Microvascular and macrovascular complications associated with type 2 diabetes are leading causes of morbidity and mortality. In fact, cardiovascular (CV) complications account for a major portion of health care costs in patients with diabetes. Although lifestyle changes and pharmacotherapy aimed at CV disease risk factor modification are the mainstay of medical treatment, revascularization (surgical and endovascular) is often necessary to restore tissue perfusion and function. Indeed, nearly 25% of coronary revascularization procedures performed in the U.S. occur in individuals with diabetes (1). Furthermore, diffuse atherosclerosis, impaired ability to form vascular collaterals, and restenosis contribute to higher rates of repeat revascularization in these patients (1). Consequently, cell-based therapy with bone marrow cells and endothelial progenitor cells (EPCs) aimed at inducing angiogenesis and reendothelialization is a promising strategy that is under active investigation in both preclinical and clinical studies (2,3).
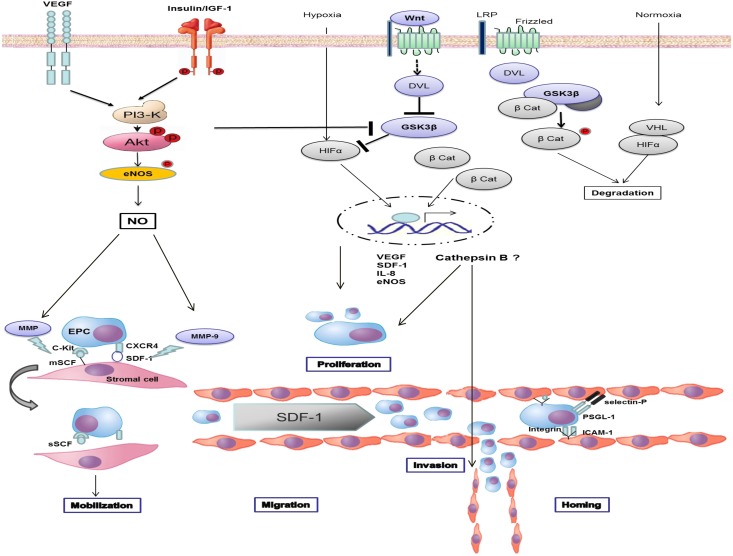
EPCs constitute a very small subset of circulating blood cells. They are progenitors of endothelial cells and have the ability to proliferate and differentiate to form perfused blood vessels in vivo and tube-like structures in vitro (4). EPC-induced neovascularization in response to tissue hypoxia and injury is a highly coordinated, temporally regulated, and complex set of events that involves mobilization, migration, and homing of EPCs to the target tissue (5,6). Endothelial injury and hypoxia activate the transcription factor hypoxia-induced factor (HIF) to initiate the expression and release of growth factors and chemokines. These include stromal cell–derived factor 1 (SDF-1), vascular endothelial growth factor (VEGF), c-Kit ligand (or SCF), angiopoietin, and interleukin-8 (IL-8), among others (5,6). Platelet aggregation leads to high levels of platelet-derived SDF-1 at the site of endothelial injury (7). EPCs are retained in the bone marrow in distinct niches by their interaction with stromal cells. Circulating SDF-1 and VEGF stimulate production of nitric oxide (NO) by endothelial NO synthase, thereby activating matrix metalloproteinase-9 (MMP-9) (6). In turn, enhanced MMP-9 activity disrupts EPC-stromal cell interaction to mobilize EPCs from the marrow. Concentration gradients of SDF-1 direct circulating EPCs to the site of injury (7). Increased surface expression of integrin β2 and selectins (selectins E and P) on the endothelium interact with specific ligands on EPCs to recruit and home EPCs (5,6). These interrelationships are shown in Fig. 1.
Figure 1.
GSK3β and cathepsin B in vasculogenesis. Endothelial injury and hypoxia activates HIF to initiate the expression and release of SDF-1, VEGF, c-Kit ligand (SCF), and IL-8. Circulating SDF-1 and VEGF stimulate the production of NO by endothelial NO synthase (eNOS) to MMP-9. Increased MMP-9 activity disrupts EPC-stromal cell interaction to mobilize EPCs from the marrow. Concentration gradients of SDF-1 direct circulating EPCs to the site of injury. Increased surface expression of integrin β2 and selectins (selectins E and P) on the endothelium interact with specific ligands on EPCs to recruit and home EPCs. In the unstimulated cell, GSK3β phosphorylates and accelerates the degradation of HIF-1α and β-catenin. Inhibition of GSK3β leads to nuclear translocation of HIF-1α and β-catenin. GSK3β inhibitors induce expression of cathepsin B to increase EPC proliferation and invasion. β Cat, β-catenin; DVL, disheveled; mSCF; membrane stem cell factor; PSGL-1, P-selectin glycoprotein ligand-1; sSCF, soluble SCF; LRP, low-density lipoprotein-related protein; VHL, von Hippel-Lindau protein; CXCR4, CXC chemokine receptor type 4; ICAM, intercellular adhesion molecule; and P, phosphorylation.
Phosphatidylinositol-3 kinase (PI3-K) and protein kinase B (Akt) activation not only stimulate NO production, but they also inhibit glycogen synthase kinase-3β (GSK3β) (8). Similarly, activation of canonical Wnt signaling inactivates GSK3β (9). Wnts are secreted glycoproteins known to regulate hematopoiesis and stem cell function (9). In the unstimulated cell, GSK3β phosphorylates and accelerates degradation of HIF-1α and β-catenin (9,10). Inhibition of GSK3β leads to cytosolic accumulation and nuclear translocation of these transcription factors in a manner that increases EPC survival, proliferation, differentiation, mobilization, and adhesion (11–13). EPCs pretreated with GSK inhibitors or EPCs that are genetically modified to overexpress VEGF or inactive GSK3β enhance vasculogenesis, augment reendothelialization, and reduce neointimal formation (11–13).
Diabetes is associated with reduced endothelial NO bioavailability and PI3-K/Akt activity, and EPCs are defective and reduced in number in these patients. Indeed, diabetes is associated with reduced mobilization, migration, and homing of EPCs (14). Thus, EPC dysfunction and reduced number significantly limit both the quantity and quality of available EPCs for autologous transplantation in diabetic patients. Consequently, various strategies to expand the pool of available EPCs for cell-based vasculogenesis are being developed (4). In this issue, Hibbert et al. (15) examined the therapeutic efficacy of GSK3β inhibitors on EPCs from diabetic patients (D-EPC). The study addressed two important questions: 1) Does ex vivo treatment of D-EPCs with GSK3β inhibitors increase EPC yield and attenuate EPC dysfunction, and 2) What intracellular proteins mediate the salutary effects of GSK3β inhibitors?
To that end, Hibbert et al. (15) confirm prior findings of reduced EPC number and increased apoptosis in subjects with diabetes. However, for the first time, they also demonstrate increased GSK3β and phosphorylated β-catenin levels in D-EPCs. As expected, treatment of D-EPCs with GSK3β inhibitors reduced apoptosis, increased VEGF secretion, and enhanced EPC invasive capacity in vitro. A proteomic approach was used to analyze proteins that are differentially expressed in healthy EPCs, D-EPCs, and D-EPCs treated with GSK3β inhibitors. Among the 37 nonredundant, differentially regulated proteins, cathepsin B, a lysosomal cysteine protease, was downregulated in D-EPCs relative to EPCs from healthy individuals. Interestingly, GSK3β inhibition in D-EPCs enhanced cathepsin B expression and activity. The new report also demonstrated that increased survival and enhanced invasive ability of EPCs following GSK3β inhibition were mediated by increased cathepsin B activity. Finally, infusion of D-EPCs pretreated with GSK3β inhibitors attenuated neointima formation in a mouse model of femoral artery injury, an effect lost with concomitant inhibition of cathepsin B activity. Taken together, these intriguing findings suggest a previously unidentified role for cathepsin B in mediating the proliferative and vasculogenic effects of GSK3β inhibitors.
As with any good study, this study raises many interesting questions. How do GSK3β inhibitors increase endothelial cathepsin B expression? Is cathepsin B a Wnt/β-catenin target gene in the endothelium? What are the cellular mechanisms mediating the prosurvival effects of increased cathepsin B activity? These questions remain unanswered in this study. Recent reports in other cell systems appear to support some of the new data from Hibbert et al. (15). For example, activation of the Wnt/β-catenin pathway during human mesenchymal stem cell differentiation is, indeed, associated with increased expression of cathepsin B (16). Similarly, treatment of murine mesenchymal stem cells with lithium chloride, a GSK3β inhibitor and Wnt/β-catenin pathway mimetic, induces cathepsin H expression (17). Thus, increased GSK3β activity as observed in D-EPCs may play a role in downregulating cathepsin B expression.
High glucose is known to attenuate the expression of cathepsin B and cathepsin L, another cysteine protease (18,19). In fact, cathepsin L activity of EPCs was inversely related with A1C levels in diabetic subjects (19). Cathepsin L in EPCs is essential for their invasive capacity and plays a critical role in EPC-induced neovascularization (20). Cathepsins L, H, and O (but not B) are highly expressed in EPCs compared with mature endothelial cells (20). In Hibbert et al. (15), expression of cathepsin L was not reported, and the role of cathepsin B in GSK3β inhibitor–treated EPCs was inferred solely from inhibitor studies with CA074. However, CA074 may not be entirely specific to cathepsin B (21), suggesting that experiments with genetic ablation of cathepsin B are needed to confirm the role of cathepsin B in EPC function. In addition, the effect of GSK3β inhibitors on differential expression of cathepsins in EPCs may offer additional insights. Finally, increased cathepsin B expression may negatively affect EPC survival. Indeed, cathepsin B increases EPC senescence by accelerating the proteolytic cleavage of sirtuin 1 (SIRT1), a key player in EPC self-renewal and survival (22).
In conclusion, the novel findings by Hibbert et al. (15) highlight the role of cathepsin B in GSK3β-mediated modulation of EPC dysfunction in patients with diabetes. Whether cathepsin B can be targeted to improve therapeutic angiogenesis remains to be determined. However, ex vivo treatment with GSK3β inhibitor attenuates diabetes-induced EPC dysfunction to increase the yield of functional EPCs for autologous cell-based therapy.
Article Information
Funding. This work was supported by the Intramural Research Program of National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. J.R.S. is supported by National Institutes of Health (R01-HL-73101 and R01-HL-107910) and Veterans Affairs Merit System 0018.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 1410.
References
- 1.Smith SC, Jr, Faxon D, Cascio W, et al. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group VI: revascularization in diabetic patients. Circulation 2002;105:e165–e169 [DOI] [PubMed] [Google Scholar]
- 2.Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J 2011;32:1197–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raval Z, Losordo DW. Cell therapy of peripheral arterial disease: from experimental findings to clinical trials. Circ Res 2013;112:1288–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asahara T, Kawamoto A, Masuda H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells 2011;29:1650–1655 [DOI] [PubMed] [Google Scholar]
- 5.Silvestre JS, Smadja DM, Lévy BI. Postischemic revascularization: from cellular and molecular mechanisms to clinical applications. Physiol Rev 2013;93:1743–1802 [DOI] [PubMed] [Google Scholar]
- 6.Dimmeler S. Regulation of bone marrow-derived vascular progenitor cell mobilization and maintenance. Arterioscler Thromb Vasc Biol 2010;30:1088–1093 [DOI] [PubMed] [Google Scholar]
- 7.Massberg S, Konrad I, Schürzinger K, et al. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med 2006;203:1221–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995;378:785–789 [DOI] [PubMed] [Google Scholar]
- 9.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science 2002;296:1644–1646 [DOI] [PubMed] [Google Scholar]
- 10.Sodhi A, Montaner S, Miyazaki H, Gutkind JS. MAPK and Akt act cooperatively but independently on hypoxia inducible factor-1alpha in rasV12 upregulation of VEGF. Biochem Biophys Res Commun 2001;287:292–300 [DOI] [PubMed] [Google Scholar]
- 11.Hibbert B, Ma X, Pourdjabbar A, et al. Inhibition of endothelial progenitor cell glycogen synthase kinase-3beta results in attenuated neointima formation and enhanced re-endothelialization after arterial injury. Cardiovasc Res 2009;83:16–23 [DOI] [PubMed] [Google Scholar]
- 12.Kim HS, Skurk C, Thomas SR, et al. Regulation of angiogenesis by glycogen synthase kinase-3beta. J Biol Chem 2002;277:41888–41896 [DOI] [PubMed] [Google Scholar]
- 13.Choi JH, Hur J, Yoon CH, et al. Augmentation of therapeutic angiogenesis using genetically modified human endothelial progenitor cells with altered glycogen synthase kinase-3beta activity. J Biol Chem 2004;279:49430–49438 [DOI] [PubMed] [Google Scholar]
- 14.Jarajapu YP, Grant MB. The promise of cell-based therapies for diabetic complications: challenges and solutions. Circ Res 2010;106:854–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbert B, Lavoie JR, Ma X, et al. Glycogen synthase kinase-3β inhibition augments diabetic endothelial progenitor cell abundance and functionality via cathepsin B: A novel therapeutic opportunity for arterial repair. Diabetes 2014;63:1410–1421 [DOI] [PubMed] [Google Scholar]
- 16.Herencia C, Martínez-Moreno JM, Herrera C, et al. Nuclear translocation of β-catenin during mesenchymal stem cells differentiation into hepatocytes is associated with a tumoral phenotype. PLoS One 2012;7:e34656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishimoto KN, Itoi E. Lithium chloride enhances cathepsin H Expression and BMP-4 Degradation in C3H10T1/2 Cells. Biomed Res Int 2013;2013:143742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moheimani F, Kim CH, Rahmanto AS, van Reyk DM, Davies MJ. Inhibition of lysosomal function in macrophages incubated with elevated glucose concentrations: a potential contributory factor in diabetes-associated atherosclerosis. Atherosclerosis 2012;223:144–151 [DOI] [PubMed] [Google Scholar]
- 19.Urbich C, Dernbach E, Rössig L, Zeiher AM, Dimmeler S. High glucose reduces cathepsin L activity and impairs invasion of circulating progenitor cells. J Mol Cell Cardiol 2008;45:429–436 [DOI] [PubMed] [Google Scholar]
- 20.Urbich C, Heeschen C, Aicher A, et al. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med 2005;11:206–213 [DOI] [PubMed] [Google Scholar]
- 21.Steverding D. The cathepsin B-selective inhibitors CA-074 and CA-074Me inactivate cathepsin L under reducing conditions. Open Enzyme Inhibition Journal 2011;4:11–16 [Google Scholar]
- 22.Chen J, Xavier S, Moskowitz-Kassai E, et al. Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence. Am J Pathol 2012;180:973–983 [DOI] [PMC free article] [PubMed] [Google Scholar]