Abstract
Phytochrome was examined by immunochemical and spectroscopic techniques to detect differences between the protein moieties of red- and far red-absorbing phytochrome (Pr and Pfr). No differences in the reaction of Pr and Pfr with phytochrome antibody were discernible on Ouchterlony double diffusion plates. However, the microcomplement fixation assay showed a greater degree of antibody reaction with Pfr than with Pr, indicating some difference in the surface characteristics of the two forms. Circular dichroism spectroscopy between 300 and 200 nanometers revealed differences between Pr and Pfr which may reflect differences in the protein conformation. The circular dichroism spectrum of Pr showed a negative band at 285 nanometers which was not present in the spectrum of Pfr, and the large negative circular dichroism band at 222 nanometers with Pfr, associated with the α-helical content, was shifted 2 nanometers to shorter wave length with Pr although there was no change of magnitude of this band. The absorbancy of Pr and Pfr is very nearly the same in the 280 nanometer spectral region, but sensitive difference spectra between Pr and Pfr did reveal spectra which were similar to solvent perturbation spectra obtained by others with different proteins. In total, the experiments indicate that there are conformational differences between the protein moieties of Pr and Pfr but that these differences are rather slight from a standpoint of gross structure.
Full text
PDF
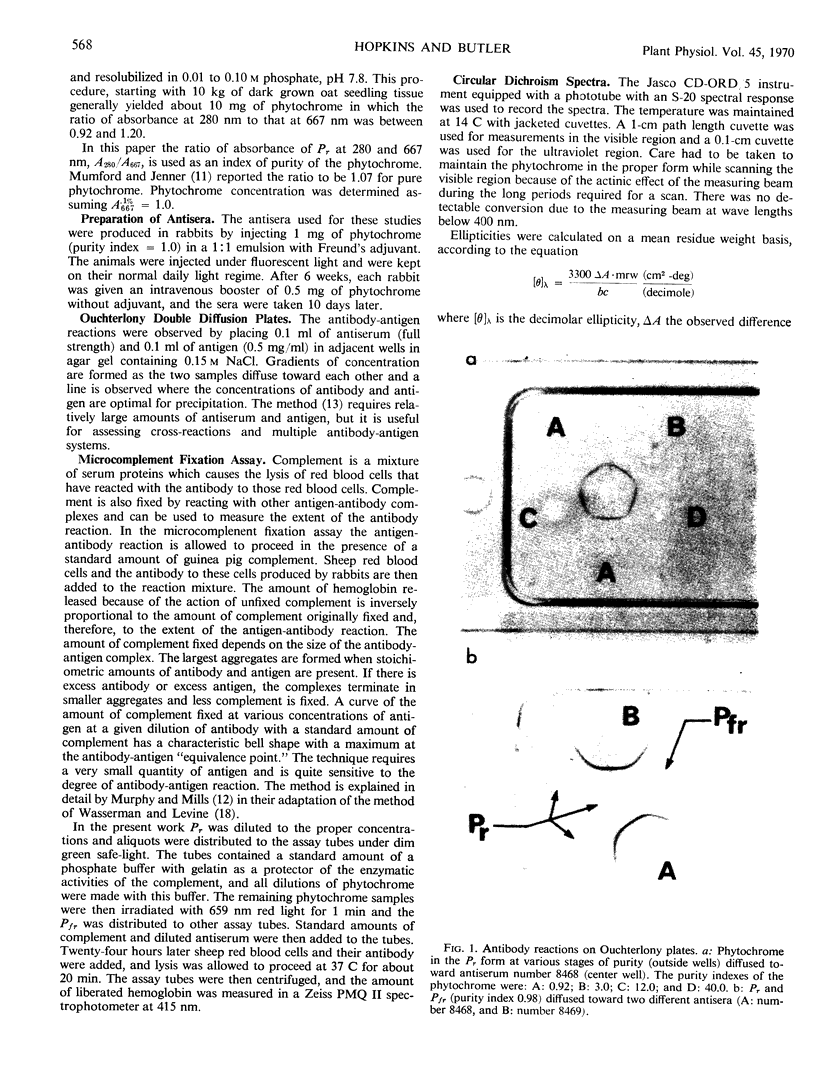
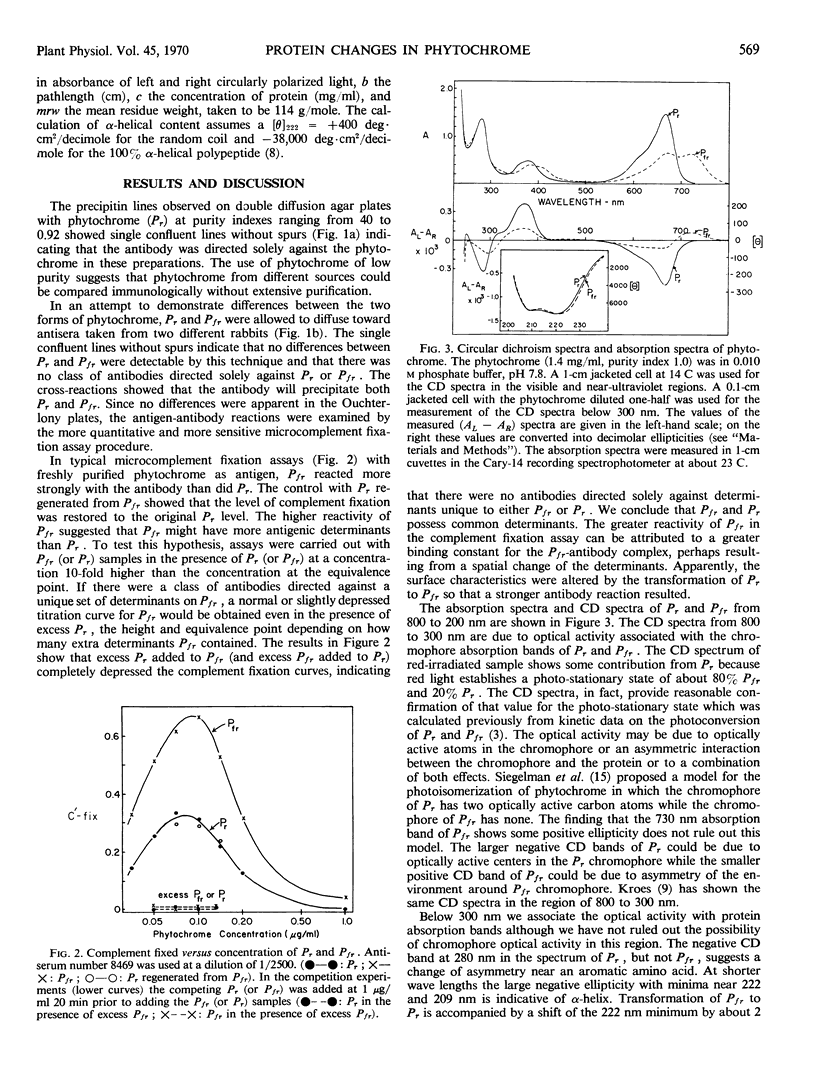
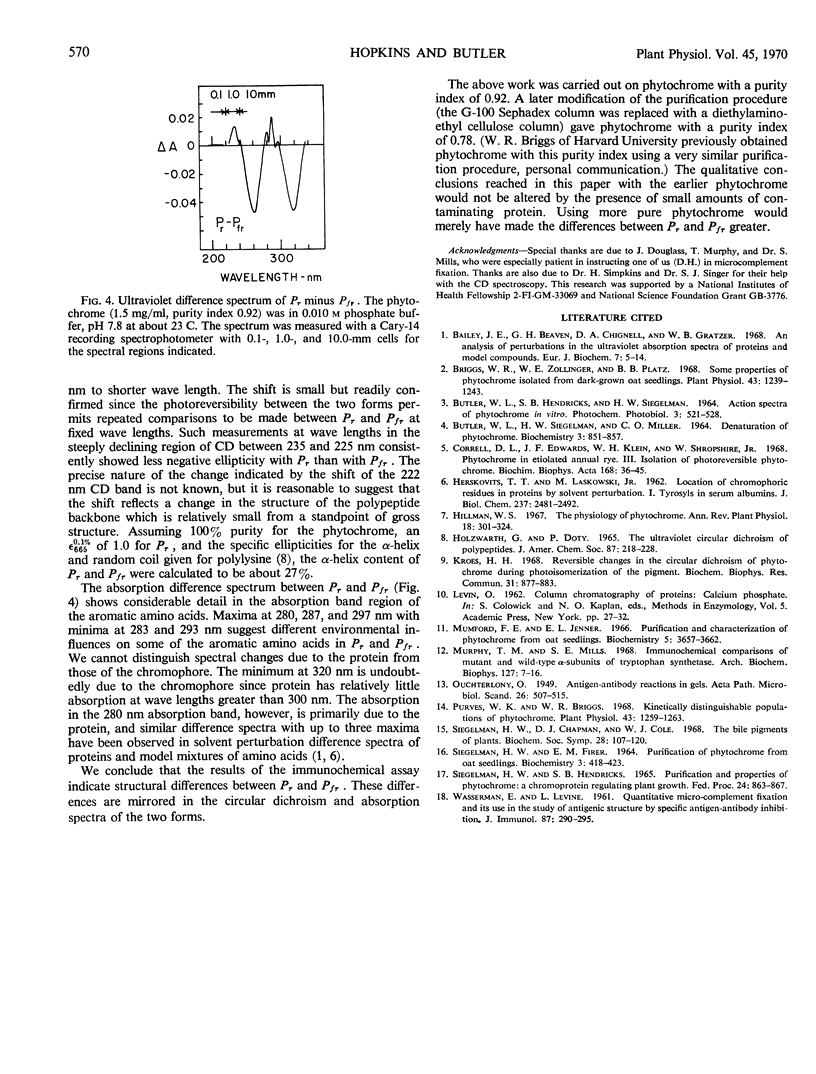
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. L., SIEGELMAN H. W., MILLER C. O. DENATURATION OF PHYTOCHROME. Biochemistry. 1964 Jun;3:851–857. doi: 10.1021/bi00894a022. [DOI] [PubMed] [Google Scholar]
- Bailey J. E., Beaven G. H., Chignell D. A., Gratzer W. B. An analysis of perturbations in the ultraviolet absorption spectra of proteins and model compounds. Eur J Biochem. 1968 Dec;7(1):8–14. doi: 10.1111/j.1432-1033.1968.tb19566.x. [DOI] [PubMed] [Google Scholar]
- Briggs W. R., Zollinger W. D., Platz B. B. Some Properties of Phytochrome Isolated From Dark-grown Oat Seedlings (Avena sativa L.). Plant Physiol. 1968 Aug;43(8):1239–1243. doi: 10.1104/pp.43.8.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll D. L., Edwards J. L., Klein W. H., Shropshire W., Jr Phytochrome in etiolated annual rye. 3. Isolation of photoreversible phytochrome. Biochim Biophys Acta. 1968 Sep 10;168(1):36–45. doi: 10.1016/0005-2795(68)90231-6. [DOI] [PubMed] [Google Scholar]
- HERSKOVITS T. T., LASKOWSKI M., Jr Location of chromophoric residues in proteins by solvent perturbation. I. Tyrosyls in serum albumins. J Biol Chem. 1962 Aug;237:2481–2492. [PubMed] [Google Scholar]
- HOLZWARTH G., DOTY P. THE ULTRAVIOLET CIRCULAR DICHROISM OF POLYPEPTIDES. J Am Chem Soc. 1965 Jan 20;87:218–228. doi: 10.1021/ja01080a015. [DOI] [PubMed] [Google Scholar]
- Kroes H. H. Reversible changes in the circular dichroism of phytochrome during photoisomerisation of the pigment. Biochem Biophys Res Commun. 1968 Jun 28;31(6):877–883. doi: 10.1016/0006-291x(68)90533-0. [DOI] [PubMed] [Google Scholar]
- Mumford F. E., Jenner E. L. Purification and characterization of phytochrome from oat seedlings. Biochemistry. 1966 Nov;5(11):3657–3662. doi: 10.1021/bi00875a039. [DOI] [PubMed] [Google Scholar]
- Murphy T. M., Mills S. E. Immunochemical comparisons of mutant and wild-type alpha-subunits of tryptophan synthetase. Arch Biochem Biophys. 1968 Sep 20;127(1):7–16. doi: 10.1016/0003-9861(68)90194-x. [DOI] [PubMed] [Google Scholar]
- Purves W. K., Briggs W. R. Kinetically distinguishable populations of phytochrome. Plant Physiol. 1968 Aug;43(8):1259–1263. doi: 10.1104/pp.43.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGELMAN H. W., FIRER E. M. PURIFICATION OF PHYTOCHROME FROM OAT SEEDLINGS. Biochemistry. 1964 Mar;3:418–423. doi: 10.1021/bi00891a019. [DOI] [PubMed] [Google Scholar]
- Siegelman H. W., Chapman D. J., Cole W. J. The bile pigments of plants. Biochem Soc Symp. 1968;28:107–120. [PubMed] [Google Scholar]
- Siegelman H. W., Hendricks S. B. Purification and properties of phytochrome: a chromoprotein regulating plant growth. Fed Proc. 1965 Jul-Aug;24(4):863–867. [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]