Abstract
Introduction
Renal scintigraphy is an important imaging modality for the diagnosis and management of a variety of renal diseases including obstruction and renovascular hypertension as well as the evaluation of absolute and relative kidney function. The goal of this work was to evaluate Al18F-NODA-butyric acid (Al18F-1) as a potential PET tracer to image the kidneys and monitor renal function by comparing its pharmacokinetic properties with those of 131I-o-iodohippurate (131I-OIH), the radioactive standard for the measurement of effective renal plasma flow.
Methods
Al18F-1 was prepared in aqueous conditions using a one-pot Al18F-radiofluorination method and its radiochemical purity was determined by HPLC. Biodistribution studies, using 131I-OIH as an internal control, were performed in normal rats and in rats with renal pedicle ligation. In vitro stability and metabolism of Al18F-1 were analyzed by HPLC. Dynamic microPET/CT studies were conducted in normal rats.
Results
Al18F-1 showed excellent stability in vitro and in vivo. Biodistribution studies in normal rats and in rats with simulated renal failure confirmed that Al18F-1 was exclusively cleared through the renal-urinary pathway and that the hepatic/gastrointestinal activity was less for Al18F-1 than for 131I-OIH both at 10 and 60 min. Dynamic PET showed a rapid transit of Al18F-1 through the kidneys into the bladder.
Conclusion
These results suggest that the easily labeled Al18F-based compounds provide a highly promising approach for the development of a PET renal radiotracer that combines superior imaging qualities with a reliable measure of effective renal plasma flow.
Keywords: Al18F-NODA-butyric acid, Aluminum fluoride, Renal radiopharmaceutical, Kidney, PET 18F
1. Introduction
Chronic kidney disease (CKD) is recognized as worldwide public health problem [1, 2]. Renal scintigraphy can help address this problem by providing a non-invasive method of evaluating a variety of known or suspected renal diseases and of monitoring renal function. Radionuclide renography also has the advantage of using a subpharmacological dose of radiotracer thus avoiding allergic reactions or toxic effects. Currently, renal scintigraphy is performed using dynamic planar gamma imaging. The best radiotracers are those labeled with technetium-99m (99mTc) since this radionuclide has almost ideal nuclear properties (t1/2 = 6 h, γ-radiation of 140 keV, no beta radiation), can be practically eluted from a 99Mo/99mTc generator, and kits for the routine preparation of 99mTc radiotracers are readily available. The most utilized 99mTc renal radiotracers (Fig. 1) are (1) 99mTc-diethylenetriamine-pentaacetic acid (99mTc-DTPA) which is cleared exclusively by glomerular filtration and can be used to measure glomerular filtration rate (GFR) [3, 4]; (2) 99mTc-mercaptoacetyltriglycine (99mTc-MAG3) which is excreted primarily by tubular secretion and has become the radiopharmaceutical of choice for evaluation of effective renal plasma flow (ERPF) [5–7]; and (3) 99mTc-dimercaptosuccinic acid (99mTc-DMSA) which is retained in the renal tubules and is used clinically for morphological evaluation of the kidneys [8, 9]. Recently, we have identified a new 99mTc renal tracer, 99mTc(CO)3-nitrilotriacetic acid (99mTc-NTA), which has showed pharmacokinetic properties almost identical to those of 131I-o-iodohippurate (131I-OIH) in rats, healthy volunteers and in patients with chronic kidney disease [10–12]. We compared 99mTc-NTA to 131I-OIH since 131I-OIH is still regarded as the radioactive standard for the non-invasive measurement of ERPF [13] even though it is no longer clinically available due to the beta emission of 131I resulting in relative high radiation dose to kidney and thyroid in patients with impaired renal function [14].
Figure 1.
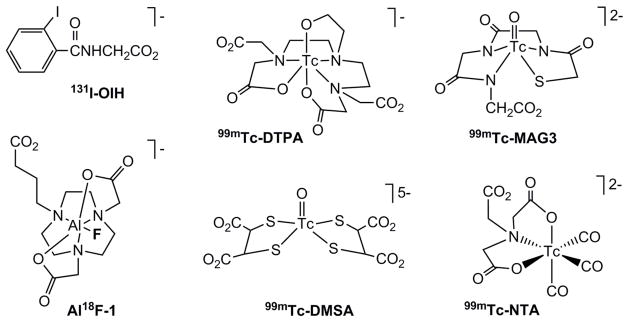
The chemical structures of known 99mTc renal radiotracers and the new 18F tracer evaluated in this study (AlF-1); tracers’ overall charges of at physiological pH are included.
The recent lack of availability of 99mTc due to a shortage of 99Mo supply [15], coupled with favorable physical properties of fluorine-18 (18F) radionuclide (t1/2 = 109.8 min, β+, 97%) and the sensitivity and spatial resolution of positron emission tomography (PET) have stimulated efforts to develop PET renal radiotracers [16–20]. Schnöckel et al. first validated an 18F-PET-based method to quantify renal function in rats using 18F-fluoride and found that PET approach could provide a reproducible and non-invasive estimation of renal function in small animals [18]; this study provided proof of concept but 18F-fluoride could not function as a dedicated renal radiopharmaceutical because of localization in the bone [21, 22]. Two other 18F-labeled agents were recently assessed in rats as potential PET renal radiopharmaceuticals [19, 20]. One of them, 18F-p-fluorohippurate (18F-PFH) was found to be rapidly and exclusively cleared by the kidneys [19] and the quality of its renogram and images obtained by dynamic PET studies were reported to be better than those obtained with 99mTc-MAG3 dynamic planar imaging studies [23]. However, the 18F-PFH synthesis was long and required a four-step, two-pot procedure using a prosthetic group, 18F-N-succinimidyl-4-fluorobenzoate (18F-SFB). The synthesis of the second tracer, 18F-m-cyano-p-fluorohippurate (18F-CNPFH), utilized the simpler process of direct onestep nucleophilic aromatic substitution reaction but biodistribution and dynamic PET imaging studies in rats showed that 18F-CNPFH would not be an effective renal imaging agent because it was eliminated by both renal and hepatobiliary pathways [20].
The development of functional PET imaging of the kidney has been slow due to the widespread availability of single photon planar renal imaging coupled with the lack of rapid and easily accessible 18F labeling procedures. Introduction of 18F-fluorine into a biomolecule usually requires formation of F-C bond; typically a challenging procedure due to multiple steps and harsh reaction conditions [24]. Recently, a new radiofluorination method using aluminum fluoride (Al18F) complex formation was reported in an attempt to address the difficulty in 18F labeling of biomolecules [25–28]. This aqueous 18F radiolabeling method via Al18F chelation is not only simple, straightforward and fast (typically 30–40 min), but it resembles the radiometal labeling process thus, it can be easily adopted to kit formulation [29]. The latest first-in-man study of Al18F-NOTA-PRGD2 (obtained from a lyophilized kit) in patients with lung cancer showed excellent in vivo stability of Al18F-based radiotracer and its suitability for PET imaging [30]. Here we describe the evaluation of a new Al18F-NODA-butyric acid complex as a potential renal PET tracer by comparing its pharmacokinetic properties to those of 131I-OIH in normal rats and in rats with renal failure.
2. Materials and Methods
2.1. General
1,4,7-triazacyclononane (TACN) was purchased from CheMatech (Dijon, France) and tert-butyl bromobutyrate was purchased from AstaTech Inc (Bristol, US). All other chemicals were purchased from Sigma/Aldrich (St. Louis, US). 2,2′-(7-(3-carboxypropyl)-1,4,7-triazacyclononane-1,4-diyl)diacetic acid (NODA-butyric acid, 1) was prepared as previously reported [31]. [18F]fluoride in water (swfi) was obtained from Emory CSI Radiopharmacy for biodistrubution studies and was purchased from PETNET Solutions at Emory University Yerkes National Primate Center for microPET imaging studies. Trap/release cartridges model DW-TRC were obtained from D&W, Inc. (Oakdale, TN). 131I-OIH was prepared as previously described [10]. 1H NMR spectra were recorded on INOVA-400 (400 MHz) spectrometer. The spectra were obtained at room temperature in CDCl3 or D2O and were referenced to the residual solvent peak. Chemical shifts (δ) were reported in ppm downfield from tetramethylsilane. HRMS were measured by the Emory University Mass Spectrometry Center and were recorded on a Thermo-Finnegan LTQ FTMS spectrometer. The synthesized compounds were characterized by 1H NMR, MS and HPLC and their purity was > 95%. The HPLC chromatograms for the 18F tracer were obtained by use of a Beckman System Gold Nouveau apparatus equipped with a model 170 radiometric detector and a model 166 ultraviolet light-visible light detector, and a Beckman C18 RP Ultrasphere octyldecyl silane column (5-μm, 4.6 × 250 mm). The flow rate of the mobile phase was 1 mL/min, the mobile phase consisted of aqueous 0.01 M trifluoroacetic acid (solvent A) and methanol (solvent B), and the gradient method used was 0% B for 10 min, 0–50% B for 10–20 min, 50–0% B for 20–23 min and 0% B for 23–25 min. Same HPLC system and conditions were used for purification and analytical analyses. Tissue/organ radioactivity was measured using an automated 2480 Wizard 2 gamma counter (Perkin Elmer) with a 3-inch NaI(Tl) detector. All animal experiments followed the principles of laboratory animal care and were approved by the Institutional Animal Care and Use Committee of Emory University.
2.2. Chemistry and radiosynthesis
2.2.1. Synthesis of Al19F-NODA-butyric acid (Al19F-1)
A solution of NODA-butyric acid, 1 (0.08 g, 0.242 mM) and AlCl3 (0.038 g, 0.289 mM) in water was adjusted to pH 4.0 using 1 M sodium acetate buffer. Reaction mixture was heated on boiling water bath for 15 min. NaF (0.05 g, 1.21 mM) was added to the above reaction mixture and heated for another 30 min. Complex formation was confirmed by ESI-Mass analysis of reaction mixture. Product was purified by RP-HPLC (Water/MeOH; 0 to 70 % of MeOH for 30 min). Collected fraction was lyophilized after removing the organic solvent to obtain white fluffy solid Al19F-3 (0.044 g, 49%). 1H NMR (D2O, 400 MHz, 25 °C): δ 1.75 (q, 2H), 2.28–2.18 (m, 2H), 3.37–2.68 (m, 14H), 3.44 (s, 1H), 3.51–3.49 (d, 2H, J = 8 Hz), 3.55 (s, 1H). HRMS calcd for C14H24AlFN3O6, 376.14646; found, 376.14611 [M + H]+.
2.2.2. 18F Labeling
An aqueous 18F solution [~ 1 mL, 1–1.85 GBq (27–50 mCi)] was loaded onto an anion exchange resin cartridge (that was prewashed with 1 mL of low 19F water), washed with 2 mL of low 19F water, and the 18F isotope was then eluted from the cartridge with a 0.9% saline solution (0.4 mL) into a sealed 2 mL vial. Al18F was prepared by adding a stock solution of AlCl3 (22 μL of 2 mM in 0.1 M sodium acetate buffer pH 4 solution) to the 18F saline solution and incubating the mixture at room temperature for 5 min. To the prepared Al18F solution, the NODA-butyric acid ligand (1) (0.5 mL of 1 mg/mL in 0.1 M acetate buffer pH 4 solution) was added and the labeling mixture was heated at 110 °C for 15 min. The labeling mixture was purified by HPLC to remove the unchelated Al18F and unlabeled ligand, and to produce the final Al18F-NODA-butyric acid complex (Al18F-1) with ~ 95% radiochemical purity as confirmed by HPLC analyses (retention time - 8 min). The fractions containing Al18F-1 were combined. The product was diluted in phosphate buffered saline (PBS, pH 7.4) to dilute any remaining organic solvent to less than 1% (v/v), and evaluated by HPLC for up to 4 h to assess complex in vitro stability. The PBS solution of Al18F-1 was used for in vivo studies. Total synthesis time was around 50–60 min including purification and formulation.
2.4. Biodistribution Studies
Al18F-1 was evaluated in two experimental groups of rats (Sprague–Dawley, 187–224 g each, Charles River, MA). Rats in both groups were anesthetized with ketamine–xylazine (2 mg/kg of body weight) injected intramuscularly, with additional supplemental anesthetic as needed. In the first group of 16 normal rats (Group A), the bladder was catheterized by use of heat-flared PE-50 tubing (Becton, Dickinson and Co.) for urine collection. The second group of 5 rats (Group B) was prepared to produce a model of renal failure. In that group, the abdomen was opened by a midline incision and both renal pedicles were identified and ligated just before radiotracer administration; thus, no urine was collected.
Each rat was injected intravenously via a tail vein with a 0.2 mL of a solution containing Al18F-1 (3.7 MBq/mL [100 μCi/mL]) and 131I-OIH (925 kBq/mL [25 μCi/mL]) in PBS pH 7.4. One additional aliquot of the 18F and 131I tracer solution (0.2 mL) for each time point was diluted to 100 mL, and three 1-mL portions of the resulting solution were used as standards.
In Group A, eight animals were sacrificed at 10 min, and eight animals were sacrificed at 60 min after injection. A blood sample was obtained, and the kidneys, heart, lungs, spleen, stomach and intestines were removed and placed in counting vials. The whole liver was weighed, and random sections were obtained for counting. Samples of blood and urine were also placed in counting vials and weighed. Each sample and the standards were counted for radioactivity by using an automated gamma-counter; counts were corrected for background radiation and physical decay. The percentage of the dose in each tissue or organ was calculated by dividing the counts in each tissue or organ by the total injected counts. The percentage injected dose in whole blood was estimated by assuming a blood volume of 6.5% of total body weight. Four rats in Group A probably became hypotensive during the study since one rat in the 10-min group and 3 rats in the 60-min group produced almost no urine and those rats were eliminated from the combined data analysis. The Group B rats were sacrificed 60 min after injection and selected organs and blood were collected and analyzed as described above for Group A.
2.5. Metabolite studies
The solution of Al18F-1 [7.4 MBq (0.2 mCi)] was injected via tail vein into two additional rats prepared as in Group A. Urine was collected for 15 min and analyzed by HPLC to determine in vivo stablility of Al18F-1.
2.6. PET/CT imaging
Small animal PET imaging was conducted according to the Emory Center for Systems Imaging (CSI) Standard Operating Procedure (SOP) and performed by CSI technical staff. Sprague-Dawley rats were initially anesthetized with ketamine/xylazine mixture injected intramuscularly and an acute intravenous catheter was placed in the rat’s tail vain for administration of radioligand. Each imaging session included one rat placed on the scanner bed of the Inveon MicroPET/CT with a nose cone used to maintain anesthesia at 1–2% isofluorane at a flow rate of 500–1000 mL/min O2. The rat was positioned in the tomograph on a resistive heating pad with rectal temperature probe feedback, and then fitted with a mini-clip for heart rate and blood oxygenation to allow physiological monitoring during the procedure. A dose of approximately 0.3–0.5 mCi/0.2mL of Al18F-1 was injected via tail vein at the start of the PET scan. Dynamic small-animal PET data were acquired over a period of 60 min after injection, followed by the acquisition of CT data over a period of 1 min. The CT was added for anatomical orientation. At the conclusion of the study, the rat was sacrificed prior to regaining consciousness. Images were reconstructed by OSEM3D/MAP using measured attenuation correction from a Co-57 point source. Image data were decay-corrected to the time of injection.
2.7. Statistical Analysis
All results are expressed as the mean ± SD. To determine statistical significance of differences between 2 groups at the same time point, comparisons were made using the 2-tailed Student t test for paired data; P < 0.05 will be considered to be statistically significant.
3. Results and Discussion
3.1. Chemistry
The synthesis of the labeling precursor 1 has been described previously [31]. Generally, NODA-butyric acid (1) was synthesized from 1,4,7-triazacyclononane via N-alkylation with tert-butyl bromoacetate to get a disubstituted intermediate by a pH-controlled workup method, followed by the reaction with tert-butyl bromobutyrate to yield the final ligand 1 as a hydrochloride salt.
The non-radioactive Al19F-1 was synthesized as a reference standard for characterizing the radioactive Al18F-NODA-butyric acid complex (Al18F-1) by HPLC. Complex Al19F-1 was obtained by reacting the ligand 1 with AlCl3 in sodium acetate buffer (pH 4.0), followed by the addition of NaF and heating. Formation of complex Al19F-1 was confirmed by a molecular ion peak by mass/ion spray positive ionization (MS/ESI+) analysis and the complex was purified by reverse phase high performance liquid chromatography (RP-HPLC). 1H NMR analysis of the purified complex Al19F-1 supported the previously reported structural analysis of the similar compound [31]. The 1H NMR spectrum of Al19F-1 showed the methylene protons of the acetate pendent arms as a pair of doublets at δ 3.57 and 3.46 ppm confirming the coordination of these two acetate moieties with aluminium fluoride during complex formation and the interaction of these protons with central fluoride atom. The –NCH2CH2N– ring protons and –NCH2CH2CH2CO2– alkyl chain protons produced overlapping multiplets centered at the region from δ 1.71 to 3.37 ppm, which shows asymmetrical methylene protons in the diastereotopic ring after complex formation as well as the unequivalent methylene protons of the dangling butyric acid arm. These multiplet patterns and chemical shifts of the chelate’s protons in aqueous solution are similar and in the range usually found in analogous Al(III) complexes [27, 31, 32].
3.2. Radiochemistry
The radioactive Al18F-1 complex (Fig. 1) was obtained through a one-pot, two-step radiolabeling process by reacting the preformed (Al18F)2+ moiety with the NODA-butyric acid ligand 1 in aqueous solution [31]. Using this approach, the laborious but critical azeotropic drying step performed in the traditional radiofluorination procedure was eliminated. The 18F radiotracer was isolated by HPLC with high radiochemical purity of > 95%, although it may be possible to develop a simpler purification step using solid phase extraction (SPE) cartridges as previously reported for other Al18F radiotracers [27, 29, 31]. The radiotracer was formed as a single species with well-established structure based on the analytic characterization of its 19F analog and its identity was confirmed by using the cold compound Al19F-1 as a reference, since the radioactive and nonradioactive AlF complexes are chemically identical and have similar retention times. Since the radiolabeling reaction and the HPLC purification took place at low pH, the collected fraction containing Al18F-1 needed to be buffered at physiological pH before using the radiotracer for in vivo animal experiments. We expected that Al18F-NODA-butyric acid complex would be monoanionic at pH 7.4 with the negative charge associated with the dangling –(CH2)3CO2− group (Fig. 1). This charge distribution is shared by 131I-OIH (Fig. 1) which shows a rapid plasma clearance, efficient tubular extraction and rapid rate of renal excretion. The simple, one-pot, aqueous 18F radiolabeling method via Al18F chelation procedure yields Al18F-1 in high radiolabeling efficiency [31]; this method will facilitate kit development since there are no organic solvents to be removed prior to clinical use and it may be possible to eliminate the HPLC purification step using SPE. A lyophilized kit, requiring only the addition of saline solution of 18F-fluoride, will not only facilitate fast and reproducible labeling procedure, but it also has the advantage of increasing the availability of 18F radiotracers for in vivo experiments by eliminating the need for hot cells or a PET radiochemistry facility.
3.3. Stability
The Al18F-NODA-butyric acid radiotracer showed high in vitro stability at physiological pH at room temperature. No measurable decomposition was observed when samples of incubated solution were analyzed by HPLC over 4 hours indicating that the tracer did not release Al18F.
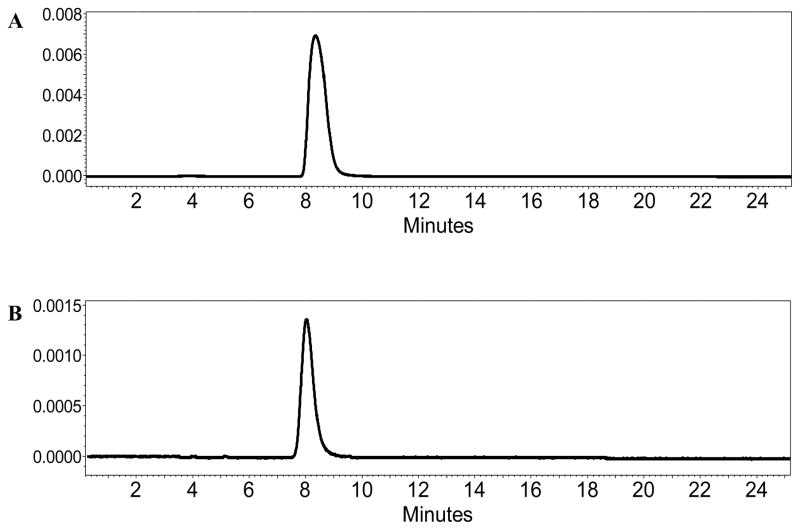
The urine of two rats collected for 15 min after the Al18F-NODA-butyric acid injection was also analyzed by HPLC to determine the radiotracer’s Al18F-1 in vivo stability and only the intact radiolabeled parent compound was observed, confirming that the tracer was excreted unchanged (Fig. 2), and that the all radioactivity was bound by Al18F-1. Our findings are in agreement with earlier reports on the in vitro and in vivo stability of Al18F radiotracers [26, 27, 30, 33, 34].
Figure 2.
HPLC of Al18F-NODA-butyric acid before injection (A) and in the rat’s urine at 15 min after injection (B).
3.4. Biodistribution
The pharmacokinetic properties of Al18F-NODA-butyric acid were compared to those of 131I-OIH, the clinical gold standard for the measurement of ERPF, in normal rats (Group A) and in a rat model with simulated complete renal failure (Group B). 18F and 131I tracers were simultaneously injected via the rat’s tail vein and animals were sacrificed at 10 or 60 min post-injection in Group A and at 60 min post-injection in Group B. The biodistribution results, expressed as a percentage of injected dose (% ID) for selected organs/tissues, blood and urine are presented in Table 1.
Table 1.
Percent injected dose of Al18F-NODA-butyric acid and 131I-OIH in blood, urine and selected organs at 10 and 60 minutes in normal ratsa (Group A) and in ratsa with renal pedicle ligatation (Group B).
| Blood
|
Kidney
|
Urine
|
Liver
|
Bowelb
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 18F | 131I-OIH | 18F | 131I-OIH | 18F | 131I-OIH | % 18F / 131I-OIH | 18F | 131I-OIH | 18F | 131I-OIH | |
| Group A | |||||||||||
| 10 min | 8.5 ± 0.8c | 5.1 ± 0.5 | 4.6 ± 0.8d | 5.3 ± 1.2 | 29.3 ± 3.7c | 50.8 ± 5.2 | 58 ± 4 | 2.3 ± 0.4c | 3.3 ± 0.2 | 1.2 ± 0.3d | 1.7 ± 0.5 |
| 60 min | 1.6 ± 0.3c | 0.8 ± 0.1 | 1.9 ± 0.4c | 0.7 ± 0.4 | 78.6 ± 4.8c | 85.4 ± 3.1 | 92 ± 3 | 0.7 ± 0.1e | 0.6 ± 0.2 | 0.7 ± 0.1c | 1.8 ± 0.3 |
| Group B | |||||||||||
| 60 min | 13.5 ± 0.7c | 15.1 ± 0.9 | 0.4 ± 0.1e | 0.5 ± 0.2 | - | - | - | 4.0 ± 0.2c | 7.1 ± 0.6 | 3.2 ± 0.6c | 7.6 ± 1.1 |
Data are presented as mean ± SD.
(10 min n=7, 60 min n=5, lig.ped. 60 min n=5).
Bowel includes intestines and stomach.
P < 0.001,
P < 0.05 and
P > 0.07, comparison of biodistribution between Al18F-NODA-butyric acid and 131I-OIH.
In normal rats, the Al18F-NODA-butyric acid tracer demonstrated high specificity for renal excretion; its urine activity, expressed as a percentage of 131I-OIH, was 58 ± 4 % at 10 min and 92 ± 3 % at 60 min (Table 1). Al18F-1 was cleared rapidly from the blood but not as rapidly as 131I-OIH; the %ID of Al18F-1 in blood at 10 min and 60 min was 8.5 ± 0.8 % and 1.6 ± 0.3 %, respectively, vs. 5.1 ± 0.5 % and 0.8 ± 0.1 %, respectively, for 131I-OIH. However, there was minimal gastrointestinal activity recorded for the Al18F tracer (1.2% at 10 min and 0.7% at 60 min) and that activity was lower at both time points than that measured for 131I-OIH (P = 0.03 for 10 min and P = 0.0007 for 60 min). Total injected activity of Al18F-1 present in the heart, lung and spleen was negligible (less than 0.3%).
Al18F-NODA-butyric acid is probably eliminated by both glomerular filtration and tubular secretion. Our previous studies of 125I-iothamalate, a GFR marker, and 131I-OIH in Sprague-Dawley rats have shown that the clearance of 125I-iothamalate is 40% of the clearance of 131I-OIH [35]. Consequently, if none of the Al18F-1 is protein bound and none of the Al18F-1 is secreted by the tubules, then the maximum Al18F-1 clearance would be 40% of the 131I-OIH clearance. The excretion of Al18F-1 in the urine at 10 min provides an indirect measurement of plasma clearance. We demonstrated that the ratio of Al18F-1 to 131I-OIH in the urine at 10 min was 58%, substantially greater than the 40% maximum from GFR alone. This result indicates that a component of Al18F-1 clearance must be due to secretion by the renal tubules. As anionic tracer, Al18F-NODA-butyric acid likely shares the same tubular transport as 131I-OIH and 99mTc-MAG3; however, this hypothesis will need to be confirmed by subsequent studies.
Al18F-NODA-butyric acid was also compared to 131I-OIH in rats with ligated renal pedicles to determine if complete renal failure would accelerate elimination via hepatobiliary excretion. As expected, renal failure slightly increased the liver and bowel activity of both tracers at 60 min but the new Al18F radiotracer had significantly less liver and bowel activity than the 131I-OIH gold standard (P < 0.001) (Table 1). 131I-OIH had higher retention in the blood than Al18F-1 (P = 0.009) in the rats with renal pedicle ligation; this higher retention may be related to a higher plasma protein binding of 131I-OIH compared to Al18F-1 resulting in lower volume of distribution for 131I-OIH and a higher plasma concentration although this is speculative since plasma protein binding was not measured in this study. It is possible that a relatively low uptake of free 18F or Al18F-1 in the bone may have resulted in a larger volume of distribution of Al18F-1 due to the relatively large bone mass and may explain the lower plasma concentration in the rats with ligated renal pedicles (see below PET/CT section). Future studies of second generation Al18F-NODA derivatives should include measurements of plasma protein binding and bone activity to investigate this possibility. The percent injected dose remaining in the spleen, heart and lung for both 18F and 131I radiotracers were all less than 1%.
The potential of Al18F-NODA-butyric acid as a renal tracer was previously suggested based on biodistribution studies in mice which revealed high uptake in the kidney at 10 min and substantial reduction at 60 min [31, 36]. Our studies extend this observation by direct comparison with the standard for evaluation of effective renal plasma flow, 131I-OIH, showing that Al18F-1 has suitable pharmacokinetic properties as the renal PET agent. Results obtained from the simulated renal failure model are also important since they show that Al18F-1 retains its high specificity for renal excretion in renal failure and elimination from the body does not shift to hepatobiliary excretion. If a renal tracer is not highly specific for renal excretion in patients with impaired renal function, the clearance of that tracer will not provide an accurate measurement of renal function; moreover, activity in the liver, gallbladder and intestine can compromise scan interpretation [37].
3.5. PET/CT
Fused PET and CT scans are shown in Figs. 3 and 4. The preliminary small-animal dynamic PET performed on normal rats with the Al18F-NODA-butyric acid tracer supported the biodistribution data and confirmed that the radiotracer was rapidly taken up by the kidneys and excreted into bladder (Fig. 3). Additional images with the intensity adjusted to emphasize background activity and activity concentration in organs with low levels of uptake (Fig. 4) demonstrate minimal uptake in the spine which may be related to blood pool, marrow uptake, uptake in the bony matrix or a combination of the above. Both 18F-fluoride and non-chelated Al18F are known for high bone uptake [21, 22, 33].
Figure 3.
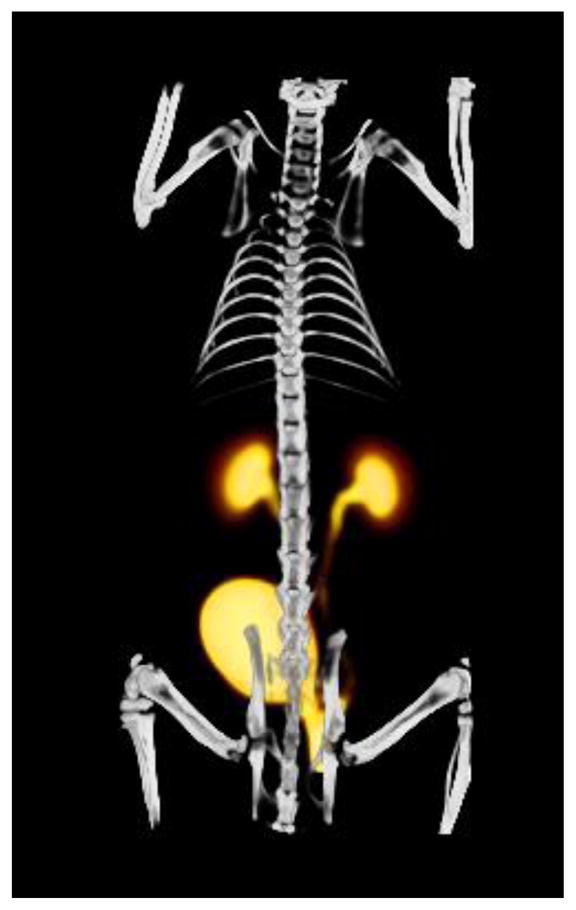
Fused volume rendering of PET and CT of a normal rat injected with Al18F-NODA-butyric acid. The PET image represents data summed from 5–60 min post-injection.
Figure 4.
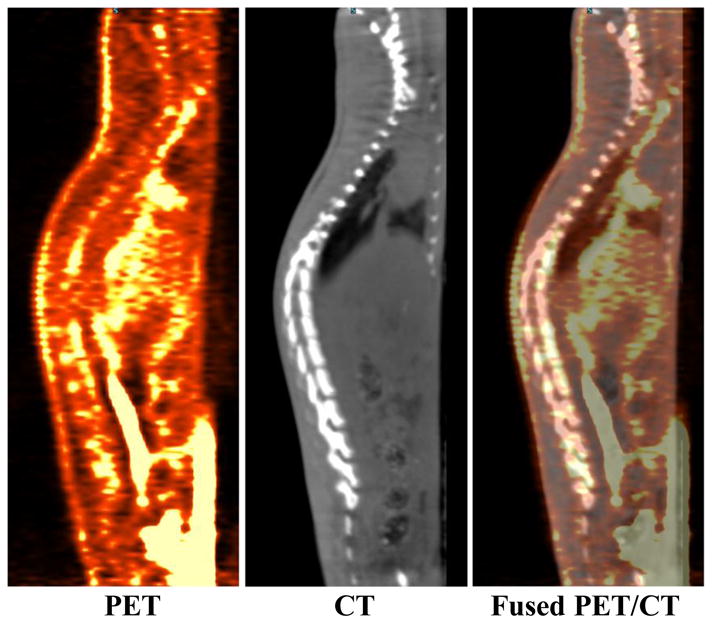
PET (left), CT (middle) and fused PET/CT (right) sagittal images of Al18F-NODA-butyric acid with intensity scaled to allow imaging of organs with low levels of activity. The PET image, obtained by summing data from 5–60 min post-injection, demonstrates activity in the spine however this may consists of blood pool activity, marrow activity, activity in the bony matrix or a combination of the above.
4. Conclusions
Al18F-NODA-butyric acid (Al18F-1) was successfully prepared and its identity was confirmed by comparison with the fully characterized cold analog, Al19F-NODA-butyric acid (Al19F-1). The radiolabeled complex Al18F-1 was isolated by HPLC with high radiochemical purity and was proven to be stable at physiological conditions for the duration of the animal experiments. Metabolite studies confirm that the tracer was excreted intact into the urine. Although the renal excretion of Al18F-1 is less than that of 131I-OIH at 10 minutes in normal rats, the urine activity of these two tracers is nearly equal at 60 min. Biodistribution studies also show that Al18F-1 is cleared rapidly from the blood but more slowly than is 131I-OIH. However, there is minimal gastrointestinal activity from the Al18F-1 tracer; lower at both 10 and 60 minutes time points than that from 131I-OIH. Studies in rats with ligated renal pedicles showed higher liver and intestinal activities for both 18F and 131I tracers, but those increases are lower for Al18F-1 suggesting that renal failure results in less hepatobiliary excretion or intestinal secretion of the Al18F tracer compared to 131I-OIH. The results from microPET/CT imaging studies confirm the biodistribution results. In summary, we have demonstrated that Al18F-NODA-butyric acid provides a facile labeling approach to the development of a PET renal tracer that has acceptable pharmacokinetic and chemical properties as a renal imaging agent. Further investigation of Al18F-based compounds as PET radiotracers may offer a superior PET tracer that combines high quality images with a reliable measurement of effective renal plasma flow.
Acknowledgments
This work was funded by the National Institute of Health (NIH/NIDDK) grant R37 DK038842 (PI: Taylor) and partially by P50 CA128301-0002 (PI: Shim). MicroPET imaging was supported by the Emory Center for Systems Imaging (CSI) pilot grant (PI: Lipowska). The authors thank Eugene Malveaux for his excellent technical assistance with all animal experiments and the Emory CSI Radiopharmacy for 18F-fluoride solution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prigent A. Monitoring renal function and limitations of renal function tests. Semin Nucl Med. 2008;38:32–46. doi: 10.1053/j.semnuclmed.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Lane BR, Poggio ED, Herts BR, Novick AC, Campbell SC. Renal function assesment in the era of chronic kidney disease: renewed emphasis on renal function centered patient care. J Urol. 2009;182:435–44. doi: 10.1016/j.juro.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Gates HF. Split renal function testing using Tc-99m DTPA: a rapid technique for determining differential glomerular filtration. Clin Nucl Med. 1983;8:400–7. doi: 10.1097/00003072-198309000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Shore RM, Koff SA, Mentser M, Hayes JR, Smith SP, Smith JP, et al. Glomerular filtration rate in children: determination from the Tc-99m-DTPA renogram. Radiology. 1984;151:627–33. doi: 10.1148/radiology.151.3.6371888. [DOI] [PubMed] [Google Scholar]
- 5.Fritzberg AR, Kasina S, Eshima D, Johson DL. Synthesis and biological evaluation of technetium-99m MAG3 as a hippuran replacement. J Nucl Med. 1986;27:111–6. [PubMed] [Google Scholar]
- 6.Taylor A, Eshima D, Fritzberg AR, Christian PE, Kasina S. Comparison of iodine-131 OIH and technetium-99m MAG3 renal imaging in volunteers. J Nucl Med. 1986;27:795–803. [PubMed] [Google Scholar]
- 7.Esteves FP, Taylor A, Manatunga A, Folks RD, Krishnan M, Garcia EV. 99mTc-MAG3 renography: normal values for MAG3 clearance and curve parameters, excretory parameters and residual urine volume. AJR. 2006;187:W610–W7. doi: 10.2214/AJR.05.1550. [DOI] [PubMed] [Google Scholar]
- 8.Shanon A, Feldman W, McDonald P, Martin DJ, Matzinger MA, Shillinger JF, et al. Evaluation of renal scars by technetium-labelled dimercaptosuccinic acid scan, intravenous urography and ultrasonography: a comparative study. J Pediatr. 1992;120:399–403. doi: 10.1016/s0022-3476(05)80904-7. [DOI] [PubMed] [Google Scholar]
- 9.Pipesz A, Blaufox MD, Gordon I, Granerus G, Majd M, O’Reilly P, et al. Consensus on renal cortical scintigraphy in children with urinary tract infection. Scientific Committee of Radionuclides in Nephrourology. Semin Nucl Med. 1999;29:160–74. doi: 10.1016/s0001-2998(99)80006-3. [DOI] [PubMed] [Google Scholar]
- 10.Lipowska M, Marzilli LG, Taylor AT. 99mTc(CO)3-nitrilotriacetic acid: a new renal radiopharmaceutical showing pharmacokinetic properties in rats comparable to those of 131I-OIH. J Nucl Med. 2009;50:454–60. doi: 10.2967/jnumed.108.058768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor AT, Lipowska M, Marzilli LG. 99mTc(CO)3(NTA): a 99mTc renal tracer with pharmacokinetic properties comparable to those of 131I-OIH in healthy volunteers. J Nucl Med. 2010;51:391–6. doi: 10.2967/jnumed.109.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor AT, Lipowska M, Cai H. 99mTc(CO)3(NTA) and 131I-OIH: comparable plasma clearances in patients with chronic kidney disease. J Nucl Med. 2013;54:578–84. doi: 10.2967/jnumed.112.108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eshima D, Fritzberg AR, Taylor A. Tc-99m renal tubular function agents: current status. Semin Nucl Med. 1990;20:28–40. doi: 10.1016/s0001-2998(05)80174-6. [DOI] [PubMed] [Google Scholar]
- 14.Marcus CS, Kuperus JH. Pediatric renal iodine-123 orthoiodohippurate dosimetry. J Nucl Med. 1985;26:1211–4. [PubMed] [Google Scholar]
- 15.Lewis DM. 99Mo supply - the times they are a-changing. Eur J Nucl Med. 2009;36:1371–4. doi: 10.1007/s00259-009-1171-4. [DOI] [PubMed] [Google Scholar]
- 16.Szabo Z, Xia J, Mathews WB, Brown PR. Future direction of renal positron emission tomography. Semin Nucl Med. 2006;36:36–50. doi: 10.1053/j.semnuclmed.2005.08.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabo Z, Xia J, Mathews WB. Radiopharmaceuticals for renal positron emission tomography imaging. Semin Nucl Med. 2008;38:20–31. doi: 10.1053/j.semnuclmed.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Schnöckel U, Reuter S, Stegger L, Schlatter E, Schäfers KP, Hermann S, et al. Dynamic 18F-fluoride small animal PET to noninvasively assess renal function in rats. Eur J Nucl Med. 2008;35:2267–74. doi: 10.1007/s00259-008-0878-y. [DOI] [PubMed] [Google Scholar]
- 19.Awasthi V, Pathuri G, Agashe HB, Gali H. Synthesis and in vivo evaluation of p-18F-fluorohippurate as a new radiopharmaceutical for assessment of renal function by PET. J Nucl Med. 2011;52:147–53. doi: 10.2967/jnumed.110.075895. [DOI] [PubMed] [Google Scholar]
- 20.Pathuri G, Hedrick AF, Awasthi V, Gali H. Single-step radiosynthesis and in vivo evaluation of novel fluorine-18 labeled hippurate for use as a PET renal agent. Nucl Med Biol. 2012;39:1195–201. doi: 10.1016/j.nucmedbio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Schirrmeister H, Guhlmann A, Elsner K, Kotzerke J, Glatting G, Rentschler M, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623–9. [PubMed] [Google Scholar]
- 22.Even-Sapir E, Metser U, Flusser G, Zuriel L, Kollender Y, Lerman H, et al. Assessment of malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride PET and 18F-fluoride PET/CT. J Nucl Med. 2004;45:272–8. [PubMed] [Google Scholar]
- 23.Pathuri G, Sahoo K, Awasthi V, Gali H. Renogram comparison of p-[18F]fluorohippurate with o-[125I]iodohippurate and [99mTc]MAG3 in normal rats. Nucl Med Commun. 2011;32:908–12. doi: 10.1097/MNM.0b013e32834a6db6. [DOI] [PubMed] [Google Scholar]
- 24.Smith GE, Sladen HL, Biagini SCG, Blower PJ. Inorganic approaches for radiolabelling biomolecules with fluorine-18 for imaging with Positron Emission Tomography. Dalton Trans. 2011;40:6196–205. doi: 10.1039/c0dt01594f. [DOI] [PubMed] [Google Scholar]
- 25.McBride WJ, Sharkey RM, Karacay H, D’Souza CA, Rossi EA, Laverman P, et al. A Novel Method of 18F Radiolabeling for PET. J Nucl Med. 2009;50:991–8. doi: 10.2967/jnumed.108.060418. [DOI] [PubMed] [Google Scholar]
- 26.McBride WJ, D’Souza CA, Sharkey RM, Karacay H, Rossi EA, Chang CH, et al. Improved 18F labeling of peptides with a fluoride-aluminum-chelate complex. Bioconjugate Chem. 2010;21:1331–40. doi: 10.1021/bc100137x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Souza CA, McBride WJ, Sharkey RM, Todaro LJ, Goldenberg DM. High-yielding aqueous 18F-labeling of peptides via Al18F chelation. Bioconjugate Chem. 2011;22:1793–803. doi: 10.1021/bc200175c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBride WJ, Sharkey RM, Goldenberg DM. Radiofluorination using aluminum-fluoride (Al18F) EJNMMI Research [Online] 2013;3:36. doi: 10.1186/2191-219X-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McBride WJ, D’Souza CA, Karacay H, Sharkey RM, Goldenberg DM. New Lyophilized Kit for Rapid Radiofluorination of Peptides. Bioconjugate Chem. 2012;23:538–47. doi: 10.1021/bc200608e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan W, Guo N, Pan D, Yu C, Weng Y, Luo S, et al. First experience of 18F-Alfatide in lung cancer patients using a new lyophilized kit for rapid radiofluorination. J Nucl Med. 2013;54:691–8. doi: 10.2967/jnumed.112.113563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shetty D, Choi SY, Jeong JM, Lee JY, Hoigebazar L, Lee YS, et al. Stable aluminium fluoride chelates with triazacyclononane derivatives proved by X-ray crystallography and 18F-labeling study. Chem Commun. 2011;47:9732–4. doi: 10.1039/c1cc13151f. [DOI] [PubMed] [Google Scholar]
- 32.de Sá A, Prata MIM, Geraldes CFGC, André JP. Triaza-based amphiphilic chelators: Synthetic route, in vitro characterization and in vivo studies of their Ga(III) and Al(III) chaelates. J Inorg Chem. 2010;104:1051–62. doi: 10.1016/j.jinorgbio.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Laverman P, McBride WJ, Sharkey RM, Eek A, Joosten L, Oyen WJG, et al. A novel facile method of labeling octreotide with 18F-Fluorine. J Nucl Med. 2010;51:454–61. doi: 10.2967/jnumed.109.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shetty D, Jeong JM, Kim YJ, Lee JY, Hoigebazar L, Lee YS, et al. Development of a bifunctional chelating agent containing isothiocyanate residue for one step F-18 labeling of peptides and application for RGD labeling. Bioorg Med Chem. 2012;20:5941–7. doi: 10.1016/j.bmc.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 35.Taylor A, Jr, Eshima D. Effects of altered physiologic states on clearance and biodistribution of technetium-99m MAG3, iodine-131 OIH, and iodine-125 iothalamate. J Nucl Med. 1988;29:669–75. [PubMed] [Google Scholar]
- 36.Shetty D, Jeong JM, Hoigebazar L, Lee Y, Lee D, Chung J, et al. Development of Al-F-18-NO2A-butyric acid complex as renal imaging radiopharmaceutical. J Nucl Med. 2011;52 (Suppl. 2):170P. [Google Scholar]
- 37.Rosen JM. Gallblader uptake simulating hydronephrosis of Tc-99m-MAG3 scintigraphy. Clin Nucl Med. 1993;18:713–4. doi: 10.1097/00003072-199308000-00020. [DOI] [PubMed] [Google Scholar]