Abstract
Control of gene expression following activation of membrane receptors results from the regulation of intracellular signaling pathways and transcription factors. Accordingly, research to elucidate the regulatory control circuits and cellular data processing mechanisms focuses on intracellular mechanisms. While autocrine and paracrine signaling are acknowledged in endocrinology, secreted factors are not typically recognized as fundamental components of the pathways connecting cell surface receptors to gene control in the nucleus. Studies of the gonadotrope suggest that extracellular regulatory loops may play a central role in the regulation of gonadotropin gene expression by gonadotropin-releasing hormone (GnRH) receptor activation. We review emerging evidence for this phenomenon, which we refer to as exosignaling, in gonadotropin gene control and in other receptor-mediated signaling systems. We propose that basic signaling circuit modules controlling gene expression can be seamlessly distributed across intracellular and exosignaling components that together orchestrate the precise physiological control of gene expression.
Keywords: exosignaling, GnRH, autocrine, prostaglandins, PACAP, inhibin α
1. Introduction
Gonadotropin-releasing hormone (GnRH) controls mammalian reproductive function through the stimulation of the synthesis and production of pituitary gonadotropins luteinizing hormone (LH) and follicle stimulating hormone (FSH). GnRH is secreted in brief pulses of several minutes duration and the pattern of pulse secretion controls the differential synthesis and secretion of pituitary gonadotropins throughout the various stages of human reproductive development, e.g. puberty, and during women’s reproductive cycle (Marshall et al., 2001,Marshall and Kelch, 1986).
FHSβ is largely constitutively secreted and its physiological control largely occurs at the level of gene expression (Bernard et al., 2010). One salient and puzzling aspect of the gonadotrope signaling system is the preferential induction of FSHβ gene expression by low frequency GnRH pulses. Despite detailed studies of the gonadotrope signaling pathways (Fink et al., 2010) and various proposed mechanisms for frequency decoding (Ciccone et al., 2010,Mistry et al., 2011,Reddy et al., 2012,Thompson et al., 2013,Tsutsumi et al., 2010), the mechanisms by which the gonadotrope recognizes low frequency GnRH pulses and in response augments FSHβ gene synthesis are incompletely understood.
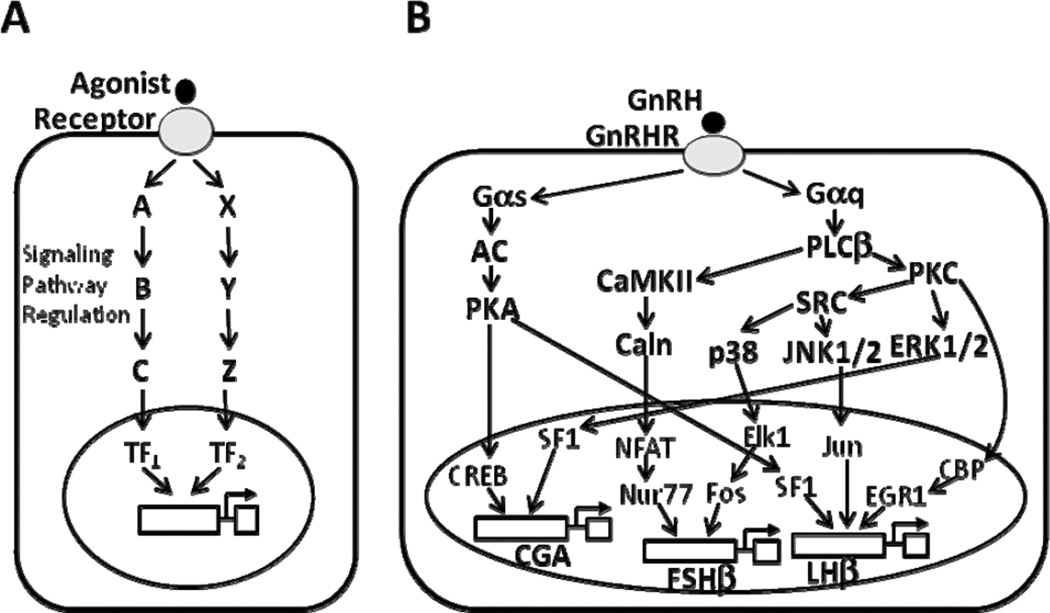
Implicit in most of the research in this area for the past several decades is the reasonable assumption that the major pathways mediating the control of gonadotropin subunit gene transcription in response to GnRH acting at cell surface receptors consist of intracellular and nuclear components (Fig. 1A). This standard paradigm has largely guided research in the field for decades and has led to detailed insight into the complex signaling pathways and transcription factors involved in gonadotropin gene regulation (Fink et al., 2010,Naor, 2009). The GnRH receptor (GnRHR) is a member of the rhodopsin-like G protein-coupled receptor (GPCR) family (Sealfon et al., 1997), and among its downstream components are phospholipase Cβ (PLCβ), with the activation of protein kinase C (PKC) and calcium/calmodulin-dependent protein kinase II (CaMKII) on the one hand, and the protein kinase A (PKA)-dependent pathway on the other hand. Additionally, PKC activates mitogen-activated protein kinase (MAPK)-dependent signaling cascades. All of these pathways are implicated in the regulation of gonadotropin subunit gene expression (Fig. 1B). Despite decades of intense research, however, a convincing explanation for key physiologically important aspects of GnRH signal processing, such as the low pulse-frequency sensitivity of the FSHβ gene, has not been established.
Fig. 1. Model of plasma membrane receptor-mediated intracellular signaling.
A, General schematic of the classical view of cell surface receptor-mediated intracellular signaling. Following the binding of an agonist to its cell surface receptor, the receptor activates several cytoplasmic signaling cascades (A, B, C, and X, Y, Z, respectively) that lead to the activation of transcription factors (TF1 and TF2, respectively), which in turn stimulate the transcription of target gene(s) in the nucleus. B, Schematic representation of the GnRH receptor intracellular signaling network. This simplified view illustrates the activation of multiple intracellular signaling cascades by the GnRH receptor (GnRHR) in response to GnRH stimulus, ultimately resulting in the induction of gonadotropin subunit gene expression in the nucleus via various transcription factors, as shown. The GnRHR is coupled to Gαq and Gαs proteins, which subsequently activate phospholipase Cβ (PLCβ) and adenylate cyclase (AC), respectively. Stimulation of AC leads to protein kinase A (PKA) activation, which notably phosphorylates cAMP responsive element binding protein (CREB). Stimulation of PLCβ leads to activation of the calcium/calmodulin-dependent protein kinase II (CaMKII)-dependent pathway and of protein kinase C (PKC), which itself mediates the activation of various mitogen-activated protein kinase (MAPK) cascades: extracellular signal-regulated kinase (ERK), jun-N-terminal kinase (JNK), and p38. Caln, calcineurin; CBP, CREB-binding protein; CGA, common α- glycoprotein subunit; EGR1, early growth response 1; FSHβ, follicle-stimulating hormone β-subunit; LHβ, luteinizing hormone β-subunit; NFAT, nuclear factor of activated T cells; SF1, steroidogenic factor 1.
There is now emerging evidence that extracellular regulatory loops acting in an autocrine/paracrine fashion participate in creating the basic regulatory motifs controlling gonadotropin gene expression, and thus may be critical in the control of reproductive functions. The role of extracellular factors in modulating intracellular responses has previously been referred to as “inside-out signaling” (Naor et al., 2007). Because this phrase has been used to describe several unrelated concepts in the research literature, we introduce the term exosignaling to refer to the extracellular location of regulatory components that behave in a fashion analogous to that of intracellular signaling components to regulate intracellular processes. We review several examples of exosignaling in various signaling systems and in the gonadotrope.
2. Autoreceptors mediate negative feedback loops in the central nervous system
The nervous system has evolved the most specialized mechanisms for complex information transmission and processing. Local synaptic signaling, which largely relies on neurotransmitter release, can be conceptualized as a special example of paracrine and autocrine signaling. The release of neurotransmitters from neuronal terminals, which regulate receptors on nearby neurons, is analogous to paracrine signaling. Many released transmitters regulate autoreceptors located on terminals, which is similar to autocrine signaling outside of the nervous system and is an example of exosignaling.
Autoreceptors are recognized to play important regulatory roles for nearly all neurotransmitters. One particularly well characterized example is the dopamine autoreceptor. Dopamine signaling in the central nervous system is involved in many aspects of behavior, including locomotion and reward (for review, see (Missale et al., 1998)). Dopamine releasing neurons express D2 type dopamine autoreceptors that exert negative feedback regulation to reduce dopamine neuron firing and dopamine synthesis and release and to regulate dopamine reuptake (Fig. 2A) (Bello et al., 2011,Bolan et al., 2007,Jones et al., 1999,Zhang and Sulzer, 2012). This regulatory mechanism has been implicated in normal and pathophysiological behaviors as well as in the effects of therapeutic drugs (Jones et al., 1999) (Sotnikova et al., 2006).
Fig. 2. Examples of other exosignaling systems.
A, Dopamine D2 autoreceptor (D2R)-mediated negative feedback loop. Following its presynaptic release through exocytosis, dopamine (DA) binds to D2R, which in turn inhibits the synthesis and release of DA as well as stimulates DA reuptake by plasma membrane DA transporters (DAT). B, Sphingosine-1-phosphate (S1P) secretion as a mediator of growth factors’ actions. Platelet-derived growth factor receptor (PDGFR) stimulates sphingosine kinase (SphK1), which promotes the formation and secretion of S1P. Secreted S1P binds to its receptor, S1P1 in an autocrine/paracrine manner, which leads to activation of the small GTPase Rac and subsequent increased cell motility.
3. Sphingosine-1-phosphate, an autocrine/paracrine mediator of the actions of cytokines and growth factors
A number of agonists activate sphingosine kinases that catalyze the formation of sphingosine-1-phosphate (S1P), a sphingolipid metabolite; in turn, S1P can either function intracellularly as a second messenger, or be secreted outside the cell and act in an autocrine/paracrine manner to activate S1P receptors present on the surface of the same cells or adjacent neighboring cells (for reviews, see (Alvarez et al., 2007,Takabe et al., 2008)). S1P has been implicated in a wide variety of biological responses, including cell proliferation, survival and motility (Spiegel and Milstien, 2003). Interestingly, secreted S1P regulates the effects of many growth factors and cytokines (for review, see (Takabe et al., 2008)). Because S1P may be implicated in various diseases, including cancer, inflammation, and autoimmune disorders, S1P has been proposed as a target for the treatment of these illnesses.
S1P can be released by platelet-derived growth factor (PDGF) receptor activation. PDGF induces cell migration in human arterial smooth muscle cells (Bornfeldt et al., 1995) and in mouse embryonic fibroblasts (Goparaju et al., 2005). The activation of S1P receptors triggers an intracellular signaling cascade that is crucial for cell motility (Fig. 2B). Although a role for S1P in the gonadotropes has not been described thus far, a recent study showed that the lipid mediator activated porcine Gpr3, whose expression was detected notably in the pituitary (Zhang et al., 2012). These intriguing results suggest that S1P could be active in the gonadotropes.
4. Matrix metalloproteinase-dependent transactivation of epidermal growth factor receptor by G-protein-coupled receptors: a paradigm in GnRHR signaling
About a decade ago, GPCRs were found to transactivate receptor tyrosine kinases, principally the epidermal growth factor receptor (EGFR) (Kalmes et al., 2001). EGFR transactivation by GPCRs occurs in different cell types and is mediated by heparin-binding EGF-like growth factor (HB-EGF). An example of multiple cascades of exosignaling was described in breast cancer cells, where estradiol (E2) induced the release of S1P, which consecutively activated its receptor, leading to EGFR transactivation in a matrix metalloproteinase (MMP)-dependent manner (Sukocheva et al., 2006). The coordination of multiple intracellular signaling and exosignaling processes illustrates how cellular information processing can be distributed across intracellular and extracellular pathways (Fig. 3A).
Fig. 3. Model of plasma membrane receptor-mediated exosignaling.
A, General schematic of the concept of exosignaling. In addition to intracellular signaling pathways activated by a cell surface receptor in response to agonist stimulation (see Fig. 1A), the receptor promotes the secretion of autocrine/paracrine factors, e.g. activator(s) and inhibitor(s), which regulate the transcription of target gene(s) in the nucleus via specific cell membrane receptors and the downstream activation of intracellular signaling mediators and transcription factor(s) (e.g. TF3). B, Schematic view of GnRHR exosignaling mechanisms. Besides the intracellular signaling pathways activated by the GnRHR in response to GnRH stimulation (see Fig. 1B), the receptor promotes the secretion of matrix metalloproteinases 2/9 (MMP2/9), pituitary adenylate cyclase-activating polypeptide (PACAP), prostaglandins (PGs), and inhibin α(INHA), which in turn regulate the transcription of gonadotropin subunit genes and the GnRHR gene in an autocrine/paracrine fashion. MMPs mediate the transactivation of epidermal growth factor receptor (EGFR) by the GnRHR, which may result in the modulation of gonadotropin subunit genes (dashed line); PACAP stimulates LHβ and FSHβ gene expression via its receptor PAC1R. PGs inhibit GnRHR and LHβgene expression via their receptors (PG Rc). INHA, which is activated by the GnRHR via Gαs, prevents activin from binding to its activin type II receptor (ActRII), thereby inhibiting FSHβ gene expression. HB-EGF, heparin-binding EGF-like growth factor.
In pituitary gonadotropes, binding of GnRH to its receptor stimulates the PKC signaling pathway, which induces the release of active MMP2 and MMP9, which in turn mediate EGFR transactivation (Roelle et al., 2003) (Fig. 3B). Because EGFR transactivation leads to engagement of the extracellular signal-regulated kinase (ERK) cascade and subsequent induction of early genes c-Fos and c-Jun, MMPs play a role in GnRH signaling and the regulation of reproductive functions. Similarly, in GnRH-producing hypothalamic neurons (GT1-7 neurons), activation of ERK1/2 in response to GnRHR stimulation depends on sequential activation of PKC, MMPs, and EGFR transactivation (for a more extensive review, see (Shah and Catt, 2004)).
5. Crosstalk between GnRHR and prostaglandin receptors
The release of prostaglandins (PGs) following GnRHR activation is an exosignaling gonadotrope pathway identified several years earlier. GnRH was shown to stimulate PG synthesis in the gonadotrope via induction of COX-2 by the PKC/MAPK-dependent pathway. In turn, the released PGs inhibited GnRHR gene expression and selectively reduced GnRH-induced LHβ gene expression in an autocrine/paracrine fashion (Fig. 3B; (Naor et al., 2007,Naidich et al., 2010). The transcriptional regulation of GnRHR and gonadotropin subunit genes was analyzed by transfecting immortalized gonadotropes with the corresponding promoter constructs. The inhibitory effects of PGs on LH (but not FSH) secretion were confirmed ex-vivo in rat pituitaries. Additionally, PGs inhibited GnRHR gene expression through inhibition of phosphoinositide turnover. Hence, locally produced PGs may contribute to the differential expression of gonadotropin subunit genes.
6. PACAP, an autocrine factor potentially involved in GnRH pulse frequency-induced FSHβ expression
An important and puzzling aspect of gonadotrope signaling is the differential induction of the gonadotropin genes by different pulse frequencies of GnRH stimulation. GnRH is released in brief pulses by the hypothalamus. Increasing GnRH pulse frequency stimulation leads to increasing levels of LHβ mRNA expression. FSHβ mRNA, in contrast, is preferentially induced by low frequency GnRH pulses, at an interval of about one pulse every 2 h in mouse and rat gonadotrope cells (Bedecarrats and Kaiser, 2003,Kaiser et al., 1997). This pattern of gene control is important for maintaining normal reproductive physiology.
Several signaling components and transcription factors have been identified as possibly contributing to the GnRH frequency-dependent gene regulation. The GnRHR can activate both Gαq/11 (Grosse et al., 2000,Hsieh and Martin, 1992,Naor et al., 1986) and Gαs (Tsutsumi et al., 2010,Liu et al., 2002) in response to GnRH stimulation. While Gαq pathway undergoes desensitization in response to pulse stimulation, the Gαs-cyclic adenosine monophosphate (cAMP)-PKA-dependent pathway is more sensitive to high GnRH pulse frequency (Tsutsumi et al., 2010). Differential expression of AP1 factors at slow GnRH pulses and of corepressors SKIL and TGIF1 at faster pulses has been proposed to mediate the sensitivity of FSHβ gene to GnRH pulse frequency (Mistry et al., 2011). On the other hand, the PKA-dependent signaling cascade has been shown to mediate GnRH activation of cAMP responsive element binding protein (CREB) at low pulse frequency, thus resulting in the stimulation of FSHβ transcription (Thompson et al., 2013). Data from the same group suggest that cAMP early repressor (ICER) counteracts the preferential stimulation of FSHβ gene expression at high GnRH pulse frequency by reducing CREB occupation of the rat FSHβ promoter (Ciccone et al., 2010). Another study reports that during low GnRH pulse frequency stimulation, GnRH induces c-Fos phosphorylation, which prolongs c-Fos half-life and increases its transcriptional activity, thereby causing stronger activation of FSHβ expression (Reddy et al., 2012).
According to other studies, differences in ERK phosphorylation pattern in response to distinct GnRH pulse regimes are linked to different expression patterns of the MAPK phosphatase MKP1 following high vs. low frequencies of GnRH pulse stimulation; this mechanism may also contribute to the differential control of gonadotropin subunit expression. Initial observations by Kanasaki et al. revealed distinct patterns of ERK1/2 phosphorylation in response to slow vs. fast GnRH pulses, suggesting the involvement of an ERK dephosphorylation enzyme working in a different way under each condition (Kanasaki et al., 2005). Later, the feedback activity of MKP1, which inactivates ERK by dephosphorylation, was shown to modulate GnRH-induced ERK activation and gonadotropin response to GnRH (Lim et al., 2009,Nguyen et al., 2010). MKP1 was mainly expressed following high frequency GnRH pulses, suggesting its implication in the GnRH pulse frequency-dependent regulation of gonadotropin subunit genes (Purwana et al., 2011).
Although these studies have been enlightening, most have not established a clear link between sensitivity of the endogenous FSHβ gene to GnRH pulse frequency and the various candidates. Notably, several exosignaling regulatory mechanisms have been implicated in gonadotropin gene regulation and GnRH frequency-sensitivity.
Pituitary adenylate cyclase-activating polypeptide (PACAP) was originally isolated from ovine hypothalami as a stimulator of cAMP in pituitary cells (Miyata et al., 1989). The peptide was later shown to be produced in pituitary gonadotropes (Koves et al., 1998,Moore et al., 2005). PACAP acts alone or in synergy with GnRH to stimulate LH and FSH gene expression and secretion in rat pituitary cells (Culler and Paschall, 1991). In LβT2 pituitary gonadotropes, GnRH stimulates PACAP and PACAP type 1 receptor (PAC1R) expression (Grafer et al., 2009) (Fig. 3B), and lower frequencies of GnRH pulses stimulate higher expression levels of PACAP and its receptor than higher frequencies (Kanasaki et al., 2011). Additionally, a PACAP antagonist inhibits GnRH-induced FSHβ mRNA expression, suggesting that PACAP acts as an autocrine regulator of GnRH pulse frequency-dependent gonadotropin expression (Kanasaki et al., 2011,Kanasaki et al., 2012). Therefore, locally produced PACAP may be involved in the fine-tuning of gonadotrope function, which is necessary for normal reproductive function.
7. Feedback suppression of FSHβ expression by inhibin α in response to GnRH
An additional member of the exosignaling network implicated in the regulation of gonadotropin expression is inhibin α (INHA) (Bilezikjian et al., 2004,Choi et al., 2012). Gαs activation by GnRH was recently reported to promote LHβ expression and to suppress FSHβ expression in LβT2 cells (Choi et al., 2012). The differential effects of Gαs activation on gonadotropin subunit expression were mediated via the secretion of autocrine/paracrine factors that included INHA (Fig. 3B). Notably, the induction of INHA expression showed a GnRH pulse frequency sensitivity that was opposite to that of FSHβ. Whereas FSHβ was preferentially induced by low frequency GnRH pulses of ~ one 5-min pulse every 2 h, INHA was preferentially induced by high frequency pulses. Thus, at higher frequencies the inhibitory autocrine suppression of FSHβ by INHA would be increased, and this could contribute to the lower frequency preference for maximizing FSHβ expression. This study also demonstrated that conditioned media from LβT2 gonadotrope cells in which Gαs had been reduced by siRNA had a stimulatory effect on FSHβ expression. Exosignaling pathways involving MMPs, PGs, PACAP, and INHA likely represent only part of the complex extracellular signaling systems contributing to gonadotrope function. We hypothesize that this exosignaling network and intracellular components form the regulatory signaling modules underlying the characteristic gene regulatory responses of the gonadotrope.
8. Conclusions and future perspectives
Overall, the literature suggests that exosignaling is an important component of gonadotrope signaling. Many of the regulatory pathways needed to understand GnRH frequency decoding and gonadotropin gene regulation may be outside the cell. Emerging studies in gonadotropes reveal a surprisingly dense exosignaling network comprising MMPs, PGs, PACAP, INHA and other factors that are beginning to rival the intracellular pathways in complexity. In transmitting information from the cell surface GnRHR to the regulatory apparatus of gonadotropin genes, the cell is not restricted by cell membrane boundaries and uses a data processing network that is distributed across both the intracellular and extracellular space.
There are potential advantages in the use of exosignaling mechanisms to regulate gonadotropin genes. Exosignaling may help coordinate responses across cells. Exosignaling may also increase the robustness of the gonadotrope responses in the presence of random fluctuations in the GnRH secretion pattern. Intriguingly, exosignaling may act in concert with steroid regulation of gonadotropins. For instance, activins and androgens synergistically regulate FSHβ gene expression, presumably via an interaction between SMAD3 and the androgen receptor (Hayes et al., 2001,Thackray and Mellon, 2008).
Exosignaling regulatory pathways are not restricted to the gonadotrope and may prove to be important in various endocrine systems. The evolution of this distributed signaling system provides useful benefits in coordinating behavior across cells. The paradigm that cell signaling occurs inside and outside the cell may lead to an improved understanding of how cells process information and to the identification of new therapeutic targets. In the gonadotrope, much work remains to characterize more components of this extracellular network, to determine which intracellular and extracellular elements are most important for particular aspects of gonadotropin gene regulation and to establish an experimentally validated model of the control of gonadotropin gene expression via intracellular and extracellular regulatory pathways.
Highlights.
-
-
The existence of a complex regulatory network of locally secreted factors has implications for physiology and pathophysiology.
-
-
Exosignaling is emerging as a novel key mechanism in the regulation of gonadotropin expression.
-
-
Exosignaling may play an important role in GnRH pulse frequency decoding by the gonadotrope.
Acknowledgements
Our gonadotrope research is supported by the National Institute of Health Grant DK46943.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- FSH
follicle stimulating hormone
- GnRH
gonadotropin-releasing hormone
- GnRHR
gonadotropin-releasing hormone receptor
- GPCR
G protein-coupled receptor
- INHA
inhibin α
- LH
luteinizing hormone
- MAPK
mitogen-activated protein kinase
- MKP
mitogen-activated protein kinase phosphatase 1
- MMP
matrix metalloproteinase
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PGs
prostaglandins
- PKA
protein kinase A
- PKC
protein kinase C
- S1P
sphingosine-1-phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hanna Pincas, Email: hanna.pincas@mssm.edu.
Soon Gang Choi, Email: soon-gang.choi@mssm.edu.
Qian Wang, Email: qian.wang@mssm.edu.
Jingjing Jia, Email: jingjing.jia@mssm.edu.
Judith L. Turgeon, Email: jlturgeon@ucdavis.edu.
Stuart C. Sealfon, Email: stuart.sealfon@mssm.edu.
References
- 1.Marshall JC, Eagleson CA, McCartney CR. Hypothalamic dysfunction. Mol Cell Endocrinol. 2001;183:29–32. doi: 10.1016/s0303-7207(01)00611-6. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JC, Kelch RP. Gonadotropin-releasing hormone: role of pulsatile secretion in the regulation of reproduction. N Engl J Med. 1986;315:1459–1468. doi: 10.1056/NEJM198612043152306. [DOI] [PubMed] [Google Scholar]
- 3.Bernard DJ, Fortin J, Wang Y, Lamba P. Mechanisms of FSH synthesis: what we know, what we don't, and why you should care. Fertil Steril. 2010;93:2465–2485. doi: 10.1016/j.fertnstert.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Fink MY, Pincas H, Choi SG, Nudelman G, Sealfon SC. Research resource: Gonadotropin-releasing hormone receptor-mediated signaling network in LbetaT2 cells: a pathway-based web-accessible knowledgebase. Mol Endocrinol. 2010;24:1863–1871. doi: 10.1210/me.2009-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciccone NA, Xu S, Lacza CT, Carroll RS, Kaiser UB. Frequency-dependent regulation of follicle-stimulating hormone beta by pulsatile gonadotropin-releasing hormone is mediated by functional antagonism of bZIP transcription factors. Mol Cell Biol. 2010;30:1028–1040. doi: 10.1128/MCB.00848-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mistry DS, Tsutsumi R, Fernandez M, Sharma S, Cardenas SA, Lawson MA, Webster NJ. Gonadotropin-releasing hormone pulse sensitivity of follicle-stimulating hormone-beta gene is mediated by differential expression of positive regulatory activator protein 1 factors and corepressors SKIL and TGIF1. Mol Endocrinol. 2011;25:1387–1403. doi: 10.1210/me.2011-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy GR, Xie C, Lindaman LL, Coss D. GnRH Increases c-Fos Half-Life Contributing to Higher FSHbeta Induction. Mol Endocrinol. 2012 doi: 10.1210/me.2012-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson IR, Ciccone NA, Xu S, Zaytseva S, Carroll RS, Kaiser UB. GnRH Pulse Frequency-Dependent Stimulation of FSHbeta Transcription Is Mediated via Activation of PKA and CREB. Mol Endocrinol. 2013;27:606–618. doi: 10.1210/me.2012-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsutsumi R, Mistry D, Webster NJ. Signaling responses to pulsatile gonadotropin-releasing hormone in LbetaT2 gonadotrope cells. J Biol Chem. 2010;285:20262–20272. doi: 10.1074/jbc.M110.132662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol. 2009;30:10–29. doi: 10.1016/j.yfrne.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Sealfon SC, Weinstein H, Millar RP. Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr Rev. 1997;18:180–205. doi: 10.1210/edrv.18.2.0295. [DOI] [PubMed] [Google Scholar]
- 12.Naor Z, Jabbour HN, Naidich M, Pawson AJ, Morgan K, Battersby S, Millar MR, Brown P, Millar RP. Reciprocal cross talk between gonadotropin-releasing hormone (GnRH) and prostaglandin receptors regulates GnRH receptor expression and differential gonadotropin secretion. Mol Endocrinol. 2007;21:524–537. doi: 10.1210/me.2006-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 14.Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, Sen N, Urizar E, Gomes I, Devi LA, Ramamoorthy S, Javitch JA, Zapata A, Shippenberg TS. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol. 2007;71:1222–1232. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- 16.Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, Caron MG. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Sulzer D. Regulation of striatal dopamine release by presynaptic auto- and heteroreceptors. Basal Ganglia. 2012;2:5–13. doi: 10.1016/j.baga.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sotnikova TD, Beaulieu JM, Gainetdinov RR, Caron MG. Molecular biology, pharmacology and functional role of the plasma membrane dopamine transporter. CNS Neurol Disord Drug Targets. 2006;5:45–56. doi: 10.2174/187152706784111579. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Takabe K, Paugh SW, Milstien S, Spiegel S. "Inside-out" signaling of sphingosine- 1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 22.Bornfeldt KE, Graves LM, Raines EW, Igarashi Y, Wayman G, Yamamura S, Yatomi Y, Sidhu JS, Krebs EG, Hakomori Sand, et al. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J Cell Biol. 1995;130:193–206. doi: 10.1083/jcb.130.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, Spiegel S. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol. 2005;25:4237–4249. doi: 10.1128/MCB.25.10.4237-4249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang BL, Li Y, Ding JH, Dong FL, Hou YJ, Jiang BC, Shi FX, Xu YX. Sphingosine 1-phosphate acts as an activator for the porcine Gpr3 of constitutively active G protein-coupled receptors. J Zhejiang Univ Sci B. 2012;13:555–566. doi: 10.1631/jzus.B1100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalmes A, Daum G, Clowes AW. EGFR transactivation in the regulation of SMC function. Ann N Y Acad Sci. 2001;947:42–54. doi: 10.1111/j.1749-6632.2001.tb03929.x. discussion 54–5; [DOI] [PubMed] [Google Scholar]
- 26.Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, Bernal A, Derian CK, Ullrich A, Vadas MA, Xia P. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol. 2006;173:301–310. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roelle S, Grosse R, Aigner A, Krell HW, Czubayko F, Gudermann T. Matrix metalloproteinases 2 and 9 mediate epidermal growth factor receptor transactivation by gonadotropin-releasing hormone. J Biol Chem. 2003;278:47307–47318. doi: 10.1074/jbc.M304377200. [DOI] [PubMed] [Google Scholar]
- 28.Shah BH, Catt KJ. Matrix metalloproteinases in reproductive endocrinology. Trends Endocrinol Metab. 2004;15:47–49. doi: 10.1016/j.tem.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Naidich M, Shterntal B, Furman R, Pawson AJ, Jabbour HN, Morgan K, Millar RP, Jia J, Tomic M, Stojilkovic S, Stern N, Naor Z. Elucidation of mechanisms of the reciprocal cross talk between gonadotropin-releasing hormone and prostaglandin receptors. Endocrinology. 2010;151:2700–2712. doi: 10.1210/en.2009-1335. [DOI] [PubMed] [Google Scholar]
- 30.Bedecarrats GY, Kaiser UB. Differential regulation of gonadotropin subunit gene promoter activity by pulsatile gonadotropin-releasing hormone (GnRH) in perifused L beta T2 cells: role of GnRH receptor concentration. Endocrinology. 2003;144:1802–1811. doi: 10.1210/en.2002-221140. [DOI] [PubMed] [Google Scholar]
- 31.Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology. 1997;138:1224–1231. doi: 10.1210/endo.138.3.4968. [DOI] [PubMed] [Google Scholar]
- 32.Grosse R, Schmid A, Schoneberg T, Herrlich A, Muhn P, Schultz G, Gudermann T. Gonadotropin-releasing hormone receptor initiates multiple signaling pathways by exclusively coupling to G(q/11) proteins. J Biol Chem. 2000;275:9193–9200. doi: 10.1074/jbc.275.13.9193. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh KP, Martin TF. Thyrotropin-releasing hormone and gonadotropin-releasing hormone receptors activate phospholipase C by coupling to the guanosine triphosphate-binding proteins Gq and G11. Mol Endocrinol. 1992;6:1673–1681. doi: 10.1210/mend.6.10.1333052. [DOI] [PubMed] [Google Scholar]
- 34.Naor Z, Azrad A, Limor R, Zakut H, Lotan M. Gonadotropin-releasing hormone activates a rapid Ca2+-independent phosphodiester hydrolysis of polyphosphoinositides in pituitary gonadotrophs. J Biol Chem. 1986;261:12506–12512. [PubMed] [Google Scholar]
- 35.Liu F, Usui I, Evans LG, Austin DA, Mellon PL, Olefsky JM, Webster NJ. Involvement of both G(q/11) and G(s) proteins in gonadotropin-releasing hormone receptor-mediated signaling in L beta T2 cells. J Biol Chem. 2002;277:32099–32108. doi: 10.1074/jbc.M203639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanasaki H, Bedecarrats GY, Kam KY, Xu S, Kaiser UB. Gonadotropin-releasing hormone pulse frequency-dependent activation of extracellular signal-regulated kinase pathways in perifused LbetaT2 cells. Endocrinology. 2005;146:5503–5513. doi: 10.1210/en.2004-1317. [DOI] [PubMed] [Google Scholar]
- 37.Lim S, Pnueli L, Tan JH, Naor Z, Rajagopal G, Melamed P. Negative feedback governs gonadotrope frequency-decoding of gonadotropin releasing hormone pulse-frequency. PLoS One. 2009;4:e7244. doi: 10.1371/journal.pone.0007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen KA, Intriago RE, Upadhyay HC, Santos SJ, Webster NJ, Lawson MA. Modulation of gonadotropin-releasing hormone-induced extracellular signal-regulated kinase activation by dual-specificity protein phosphatase 1 in LbetaT2 gonadotropes. Endocrinology. 2010;151:4882–4893. doi: 10.1210/en.2009-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purwana IN, Kanasaki H, Mijiddorj T, Oride A, Miyazaki K. Induction of dual-specificity phosphatase 1 (DUSP1) by pulsatile gonadotropin-releasing hormone stimulation: role for gonadotropin subunit expression in mouse pituitary LbetaT2 cells. Biol Reprod. 2011;84:996–1004. doi: 10.1095/biolreprod.110.088526. [DOI] [PubMed] [Google Scholar]
- 40.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 41.Koves K, Kantor O, Scammell JG, Arimura A. PACAP colocalizes with luteinizing and follicle-stimulating hormone immunoreactivities in the anterior lobe of the pituitary gland. Peptides. 1998;19:1069–1072. doi: 10.1016/s0196-9781(98)00049-7. [DOI] [PubMed] [Google Scholar]
- 42.Moore JP, Jr., Burger LL, Dalkin AC, Winters SJ. Pituitary adenylate cyclase activating polypeptide messenger RNA in the paraventricular nucleus and anterior pituitary during the rat estrous cycle. Biol Reprod. 2005;73:491–499. doi: 10.1095/biolreprod.105.041624. [DOI] [PubMed] [Google Scholar]
- 43.Culler MD, Paschall CS. Pituitary adenylate cyclase-activating polypeptide (PACAP) potentiates the gonadotropin-releasing activity of luteinizing hormone-releasing hormone. Endocrinology. 1991;129:2260–2262. doi: 10.1210/endo-129-4-2260. [DOI] [PubMed] [Google Scholar]
- 44.Grafer CM, Thomas R, Lambrakos L, Montoya I, White S, Halvorson LM. GnRH stimulates expression of PACAP in the pituitary gonadotropes via both the PKA and PKC signaling systems. Mol Endocrinol. 2009;23:1022–1032. doi: 10.1210/me.2008-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanasaki H, Purwana IN, Mijiddorj T, Oride A, Miyazaki K. Possible involvement of PACAP and PACAP type 1 receptor in GnRH-induced FSH beta-subunit gene expression. Regul Pept. 2011;167:227–232. doi: 10.1016/j.regpep.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Kanasaki H, Purwana IN, Miyazaki K. Possible Role of PACAP and its PAC1 Receptor in the Differential Regulation of Pituitary LHbeta- and FSHbeta-Subunit Gene Expression by Pulsatile GnRH Stimulation. Biol Reprod. 2012 doi: 10.1095/biolreprod.112.105601. [DOI] [PubMed] [Google Scholar]
- 47.Bilezikjian LM, Blount AL, Leal AM, Donaldson CJ, Fischer WH, Vale WW. Autocrine/paracrine regulation of pituitary function by activin, inhibin and follistatin. Mol Cell Endocrinol. 2004;225:29–36. doi: 10.1016/j.mce.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Choi SG, Jia J, Pfeffer RL, Sealfon SC. G proteins and autocrine signaling differentially regulate gonadotropin subunit expression in pituitary gonadotrope. J Biol Chem. 2012;287:21550–21560. doi: 10.1074/jbc.M112.348607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayes SA, Zarnegar M, Sharma M, Yang F, Peehl DM, ten Dijke P, Sun Z. SMAD3 represses androgen receptor-mediated transcription. Cancer Res. 2001;61:2112–2118. [PubMed] [Google Scholar]
- 50.Thackray VG, Mellon PL. Synergistic induction of follicle-stimulating hormone beta-subunit gene expression by gonadal steroid hormone receptors and Smad proteins. Endocrinology. 2008;149:1091–1102. doi: 10.1210/en.2007-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]