Abstract
Efforts to reduce the burden of type 2 diabetes include attempts to prevent or delay the onset of the disease. Landmark clinical trials have shown that lifestyle modification programs focused on weight loss can delay the onset of type 2 diabetes in subjects at high risk of developing the disease. Building on this knowledge, many community-based studies have attempted to replicate the trial results and, simultaneously, payers have begun to cover diabetes prevention services. This article focuses on the evidence supporting the premise that community prevention efforts will be successful. Unfortunately, no study has shown that diabetes can be delayed or prevented in a community setting, and efforts to replicate the weight loss achieved in the trials have been mostly disappointing. Furthermore, both the clinical trials and the community-based prevention studies have not shown a beneficial effect on any diabetes-related clinical outcome. While the goal of diabetes prevention is extremely important, the absence of any persuasive evidence for the effectiveness of community programs calls into question whether the use of public funds or national prevention initiatives should be supported at this time.
Introduction
Landmark clinical trials have shown that we can delay and possibly prevent the onset of diabetes in many individuals at high risk (1,2). Encouraged by these results, there have been many attempts to translate the prevention trials into community-based programs. There have also been requests to expand diabetes prevention services considerably (3–5). While diabetes prevention would be an invaluable benefit to society, we must have confidence in our ability to translate the trials’ results using far fewer resources and in real-world settings. Clinical trial results show what is possible, but it cannot simply be assumed that their results can easily or cost-effectively be translated into practice. In this article, we review many of the important factors that must be considered before embarking on a nationwide effort to delay or prevent the onset of diabetes. We show not only that the magnitude of the task is daunting, but also that the efforts so far have been disappointing and that essential information is missing.
An important issue to appreciate is the magnitude of the effort we will undertake. Table 1 shows that between 6 and 38% of Americans (12.8–82 million people) have “prediabetes,” depending on where the lower boundary is drawn and which test is used to make the diagnosis. All of the cut points shown are arbitrary, since there is no biological basis for any test value at which prediabetes begins (6). Even at the higher end of the range of prediabetes (people with an A1C between 6.1–6.4%), how this large population will be identified and the cost of the intervention itself become very important. Thus, if nationwide diabetes prevention efforts are to be initiated, our first consideration is to realize that the at-risk population is very large and the cost of prevention could amount to a great deal of money.
Table 1.
Prevalence of prediabetes 2009–2010
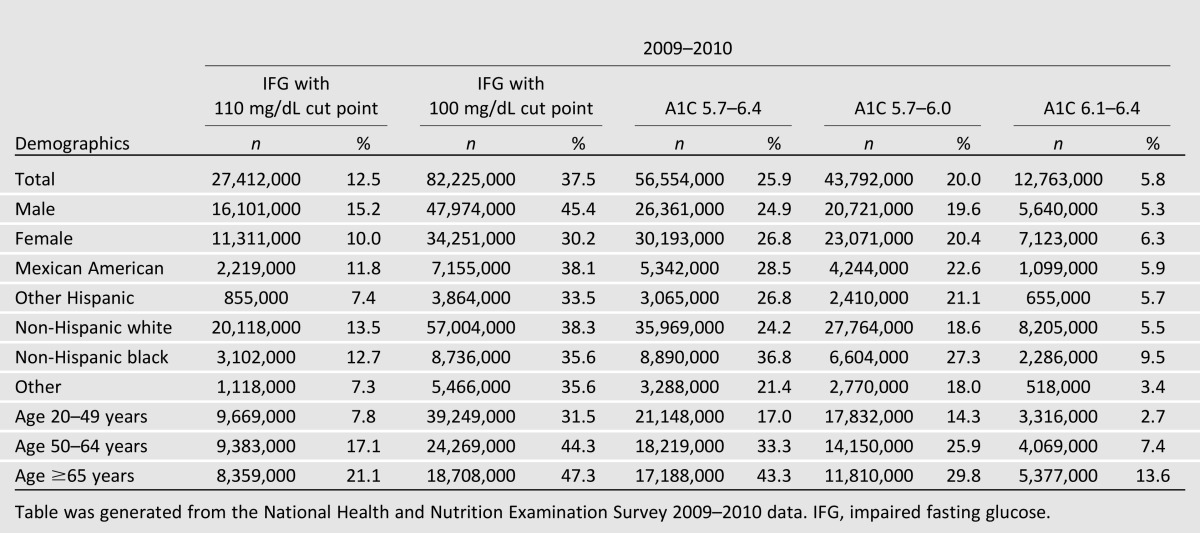
Although the above discussion implies a nationwide effort to prevent diabetes, for individuals who in the course of an office visit are advised to lose weight and do so on their own, the intervention might obviously be well worth their own money, if any is spent. But there is no value to implementing a widespread prevention program that does very little if anything to reduce the development of diabetes. Initiating diabetes prevention should require confidence that society will benefit and that the cost of such programs will be money well spent. Thus, it is imperative to ask whether there is evidence that community-based interventions will be effective.
Can We Prevent or Delay Diabetes Outside of a Clinical Trial?
Lifestyle Modification
The major prevention trials used either “lifestyle modification” or pharmacotherapy to significantly reduce the incidence of diabetes. Lifestyle modification was neither simple nor straightforward. In the Diabetes Prevention Program (DPP), the primary goal was a reduction of at least 7% of initial body weight and an enhanced level of physical activity in overweight or obese people at high risk of diabetes (1,7). In a post hoc study analysis of the DPP, weight loss was by far the most important contributor to prevention (8). Reduction of calories from fat and increased physical activity predicted and helped sustain weight loss (1,8). In the follow-up study of the DPP, termed the DPP Outcomes Study (DPPOS), the contribution of exercise to prevention went unmentioned, implying that this intervention was of marginal value in preventing diabetes (9). The Finnish Diabetes Prevention Study (DPS) not only strived for weight loss and increased physical activity in a similar population but also sought many changes in diet composition, such as a reduction in saturated fat and an increase in fiber intake (2). When all the variables were analyzed simultaneously “the only significant association was between weight reduction and diabetes risk” (10). Although dietary composition and physical activity were important, “their effect was mediated through resulting weight reduction” (10). Other randomized trials also show that weight loss is the key, if not critical, intervention to reduce diabetes risk (11–13). Thus, weight loss appears to take center stage when it comes to lifestyle modification delaying the onset of diabetes (14).
The Chinese Da Qing Diabetes Prevention study also showed great benefit in preventing diabetes through lifestyle modification (15), although the interventions were of questionable value. In that study, weight loss was minimal (∼2 lbs) in the combined diet-exercise arm after the initial 6-year intervention; weight loss was actually greater in the control arm after 20 years of follow-up (16). Individuals randomized to the exercise-only arm showed a 46% reduction in the incidence of diabetes after 6 years (15). At baseline, however, the exercise group was already doing 42% more exercise than control subjects (casting some doubt on the randomization process); after the 6-year follow-up, their exercise level did not improve significantly from their baseline level. In the 20-year follow-up report, no information related to the exercise-only group was reported (16).
Despite the impressive results of the Chinese study (43% relative risk reduction in the lifestyle group after 20-years follow-up), it is impossible to translate the intervention used in this study since neither a complete description of the lifestyle changes that occurred nor the identification of the active component(s) are known. This point was made by the investigators in their discussion of the results (16).
In summary, it appears that weight loss is the key factor that reduces diabetes risk, and thus all efforts to translate the prevention trials to a community setting have focused on weight reduction. While exercise may be important, aside from the Chinese study results, which are inconclusive, we have no randomized controlled studies showing the benefit of exercise alone on diabetes prevention or what type of exercise or for how long (i.e., over months or years) is key. Ali et al. (17) recently summarized all the lifestyle interventions initiated in real-world settings that have been modeled on the DPP. They identified 28 studies but only 4 were carried out for 12 months or longer, and none of those reported the incidence of diabetes.
In addition to the four longer-term studies identified by Ali et al. (17), we performed a broad literature search using PubMed and Embase to identify randomized, controlled trials demonstrating the effectiveness of delivering behavioral weight loss interventions to overweight or obese adults in a community setting that had at least 1 year of follow-up (18–30). Table 2 shows the degree of weight loss seen in the intervention group over that seen in the control group in all the community studies we identified (18–30) and in the two major prevention trials. There are two important points to be gleaned from these studies. One is that where data were collected beyond 1 year, weight regain begins, commencing about that time. Of note, however, in the studies by Katula et al. (23,24), subjects regained virtually no weight in year 2, which is surprising given the fact that no other weight loss study using lifestyle modification was nearly as successful (1,2,21,22,27,31). The results from Katula et al. (23,24) may, however, have been an artifact due to the fact the year 2 weight assessment was done on those returning for that examination (n = 127 or a 16% drop-out rate in the intervention arm; 11% drop-out rate in control subjects), whereas the baseline assessment was made on the entire starting cohort (n = 151).
Table 2.
Percent of weight loss over that achieved in standard weight-loss program control subjects
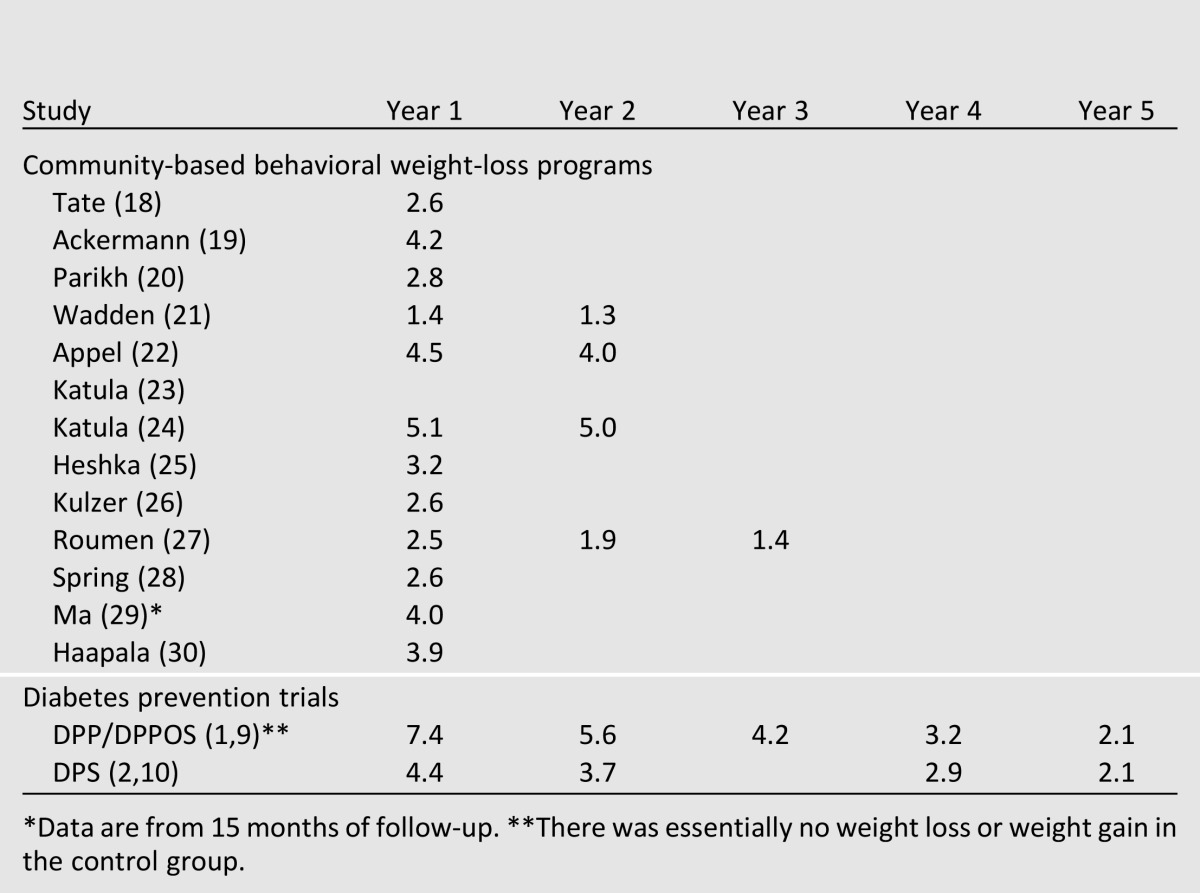
Weight regain is very common in weight loss studies that use a behavior intervention. Thus, it is extremely difficult to maintain weight loss, even in studies where the intervention is still in full force and the enrollees are extremely well motivated. Weight regain is thought to occur because of the physiologic drive to return to one’s previous weight (32–34).
The other key finding shown in Table 2 is that the degree of weight loss in the first year of the vast majority of the community programs was less than that achieved in the prevention trials at the equivalent period. Overall, weight loss in most community settings at year 1 was about the same as that achieved in the later years of the major trials. The most notable exception again was the studies by Katula et al. (23,24), in which weight loss at year 2 was the closest to what was achieved in the study it attempted to translate (i.e., DPP). Notwithstanding potential issues related to the measurement of weight loss over the 2-year follow-up, as mentioned above, the results of the Katula studies’ (23,24) intervention may have promise if the weight loss achieved at year 2 can be sustained and replicated.
There are other reports that are relevant to weight loss in community settings. Finland has initiated a large-scale nationwide diabetes prevention program modeled after the DPS (35). The interim report of that effort showed that after 1 year, participants on average lost very little weight (mean 1.3%); only 18% of the cohort lost ≥5% of their body weight compared with nearly 50% of participants in the original DPS (2). Thus, the translation study could not achieve the same degree of weight loss as in the prevention trial. Compared with those who maintained their weight, people losing 2.5–4.9% body weight had only an 18% risk reduction, compared with a 58% relative risk reduction in the DPS after 3 years of follow-up. Also, there are widely available commercial weight loss programs that could be considered community-based interventions. These reports show that some participants can lose 3–4% of their body weight in the first year of enrollment (36,37).
The inability of nearly all the community studies to come close to replicating the weight loss achieved in year 1 of the prevention trials and the steady weight regain thereafter is troublesome. Moreover, as the lifestyle intervention in all the community studies was provided free, subjects received a great deal of staff-directed encouragement throughout the study and were likely to be a more highly motivated group. Such important benefits are unlikely to be provided outside of a study environment.
How Much Weight Loss Protects Against Diabetes?
There appear to be no data directly documenting the relationship between weight loss and diabetes prevention. Hammen et al. (8) reported that every kilogram of weight loss in the DPP resulted in a 16% reduction in risk, but their results were based entirely on data from the initial 3 years of the study. As the mean weight loss diminishes with time, so would the relationship between absolute weight reduction and risk of diabetes. In the DPS (10), the relative risk reduction in the cumulative incidence of diabetes was 36% when only the 3-year postintervention period was analyzed, which narrowly made statistical significance (P < 0.04). In the first year of the 3-year postintervention period (which corresponds to DPS year 5 data in Table 2), weight reduction was 2.1% relative to control subjects. It is very possible that as weight regain occurred in DPS (and DPP too); the cumulative incidence data over the initial years mask a much lower difference between groups in the annual incidence of diabetes in subsequent years. In fact, at year 4 of the DPS, fasting plasma glucose levels had returned to baseline values and continued to increase thereafter (38).
Other data support the hypothesis that weight loss of at least 3–4% over an extended period of time is necessary to achieve appreciable diabetes prevention. At the start of the DPPOS, the weight of individuals in the lifestyle arm was about 3–4% less than control subjects (9), and the incidence of diabetes was no different thereafter between the lifestyle and control arms. It was not entirely clear, however, whether the absence of a protective effect was due to the smaller percentage of weight lost relative to the DPP or due to other factors identified in the follow-up report (9). Also in the DPP/DPPOS (1,9), the mean A1C level in the lifestyle arm was lowest when weight loss was at its maximum (i.e., year 1); A1C levels increased thereafter, seeming to correlate with the degree of weight regain (9).
In a computer simulation of the DPP (39) that relied only on the baseline data of the DPP participants and the degree of weight loss, the incidence of diabetes mimicked the results of the DPP (1) and DPPOS (9). The simulation assumed a mean weight loss of 7% in year 1, gradually decreasing to 4% weight loss after 3 years (as seen in the DPP), and held at that level for decades. After 10 years of follow-up in the simulation, the incidence of diabetes in the lifestyle group was virtually the same as that achieved in the DPP. If the 4% weight loss in the simulation was maintained for 30 years, the risk of diabetes was reduced by a relative 15%. These data confirm that the benefit of lifestyle modification was due mainly to weight loss and that a 4% weight reduction for life would be beneficial.
Finally, in a study to determine whether bariatric surgery could prevent the onset of type 2 diabetes, after 15 years of follow-up there was an 83% relative reduction in the incidence of diabetes, and that corresponded to a relative 17–26% loss in body weight over the entire follow-up period (40).
Although the information above on the relationship between weight loss and diabetes prevention is not definitive, it does suggest that the delay in developing diabetes is relatively proportional to weight loss. Since nearly all the real-world studies could only achieve a weight loss in year 1 that corresponded to years 3–4 in the prevention trials and weight regain after year 1 is assured, it is likely that very few cases of diabetes would be prevented in community programs, particularly over more than just a couple of years. Unfortunately, none of the community studies were designed to determine the extent to which diabetes is prevented; all report only surrogate end points. So, in fact, we will not know the clinical benefit of modest weight loss anytime soon, but all the indirect evidence points to a greatly diminished, or absent, long-term benefit.
Pharmacotherapy
Many studies show that glucose-lowering drugs can delay the onset of diabetes (41). With the exception of metformin, none of the drugs have been given for more than 3 years. After their discontinuation, the incidence of diabetes increases. In the DPP, metformin was given for 3 years and resulted in a 31% reduction in the incidence of diabetes (1). The delay to the onset of diabetes was estimated to be half that achieved in the lifestyle group (9). About 75% of the participants in the metformin arm took what was considered to be the prescribed dose, but in the follow-up study the rate of adherence dropped to 57% (9). Considering safety and cost, metformin appears to be the best drug to reduce the incidence of diabetes, but success requires adherence to long-term treatment, which appears problematic and even less likely among individuals not enrolled in a clinical trial. While some investigators suggest using a combination of glucose-lowering drugs (42), such regimens have not been tested. Newer drugs (43) hold promise in their ability to promote weight loss and improve cardiovascular risk factors, but they are very expensive and have not been given to individuals with prediabetes for long periods of time.
Despite the above considerations, if drugs are to be routinely used in people with prediabetes, then essentially we have moved the diagnostic cut point for diabetes to a lower level. While that may be warranted, all the ramifications of such a change deserve considerable discussion.
The Impact of Diabetes Prevention on Clinical Outcomes
Lifestyle intervention in the DPP and DPS resulted in an overall mean delay in the development of diabetes of about 4–5 years; pharmacotherapy, 2 years (9). Since the primary goal of diabetes prevention is a reduction in diabetes-related complications, it is important to know how valuable a 4–5 year delay is in preventing the clinical outcomes of interest. In their initial intervention periods (∼3 years), both the DPS (2) and DPP (44) observed significant reductions in some surrogate biomarkers. Systolic blood pressure and triglyceride levels were significantly reduced but not total cholesterol or LDL. In the DPPOS (9,45), the risk factor reductions initially observed were not maintained by the end of the extended follow-up period. More important, after 7 years of follow-up in the DPS and 10 years follow-up in the DPP, no significant change in any clinical outcomes between the intervention and control arms have been reported. With one exception, no clinical outcome benefits using pharmacotherapy to prevent diabetes have been reported. In the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) trial, a reduction in cardiovascular events was reported, but the overall event rate was very low and the finding was not considered important to even mention by those reviewing pharmacologic options to prevent diabetes (14,42).
Two other studies provide evidence that a considerable degree of weight loss is necessary to see clinical value. In the Action for Health in Diabetes (Look AHEAD) trial (31), a study to determine if weight loss in people with diabetes will reduce cardiovascular events, weight loss after the first year was substantial (7.9% relative to control subjects) and at year 4 the intervention group had lost 3.6% of their initial body weight relative to control subjects. Nevertheless, after many years of follow-up, the study was halted because there was no benefit of weight reduction on cardiovascular events (46).
Conversely, following bariatric surgery that resulted in a 17% mean loss in body weight after a median 15 years of follow-up, first-time fatal and nonfatal cardiovascular events declined 33% and overall cardiovascular deaths declined by 53% compared with nonsurgical control subjects (47). Although there was an approximate 20% reduction in weight 1 year following surgery, the benefit in outcomes between the two groups was not seen until year 6. In the modeling study referred to previously (39), a 4% weight loss maintained for decades resulted in a small but important reduction in diabetes-related complications.
As the weight loss achieved in community-based programs is usually much less than was achieved in DPP, DPS, or Look AHEAD, many-fold less than in the surgical study, and very likely not maintained for many years, the odds of a community diabetes prevention program having a favorable impact on cardiovascular disease or other diabetes-related complications seem slim. There may be other potential benefits of a community prevention program independent of preventing diabetes or its complications, such as work productivity, but there is no evidence of such benefits or if they would remain once weight regain occurs.
Is Diabetes Prevention Cost-effective?
Some participants in community-based programs will surely not progress to diabetes, but what matters most is whether a few successes make the entire effort cost-effective. There are many reports claiming that diabetes prevention programs can be or are cost-effective (39,48–51). In order to achieve such a benefit, all of the reports posit that the lifestyle intervention would cost far less than it did in the DPP and, more remarkably, achieve weight loss results equivalent to the DPP (7% initially declining to ∼4%, relative to control subjects) that would not diminish over at least 30 years. As discussed previously, that has not yet happened in real life. Also, cost-effectiveness is achieved when the time horizon is about 30 years or more, but not in the first 10–15 years of the program. As a result, these studies do not actually represent an outcome that health plans or a national program would actually experience in a time frame more familiar to planning and budgeting or to the clinical outcomes people are likely to experience. Also, with one exception (39), all the modeling studies use a Markov model, which represents diseases as a set of discrete clinical “states,” progression of disease as annual transitions between states, and the effects of treatments as changes in the likelihood of transitions between states. In order to fit a complex disorder such as diabetes into the Markov structure, a great many assumptions and simplifications must be made that are unrealistic (see appendix to ref. 39). In the only cost-effectiveness study (39) that used a model validated against a great many clinical trials, the results indicated that cost-effectiveness after 30 years required an intervention costing no more than about $200/year and weight loss of 4% over that entire period.
The above critique highlights that a claim of cost-effectiveness for a program achieving hypothetical results over an unrealistic time frame and using, as in most studies, many unrealistic assumptions is at best an abstraction. An analysis of actual costs was recently reported by the DPP/DPPOS investigators (52). Their data show that the total cost of medical care during a median 8.5 years follow-up was modestly higher in the placebo group than in the lifestyle group (per capita difference of $291/year), but the cost of the intervention itself wiped out this benefit. If the cost of the lifestyle intervention had been much less (and assuming there would be the same degree of weight loss) or if metformin were used, the total cost might favor diabetes prevention services. Of course, by far and away the costly part of diabetes is in the treatment of its complications, and in the initial 8.5 years of the DPP/DPPOS very few serious diabetes-related complications occurred in any group (9). Without knowing whether the DPP interventions reduce the costly events associated with diabetes, it is too premature to conclude that the DPP, or more important a less robust DPP-like intervention as experienced in community programs, is cost-effective.
The Future of Diabetes Prevention
Although we know how to postpone the onset of diabetes, the resulting benefit is not altogether clear and translating the information to a community setting has been elusive. Although some people can lose weight and keep it off even without any structured programmatic help and some may be able to maintain a lifestyle modification program that, by whatever means, delays the onset of diabetes, the success rate of either appears to be very low in the real world. And if the diagnosis of diabetes is delayed by 2–5 years as in the trials, it is still uncertain that such a delay, absent greater weight loss maintained for a very long time (39), will reduce the rate of serious adverse clinical events. Although other components of a lifestyle modification may be valuable (e.g., exercise or a change in diet composition), we do not have good trial data showing how they would be effective in changing the course of prediabetes. Nor do we know if such changes in of themselves as elements of a diabetes prevention intervention have long-term clinical benefits that would ultimately make prevention services beneficial in other ways.
Too much information is missing to implement nationwide, community-based diabetes prevention programs, as has been suggested (3,4,50,51). We should at least have good evidence that a specific lifestyle or pharmacologic intervention will lead to substantial weight reduction maintained for at least 10 years. Then we need realistic cost-effectiveness studies. Given the evidence to date, it also seems unlikely that current lifestyle modification programs can both overcome our obesogenic environment and remain effective for very long.
The previous discussion does not at all mean we should abandon efforts to replicate the results of the prevention trials. Nor should we discourage individuals from attempting weight loss using their own resources, even knowing that relatively few will be successful at sustaining meaningful weight loss. Indeed, providers should routinely encourage their overweight/obese patients to lose weight and provide referral to institutions that may be of benefit. In addition, we certainly have an imperative to prevent diabetes and to continue experimenting with various approaches to translating the major clinical trials. Equally important, we need to better understand the clinical value of various amounts of weight loss. Since most studies had participants who achieved and maintained considerable weight loss, it would be valuable to be able to identify such individuals before an intervention is given to an entire population. Also, we should experiment with novel approaches to behavior modification (53). But our failure so far to come close toward replicating the major prevention trials in community-based settings strongly suggests that it is premature to siphon off precious national health care resources or revenue going into health plans toward an intervention that has no clear clinical benefit.
Although bariatric surgery in the obese is very effective in preventing diabetes (40) and some important diabetes-related complications (48), surgery is an impractical choice for most people with prediabetes. Finally, it may be more beneficial to achieve diabetes prevention by attacking the problem through national policies that reduce our overall consumption of food (54,55). In the long run, a societal solution (not a medical one) to the obesity/diabetes epidemic may end up being the best option.
Article Information
Acknowledgments. The authors thank Deyu Pan, Charles R. Drew University, for analyzing the National Health and Nutrition Examination Survey data shown in Table 1.
Funding. M.B.D. was supported in part by National Institutes of Health (NIH) National Institute on Minority Health and Health Disparities grant U54-MD-007598 (AXIS grant, formerly U54–RR-026138) and NIH National Center for Advancing Translational Sciences grant UL1-TR-000124.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.K. and M.B.D. wrote and edited the manuscript.
Footnotes
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 3.Fradkin JE, Roberts BT, Rodgers GP. What’s preventing us from preventing type 2 diabetes? N Engl J Med 2012;367:1177–1179 [DOI] [PubMed] [Google Scholar]
- 4.Thorpe KE. Analysis & commentary: The Affordable Care Act lays the groundwork for a national diabetes prevention and treatment strategy. Health Aff (Millwood) 2012;31:61–66 [DOI] [PubMed] [Google Scholar]
- 5.The Medicare Diabetes Prevention Act of 2012. http://www.franken.senate.gov/files/documents/120731_Medicare_Diabetes_Prevention_Act.pdf Accessed 28 December 2012
- 6.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Diabetes Prevention Program The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowler WC, Fowler SE, Hamman RF, et al. Diabetes Prevention Program Research Group 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Finnish Diabetes Prevention Study Group Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 11.Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care 1998;21:350–359 [DOI] [PubMed] [Google Scholar]
- 12.Sjöström CD, Lissner L, Wedel H, Sjöström L. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 1999;7:477–484 [DOI] [PubMed] [Google Scholar]
- 13.Heymsfield SB, Segal KR, Hauptman J, et al. Effects of weight loss with orlistat on glucose tolerance and progression to type 2 diabetes in obese adults. Arch Intern Med 2000;160:1321–1326 [DOI] [PubMed] [Google Scholar]
- 14.Ratner RE, Sathasivam A. Treatment recommendations for prediabetes. Med Clin North Am 2011;95:385–395, viii–ix [DOI] [PubMed] [Google Scholar]
- 15.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 16.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 17.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012;31:67–75 [DOI] [PubMed] [Google Scholar]
- 18.Tate DF, Jackvony EH, Wing RR. Effects of Internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: a randomized trial. JAMA 2003;289:1833–1836 [DOI] [PubMed] [Google Scholar]
- 19.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med 2008;35:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parikh P, Simon EP, Fei K, Looker H, Goytia C, Horowitz CR. Results of a pilot diabetes prevention intervention in East Harlem, New York City: Project HEED. Am J Public Health 2010;100(Suppl 1):S232–S239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med 2011;365:1969–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med 2011;365:1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katula JA, Vitolins MZ, Rosenberger EL, et al. One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes Care 2011;34:1451–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katula JA, Vitolins MZ, Morgan TM, et al. The Healthy Living Partnerships to Prevent Diabetes study: 2-year outcomes of a randomized controlled trial. Am J Prev Med 2013;44(Suppl 4):S324–S332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heshka S, Anderson JW, Atkinson RL, et al. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA 2003;289:1792–1798 [DOI] [PubMed] [Google Scholar]
- 26.Kulzer B, Hermanns N, Gorges D, Schwarz P, Haak T. Prevention of Diabetes Self-management Program (PREDIAS): effects on weight, metabolic risk factors, and behavioral outcomes. Diabetes Care 2009;32:1143–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roumen C, Corpeleijn E, Feskens EJ, Mensink M, Saris WHM, Blaak EE. Impact of 3-year lifestyle intervention on postprandial glucose metabolism: the SLIM study. Diabet Med 2008;25:597–605 [DOI] [PubMed] [Google Scholar]
- 28.Spring B, Duncan JM, Janke EA, et al. Integrating technology into standard weight loss treatment: a randomized controlled trial. JAMA Intern Med 2013;173:105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma J, Yank V, Xiao L, et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med 2013;173:113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haapala I, Barengo NC, Biggs S, Surakka L, Manninen P. Weight loss by mobile phone: a 1-year effectiveness study. Public Health Nutr 2009;12:2382–2391 [DOI] [PubMed] [Google Scholar]
- 31.Wing RR, Look AHEAD Research Group Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med 2010;170:1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heymsfield SB, Harp JB, Reitman ML, Beetsch JW, Schoeller DA, Erondu N, Pietrobelli A. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. Am J Clin Nutr 2007;85:346–354 [DOI] [PubMed] [Google Scholar]
- 33.Hall KD, Sacks G, Chandramohan D, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011;378:826–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guyenet SJ, Schwartz MW. Clinical review: Regulation of food intake, energy balance, and body fat mass: implications for the pathogenesis and treatment of obesity. J Clin Endocrinol Metab 2012;97:745–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saaristo T, Moilanen L, Korpi-Hyövälti E, et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010;33:2146–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med 2005;142:56–66 [DOI] [PubMed] [Google Scholar]
- 37.Jebb SA, Ahern AL, Olson AD, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet 2011;378:1485–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindström J, Peltonen M, Eriksson JG, et al. Finnish Diabetes Prevention Study (DPS) Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia 2013;56:284–293 [DOI] [PubMed] [Google Scholar]
- 39.Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med 2005;143:251–264 [DOI] [PubMed] [Google Scholar]
- 40.Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704 [DOI] [PubMed] [Google Scholar]
- 41.Phung OJ, Sood NA, Sill BE, Coleman CI. Oral anti-diabetic drugs for the prevention of type 2 diabetes. Diabet Med 2011;28:948–964 [DOI] [PubMed] [Google Scholar]
- 42.DeFronzo RA, Abdul-Ghani M. Type 2 diabetes can be prevented with early pharmacological intervention. Diabetes Care 2011;34(Suppl. 2):S202–S209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Astrup A, Carraro R, Finer N, et al. NN8022-1807 Investigators. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide [published correction appears in Int J Obes 2012;37:322]. Int J Obes 2012;36:843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratner R, Goldberg R, Haffner S, et al. Diabetes Prevention Program Research Group Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care 2005;28:888–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orchard TJ, Temprosa M, Barrett-Connor E, et al. Diabetes Prevention Program Outcomes Study Research Group Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med 2013;30:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wing RR, Bolin P, Brancati FL, et al. Look AHEAD Research Group Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56–65 [DOI] [PubMed] [Google Scholar]
- 48.Herman WH, Hoerger TJ, Brandle M, et al. Diabetes Prevention Program Research Group The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005;142:323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colagiuri S, Walker AE. Using an economic model of diabetes to evaluate prevention and care strategies in Australia. Health Aff (Millwood) 2008;27:256–268 [DOI] [PubMed] [Google Scholar]
- 50.Thorpe KE, Yang Z. Enrolling people with prediabetes ages 60-64 in a proven weight loss program could save Medicare $7 billion or more. Health Aff (Millwood) 2011;30:1673–1679 [DOI] [PubMed] [Google Scholar]
- 51.Zhuo X, Zhang P, Gregg EW, et al. A nationwide community-based lifestyle program could delay or prevent type 2 diabetes cases and save $5.7 billion in 25 years. Health Aff 2012;31:50–60 [DOI] [PubMed] [Google Scholar]
- 52.Diabetes Prevention Program Research Group The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care 2012;35:723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marteau TM, Hollands GJ, Fletcher PC. Changing human behavior to prevent disease: the importance of targeting automatic processes. Science 2012;337:1492–1495 [DOI] [PubMed] [Google Scholar]
- 54.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011;378:804–814 [DOI] [PubMed] [Google Scholar]
- 55.Lewis KH, Rosenthal MB. Individual responsibility or a policy solution—cap and trade for the U.S. diet? N Engl J Med 2011;365:1561–1563 [DOI] [PubMed] [Google Scholar]


