Abstract
OBJECTIVE
To assess racial differences in diabetes processes and intermediate outcomes of care in an internal medicine, patient-centered medical home (PCMH) group practice.
RESEARCH DESIGN AND METHODS
We conducted a retrospective cohort study of 1,457 adults with diabetes receiving care from 89 medical providers within a PCMH-designated academic practice between 1 July 2009 and 31 July 2010. We used mixed models to assess independent associations between patient race (non-Hispanic white or black) and 1) receipt of processes of care (A1C and LDL testing, foot and retinal examination, and influenza and pneumococcal vaccination) and 2) achievement of intermediate outcomes (LDL <100 mg/dL, blood pressure [BP] <140/90 mmHg, A1C <7.0% [<53 mmol/mol], and A1C >9.0% [>75 mmol/mol]), controlling for sociodemographic factors, health status, treatment intensity, and clinical continuity.
RESULTS
Compared with non-Hispanic white patients, black patients were younger, were more often single, had lower educational attainment, and were less likely to have commercial insurance. In unadjusted analyses, fewer black patients received a retinal examination and influenza vaccination during the study period or any lifetime pneumococcal vaccination (P < 0.05 [all comparisons]). Fewer black patients achieved an LDL <100 mg/dL, BP <140/90 mmHg, or A1C <7.0% (<53 mmol/mol), while more black patients had an A1C >9.0% (>75 mmol/mol) (P < 0.05 [all comparisons]). In multivariable models, black patients were less likely to receive A1C testing (odds ratio [OR] 0.57 [95% CI 0.34–0.95]) or influenza vaccination (OR 0.75 [95% CI 0.57–0.99]) or to achieve an LDL <100 mg/dL (OR 0.74 [95% CI 0.55–0.99]) or BP <140/90 mmHg (OR 0.64 [95% CI 0.49–0.84]).
CONCLUSIONS
Racial differences in processes and intermediate outcomes of diabetes care were present within this PCMH-designated practice, controlling for differences in sociodemographic, clinical, and treatment factors.
Nearly 26 million people, or 8% of the U.S. population, have diabetes, and this number is steadily increasing (1,2). Compared with non-Hispanic whites, black Americans have a higher prevalence of diabetes, and their incidence of diabetes is increasing more rapidly (1–3). Black Americans are also more likely to experience diabetes-related hospitalizations and long-term complications, such as end-stage renal disease, retinopathy, amputations, and death (1,3–9).
Evidence-based practice guidelines for processes of care (e.g., A1C testing) and treatment goals (e.g., A1C <7.0% [<53 mmol/mol]) have been developed by specialty medical societies to improve the quality of diabetes care nationally. Unfortunately, the proportion of Americans receiving guideline-concordant diabetes care is suboptimal (10), particularly among racial and ethnic minorities (8). There are multiple factors that contribute to racial disparities in the quality of diabetes care, including differences in sociodemographic characteristics (11), health literacy and numeracy (12,13), treatment intensity (14), and the overall quality of care at the facilities where medical care is provided (15,16). Several studies have shown that these disparities may be narrowing over time, as the overall quality of diabetes care improves nationally (17,18). However, this narrowing of differences appears to be more pronounced for processes of care rather than intermediate or more long-term medical outcomes (8,17).
In 2006, The Commonwealth Fund Health Care Quality Survey demonstrated an association between having access to a regular source of medical care with components of a patient-centered medical home (PCMH) and a reduction in disparities in self-reported access to care, receipt of patient-directed preventative care reminders, and lipid screening (19). Based on these findings, the PCMH model of care, which focuses on comprehensive, patient-centered care, enhanced patient access, and quality improvement, has been endorsed as a potential model of health care delivery to address racial disparities for patients with chronic diseases, such as diabetes (20). While the number of PCMH-designated practices continues to increase nationally, no study has evaluated racial differences in processes and outcomes of diabetes care within the context of a PCMH.
The goal of our study was to assess the independent associations between patient race (i.e., non-Hispanic white vs. black) and several processes and intermediate outcomes of care for patients with diabetes who were managed in a level 3 PCMH.
Research Design and Methods
We conducted a retrospective cohort study of all adults with diabetes receiving primary care within a university-based, PCMH-designated, general internal medicine practice between 1 July 2009 and 31 July 2010. The University of Pittsburgh Institutional Review Board approved this project.
Study Site, Providers, and Patients
The University of Pittsburgh General Internal Medicine practice is a level 3 PCMH that provided comprehensive primary care for 16,536 unique patients during the study period. The practice has received recognition from the National Committee for Quality Assurance for the delivery of high-quality diabetes care, as well as a distinction for multicultural health care delivery.
During the study period, 89 primary care providers (41 [46.1%] attending physicians and 48 [53.9%] internal medicine residents) managed all patients within this practice. All providers use an advanced electronic health record (EHR) that incorporates best-practice alerts and decision aids into clinical care and is inclusive of all patient data from clinic, hospital, and emergency department encounters. The clinic also provides all physicians with quarterly reports documenting their clinical performance based on clinic and national benchmarks for recommended disease prevention and management. Patients have access to same-day appointment scheduling, a 24-h telephone helpline, and encrypted physician e-mail communication. Prior to each appointment, all patients are asked to complete a computerized clinical-intake form that tracks sociodemographic and clinical characteristics (21). A multidisciplinary team, consisting of a pharmacist, social worker, and diabetes nurse educators, is available on site, and endocrinology consultation is accessible within the university health system. Providers were not compensated for clinical performance or for phone or e-mail patient communication during the study period.
Transitions from the clinic to the hospital or emergency department are facilitated by provider-to-provider phone calls, and clinic providers are alerted by e-mail if a patient has an unscheduled hospital admission or emergency department encounter. After hospital discharge, primary care providers are forwarded discharge summaries and all patients are contacted by the outpatient clinic within 48 h to schedule a postdischarge appointment.
We included patients 18 years of age or older with a diagnosis of type 1 or 2 diabetes who had at least one primary care or urgent care appointment in the practice during the study period. Patients were defined as having diabetes based on any combination of ICD-9 diagnosis codes for diabetes (250.00–250.93) or a prescription for a diabetes medication during both the study period and preceding 12 months. To ensure that patients with polycystic ovarian syndrome without diabetes were not included in the cohort, we excluded those who met criteria for diabetes based solely on the use of metformin and had an ICD-9 code for polycystic ovarian syndrome (256.4, 256.1). We also excluded patients who died during the study period, those with missing race data, and those with race other than non-Hispanic white or black owing to small numbers.
Collection of Baseline Patient and Provider Data
We abstracted all baseline patient data from the clinical, encounter, and billing components of the EHR. Patient demographics were age, sex, race, marital status, self-reported level of education, and type of medical insurance. We preferentially used self-reported race and marital status from the clinical intake form and secondarily used administrative data from the EHR to record these variables when self-reported data were missing (race from administrative data, n = 198; marital status from administrative data, n = 228). We defined our independent variable of interest as non-Hispanic white or black.
We recorded BMI from the EHR as the sole physical examination finding. We used all pertinent nondiabetes ICD-9 diagnosis codes to quantify the extent of comorbid illness using the Charlson comorbidity index (22). We also used ICD-9 codes to quantify the number of diabetes complications (range 0–4) based on the presence of retinopathy (250.5, 362.0), neuropathy (357.2, 250.6), nephropathy (250.4), and peripheral vascular disease (250.7). Finally, using the last measured value during the study period, we assessed baseline level of self-reported social support using a single item that asks about the number of individuals available to provide tangible support (23), and we assessed quality of life using the mental and physical health composite scores of the RAND 36 Survey (24).
Diabetes Treatment Intensity and Continuity of Care
We used billing and encounter data to determine the number of completed physician appointments (resident and attending), as well as appointments attended with a pharmacist or diabetes nurse educator within the practice or an endocrinologist within the health care system. We included provider status as a measure of treatment intensity based on an a priori hypothesis that residents and attending physicians might provide different levels of care through differing clinical care skills, levels of medical knowledge, time spent in clinic, or ability and efficiency to function within a PCMH. We used total number of prescribed diabetes medication classes, including oral, injectable, and insulin agents, as well as completed appointments with an endocrinologist, as additional measures of treatment intensity. To assess treatment intensity among patients with poorly controlled LDL and blood pressure (BP), we quantified the total number of prescribed lipid-lowering and antihypertensive medications for each patient with LDL ≥130 mg/dL or BP ≥140/90 mmHg, respectively.
We defined each patient’s primary care provider as the physician who was visited most often during the study period. We measured patient continuity of care based on the percentage of appointments in which a patient was treated by his or her primary care provider, rather than another physician in the practice. We also assessed health care continuity by quantifying the number of missed physician appointments and the number of emergency department visits during the study period.
Processes of Care and Intermediate Outcomes
We used clinical, laboratory, and health maintenance data from the EHR to assess diabetes processes of care and intermediate outcomes that are recommended by National Committee for Quality Assurance practice guidelines (25). Processes of care included the receipt of A1C and LDL testing, dilated retinal examination, comprehensive foot examination, and influenza vaccination during the study period and any lifetime pneumococcal vaccination. Completion of each process of care was confirmed by the presence of electronic results within the EHR or using the EHR’s health maintenance reminder system. Health maintenance data are automatically updated based on the availability of test results or are updated by physicians and medical support staff during clinical encounters if patients report having received a given preventative service.
We used the last measured value during the study period to record the following intermediate outcomes: A1C, LDL, and BP. We used thresholds of A1C <7.0% (<53 mmol/mol), LDL <100 mg/dL, and BP <140/90 mmHg to define optimal levels for glycemic, lipid, and BP control, respectively, and A1C >9.0% (>75 mmol/mol) to define poor glycemic control.
Statistical Analysis
We used Kruskal-Wallis and χ2 tests to compare racial differences in continuous and categorical variables, respectively. For each outcome, we fit a generalized mixed model with the primary independent variable of race. We identified the following covariates a priori based on clinical importance: age, sex, marital status, education, insurance type, social support, mental and physical health composite scores, BMI, total number of diabetes complications, modified Charlson comorbidity index, treatment intensity (attending vs. resident primary care provider, total number of multidisciplinary visits, and total number of prescribed diabetes medication classes), and clinical care continuity (proportion of all physician visits seen by primary care provider and total number of missed physician appointments). All generalized mixed models included a random intercept for patients’ primary care providers to accommodate for clustering effects. Because of missing values, we performed multiple imputations prior to any modeling by using the fully conditional specification method (26). We generated 10 imputed data sets and combined the modeling results across these imputed data sets to obtain final estimates. To better understand the effect of provider type (i.e., resident vs. attending) on potential racial differences in diabetes care, we performed a sensitivity analysis to evaluate the patient characteristics, treatment intensity, clinical continuity, and primary outcomes of all patients with an attending primary care provider.
We considered a two-tailed P value <0.05 statistically significant. We used SAS, version 9.1.3 (SAS Institute, Cary, NC), or STATA, version 12.0 (StataCorp, College Station, TX), for all analyses.
Results
Of the 1,618 patients with diabetes and a physician visit during the study period, 1,457 met all eligibility criteria, of whom 868 (59.6%) were non-Hispanic white and 589 (40.4%) were black (Supplementary Fig. 1).
Baseline Patient Characteristics by Race
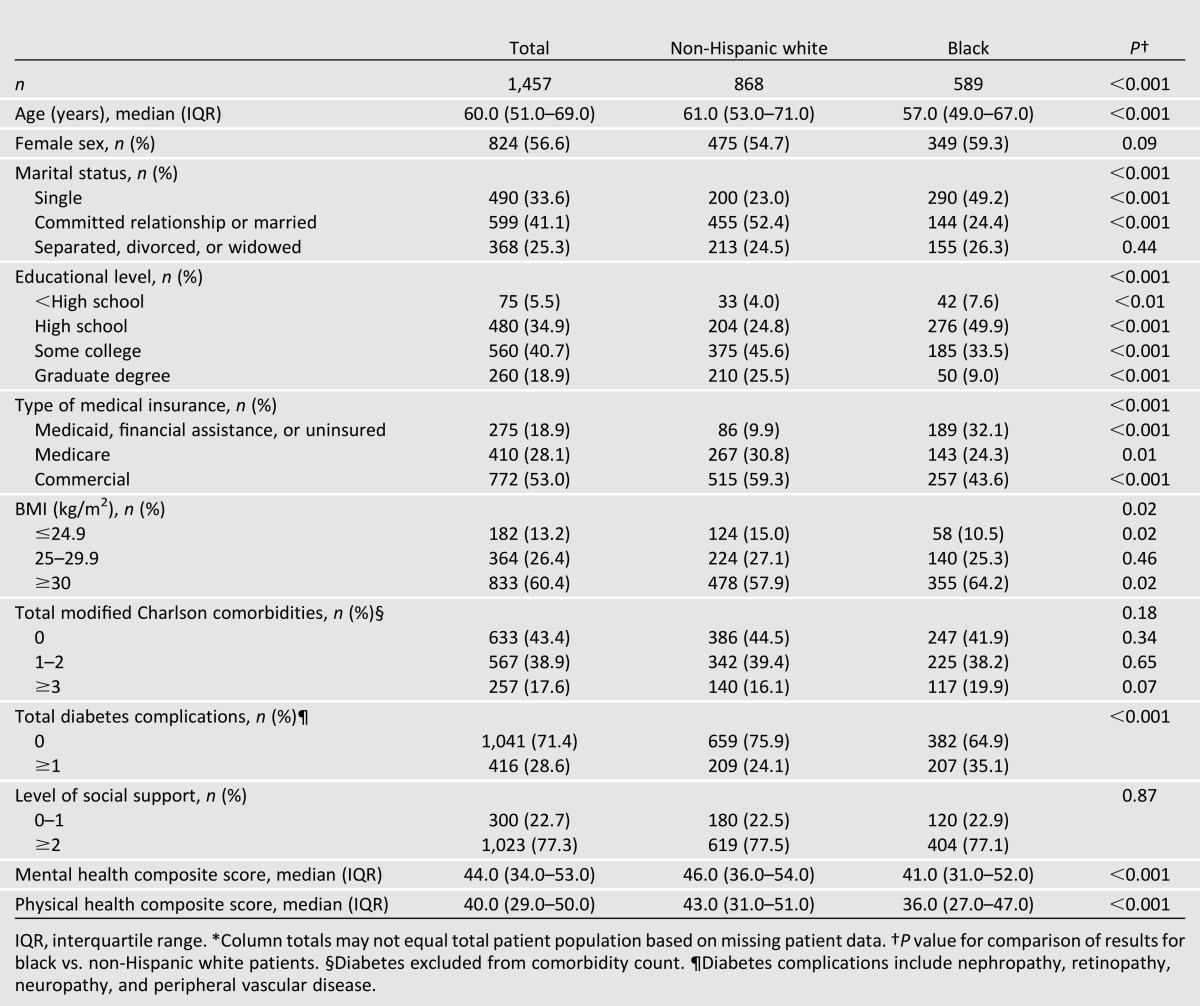
The median age of all patients was 60.0 years; 56.6% were female, 41.1% were in a committed relationship or married, 59.6% attended college or had a graduate degree, 53.0% had commercial insurance, and 86.8% had a BMI of ≥25 kg/m2 (Table 1). Compared with non-Hispanic white patients, black patients were younger (median age 57.0 vs. 61.0 years, P < 0.001), more likely to be single (49.2 vs. 23.0%, P < 0.001), and less likely to have attended college (33.5 vs. 45.6%, P < 0.001) or have commercial insurance (43.6 vs. 59.3%, P < 0.001). Black patients more often had a BMI of ≥30 kg/m2 (64.2 vs. 57.9%, P = 0.02) and had lower median SF-36 mental (41.0 vs. 46.0, P < 0.001) and physical (36.0 vs. 43.0, P < 0.001) health composite scores. Although a larger proportion of black patients had one or more diabetes complications (35.1 vs. 24.1%, P < 0.001), there were no statistically significant racial differences in the modified Charlson comorbidity index.
Table 1.
Comparison of baseline patient characteristics by race*
Diabetes Treatment Intensity and Continuity of Care by Race
Compared with non-Hispanic white patients, black patients were less likely to have an attending (rather than resident) primary care provider (69.3 vs. 94.5%, P < 0.001), had more multidisciplinary visits (mean 0.66 vs. 0.41, P < 0.001), and had fewer endocrinology visits during the study period (mean 0.29 vs. 0.46, P < 0.01) (Table 2). Although no statistically significant racial differences were observed for the total number of prescribed classes of diabetes medications, black patients were slightly more likely to be treated with insulin (32.8 vs. 28.0%, P = 0.05). Among all patients with LDL ≥130 mg/dL (n = 172), compared with non-Hispanic whites, more black patients were prescribed at least one lipid-lowering medication (75.0 vs. 59.1%, P = 0.03). Among all patients with BP ≥140/90 mmHg (n = 413), there was no racial difference in the proportion of patients who were prescribed at least one antihypertensive medication.
Table 2.
Diabetes treatment intensity and continuity of care by race during the study period
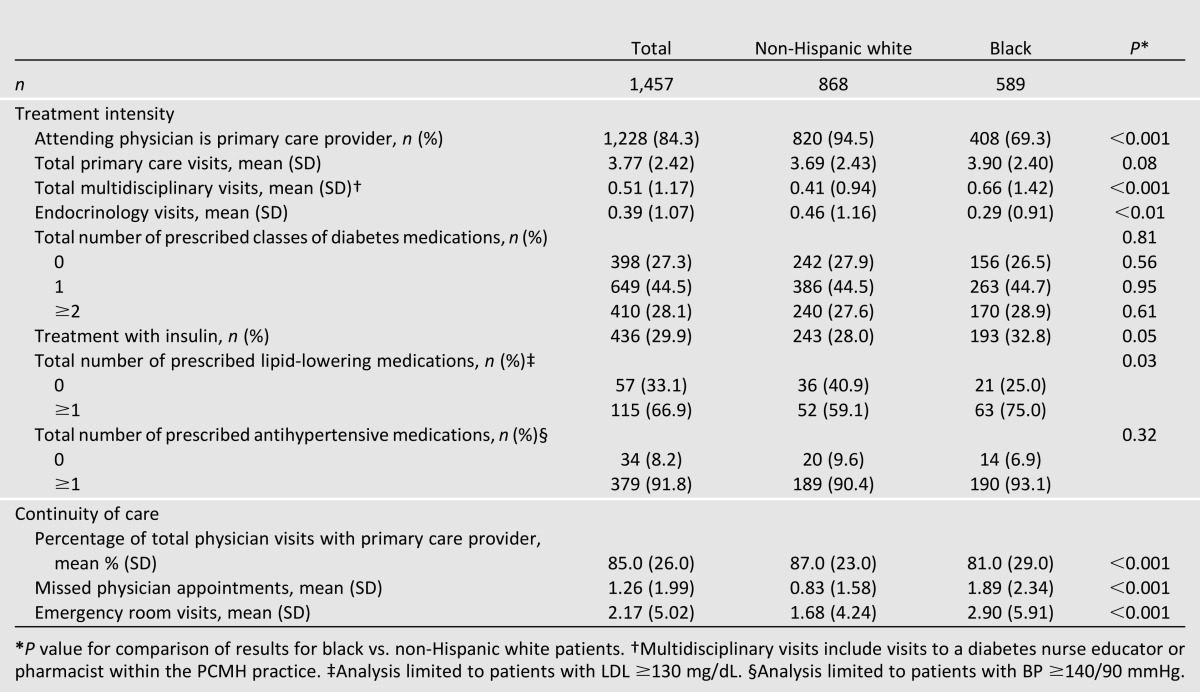
Overall, black patients had less continuity of care, with a smaller proportion of total physician visits with their primary care provider (81.0 vs. 87.0%, P < 0.001) and a larger number of missed physician appointments (mean 1.89 vs. 0.83, P < 0.001) and emergency department visits during the study period (mean 2.90 vs. 1.68, P < 0.001) (Table 2).
Diabetes Processes of Care and Intermediate Outcomes by Race
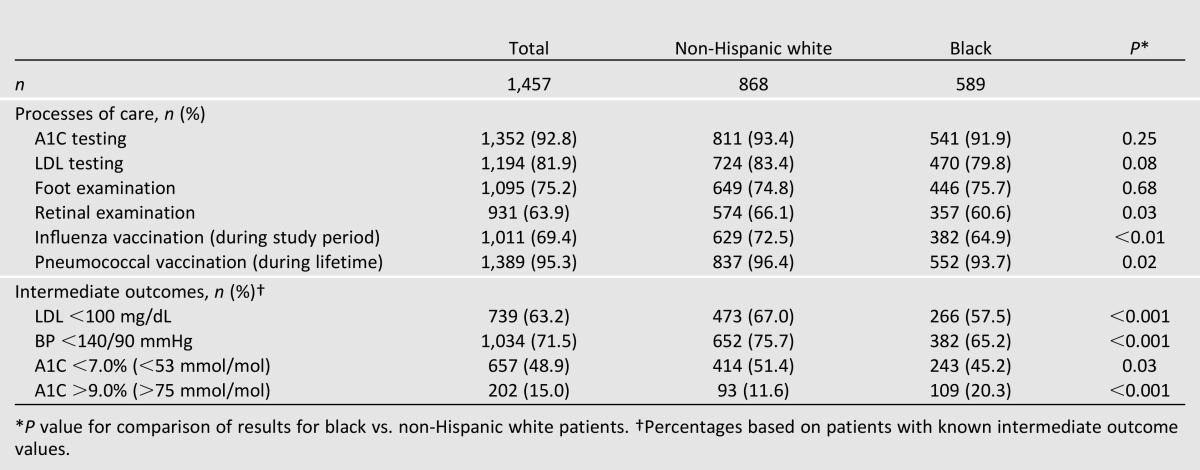
In unadjusted analyses, compared with non-Hispanic white patients, a smaller proportion of black patients received a dilated retinal examination (60.6 vs. 66.1%, P = 0.03) and influenza vaccination (64.9 vs. 72.5%, P < 0.01) during the study period, as well as any lifetime pneumococcal vaccination (93.7 vs. 96.4%, P = 0.02) (Table 3). In racial comparisons of intermediate outcomes, a smaller proportion of black patients had an LDL <100 mg/dL (57.5 vs. 67.0%, P < 0.001), BP <140/90 mmHg (65.2 vs. 75.7%, P < 0.001), and A1C<7.0% (<53 mmol/mol) (45.2% vs. 51.4%, P = 0.03), and a larger proportion of black patients had an A1C >9.0% (>75 mmol/mol) (20.3% vs. 11.6%, P < 0.001).
Table 3.
Unadjusted comparisons of diabetes processes of care and intermediate outcomes by race
In multivariable models, black patients were less likely to receive A1C testing (odds ratio [OR] 0.57 [95% CI 0.34–0.95]) and influenza vaccination during the study period (OR 0.75 [95% CI 0.57–0.99]) (Fig. 1). Black patients were also less likely to have an LDL <100 mg/dL (OR 0.74 [95% CI 0.55–0.99]) and BP <140/90 mmHg (OR 0.64 [95% CI 0.49–0.84]), though no adjusted racial differences were observed for A1C <7.0% (<53 mmol/mol) or A1C >9.0% (>75 mmol/mol).
Figure 1.
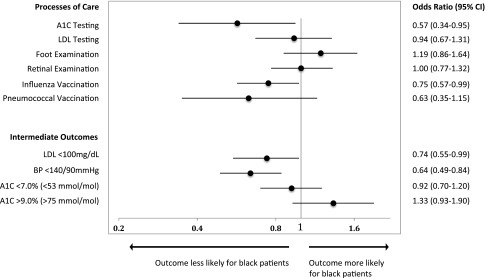
Adjusted ORs for diabetes processes and intermediate outcomes of care. Adjusted ORs for black patients represent the likelihood of receiving a process of care or achieving an intermediate outcome of diabetes care compared with non-Hispanic white patients. Black patients were less likely to receive A1C testing and influenza vaccination and were less likely to have an LDL <100 mg/dL and BP <140/90 mmHg. Model covariates include age, sex, marital status, education, insurance type, social support, mental and physical health composite scores, BMI, total number of diabetes complications, modified Charlson comorbidity index, and treatment intensity and clinical care continuity.
Variables Independently Associated With Processes of Care and Intermediate Outcomes
Complete model results for diabetes processes of care and intermediate outcomes are shown in Supplementary Tables 1 and 2, respectively. In summary, greater age, a greater number of multidisciplinary visits, the presence of at least one diabetes complication, and having a prescription for one or more classes of diabetes medications were each associated with an increased odds of receiving at least three of six processes of care. Greater age was also associated with achieving LDL <100 mg/dL and A1C <7.0% (<53 mmol/mol), while a greater number of multidisciplinary visits and the presence of diabetes complications were associated with a decreased odds of having A1C <7.0% (<53 mmol/mol) and an increased odds of having A1C >9.0% (>75 mmol/mol).
Sensitivity Analysis of Attending-Only Patients
Of 1,457 patients in the main analysis, 1,228 (84.3%) had an attending primary care provider. Baseline patient characteristics and patterns of treatment intensity and clinical continuity in this subgroup were similar compared with the cohort in the main analysis.
In unadjusted analyses of the attending-only subgroup, compared with non-Hispanic whites, a smaller proportion of black patients received an influenza vaccination during the study period (66.9 vs. 73.0%, P = 0.03) and any lifetime pneumococcal vaccination (93.4 vs. 96.5%, P = 0.02). A smaller proportion of black patients also had an LDL <100 mg/dL (56.6 vs. 67.8%, P < 0.001) and BP <140/90 mmHg (64.9 vs. 75.8%, P < 0.001), and a larger proportion of black patients had an A1C >9.0% (>75 mmol/mol) (17.1% vs. 11.2%, P < 0.01).
In multivariable models of the attending-only subgroup, there were no racial differences for any processes of care (Supplemental Fig. 2). Compared with non-Hispanic white patients, black patients remained less likely to have an LDL <100 mg/dL (OR 0.70 [95% CI 0.51–0.94]) and BP <140/90 mmHg (OR 0.65 [95% CI 0.48–0.87]). No adjusted racial differences were observed for A1C <7.0% (<53 mmol/mol) or A1C >9.0% (>75 mmol/mol).
Conclusions
We observed racial differences in disease-specific processes of care and intermediate outcomes among 1,457 black and non-Hispanic white patients with diabetes who were medically managed within a level 3 PCMH. The overall proportion of patients who received select processes of care and achieved select intermediate outcomes in this setting exceeded national benchmarks and, in many cases, approximated the 90th percentile of performance nationally (27). Black patients were, however, less likely to receive A1C testing and influenza vaccination and were less likely to have optimal control of LDL and BP. While the absolute differences in some outcomes were small, their impact over an entire clinic population is clinically relevant. Though prior studies have documented such differences in diabetes care nationally (8,17), few have addressed such outcomes within the context of a medical home (19,28).
In adjusted analyses controlling for sociodemographics, clinical characteristics, treatment intensity, and clinical continuity, black patients had 43% lower odds of receiving A1C testing. This finding has already been described in the diabetes literature (17,29) and, in this setting, may be a result of differences in health seeking and reporting behaviors. A1C testing was considered complete if done within the clinic or if patients reported receiving the test from another medical provider. Thus, black patients may have had less testing recorded if they were less likely to receive care from another provider or clinic. Medical record data may have also underestimated A1C testing in black patients if they were less likely to report having received the test or were less aware that testing was performed in an outside setting. This observation for A1C testing may also be due to racial differences in the content of primary care visits. Black patients had more missed visits and emergency department visits during the study period, which may have shifted the focus of clinic visits to acute, episodic care rather than preventative care or chronic disease management.
Given that we did not observe differences in optimal or poor glycemic control, the consequences of this difference in A1C testing are unclear. However, black patients were less likely to achieve recommended control of LDL (26% decreased odds) and BP (36% decreased odds). These findings are consistent with several prior studies demonstrating disparities in BP and lipid control, including those conducted in managed care and Medicare settings where barriers to care are presumably lessened (15,17,30,31). These differences in lipid and BP control are potentially due to differences in health behaviors (e.g., physical activity, diet, medication adherence) that are largely influenced by factors outside of the context of the traditional health care system.
Importantly, we found no evidence that differences in BP and lipid control were due to differential treatment intensity (i.e., prescribing of antihypertensive and lipid-lower medications). Equal proportions of black and non-Hispanic white patients with BP ≥140/90 mmHg were prescribed at least one antihypertensive medication, and among those with LDL ≥130 mg/dL, substantially more black patients were prescribed a lipid-lowering medication (75 vs. 59%). However, we used available data and performed a crude analysis of treatment intensity based on the mere presence or absence of prescribed medications and did not include data on dosage, dose adjustment, or medication adherence. Though this is similar to approaches used elsewhere (15,29), other studies using more sophisticated methods have suggested that treatment intensity may be partially responsible for racial and ethnic disparities in intermediate outcomes, such as BP and lipid control (31,32).
In this cohort, black patients were also less likely than non-Hispanic white patients to receive an influenza vaccination during the study period (25% decreased odds). Racial disparities in influenza vaccination in patients with and without diabetes are well documented nationally and may prove to be a unique challenge for the PCMH (29,33). Provider recommendations and vaccine-seeking behaviors may play major roles in vaccination rates (34,35). Compliance with vaccination recommendations also involves addressing patients’ beliefs and attitudes, which may not affect the uptake of other, relatively benign processes of care, such as foot examination (36). While increasing the patient-centeredness of primary care visits to address such issues is a goal of the PCMH, the extent to which it achieves this and, more important, the extent to which that translates into improved vaccination rates remain unknown.
One of the most striking findings in this study was the observed difference in continuity of medical care between black and non-Hispanic white patients. Despite a similar total number of primary care visits, black patients were less likely to meet with their primary care provider (81 vs. 87%). Notably, the effect of this discontinuity on the quality of diabetes care is unclear, and having a usual site of care may actually be more important than seeing the same provider (37–39). However, even with no appreciable difference in comorbidities, black patients were dramatically more likely to receive care in the emergency department and to miss physician appointments altogether. This disruption in primary care continuity and increased reliance on acute care may correlate with differences in health behaviors, such as compliance with treatment recommendations and medication adherence, and almost certainly affects the content of primary care visits, as well as the management of intermediate outcomes such as lipid and BP control.
To explore how these differences might be influenced by provider type, we performed a sensitivity analysis of all patients with an attending provider. In this subgroup, black patients were similarly less likely to achieve LDL <100 mg/dL (30% decreased odds) and BP <140/90 mmHg (35% decreased odds). However, in contrast to our main analysis, we observed no racial differences in A1C testing or influenza vaccination. The implications of this finding for A1C testing are unclear, especially considering that we observed no racial differences in A1C testing in unadjusted analyses and found no adjusted differences in glycemic outcomes in the sensitivity or main analyses. Given that clinic staff can complete A1C testing independent of a physician order, this finding may be due to variations in visit patterns between provider cohorts. Although we observed no differences in influenza vaccination in our attending-only subgroup, this is likely due to the decreased sample size available for the sensitivity analysis. While the OR estimate is only slightly different in the main analysis (0.75) compared with the sensitivity analysis (0.77), the upper limit of the 95% CI extends from 0.99 (main analysis) to 1.04 (sensitivity analysis), resulting in a null finding. In addition to these results, provider status was only associated with 2 of 10 primary outcomes in the main analysis (Supplementary Tables 1 and 2). Overall, these analyses are consistent with the limited available literature on this subject and suggest that provider type may not be a major determinant of quality of care in this setting (40).
This study has some limitations to consider. First, we evaluated a single internal medicine PCMH serving a patient population with a higher educational attainment than would be expected based on national census data and limited our analyses to patients who were non-Hispanic white or black. Therefore, these results may not be generalizable to other settings and populations. Second, although we included educational attainment in our model, this variable may not fully capture variance in diabetes and health-related knowledge and is not an adequate proxy for health literacy or numeracy (41). Third, this study does not include information on patient health behaviors or qualitative information regarding patient preferences and beliefs, patient-provider interactions, or care-seeking behaviors, which may be important in measuring diabetes care outcomes, particularly in vaccination compliance and hypertension management (34–36,42). Fourth, black and non-Hispanic white patients in this cohort had profound differences in their baseline characteristics and visit patterns. While we adjusted for these covariables, including patient-reported data not typically available in such analyses, it remains possible that the racial differences we observed are due to residual confounding as opposed to race. Finally, this is a retrospective cohort study and is inherently limited by its inability to establish causality and determine the impact of PCMH implementation on racial differences in diabetes care.
In summary, despite the implementation of a highly innovative PCMH, we observed racial differences in both processes of care and intermediate outcomes for patients with diabetes. Racial disparities in the management and outcomes of chronic diseases, such as diabetes, remain a substantial problem for the quality and equity of health care nationally. Though the PCMH has the potential to address these disparities, the efficacy of the model, as well as the particular components of the PCMH that are most effective in doing so, remains unclear.
Supplementary Material
Article Information
Funding. This project was supported by National Institutes of Health grants UL1-RR-024153 and UL1-TR-000005.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.A.S., M.J.F., and R.H. were involved in study design, data analysis, and manuscript writing. Y.-F.C. was involved in manuscript writing and performed all statistical analyses for this project. D.S. was involved in study design and data analysis. J.A.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-1332/-/DC1.
A slide set summarizing this article is available online.
References
- 1.Centers for Disease Control and Prevention National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 2.Honeycutt AA, Boyle JP, Broglio KR, et al. A dynamic Markov model for forecasting diabetes prevalence in the United States through 2050. Health Care Manage Sci 2003;6:155–164 [DOI] [PubMed] [Google Scholar]
- 3.McBean AM, Li S, Gilbertson DT, Collins AJ. Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ethnic groups: whites, blacks, hispanics, and asians. Diabetes Care 2004;27:2317–2324 [DOI] [PubMed] [Google Scholar]
- 4.Davis SK, Liu Y, Gibbons GH. Disparities in trends of hospitalization for potentially preventable chronic conditions among African Americans during the 1990s: implications and benchmarks. Am J Public Health 2003;93:447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care 2003;26:2392–2399 [DOI] [PubMed] [Google Scholar]
- 6.Harris MI, Klein R, Cowie CC, Rowland M, Byrd-Holt DD. Is the risk of diabetic retinopathy greater in non-Hispanic blacks and Mexican Americans than in non-Hispanic whites with type 2 diabetes? A U.S. population study. Diabetes Care 1998;21:1230–1235 [DOI] [PubMed] [Google Scholar]
- 7.Young BA, Maynard C, Reiber G, Boyko EJ. Effects of ethnicity and nephropathy on lower-extremity amputation risk among diabetic veterans. Diabetes Care 2003;26:495–501 [DOI] [PubMed] [Google Scholar]
- 8.National Healthcare Disparities Report Rockville, MD, Agency for Healthcare Research and Quality, 2011 [Google Scholar]
- 9.National Center for Health Statistics. Health, United States, 2011: With Special Feature on Socioeconomic Status and Health. Hyattsville, MD, 2012 [PubMed] [Google Scholar]
- 10.National Healthcare Quality Report Rockville, MD, Agency for Healthcare Research and Quality, 2011 [Google Scholar]
- 11.Sequist TD, Fitzmaurice GM, Marshall R, Shaykevich S, Safran DG, Ayanian JZ. Physician performance and racial disparities in diabetes mellitus care. Arch Intern Med 2008;168:1145–1151 [DOI] [PubMed] [Google Scholar]
- 12.Osborn CY, Cavanaugh K, Wallston KA, et al. Health literacy explains racial disparities in diabetes medication adherence. J Health Commun 2011;16(Suppl. 3):268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osborn CY, Cavanaugh K, Wallston KA, White RO, Rothman RL. Diabetes numeracy: an overlooked factor in understanding racial disparities in glycemic control. Diabetes Care 2009;32:1614–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodondi N, Peng T, Karter AJ, et al. Therapy modifications in response to poorly controlled hypertension, dyslipidemia, and diabetes mellitus. Ann Intern Med 2006;144:475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heisler M, Smith DM, Hayward RA, Krein SL, Kerr EA. Racial disparities in diabetes care processes, outcomes, and treatment intensity. Med Care 2003;41:1221–1232 [DOI] [PubMed] [Google Scholar]
- 16.Hasnain-Wynia R, Kang R, Landrum MB, Vogeli C, Baker DW, Weissman JS. Racial and ethnic disparities within and between hospitals for inpatient quality of care: an examination of patient-level Hospital Quality Alliance measures. J Health Care Poor Underserved 2010;21:629–648 [DOI] [PubMed] [Google Scholar]
- 17.Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Trends in the quality of care and racial disparities in Medicare managed care. N Engl J Med 2005;353:692–700 [DOI] [PubMed] [Google Scholar]
- 18.Sequist TD, Adams A, Zhang F, Ross-Degnan D, Ayanian JZ. Effect of quality improvement on racial disparities in diabetes care. Arch Intern Med 2006;166:675–681 [DOI] [PubMed] [Google Scholar]
- 19.Beal AC, Doty MM, Hernandez SE, Shea KK, Davis K. Closing the Divide: How Medical Homes Promote Equity in Health Care—Results from the Commonwealth Fund 2006 Health Care Quality Survey, The Commonwealth Fund, June 2007 [Internet]. Available from http://www.commonwealthfund.org/Publications/Fund-Reports/2007/Jun/Closing-the-Divide–How-Medical-Homes-Promote-Equity-in-Health-Care–Results-From-The-Commonwealth-F.aspx
- 20.Rosenthal TC. The medical home: growing evidence to support a new approach to primary care. J Am Board Fam Med 2008;21:427–440 [DOI] [PubMed] [Google Scholar]
- 21.Hess R, Matthews K, McNeil M, Chang CH, Kapoor W, Bryce C. Health services research in the privacy age. J Gen Intern Med 2005;20:1045–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619 [DOI] [PubMed] [Google Scholar]
- 23.Blake RL, Jr, McKay DA. A single-item measure of social supports as a predictor of morbidity. J Fam Pract 1986;22:82–84 [PubMed] [Google Scholar]
- 24.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–483 [PubMed] [Google Scholar]
- 25.National Committee for Quality Assurance. DM technical specifications [internet]. Available from http://www.ncqa.org/tabid/1256/default.aspx Accessed 29 June 2012
- 26.Van Buuren S. Multiple imputation of multilevel data. In Handbook of Advanced Multilevel Analysis. Hox JJ, Roberts JK, Eds. New York, NY, Routledge, 2011, p. 173–196 [Google Scholar]
- 27.The state of health care quality (2012) [internet], 2012. Washington, DC, National Committee for Quality Assurance. Available from http://www.ncqa.org.reportcards/healthplans/stateofhealthcarequality.aspx Accessed 29 June 2012
- 28.Lee K, Palacio C, Alexandraki I, Stewart E, Mooradian AD. Increasing access to health care providers through medical home model may abolish racial disparity in diabetes care: evidence from a cross-sectional study. J Natl Med Assoc 2011;103:250–256 [DOI] [PubMed] [Google Scholar]
- 29.Brown AF, Gregg EW, Stevens MR, et al. Race, ethnicity, socioeconomic position, and quality of care for adults with diabetes enrolled in managed care: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care 2005;28:2864–2870 [DOI] [PubMed] [Google Scholar]
- 30.Cummings DM, Doherty L, Howard G, et al. Blood pressure control in diabetes: temporal progress yet persistent racial disparities: national results from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Diabetes Care 2010;33:798–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saffar D, Williams K, Lafata JE, Divine G, Pladevall M. Racial disparities in lipid control in patients with diabetes. Am J Manag Care 2012;18:303–311 [PMC free article] [PubMed] [Google Scholar]
- 32.Hicks LS, Fairchild DG, Horng MS, Orav EJ, Bates DW, Ayanian JZ. Determinants of JNC VI guideline adherence, intensity of drug therapy, and blood pressure control by race and ethnicity. Hypertension 2004;44:429–434 [DOI] [PubMed] [Google Scholar]
- 33.Egede LE, Zheng D. Racial/ethnic differences in adult vaccination among individuals with diabetes. Am J Public Health 2003;93:324–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singleton JA, Santibanez TA, Wortley PM. Influenza and pneumococcal vaccination of adults aged > or = 65: racial/ethnic differences. Am J Prev Med 2005;29:412–420 [DOI] [PubMed] [Google Scholar]
- 35.Lindley MC, Wortley PM, Winston CA, Bardenheier BH. The role of attitudes in understanding disparities in adult influenza vaccination. Am J Prev Med 2006;31:281–285 [DOI] [PubMed] [Google Scholar]
- 36.Hebert PL, Frick KD, Kane RL, McBean AM. The causes of racial and ethnic differences in influenza vaccination rates among elderly Medicare beneficiaries. Health Serv Res 2005;40:517–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parchman ML, Burge SK, Residency Research Network of South Texas Investigators Continuity and quality of care in type 2 diabetes: a Residency Research Network of South Texas study. J Fam Pract 2002;51:619–624 [PubMed] [Google Scholar]
- 38.Mainous AG, 3rd, Koopman RJ, Gill JM, Baker R, Pearson WS. Relationship between continuity of care and diabetes control: evidence from the Third National Health and Nutrition Examination Survey. Am J Public Health 2004;94:66–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulliford MC, Naithani S, Morgan M. Continuity of care and intermediate outcomes of type 2 diabetes mellitus. Fam Pract 2007;24:245–251 [DOI] [PubMed] [Google Scholar]
- 40.Zallman L, Ma J, Xiao L, Lasser KE. Quality of US primary care delivered by resident and staff physicians. J Gen Intern Med 2010;25:1193–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett IM, Chen J, Soroui JS, White S. The contribution of health literacy to disparities in self-rated health status and preventive health behaviors in older adults. Ann Fam Med 2009;7:204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kressin NR, Wang F, Long J, et al. Hypertensive patients’ race, health beliefs, process of care, and medication adherence. J Gen Intern Med 2007;22:768–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


