Abstract
Our objective was to correlate vascular endothelial growth factor (VEGF) genetic polymorphisms with the risk of bronchopulmonary dysplasia (BPD) development in premature newborns. Fifty-five newborns with BPD (BPD: median gestational age [GA]: 27 weeks, birthweight [BW]: 786 g) and 42 newborns without BPD (non-BPD: median GA: 29 weeks, BW: 1,165 g), who were born at <32 weeks gestational age and were admitted to Kobe University Hospital, were included. BPD was defined as oxygen dependency at 36 weeks postmenstrual age. Genomic DNA was extracted from the umbilical cord, cord blood, or buccal mucosa. Six VEGF genotypes (-1498T > C, -1154G > A, -634C > G, -7C > T, 936C > T, and 1612G > A) were determined by DNA sequencing. Clinical characteristics, and allele and genotype frequencies of VEGF in the BPD and non-BPD groups were analyzed. G allele frequencies in -634C > G of the BPD group were significantly higher than in the non-BPD group (66.4% vs. 50%, P = 0.02). -634C > G genotype distributions differed significantly between the BPD and non-BPD groups (BPD: CC 7%/CG 53%/GG 40%; non-BPD: CC 24%/CG 52%/GG 24%; P = 0.04). Multivariate logistic regression showed that duration of ventilation, VEGF-634G > C G alleles, and male gender were independent risk factors for BPD. In conclusion, polymorphism VEGF -634C > G may influence the risk of BPD.
The incidence of bronchopulmonary dysplasia (BPD), a form of chronic lung disease, is approximately 30% for infants with birth weights < 1,000 g1, and is associated with poor neurodevelopmental and medical outcomes2. Moreover, the absolute numbers of extremely premature infants surviving with BPD is rising because of increasing numbers of preterm births2.
BPD is characterized by impaired alveolarization and vascularization in developing lungs3, and its pathogenesis is multi-factorial, including fetal infection or inflammation, absence of antenatal steroids, oxidant stress, ventilator-induced lung injury, postnatal inflammation/infection, poor nutrition, abnormal growth-factor signaling, and genetic factors4,5.
Among the neonatal morbidities, significant genetic susceptibility for BPD in preterm infants has previously been identified in two separate multicenter retrospective twin studies6,7. In the study comparing the intrapair occurrence of BPD within monozygotic and dizygotic twins, sharing 100% and approximately 50% of their genome, respectively, Bhandari et al. found that the observed concordance for developing BPD was significantly higher than the expected concordance in monozygotic twins6. Similarly, Lavoie et al. reported that intrapair correlation coefficients in monozygotic pairs were greater than in dizygotic pairs for the co-occurrence of 36-week oxygen need-based BPD7. According to these studies, the heritability of moderate-severe BPD is estimated at 53–79%8.
Vascular endothelial growth factor (VEGF), a major mediator of vascular permeability, endothelial cell proliferation, and migration, is important in vasculogenesis and angiogenesis9,10. It also plays a crucial role in fetal development. For example, mice deficient in VEGF showed embryonic lethality because of defects in the development of the vascular system11,12. In humans, VEGF mRNA can be detected in fetal tissues from 16 weeks of gestation13. During the fetal period, VEGF is expressed in villous and extravillous trophoblasts14, and the expression level alters with adverse pregnancy outcomes15. Previously, a developmental increase in VEGF levels was reported in umbilical cord plasma, with premature fetuses having significantly lower mean VEGF concentrations than term fetuses16.
VEGF expression levels are particularly high in the lung, where it is essential in lung development and structural maintenance17. A previous immunohistochemical study of aborted human fetuses showed that VEGF was strongly expressed in the epithelial cells of most distal airways, suggesting that it is involved in the maturation as well as proliferation of capillary endothelial cells18. Interestingly, abnormal VEGF signaling was associated with the pathophysiology of pulmonary hypertension19,20, which is strongly linked to BPD development21,22. Infants with BPD demonstrate decreased VEGF levels in their lungs, suggesting an association between disrupted alveolar vascular development and impaired VEGF expression in BPD patients23.
The VEGF gene is located on chromosome 6q21.3, and consists of eight exons and seven introns. Several VEGF polymorphisms have been identified that influence VEGF protein production through the regulation of VEGF expression24,25,26,27. For example, some are associated with neonatal disease morbidities, especially in retinopathy of prematurity28,29. However, no studies have investigated the correlation between VEGF polymorphisms and BPD risk in the Japanese population, so the present study set out to achieve this aim.
Results
Clinical characteristics of patients in BPD and non-BPD groups
Univariate and multivariate analyses were performed of the 97 patients. The median GA of preterm infants in the BPD group was significantly younger than in the non-BPD group (27 [range, 23–31] vs. 29 [range, 25–31] weeks). The median BWs of the preterm infants also differed significantly between the groups (786 [range, 430–1,626] vs. 1,165 [range, 800–1,810] g). Furthermore, the BPD group had a significantly lower Apgar score at 5 min, and a significantly higher duration of ventilation and oxygen than the non-BPD group. Univariate analysis of categorized factors revealed that gender, maternal smoking status, SGA, respiratory distress syndrome, and use of surfactant were significant risk factors for the development of BPD. The risk of BPD was not significantly influenced by other tested factors (Table 1).
Table 1. Patient clinical characteristics of BPD and non-BPD groups.
| BPD, n = 55 | non-BPD, n = 42 | P-value | |
|---|---|---|---|
| Gestational age (weeks) | 27 (23–31) | 29 (25–31) | <0.01 |
| Birth weight (g) | 786 (430–1626) | 1165 (800–1810) | <0.01 |
| Apgar score at 5 min | 8 (1–10) | 9 (3–10) | <0.01 |
| Male (%) | 35/55 (63.6) | 17/42 (40.5) | <0.05 |
| Multiple pregnancy (%) | 15/55 (27.3) | 10/42 (23.8) | 0.70 |
| Cesarean section (%) | 52/55 (94.6) | 37/42 (88.1) | 0.25 |
| Antenatal steroids (%) | 22/55 (40) | 21/41 (51.2) | 0.27 |
| Maternal age (years) | 32 (19–42) | 33 (21–43) | 0.13 |
| Primiparity (%) | 25/55 (45.5) | 12/42 (28.6) | 0.09 |
| Histological chorioamnionitis (%) | 18/54 (33.3) | 12/38 (31.6) | 0.86 |
| Maternal smoking (%) | 6/54 (11.1) | 11/41 (26.8) | <0.05 |
| Assisted reproductive technology (%) | 12/54 (22.2) | 11/42 (26.2) | 0.65 |
| Pregnancy-induced hypertension (%) | 13/55 (23.6) | 9/42 (21.4) | 0.80 |
| Premature rupture of the membranes (%) | 13/55 (23.6) | 7/42 (16.7) | 0.40 |
| Small-for-gestational age (%) | 31/55 (56.4) | 12/42 (28.6) | <0.01 |
| Symptomatic patent ductus arteriosus (%) | 17/48 (35.4) | 9/42 (21.4) | 0.14 |
| Respiratory distress syndrome (%) | 51/55 (92.7) | 32/42 (76.2) | <0.05 |
| Use of surfactant (%) | 52/55 (94.5) | 30/42 (71.4) | <0.01 |
| Duration of ventilation (days) | 61 (2–129) | 25 (0–60) | <0.01 |
| Duration of oxygen (days) | 75 (33–143) | 33 (7–67) | <0.01 |
| Culture-proven sepsis (%) | 5/55 (9.1) | 2/42 (4.8) | 0.41 |
| Serum IgM at birth ≥ 20 mg/dL (%) | 1/54 (1.9) | 2/41 (4.9) | 0.40 |
Data are expressed as medians (range) or numbers (%).
VEGF allele and genotype frequencies of the BPD and non-BPD groups
The observed genotype frequencies did not deviate from Hardy-Weinberg equilibrium. The VEGF polymorphisms -1498 T > C, -1154 G > A, -634 C > G, -7 C > T, 936 C > T, and 1612 G > A in the BPD and non-BPD groups are shown in Table 2. The distribution of allele (BPD: C 33.6%/G 66.4%; non-BPD: C 50%/G 50%; P = 0.02) and genotype (BPD: CC 7%/CG 53%/GG 40%; non-BPD: CC 24%/CG 52%/GG 24%; P = 0.04) frequencies of the VEGF -634C > G polymorphism differed significantly between the BPD and non-BPD groups (Table 2).
Table 2. VEGF allele and genotype frequencies of BPD and non-BPD groups.
| Polymorphism | Genotype | BPD, n = 55 | non-BPD, n = 42 | P-value |
|---|---|---|---|---|
| -1498T > C | TT/TC/CC | 24/23/8 | 22/14/6 | 0.66 |
| -1154G > A | GG/GA/AA | 38/15/2 | 25/16/1 | 0.51 |
| -634C > G | CC/CG/GG | 4/29/22 | 10/22/10 | 0.04 |
| -7C > T | CC/CT/TT | 34/20/1 | 33/9/0 | 0.17 |
| 936C > T | CC/CT/TT | 27/27/1 | 26/15/1 | 0.42 |
| 1612G > A | GG/GA/AA | 40/15/0 | 33/9/0 | 0.51 |
VEGF haplotypes
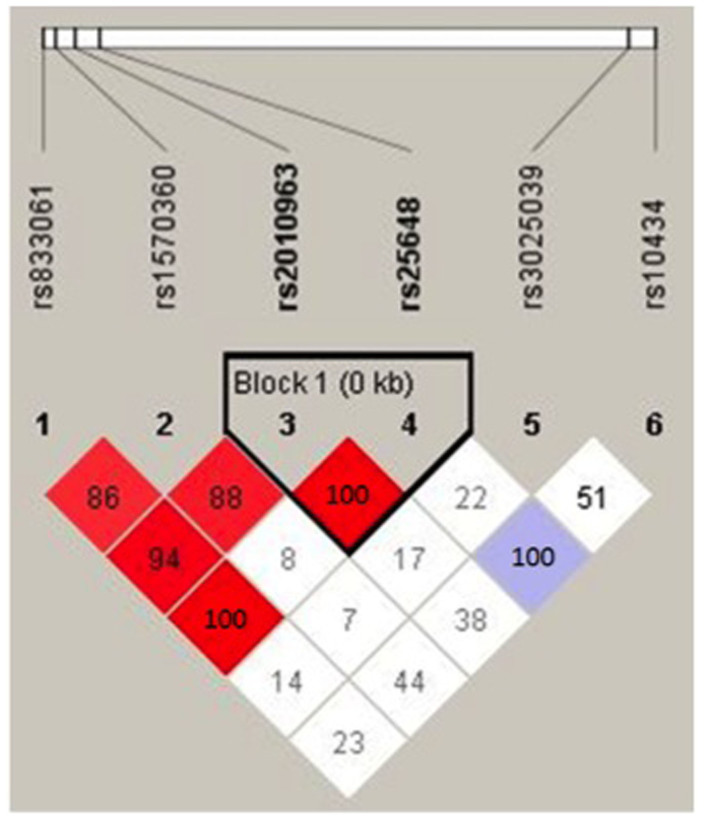
Linkage disequilibrium (LD) plots from the analysis of VEGF SNPs are shown in Figure 1. Strong LD was observed between the -634C > G (rs2010963) polymorphism and the three SNPs in the promoter and 5′ UTR region. Haploview analysis based on the four-gamete rule44 identified one block (<1 kb) containing -634C > G (rs2010963) and -7C > T (rs25648) in the 5′ UTR. Table 3 shows the two-locus [rs2010963 and rs25648] VEGF haplotype. As shown, the -634G/-7C haplotype was significantly decreased in patients with BPD compared with those without (P = 0.02).
Figure 1. Haploview linkage disequilibrium (LD) plots of the six VEGF SNPs analyzed.
Red blocks, D′ (normalized linkage disequilibrium measure or D) ≤ 1.0, with logarithm of odds (LOD) score ≥ 2.0; white blocks, D′ < 1.0 with LOD < 2.0; blue blocks, D′ = 1.0 with LOD < 2.0. Numbers in blocks denote D′ values. The genomic organization is described above the LD plot. LOD was defined as log10(L1/L0), where L1 = likelihood of the data under linkage disequilibrium, and L0 = likelihood of the data under linkage equilibrium. D′ was calculated as follows: D′ = (D) divided by the theoretical maximum for the observed allele frequencies.
Table 3. VEGF haplotype frequencies of BPD and non-BPD groups.
| Frequency | BPD, n = 110 | non-BPD, n = 84 | χ2 | P-value | |
|---|---|---|---|---|---|
| -634G/-7C | 0.433 | 51 (46.4%) | 33 (39.3%) | 0.972 | 0.32 |
| -634C/-7C | 0.407 | 37 (33.6%) | 42 (50.0%) | 5.283 | 0.02 |
| -634G/-7T | 0.16 | 22 (20.0%) | 9 (10.7%) | 3.059 | 0.08 |
Multivariate stepwise logistic regression analysis of factors associated with BPD development
Stepwise logistic regression analysis was performed to determine whether G alleles in VEGF-634C > G were independently associated with the development of BPD based on factors that showed a significant difference between groups, including gestational age, birth weight, Apgar score at 5 min, gender, maternal smoking status, SGA, respiratory distress syndrome, use of surfactant, and duration of ventilation. Based on the finding by Awata et al. that healthy Japanese subjects without VEGF-634C > G G alleles showed significantly higher serum VEGF levels than those with G alleles35, we assumed a dominant model in this analysis. Because BPD was defined in terms of oxygen requirement, the duration of oxygen supplementation was not included as an independent variable in this analysis45. This revealed that duration of ventilation (P < 0.01), the carrying of G alleles in VEGF-634C > G (P = 0.03), and male gender (P = 0.02) were significant independent risk factors for the development of BPD (Table 4).
Table 4. Multivariate stepwise logistic regression analysis of BPD risk factors.
| Likelihood ratio chi2 | R2 | P-value | |
|---|---|---|---|
| Duration of ventilation | 43.57 | 0.34 | <0.01 |
| Any G -634C > G | 4.53 | 0.37 | 0.03 |
| Male gender | 5.20 | 0.41 | 0.02 |
Discussion
Because BPD has a multifactorial etiology5, it is important to collect major causative factors to explore the effects of genetic factors on its development6. We collected details of antenatal and postnatal causative factors from our population that was treated uniformly at a single institution, and used multivariate analysis to show that VEGF-634C > G, the duration of mechanical ventilation, and male gender were independent risk factors. Mechanical ventilation46,47 and male gender48,49 have previously been described as risk factors of BPD; however, the causative effect of VEGF-634C > G on the development of BPD was elucidated for the first time in this study.
Recently, an imbalance of pro-inflammatory and anti-inflammatory cytokines leading to the activation of cellular death pathways in the lung, followed by healing and repair was proposed to be a pathologic hallmark of BPD. Interestingly, each of these steps is closely associated with genetic susceptibility, and BPD is considered to develop from gene-environment interactions, not as secondary to environmental factors6,50. Additionally, many growth factors under genetic regulation were found to be associated with the disturbance of late lung development51. Thus, we believe that a genetic predisposition is intriguing in consideration of the development of BPD.
Lung angiogenesis is largely regulated by VEGF52, and the importance of VEGF in the development of BPD has been reported in several previous studies. Bhatt et al. carried out a lung comparison study between infants dying with BPD or nonpulmonary causes, and found that the former group had decreased VEGF mRNA levels and decreased VEGF immunostaining compared with the latter group23. Another study investigated VEGF in tracheal aspirates, and revealed that preterm infants who developed BPD had lower VEGF levels during the early postnatal days than those without the disease, suggesting a prolonged and more severe respiratory distress53. Meanwhile, Levesque et al. reported that low VEGF protein levels in infant urine and the need for mechanical ventilation were both associated with BPD development; however, the VEGF protein association was no longer significant once the model was adjusted for respiratory support54. Therefore, the correlation between BPD development and systemic VEGF levels remains unclear. Nevertheless, we speculate that pathogenic lung angiogenesis in BPD is mostly driven by local VEGF synthesis.
Various VEGF genetic polymorphisms have been identified that influence the levels of VEGF expression24,27. Our results showed that there was a significant difference in distribution of the -634C > G allele and genotype in premature Japanese infants with BPD. Additionally, one haploblock that included the -634C > G allele was identified, while the frequency of haplotype -634C/-7C that contains only C alleles showed a significant decrease in the BPD compared with the non-BPD population. The -634 C > G polymorphism is located in the 5′ UTR of the VEGF gene, and is predicted to lie within a potential myeloid zinc finger protein (MZF1) binding site, which controls the binding specificity of this motif24. Adult carriers of the -634 C > G polymorphism CC genotype were found to have significantly higher levels of plasma VEGF35, while Lambrechts et al. showed that the G allele reduces internal ribosome entry site-mediated VEGF expression and translation of the large L-VEGF isoform55. However, no data are available on the genetic effects on VEGF levels in immature and developing lungs. We predict that Japanese premature newborns with the VEGF -634 G allele will show decreased VEGF levels in their lungs and will be susceptible to BPD.
Multiple testing procedures usually increase the probability of obtaining false-positives unless statistical adjustments such as Sidak or Bonferroni corrections are employed. However, such corrections are based on the hypothesis that each testing is completely independent, and would remarkably overcorrect for the false-positive rate if each test correlated, resulting in a reduction in power56. Indeed, the types of correction to be adapted for a genetic polymorphism study remain controversial. In the current study, we observed remarkable LD in our six VEGF SNPs studied, so we attempted to assess the statistical significance of haplotype frequency differences by performing permutation tests using Haploview instead of conservative correction, following previous reports57,58,59. We observed a single χ2 value greater than 5.283 392 times (empirical P = 0.0392) from 10,000 permutation tests.
In contrast with our results and others, Kwinta et al. found no association between the allele state of VEGF -634 C > G and risk of BPD in a Polish population60. The first genome-wide association study (GWAS) for BPD that included only Caucasian and African ethnic infants identified SPOCK2 SNPs as genetic risks, but not VEGF -634 C > G61. Moreover, the recent GWAS for BPD could not identify SNPs associated with BPD at a genome-wide significance level8. However, we cannot compare our results with these directly because of the study differences with respect to ethnic background, since ethnicity greatly influences the distribution of gene polymorphisms. We were interested in studying the VEGF -634 C > G polymorphism in the Japanese population because it has a homogeneous ethnic origin compared with the more heterogeneous characteristics of ethnic groups examined in previous studies8,60.
One limitation of the present study is its relatively small sample size as it was a retrospective study conducted at a single center. Although this sample size is much smaller than those of previous studies8,61, the first GWAS did not include an Asian population61 while the more recent GWAS included a population of 167 Asian/Pacific islanders that was only 9.6% of the total cohort8. We therefore believe that our results based on 97 infants of single Japanese ethnicity that were uniformly treated in a single institution are meaningful considering the genetic risks of BPD in Asian populations. Further studies of larger sample sizes are therefore necessary to clarify the observed VEGF genotype-specific effects on BPD development.
In conclusion, we identified VEGF -634 C > G as an independent risk factor for the development of BPD using a single center study of Japanese infants less than 32 weeks' gestational age; those infants with the -634 G allele were shown to have a higher susceptibility for BPD.
Methods
Study design and patient groups
This retrospective study was conducted under the approval of the ethical committee of Kobe University Graduate School of Medicine with written informed consent acquired from the parents of the patients. This study was also performed in accordance with the ethics guidelines for human genome/gene analysis research from the Ministries of Education, Culture, Sports, Science and Technology, Health, Labour and Welfare, and Economy, Trade and Industry in Japan.
Ninety-seven infants who were born at our center between 2002 and 2011 with a gestational age (GA) less than 32 weeks were included in the study. All infants were treated at the neonatal intensive care unit of Kobe University Hospital. Patients with congenital or chromosomal anomalies were excluded. Study patients were classified into newborns with BPD (BPD group, n = 55) and newborns without BPD (non-BPD group, n = 42), according to their level of oxygen dependency at a postnatal gestational age of 36 weeks. Clinical, laboratory, and VEGF genetic polymorphism data between BPD and non-BPD groups were analyzed.
Patient clinical data
Clinical data were collected from the patient's record and included GA, birth weight (BW), Apgar score (a numerical expression of an infant's condition after birth, usually determined from heart rate, respiratory efforts, muscle tone, reflex irritability, and color) at 5 min30, gender, antenatal steroid uses, maternal age, primiparity, chorioamnionitis histology, maternal smoking status, the presence or absence of assisted reproductive technology, pregnancy-induced hypertension (maternal systolic blood pressure > 140 mmHg and/or diastolic pressure > 90 mmHg during pregnancy), premature rupture of the membranes (rupture of the membranes more than 24 h before delivery), small for gestational age (SGA; BW less than the 10th percentile of mean BW at the same gestational age in Japanese newborns)31, symptomatic patent ductus arteriosus (therapeutic use of indomethacin or surgical ligation), respiratory distress syndrome diagnosed by chest X-ray findings and microbubble test, use of surfactant, duration of ventilation (regardless of the mode of ventilation), duration of oxygen, culture-proven sepsis and increased serum IgM concentration at birth (≥20 mg/dL), and details of multiple pregnancies and birth by Cesarean section28. BPD was diagnosed when the infants required oxygen therapy at a corrected postnatal gestational age of 36 weeks in association with characteristic radiologic findings32.
VEGF polymorphism genotyping
Genomic DNA was extracted from the umbilical cord, cord blood, or buccal mucosa using a DNeasy Tissue Kit (Qiagen, Hilden, Germany) or QIAamp DNA Mini Kit (Qiagen), according to the manufacturer's instructions. Six VEGF candidate polymorphisms were selected: -1498T > C (rs833061) and -1154G > A (rs1570360) in the promoter region, -634C > G (rs2010963) and -7C > T (rs25648) in the 5′ UTR, and 936C > T (rs3025039) and 1612G > A (rs10434) in the 3′ UTR. Among the polymorphisms that showed a minor allele frequency of at least 0.10 in the Japanese population, we chose these six single nucleotide polymorphisms (SNPs) because they have been shown to influence gene expression27,33, protein production24,25, and plasma levels26, and were also previously investigated in terms of their association with disease or clinical situations in Japanese populations34,35,36,37,38, according to previous reports28,39. They were identified by PCR, and restriction fragment length polymorphism analysis or direct sequencing28.
Statistical analysis
Data are expressed as the median (range) or number (%). Univariate analyses were performed using the Mann-Whitney nonparametric rank test, chi-square test, or Fisher's exact test as appropriate to compare data between the two groups. To prevent the possible effects of confounding factors, significant factors identified in univariate analysis underwent further analyses, including stepwise logistic regression. Differences were deemed statistically significant when P < 0.05. Analyses were performed using GraphPad Prism 5.0 software (Graphpad Software, Inc., San Diego, CA, USA) and JMP version 8.0.2 (SAS Institute Japan., Tokyo, Japan).
To test whether the genotypes were in Hardy-Weinberg equilibrium, we compared the observed genotype frequencies with expected frequencies at equilibrium based on the chi-square test, according to previous reports40,41. LD analysis and haplotype reconstruction were performed using Haploview 4.2 (http://www.broadinstitute.org/scientific-community/science/programs/medical-and-population-genetics/haploview/haploview)42. Sample size calculations were performed using G*Power 3.1 (http://www.gpower.hhu.de/)43, based on our previous report that showed the frequency of common alleles of the six VEGF SNPs to range between 60 and 90% and the prevalence of BPD to be approximately 50% in the studied population28. If a common allele frequency was calculated to differ by 20% between BPD and non-BPD groups, this would result in an 80% chance of detecting this difference with a type I risk error of 5% with 50 infants (100 alleles) in each group.
Author Contributions
K.F. and I.M. designed the study, carried out the statistical analyses and wrote the manuscript. All authors contributed to the intellectual content of this manuscript. K.F., A.S., T.Y., T.K., M.N., K.I. and I.M., were pediatricians who treated the patients and were collected the clinical data and samples. H.Y. were obstetricians and collected the cord blood samples of the patients. K.F., M.Y. and Y.T. analyzed the VEGF polymorphism genotyping.
Acknowledgments
This study was supported by the Support Program for Improving Graduate School Education from the Ministry of Education, Culture, Sports, Science and Technology in Japan (K.F.), and grants for Scientific Research from the Morinaga Hoshi-kai Foundation 2011 (K.F.), and the Ministry of Education, Culture, Sports, Science and Technology in Japan (K.F., #23791225 and I.M., #23791224).
References
- Stevenson D. K. et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1993 through December 1994. Am J Obstet Gynecol. 179, 1632–1639 (1998). [DOI] [PubMed] [Google Scholar]
- Shepherd E. G. et al. An interdisciplinary bronchopulmonary dysplasia program is associated with improved neurodevelopmental outcomes and fewer rehospitalizations. J Perinatol. 32, 33–38 (2012). [DOI] [PubMed] [Google Scholar]
- Tang J. R. et al. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol. 287, L344–351 (2004). [DOI] [PubMed] [Google Scholar]
- Egawa T. et al. Ureaplasma urealyticum and Mycoplasma hominis presence in umbilical cord is associated with pathogenesis of funisitis. Kobe J Med Sci. 53, 241–249 (2007). [PubMed] [Google Scholar]
- Kinsella J. P., Greenough A. & Abman S. H. Bronchopulmonary dysplasia. Lancet. 367, 1421–1431 (2006). [DOI] [PubMed] [Google Scholar]
- Bhandari V. et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 117, 1901–1906 (2006). [DOI] [PubMed] [Google Scholar]
- Lavoie P. M., Pham C. & Jang K. L. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics. 122, 479–485 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. A genome-wide association study (GWAS) for bronchopulmonary dysplasia. Pediatrics. 132, 290–297 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H. & Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond). 109, 227–241 (2005). [DOI] [PubMed] [Google Scholar]
- Breier G., Albrecht U., Sterrer S. & Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 114, 521–532 (1992). [DOI] [PubMed] [Google Scholar]
- Carmeliet P. et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 380, 435–439 (1996). [DOI] [PubMed] [Google Scholar]
- Ferrara N. et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 380, 439–442 (1996). [DOI] [PubMed] [Google Scholar]
- Shifren J. L., Doldi N., Ferrara N., Mesiano S. & Jaffe R. B. In the human fetus, vascular endothelial growth factor is expressed in epithelial cells and myocytes, but not vascular endothelium: implications for mode of action. J Clin Endocrinol Metab. 79, 316–322 (1994). [DOI] [PubMed] [Google Scholar]
- Clark D. E., Smith S. K., Sharkey A. M. & Charnock-Jones D. S. Localization of VEGF and expression of its receptors flt and KDR in human placenta throughout pregnancy. Hum Reprod. 11, 1090–1098 (1996). [DOI] [PubMed] [Google Scholar]
- Andraweera P. H., Dekker G. A., Laurence J. A. & Roberts C. T. Placental expression of VEGF family mRNA in adverse pregnancy outcomes. Placenta. 33, 467–472 (2012). [DOI] [PubMed] [Google Scholar]
- Lassus P. et al. Vascular endothelial growth factor and angiogenin levels during fetal development and in maternal diabetes. Biol Neonate. 84, 287–292 (2003). [DOI] [PubMed] [Google Scholar]
- Voelkel N. F., Vandivier R. W. & Tuder R. M. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 290, L209–221 (2006). [DOI] [PubMed] [Google Scholar]
- Maeda S. et al. Analysis of intrapulmonary vessels and epithelial-endothelial interactions in the human developing lung. Lab Invest. 82, 293–301 (2002). [DOI] [PubMed] [Google Scholar]
- Fujita M. et al. Pulmonary hypertension in TNF-alpha-overexpressing mice is associated with decreased VEGF gene expression. J Appl Physiol. 93, 2162–2170 (2002). [DOI] [PubMed] [Google Scholar]
- Grover T. R. et al. Intrauterine hypertension decreases lung VEGF expression and VEGF inhibition causes pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 284, L508–517 (2003). [DOI] [PubMed] [Google Scholar]
- Jobe A. H. & Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 163, 1723–1729 (2001). [DOI] [PubMed] [Google Scholar]
- Goodman G. et al. Pulmonary hypertension in infants with bronchopulmonary dysplasia. J Pediatr. 112, 67–72 (1988). [DOI] [PubMed] [Google Scholar]
- Bhatt A. J. et al. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 164, 1971–1980 (2001). [DOI] [PubMed] [Google Scholar]
- Watson C. J., Webb N. J., Bottomley M. J. & Brenchley P. E. Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine. 12, 1232–1235 (2000). [DOI] [PubMed] [Google Scholar]
- Shahbazi M. et al. Vascular endothelial growth factor gene polymorphisms are associated with acute renal allograft rejection. J Am Soc Nephrol. 13, 260–264 (2002). [DOI] [PubMed] [Google Scholar]
- Renner W., Kotschan S., Hoffmann C., Obermayer-Pietsch B. & Pilger E. A common 936 C/T mutation in the gene for vascular endothelial growth factor is associated with vascular endothelial growth factor plasma levels. J Vasc Res. 37, 443–448 (2000). [DOI] [PubMed] [Google Scholar]
- Yamamori M. et al. Association of VEGF genotype with mRNA level in colorectal adenocarcinomas. Biochem Biophys Res Commun. 325, 144–150 (2004). [DOI] [PubMed] [Google Scholar]
- Yagi M. et al. VEGF 936C > T is Predictive of Threshold Retinopathy of Prematurity in Japanese Infants with Gestational Age of 30 weeks or less. Res Rep Neonatol. 1, 5–11 (2011). [Google Scholar]
- Cooke R. W., Drury J. A., Mountford R. & Clark D. Genetic polymorphisms and retinopathy of prematurity. Invest Ophthalmol Vis Sci. 45, 1712–1715 (2004). [DOI] [PubMed] [Google Scholar]
- Salmon J. Fetal alcohol spectrum disorder: New Zealand birth mothers' experiences. Can J Clin Pharmacol. 15, e191–213 (2008). [PubMed] [Google Scholar]
- Itabashi K. et al. Revised Birth size standards by gestational age for Japanese neonates. J Jpn Pediatr Soc. 114, 1271–1293 (In Japanese) (2010). [Google Scholar]
- Shennan A. T., Dunn M. S., Ohlsson A., Lennox K. & Hoskins E. M. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 82, 527–532 (1988). [PubMed] [Google Scholar]
- Hankinson J. The role of VEGF in lung function. The University of Manchester Library, 2013. 1–223 (2013). [Google Scholar]
- Morohashi K., Takada T., Omori K., Suzuki E. & Gejyo F. Vascular endothelial growth factor gene polymorphisms in Japanese patients with sarcoidosis. Chest. 123, 1520–1526 (2003). [DOI] [PubMed] [Google Scholar]
- Awata T. et al. A common polymorphism in the 5′-untranslated region of the VEGF gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes. 51, 1635–1639 (2002). [DOI] [PubMed] [Google Scholar]
- Yamamori M. et al. VEGF T-1498C polymorphism, a predictive marker of differentiation of colorectal adenocarcinomas in Japanese. Int J Med Sci. 5, 80–86 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara T. et al. Effect of polymorphisms in the 3′-untranslated region (3′-UTR) of VEGF gene on gastric pre-malignant condition. Anticancer Res. 29, 485–489 (2009). [PubMed] [Google Scholar]
- Masago K. et al. Effect of vascular endothelial growth factor polymorphisms on survival in advanced-stage non-small-cell lung cancer. Cancer Sci. 100, 1917–1922 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaeda T. et al. VEGF G-1154A is predictive of severe acute toxicities during chemoradiotherapy for esophageal squamous cell carcinoma in Japanese patients. Ther Drug Monit. 30, 497–503 (2008). [DOI] [PubMed] [Google Scholar]
- Hadchouel A. et al. Matrix metalloproteinase gene polymorphisms and bronchopulmonary dysplasia: identification of MMP16 as a new player in lung development. PLoS One. 3, e3188 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez S., Gaunt T. R. & Day I. N. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol. 169, 505–514 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Fry B., Maller J. & Daly M. J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 21, 263–265 (2005). [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Buchner A. & Lang A. G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 41, 1149–1160 (2009). [DOI] [PubMed] [Google Scholar]
- Wang N., Akey J. M., Zhang K., Chakraborty R. & Jin L. Distribution of recombination crossovers and the origin of haplotype blocks: the interplay of population history, recombination, and mutation. Am J Hum Genet. 71, 1227–1234 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers K. M., Gibson A. T., Russell J. M. & Powers H. J. Antioxidant activity, packed cell transfusions, and outcome in premature infants. Arch Dis Child Fetal Neonatal Ed. 78, F214–219 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Marter L. J. et al. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network. Pediatrics. 105, 1194–1201 (2000). [DOI] [PubMed] [Google Scholar]
- Young T. E., Kruyer L. S., Marshall D. D. & Bose C. L. Population-based study of chronic lung disease in very low birth weight infants in North Carolina in 1994 with comparisons with 1984. The North Carolina Neonatologists Association. Pediatrics. 104, e17 (1999). [DOI] [PubMed] [Google Scholar]
- Peacock J. L., Marston L., Marlow N., Calvert S. A. & Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res. 71, 305–310 (2012). [DOI] [PubMed] [Google Scholar]
- Kraybill E. N., Runyan D. K., Bose C. L. & Khan J. H. Risk factors for chronic lung disease in infants with birth weights of 751 to 1000 grams. J Pediatr. 115, 115–120 (1989). [DOI] [PubMed] [Google Scholar]
- Bhandari A. & Bhandari V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics. 123, 1562–1573 (2009). [DOI] [PubMed] [Google Scholar]
- Madurga A., Mizikova I., Ruiz-Camp J. & Morty R. E. Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 305, L893–905 (2013). [DOI] [PubMed] [Google Scholar]
- Acarregui M. J., Penisten S. T., Goss K. L., Ramirez K. & Snyder J. M. Vascular endothelial growth factor gene expression in human fetal lung in vitro. Am J Respir Cell Mol Biol. 20, 14–23 (1999). [DOI] [PubMed] [Google Scholar]
- Lassus P. et al. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med. 164, 1981–1987 (2001). [DOI] [PubMed] [Google Scholar]
- Levesque B. M. et al. Low urine vascular endothelial growth factor levels are associated with mechanical ventilation, bronchopulmonary dysplasia and retinopathy of prematurity. Neonatology. 104, 56–64 (2013). [DOI] [PubMed] [Google Scholar]
- Lambrechts D. et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 34, 383–394 (2003). [DOI] [PubMed] [Google Scholar]
- Nyholt D. R. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 74, 765–769 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov I. et al. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA. 99, 13675–13680 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallin D. et al. Genetic analysis of case/control data using estimated haplotype frequencies: application to APOE locus variation and Alzheimer's disease. Genome Res. 11, 143–151 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll M. et al. Genetic variation in DLG5 is associated with inflammatory bowel disease. Nat Genet. 36, 476–480 (2004). [DOI] [PubMed] [Google Scholar]
- Kwinta P. et al. Genetic risk factors of bronchopulmonary dysplasia. Pediatr Res. 64, 682–688 (2008). [DOI] [PubMed] [Google Scholar]
- Hadchouel A. et al. Identification of SPOCK2 as a susceptibility gene for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 184, 1164–1170 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]