Abstract
OBJECTIVE
The Diabetes Prevention Program (DPP) trial investigated rates of progression to diabetes among adults with prediabetes randomized to treatment with placebo, metformin, or intensive lifestyle intervention. Among women in the DPP, diabetes risk reduction with metformin was greater in women with prior gestational diabetes mellitus (GDM) compared with women without GDM but with one or more previous live births.
RESEARCH DESIGN AND METHODS
We asked if genetic variability could account for these differences by comparing β-cell function and genetic risk scores (GRS), calculated from 34 diabetes-associated loci, between women with and without histories of GDM.
RESULTS
β-Cell function was reduced in women with GDM. The GRS was positively associated with a history of GDM; however, the GRS did not predict progression to diabetes or modulate response to intervention.
CONCLUSIONS
These data suggest that a diabetes-associated GRS is associated with development of GDM and may characterize women at risk for development of diabetes due to β-cell dysfunction.
Introduction
In the Diabetes Prevention Program (DPP), women with prediabetes and prior gestational diabetes mellitus (GDM) were 71% more likely to develop diabetes compared with women without prior GDM and one or more previous live births. Interestingly, intensive lifestyle intervention (ILS) was equally effective at preventing progression to diabetes in both groups of women compared with placebo (53 vs. 49% risk reduction), whereas metformin was more effective in women with a history of GDM (50 vs. 14% risk reduction) (1). Individual genetic risk scores (GRS), developed from a composite of single nucleotide polymorphisms (SNPs) at loci associated with type 2 diabetes, predicted progression to diabetes among DPP participants (2). Here, we compared β-cell function between GDM and non-GDM women in the DPP and examined the utility of this GRS and its individual risk alleles in predicting progression to diabetes and response to intervention in women with or without prior GDM.
Research Design and Methods
The DPP trial design and baseline characteristics have been described in detail previously (3,4). In brief, across 27 U.S. clinical centers, 3,234 participants aged ≥25 years with impaired glucose tolerance, elevated fasting glucose (95–125 mg/dL), and BMI ≥24 kg/m2 were randomized to placebo, metformin, or ILS. Primary study end point was development of diabetes. At the time of enrollment, all women completed a questionnaire regarding gravidity, parity, and GDM history.
Among 1,416 women with one or more live births and without GDM and 350 with a history of GDM, a subset (n = 1,102 without GDM; n = 281 with GDM) underwent genotyping. DNA samples were extracted from peripheral leukocytes, and 34 type 2 diabetes–associated SNPs were genotyped, as described previously (2). A GRS for each participant was calculated using the 34 loci by weighting each risk allele by its effect size (β-estimate) on diabetes risk and summing these values, with a theoretical range of 0–68 (2).
Statistical Analyses
Similar numbers of GDM and non-GDM women who underwent genotyping were assigned to placebo, metformin, or ILS (Table 1). Insulinogenic index calculated from the 75-g, 2-h oral glucose tolerance test [log(∆insulin/∆glucose)(0-30 min)] (5) was used as a measure of β-cell function in general linear models at baseline and at 1 year to determine differences between GDM and non-GDM women. Independent variables included GDM status, race/ethnicity, age at randomization, parity, and intervention (at 1 year only).
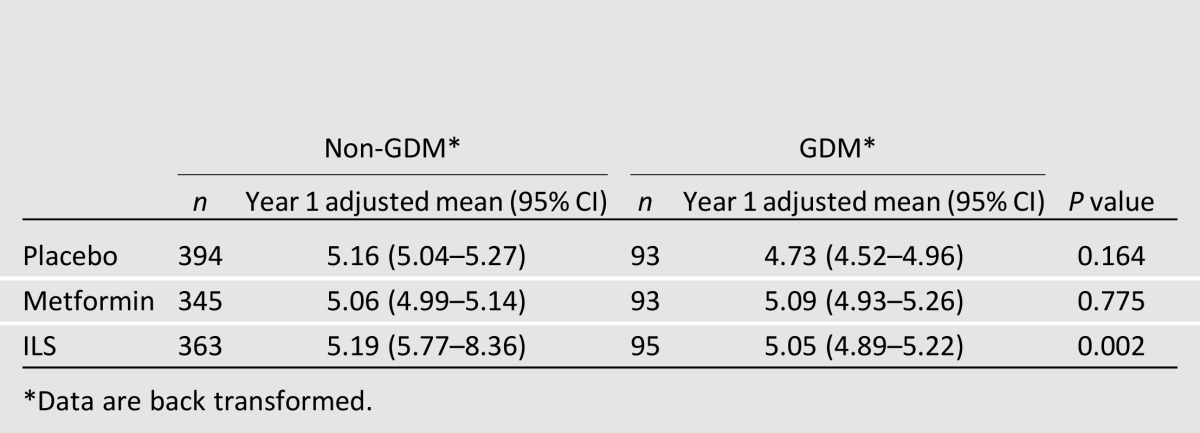
Table 1.
β-Cell function [insulinogenic index: log(∆insulin/∆glucose)(0–30 min)] at 1 year, after adjustment for baseline β-cell function, race/ethnicity, age at randomization, and parity
Logistic regression was used to evaluate the association between GRS and baseline history of GDM adjusted for ethnicity, age, and parity. We examined the GRS, treatment interventions, and history of GDM in Cox regression models as independent variables predicting diabetes incidence. Models were adjusted for ethnicity and parity. Next, ANCOVA was used to assess the effect of DPP treatments, GRS, and history of GDM on β-cell function adjusted for concomitant insulin sensitivity, as measured by the oral disposition index (DIo) (2,6) at 1 year. In contrast to the insulinogenic index, DIo captures β-cell function adjusted for insulin sensitivity and therefore takes into account physiological compensation (2,6). Models were adjusted for baseline DIo, ethnicity, age, and parity. Individual effects of SNPs were tested in similar ANCOVA models using SNP as an additive term. Genotype, treatment, and GDM three-way and two-way interaction tests were performed for all models testing postrandomization outcomes. Treatment groups were analyzed together if there were no significant interactions. Analyses were also performed after adjustment for waist circumference, which was a significant predictor of development of diabetes in the DPP cohort (2). Nominal two-sided P values are reported.
Results
At baseline, β-cell function (insulinogenic index) was decreased in GDM (mean 4.19 [95% CI 4.10–4.29]) versus non-GDM (mean 4.35 [4.30–4.40]) women (P < 0.01). At 1 year, there was a significant interaction between treatment group and GDM status (P = 0.02); therefore, analysis was stratified by GDM status and treatment group. After adjustments, 1-year insulinogenic index was also lower in GDM compared with non-GDM women (P < 0.01) (Table 1).
Adjusted for ethnicity and age, the GRS was positively associated with GDM history (odds ratio 1.05 [95% CI 1.00–1.08], P = 0.04), such that for every one unit increase in the GRS, the odds of GDM increased by 5%. This association was unaffected by additional adjustment for parity (1.04 [1.00–1.08], P = 0.04); however, it was no longer significant after adjustment for waist circumference (1.04 [1.00–1.08], P = 0.07). There was no difference in the hazard ratios (HRs) for the GRS predicting progression to diabetes in women with GDM compared with women without GDM after adjustment for ethnicity, age, and treatment arm (P = 0.09).
Because β-cell function as measured by insulinogenic index was lower in GDM than in non-GDM women, we next examined the relationship of individual SNPs within the GRS with β-cell function after adjustment for concomitant insulin sensitivity, as measured by DIo. Four of the 34 SNPs comprising the GRS are primarily associated with insulin resistance (KLF14, rs972283; PPARG, rs1801282; IRS1, rs7578326; GCKR, rs780094) (7) and thus were excluded from this analysis in order to isolate any genetic component of β-cell function in GDM women. After adjusting for ethnicity, age, and parity, none of the remaining 30 SNPs comprising the GRS independently associated with DIo in women with GDM compared with women without GDM.
Conclusions
We found that among parous women in the DPP, β-cell function defined by insulinogenic index was reduced in women with a history of GDM compared with women without prior GDM. This is consistent with the reduced insulin-to-glucose ratio previously reported in GDM women in this cohort (1). Further, a GRS calculated using SNPs strongly associated with type 2 diabetes was higher in women with GDM compared with women without GDM; thus, GRS is positively associated with prior GDM in DPP women. This association remained significant after adjustment for age, ethnicity, and parity, consistent with epidemiological data demonstrating that increasing parity or gravidity do not alter future diabetes risk in women with (8) or without (9) previous GDM who have been pregnant.
On the other hand, these data suggest the GRS is not associated with progression to diabetes in high-risk women either with or without a GDM history, in any of the study arms. This is of interest because a prior analysis from the DPP showed that risk reduction for progression to diabetes in response to metformin was greater among women with GDM compared with women without GDM (1), leading us to hypothesize that genetic variability may play a role. Our data showed that GRS predicted the presence of GDM but not progression to diabetes among affected women. That said, the HR for the GRS predicting progression to diabetes in this small cohort of female DPP participants with prior GDM (HR 1.04 [95% CI 1.00–1.08]) is similar to the significant HR for the GRS predicting progression to diabetes among the entire DPP cohort with genetic information (1.02 [1.02–1.03]) (2), indicating that analysis of a larger population of prediabetic women with prior GDM may strengthen the relationship between this GRS and diabetes progression.
Limitations of our study include small sample size and a relatively long diabetes-free interval (mean 12 years) since the index pregnancy among women with GDM at enrollment. This suggests the DPP excluded GDM women with the highest risk for diabetes progression, possibly diminishing differences in GRS between women with and without GDM.
β-Cell function is reduced in women with GDM compared with women without GDM. Accordingly, GRS comprising 34 diabetes-associated loci is higher in women with prediabetes and histories of GDM compared with women with prediabetes without GDM and one or more prior live births. This GRS does not, however, differentiate diabetes risk or response to treatment with metformin or ILS between high-risk women with and without GDM.
Supplementary Material
Article Information
Acknowledgments. The authors gratefully acknowledge the commitment and dedication of the participants in the DPP.
Funding and Duality of Interest. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the coordinating center for the design and conduct of the study and the collection, management, analysis, and interpretation of the data (U01-DK-048489). The Southwestern American Indian Centers were supported directly by the NIDDK and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, supported data collection at many of the clinical centers. Funding for data collection and participant support was also provided by the Office of Research on Minority Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Aging, the Centers for Disease Control and Prevention, the Office of Research on Women’s Health, and the American Diabetes Association. This research was also supported, in part, by the intramural research program of the NIDDK. Genotyping for this project was supported by NIDDK R01-DK-072041 to J.C.F. and K.A.J.
Bristol-Myers Squibb and Parke-Davis provided the medication. LifeScan, Health o meter, Hoechst Marion Roussel, Merck-Medco Managed Care, Merck, Nike Sports Marketing, Slim-Fast, and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson Corp., Matthews Media Group, and the Henry M. Jackson Foundation provided support services under subcontract with the coordinating center. P.W.F. was supported by grants from Novo Nordisk, the Swedish Research Council, the Swedish Heart-Lung Foundation, and the Swedish Diabetes Association. J.C.F. has received consulting honoraria from Eli Lilly and Company and Pfizer. S.D.-J. has received research grants from AstraZeneca, Bristol-Myers Squibb, Novo Nordisk, and Boehringer Ingelheim (all under contracts with his employer) and has received honoraria for serving as a consultant for Merck, Santarus, Amylin Pharmaceuticals, and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
The opinions expressed in this article are those of the investigators and do not necessarily reflect the views of the Indian Health Service or other funding agencies.
Author Contributions. S.D.S. planned the analysis and wrote, reviewed, and edited the manuscript. K.A.J. planned the analysis, performed statistical analyses, and wrote, reviewed, and edited the manuscript. J.C.F. planned the analysis, supervised the genotyping, and reviewed and edited the manuscript. D.D., P.W.F., C.K., and C.A.C. reviewed and edited the manuscript. S.D.-J. and W.C.K. reviewed and edited the manuscript and planned and performed the clinical trial. R.R. planned the analysis, reviewed and edited the manuscript, and planned and performed the clinical trial. The full DPP Research Group planned and performed the clinical trial. S.D.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
A complete list of centers, investigators, staff, and members of the Diabetes Prevention Program Research Group can be found in the Supplementary Data online.
Clinical trial reg. no. NCT00004992, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0700/-/DC1.
References
- 1.Ratner RE, Christophi CA, Metzger BE, et al. Diabetes Prevention Program Research Group Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hivert MF, Jablonski KA, Perreault L, et al.; DIAGRAM Consortium ; Diabetes Prevention Program Research Group. An updated genetic score based on 34 confirmed type 2 diabetes loci is associated with diabetes incidence and regression to normoglycemia in the diabetes prevention program. Diabetes 2011;60:1340–1348 [DOI] [PMC free article] [PubMed]
- 3.The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Diabetes Prevention Program: baseline characteristics of the randomized cohort. The Diabetes Prevention Program Research Group. Diabetes Care 2000;23:1619–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 6.Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009;32:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voight BF, Scott LJ, Steinthorsdottir V, et al. MAGIC investigators. GIANT Consortium Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 2010;42:579–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes Care 2007;30:878–883 [DOI] [PubMed] [Google Scholar]
- 9.Charles MA, Pettitt DJ, McCance DR, Hanson RL, Bennett PH, Knowler WC. Gravidity, obesity, and non-insulin-dependent diabetes among Pima Indian women. Am J Med 1994;97:250–255 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


