hartmannella vermiformis as a potential receptor for attachment and invasion by the Legionnaires’ disease bacterium. J Exp Med. 1997;186:537–547. doi: 10.1084/jem.186.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman C, Kwaik YA. Signal transduction in the protozoan host Hartmannella vermiformis upon attachment to Legionella pneumophila. Microbes Infect. 2000;2:867–875. doi: 10.1016/s1286-4579(00)00387-7. [DOI] [PubMed] [Google Scholar]
- Ventura C, Maioli M. Protein kinase C control of gene expression. Crit Rev Eukaryot Gene Expr. 2001;11:243–267. [PubMed] [Google Scholar]
- Verstrepen L, Bekaert T, Chau TL, Tavernier J, Chariot A, et al. TLR-4, IL-1R and TNF-R signaling to NF-kappaB: variations on a common theme. Cell Mol Life Sci. 2008;65:2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, et al. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem Biophys Res Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- Weinstein SL, Sanghera JS, Lemke K, DeFranco AL, Pelech SL. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J Biol Chem. 1992;267:14955–14962. [PubMed] [Google Scholar]
- Welsh CT, Summersgill JT, Miller RD. Increases in c-Jun N-terminal kinase/stressactivated protein kinase and p38 activity in monocyte-derived macrophages following the uptake of Legionella pneumophila. Infect Immun. 2004;72:1512–1518. doi: 10.1128/IAI.72.3.1512-1518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Liu M. Atypical protein kinase C in cell motility. Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-1192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Zhou B, Xie L. Modulation of protein kinase signaling by protein phosphatases and inhibitors. Pharmacol Ther. 2002;93:307–317. doi: 10.1016/s0163-7258(02)00199-7. [DOI] [PubMed] [Google Scholar]
- Zhu JW, Brdicka T, Katsumoto TR, Lin J, Weiss A. Structurally distinct phosphatases CD45 and CD148 both regulate B cell and macrophage immunoreceptor signaling. Immunity. 2008;28:183–196. doi: 10.1016/j.immuni.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Banga S, Tan Y, Zheng C, Stephenson R, et al. Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PLoS ONE. 2011;6:e17638. doi: 10.1371/journal.pone.0017638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckman DM, Hung JB, Roy CR. Pore-forming activity is not sufficient for Legionella pneumophila phagosome trafficking and intracellular growth. Mol Microbiol. 1999;32:990–1001. doi: 10.1046/j.1365-2958.1999.01410.x. [DOI] [PubMed] [Google Scholar]