Abstract
Electrochemical deposition of crosslinked oxo-cyanoruthenate, Ru-O/CN-O, from a mixture of RuCl3 and K4Ru(CN)6 is known to yield a film on glassy carbon that promotes oxidations by a combination of electron and oxygen transfer. Layer-by-layer (LbL) deposition of this species and of a film formed by cycling of the electrode potential in a ZrO2 solution systematically increases the number of catalytically active sites of the Ru-O/CN-O on the electrode. The evaluation of the electrocatalytic activity was by cyclic voltammetric oxidation of cysteine at pH 2. Plots of the anodic peak current vs. the square root of scan rate were indicative of linear diffusion control of this oxidation, even in the absence of ZrO2, but the slopes of these linear plots increased with bilayer number, n, of (ZrO2 | Ru-O/CN-O)n. The latter observation is hypothesized to be due to an increased number of active sites for a given geometric electrode area, but proof required further study. To optimize utilization of the catalyst and to provide a size-exclusion characteristic to the electrode, the study was extended to LbL deposition of the composite in 50-nm pores of an organically modified silica film deposited by electrochemically assisted sol-gel processing using surface-bound poly(styrene sulfonate) nanospheres as a templating agent.
Keywords: Electrocatalysis, Layer-by-layer assembly, Sol-gel processing, Cysteine oxidation
1. Introduction
Cyclic voltammetry at various electrodes in acidic mixtures of RuCl3 and K4Ru(CN)6 over the range −0.2 to 1.2 V vs. Ag|AgCl results in deposition of a film of mixed-valent polynuclear ruthenium oxide with cyano crosslinks [1–5]. The initial suggestion that the film was the ruthenium analogue of Prussian Blue [1] was refined by Auger electron spectroscopy [2,3] and x-ray photoelectron spectroscopy [5], which showed than the electrochemical deposition process yielded a -Ru-O-Ru- network with cyano crosslinks, Ru-O/CN-O, where the ruthenium existed in mixed oxidation states. Contrary to iron ions in Prussian Blue, ruthenium can be readily oxidized to higher oxidation states to form catalytically active ruthenium(IV,III) oxo species that include Ru3O26+ or Ru2O5+ [2,3,5]. Further, the hexacyanoruthenate(III) anions undergo transformation to dinuclear anionic species, (CN)5-RuII-CN-RuIII-(CN)56− [6]. Strong electrostatic attractive interactions as well as the polynuclear character of deposits lead to the overall stabilization of the system. Films of Ru-O/CN-O were demonstrated to have low ohmic resistances as a result of effective charge transport by hydrogen and/or potassium ions [2].
Numerous applications of Ru-O/CN-O have been reported [7 and citations therein]. The oxidation of alcohols [2,4,5,8], inorganic [9,10] and organic [11,12] sulfur compounds, glucose [13], hydrazine [8,14], aldehydes [15], nitrosamines [16,17], insulin [18,19], and tetracycline [20] are examples. As is typical with electrode reactions involving mediated electron transfer, signal-to-background ratios with amperometry under potentiostatic conditions are preferred over potentiodynamic methodology for analytical applications. By the former approach, detection limits of nmol dm−3 in an injection are achieved [4,16,18,20]. For example, a linear calibration curve was obtained over the range 8 – 200 ng insulin in a 7.5 μL injection into a flow system with measurement of current under potentiostatic conditions [18]. Analogous to mixed metal oxides as modifiers [21,22], the efficacy of this catalyst was related to the combination of oxygen and electron transfer [12]. For example, the oxidation of cysteine at a conventional electrode results in a dimer, which can passivate the surface by adsorption, whereas controlled-potential electrolysis at an electrode modified with a film of Ru-O/CN-O yielded cysteic acid, which does not adsorb to the surface [12].
Multicomponent catalysts provide a route to improving electrocatalytic efficiency over that at single-component systems because of electronic and/or geometric factors [23]. In the case of the thermal oxide of ruthenium, mixtures with ZrO2 have been investigated by Camara and Trasatti [24]. In addition to the well-known stabilization effect of ZrO2 or TiO2 on ruthenium oxo species (leading to the formation of dimensionally stable anodes), attractive interactions with positively charge oxo-cationic interfacial zirconia species, which include polymeric forms of ZrO2+ such as [Zr4(OH)8]8+ or [Zr3(OH)4]8+, and polyanionic oxocyanoruthenate structures occurred.
Layer-by-layer (LbL) electrostatic assembly, which was first described by Decher [25], is a convenient means of obtaining multi-component composites. The metal of primary interest in the present study, ruthenium, has been incorporated into LbL assemblies in several ways. Among them are the following: ruthenium-containing LbL assemblies for electrochemical studies including a ruthenium porphyrin in combination with a polyoxometalate [26]; a cobalt porphyrinate- ruthenium bipyridine (bpy) complex with a zinc porphyrinate [27]; a ruthenium terpyridine (tpy) complex alternated with sulfonated poly(aniline) [28]; Ru(bpy)(tpy) in combination with a polyoxometalate [29]; and a ruthenium metallodendrimer and double-stranded DNA assembly [30]. These composites can exhibit synergism between the components of the layers, in part because of the high degree of inter-twining between the layers that occurs. Indeed, when fabricated of polyelectrolytes, the resulting composites have been described as “fuzzy nanoassemblies” [25]. This mixing of the individually deposited layers of a poly(aniline), poly(styrene sulfonate) co-polymer and poly(3,4-ethylenedioxythiophene), PEDOT, in an LbL assembly enhanced the activity of horseradish peroxidase, thereby facilitating the 4-electron reduction of oxygen [31]. Synergism was demonstrated in the multi-layer assembly of PEDOT and poly(N-methylpyrrole) for fabrication of supercapacitors [32]. The LbL assembly of poly(thiophene) and magnetite yielded a greater sensitivity in the amperometric determination of dopamine than that exhibited with an electrode modified by the individual components [33]. Intercalated montmorillonite and phosphotungstic acid provided increased electrocatalytic oxidation of methyl jasmonate because of the combination of the adsorption capacity of the former and the conductivity and redox characteristics of the latter in an assembly of these two components [34].
In the present study, the electrochemical catalyst, Ru-O/CN-O, is investigated as a component of an LbL assembly with ZrO2. The LbL assembly of other forms of ruthenium and zirconium has been reported. A composite containing RuII(bpy)3 and zirconium phosphate was shown to form donor-acceptor complexes between the inorganic layers and to exhibit electron transfer under photo-illumination [35]. More related to the present study, incorporation of a ruthenium phenanthroline complex with zirconium phosphate gave a porous film with properties suggesting application to electrochemical sensing of oxygen [36].
In the present study, a composite of Ru-O/CN-O and ZrO2 bilayers was fabricated by LbL assembly on a conventional glassy carbon electrode. Characterization was by cyclic voltammetry and electron microscopy. The goal was to determine whether the ZrO2 enhanced the known electrocatalytic activity of Ru-O/CN-O using the oxidation of cysteine at pH 2 as the test system. Second, whether assembly of these bilayers can be achieved in an organically modified silica sol-gel (ormosil) film on a glassy carbon electrode was investigated. Here, a previously reported procedure for depositing the ormosil with 50-nm channels presumably normal to the electrode surface was used to fabricate the film [37]. The deposition of the ormosil was by electrochemically assisted sol-gel processing [38–40] onto a surface modified by immobilization of 50-nm diameter poly(styrene sulfonate), which served to template the pores [37]. This film geometry is suited for size-exclusion electrochemical methodology [41]; the range of application of this methodology can be expanded if further modification of these electrodes by incorporating catalysts in the pore structure is achieved.
2. Experimental
2.1 Materials
Except for those noted below, chemicals were Reagent Grade. Methoxytrimethylsilane, 98%; 3-aminopropyltriethoxysilane (99%), APTES; zirconium(IV) oxide nanoparticles (5 wt. % dispersion in water); L-cysteine; and ruthenium(III) chloride hydrate were obtained from Sigma-Aldrich (St. Louis, MO). The 2-propanol, 99.5%, was from Alfa Aesar (Ward Hill, MA). Potassium hexacyanoruthenate(II) hydrate was obtained from Alfa Aesar. Sulfuric acid and potassium sulfate were bought from POCH (Poland). Poly(styrene sulfonate), PSS, in the form of spheres with a 50-nm mean diameter, was purchased as an aqueous suspension of 2.5% solids (w/v) from Polysciences, Inc. (Warrington, PA). The water was house-distilled that was further purified with Barnstead Nanopure II system. The glassy carbon (GC) electrodes, 3.0 mm diameter, were from Bioanalytical Systems (West Lafayette, IN).
2.2 Instrumentation and methods
Electrochemical experiments were performed with CH Instruments (Austin, TX) Models 760D, 400 or 660B systems. The cells were of conventional design with a glassy carbon (GC) rod as the counter electrode and Ag|AgCl, 3mol dm−3 KCl as the reference electrode (Bioanalytical Systems, West Lafayette, IN). Prior to experiments all the solutions were deaerated with nitrogen gas. The GC disk working electrodes (geometric areas, 0.071 cm2) were polished with 0.05-μm alumina and rinsed with distilled water in an ultrasonic bath prior to each experiment. Unless otherwise stated, all potentials are reported vs. an Ag|AgCl reference electrode. Measurements were made at room temperature (22°C ± 2°C). The morphology of films was monitored using a Carl Zeiss scanning electron microscope (SEM).
The modification of GC with a porous ormosil film was by our recently reported procedure [37]. Briefly, GC was first modified with sub-monolayer coverage of APTES by immersion for 15 min in a 0.5 mmol dm−3 solution. After rinsing with water the GC | APTES was treated for 30 min in a 1:1 dilution (with water) of the commercially obtained 50-nm PSS suspension. After rinsing, the ormosil was deposited by electrochemically assisted processing in a pH 5.0 solution (adjusted with 1.0 mol dm−3 HCl) comprising 5 mL of 0.1 mol dm−3 lithium perchlorate in 2-propanol, 2.5 mL of H2O, and 2.0 mL of methoxytrimethylsilane. A potential of 2.0 V, at which the hydrogen ion catalyst was generated at the surface, was applied for 30 min during which the solution was stirred. The electrode was slowly withdrawn from the processing solution while the potential was applied, and any excess solution was removed by blotting at the perimeter of the electrode area. After air-drying overnight, the PSS was dissolved with chloroform to provide the pores. Because acid-catalyzed sol-gel processing produces a compact film, subsequent electrochemical reactions were restricted to these pores. Formation of LbL assemblies of ZrO2 and Ru-O/CN-O in these pores followed the methodology described above for deposition of these assemblies on bare GC. Note: (CH3)3SiOCH3 is a potential eye hazard; the film deposition should be performed in a hood.
Electrochemical oxidation of cysteine was studied in a 1.0 mmol dm−3 solution with 0.25 mol dm−3 K2SO4 at pH 2.0 as the supporting electrolyte. A 10-min rest period was used between each voltammetric scan. When AsIII was the test system, the same procedure was employed.
3. Results and discussion
The overall goal of this study was to develop an electrode with size-exclusion electrocatalytic properties for the oxidation of biological compounds by using layer-by-layer (LbL) immobilization of the catalyst in the 50-nm pores of an ormosil film on a GC electrode. In a previous report, phosphomolybdic acid and generation-4 poly(amidoamine) dendrimer (PAMAM) comprised the assembled bilayers, and the reduction of bromate was the test system [37]. Although LbL assembly was achieved, the system was not stable; moreover, the only role of the PAMAM was to serve as a counter-layer. In the present study, Ru-O/CN-O was selected as the oxidation catalyst, and ZrO2 was the counter-layer. The choice of ZrO2 was because this metal oxide is characterized by a high interfacial population of surface hydroxyl groups and proton mobility, a large degree of porosity and rigidity, and its known favorable interactions with ruthenium groups [24,35]. The test system was the oxidation of cysteine in that it has been characterized on Ru-O/CN-O, which catalyzes the oxidation by facilitation of both electron and oxygen transfer. Prior to study in an ormosil film with 50-nm pores, fabrication of composites of Ru-O/CN-O and ZrO2 by LbL assembly was investigated at a conventional GC electrode. This system is not typical for LbL assembly, which is generally associated with layering of organic polyelectrolytes. Also of interest was to establish whether the resulting composite was synergistic in terms of catalytic behavior.
3.1 Layer-by-layer modification of glassy carbon with Ru-O/CN-O and ZrO2
In the first step of the LbL assembly, films of zirconia nanoparticles were deposited on a GC electrode (or on Ru-O/CN-O in multilayer fabrications) by potential cycling (50 cycles) at 10 mV s−1 in the range from −0.2 to 0.8 V. The modification solution comprised a 1:100 dilution of the commercial suspension of ZrO2 nanoparticles with 0.5 mol dm−3 H2SO4. The procedure for electrochemical deposition of Ru-O/CN-O onto GC | ZrO2 was as follows unless otherwise noted. The GC | ZrO2 was immersed for 30 min in a solution comprising 4 mmol dm−3 RuCl3, 4 mmol dm−3 K4Ru(CN)6, and 0.25 mol dm−3 K2SO4 at pH 2.0, and the potential was scanned over the range −0.2 to 1.2 V at 50 mV s−1. Subsequent deposition of bilayers was accomplished by repeating these steps. In all cases, the first step was deposition of a ZrO2 film on a glassy carbon electrode and the outer layer was Ru-O/CN-O.
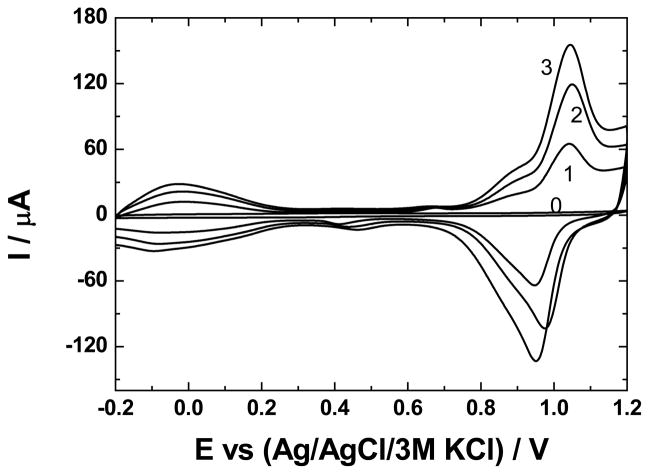
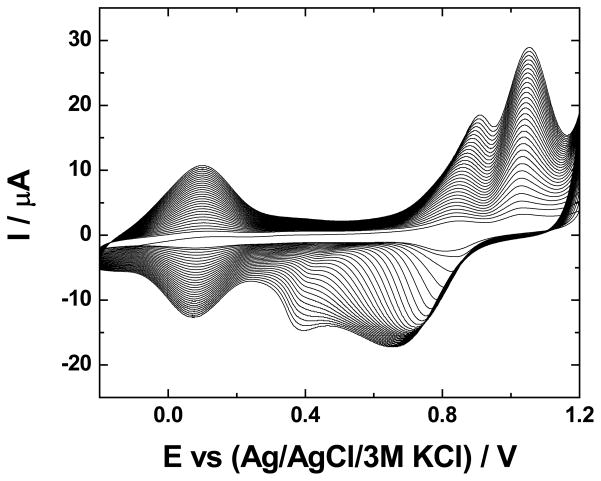
As described in the Experimental section, GC was modified sequentially with 1 – 3 bilayers, n, of ZrO2 | Ru-O/CN-O. The results are shown in Fig. 1. Voltammetry of Ru-O/CN-O agrees with previous reports when the supporting electrolyte anion during deposition was sulfate at pH 0 [42]. In addition to the –Ru-O-Ru- network with cyano crosslinks that form upon deposition in chloride media [2,3,5], some Ru(CN)64−,3− is retained in the structure. Thus, the peaks in Fig. 1 are those characteristic of Ru-oxo species, namely a RuII,III couple at potentials near 0.0 V and RuIII,IV processes in the region 0.75 V to 1.1 V, along with RuIII,IV processes related to cyanoruthenate near 0.9 V [42].
Fig. 1.
Voltammetric formation of multilayers of Ru-O/CN-O and ZrO2 on a glassy carbon electrode. n = 0, GC | ZrO2; n = 1–3, GC | (ZrO2 | Ru-O/CN-O)n. Supporting electrolyte, 0.5 mol dm−3 H2SO4; scan rate, 50 mV s−1. Deposition conditions are given in the Experimental section.
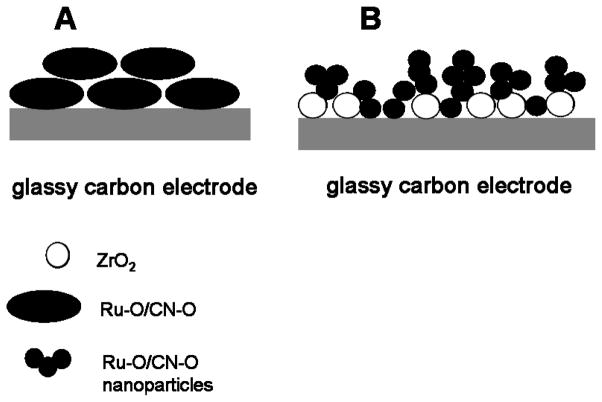
The voltammetric currents in Fig. 1 increase in a near-linear manner with n; specifically, the anodic peak currents, ipa, near 1.0 V are 65, 120, and 160 μA for n-values of 1, 2, and 3, respectively. When the electrode is GC | ZrO2, a redox process is not observed prior to the oxidation of water. The difference in the anodic and cathodic potentials, ΔEp, of these peaks and the actual values of these potentials are not a function of n. For the couple near 1.0 V, ΔEp for n = 1, 2, and 3 is 0.10, 0.08, and 0.10 V, respectively, and the corresponding anodic peak potentials are 0.94, 0.95, and 0.94 V. If the bilayers had significant resistances, ohmic losses of voltage would result in an increase of ΔEp and of the anodic peak potential with current and, hence, with n. Because ZrO2 alone is not highly conducting, the assembly is apparently comprised of compact bilayers, each of which contains a mixture of ZrO2 and Ru-O/CN-O as modeled in Fig. 2. Such a structure was expected in that even LbL assemblies of polyelectrolytes have an intertwined structure rather than existing as discrete, single-component layers [25]. This mixing of the layers is important to the electrochemical behavior in that the ability of the LbL composite to conduct charge is determined by the Ru-O/CN-O rather than by ZrO2 and the former is known to be protonically conducting because of its high population of surface hydroxyl sites thereby overcoming the limited conductivity of ZrO2. Moreover, the structure of Ru-O/CN-O results in facile transport of potassium ions, so salts thereof are well suited to serve as supporting electrolytes with this catalyst [2]. In addition, by analogy to titania, zirconia is capable of interacting specifically with ruthenium oxo centers and activating them toward various electrocatalytic oxidations [23,24,43,44].
Fig. 2.
Model of the LbL assembly of ZrO2 and Ru-O/CN-O illustrating the intermingling of layers as described by Decher [25] for polyelectrolytes.
The SEM images in Fig. 3 illustrate another important factor in the formation of a compact, conductive film. When deposited on ZrO2 (Fig. 3C) rather than directly on GC (Fig. 3B), the Ru-O/CN-O forms as agglomerates of 50 – 100 nm nanoparticles on the ZrO2, the sizes of which are 15 – 25 nm (Fig. 3A). Apparently, electrostatic interaction between the hexacyanoruthenate in the solution from which the film is deposited and the positive sites on the ZrO2 occurs so that the resulting electrochemical formation results in clusters of nanoscale particles of Ru-O/CN-O around the ZrO2 centers. The change of morphology of Ru-O/CN-O by the ZrO2 may increase the population of active sites for electrocatalysis, a conjecture that is supported by the data below.
Fig. 3.
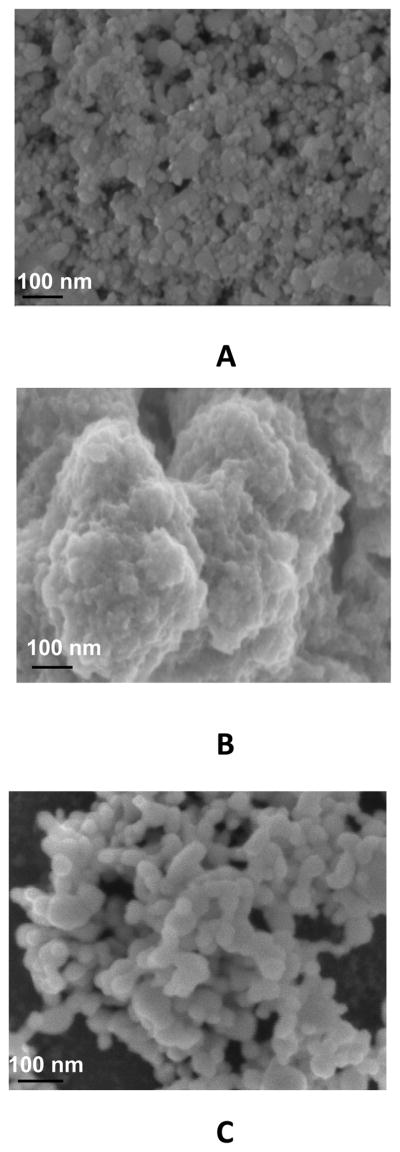
SEM images of (A) ZrO2, (B) Ru-O/CN-O, and (C) ZrO2 | Ru-O/CN-O on glassy carbon.
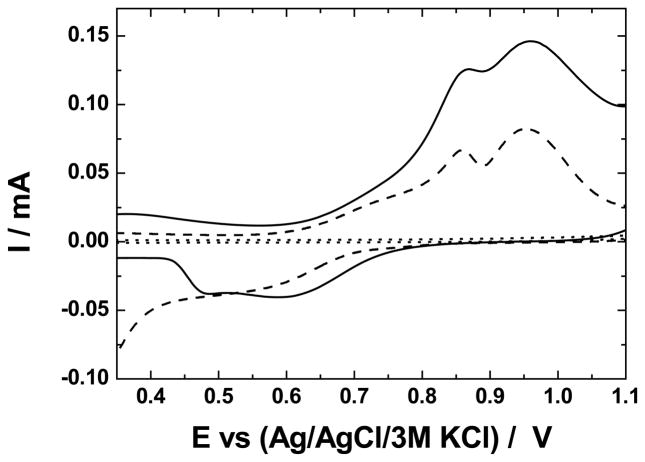
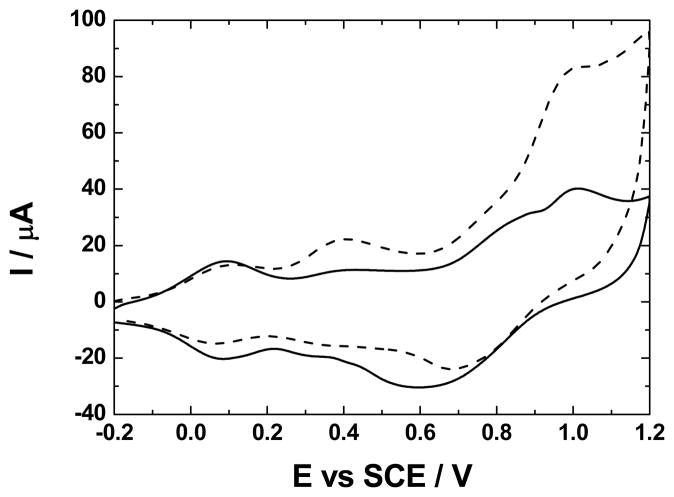
The electrocatalytic oxidation of cysteine on the electrodes in Fig. 1 was compared to that at GC | Ru-O/CN-O. Fig. 4 shows this oxidation at GC | (ZrO2 | Ru-O/CN-O)3 and at a bare GC electrode. At the bare electrode, the oxidation of cysteine passivated the surface to the extent that capacitive current is not observed (Fig. 4, broken line). This behavior is consistent with our previous report [12]. The shape of the voltammogram is the same as that at the GC | Ru-O/CN-O electrode [12], and the peak potentials were independent of n.
Fig. 4.
Oxidation of 1.0 mmol dm−3 cysteine at a GC electrode (broken line) and a GC | (ZrO2, (Ru-O/CN-O)3 electrode (solid line). The dashed line is a voltammogram of GC | (ZrO2, (Ru-O/CN-O)3 in supporting electrolyte (0.25 mol dm−3 K2SO4 at pH 2.0); v, 50 mV s−1).
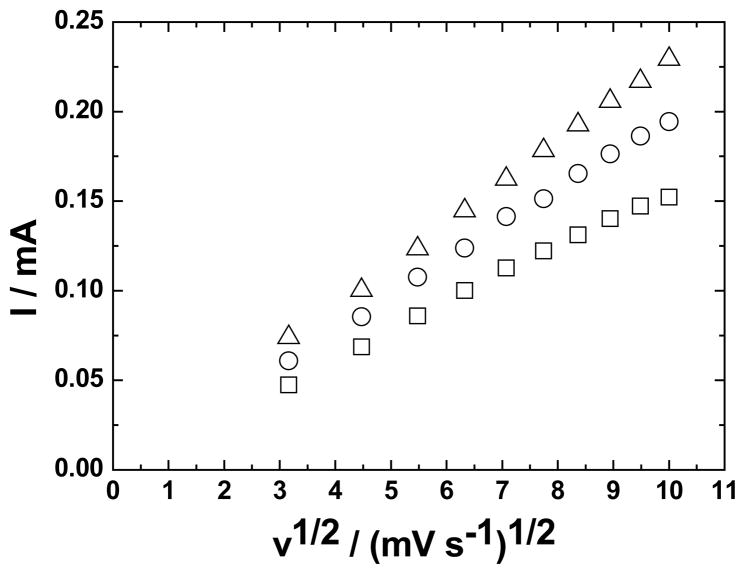
In contrast to the lack of influence of bilayer number on peak potential, the current for the oxidation of cysteine markedly increases with n. To interpret this influence, the anodic peak current, ipa, (corrected for the background current in the absence of cysteine) was measured as a function of v1/2 and n (Fig. 5). When n = 1, a departure from linearity is observed at the fastest scan rates, and R2 is 0.997. When n was 2 or 3, the plots of ipa vs. v1/2 were linear; the respective R2 values were 0.999 in both cases. Apparently, there is partial kinetic control of the oxidation of cysteine at the fastest scan rates employed when n = 1 but linear diffusion control of the current over the entire range when n is 2 or 3. In an ideal LbL assembly, a current limited by linear diffusion, which is the general case in Fig. 5, should not increase with bilayer number.
Fig. 5.
Test for the current-limiting process on GC modified with: □ Ru-O/CN-Ru; ○ ZrO2 | Ru-O/CN-Ru; △ (ZrO2 | Ru-O/CN-O)3. Test system, 1.0 mmol dm−3 cysteine in 0.25 mol dm−3 K2SO4 at pH 2.0.
From the factors in the Randles-Sevcik Equation that comprise the slope of the plot of ip vs. v1/2, two are possibly applicable to the unusual behavior in Fig. 5, namely the number of electrons transferred per mole and the effective area of the electrode. The former was considered unlikely because of previous studies of the pathway of oxidation of cysteine at Ru-O/CN-O [12]. Concerning the latter, an influence of bilayer number on the effective area of the electrode could occur in at least two instances, an increase in geometric area caused by spillover of the LbL assembly beyond the perimeter of the base GC electrode and an apparent electrode area determined by the nanoscale morphology of the active sites of the LbL assembly. Evidence of spillover was not observed, so this explanation was discounted. With regard to the active area, this factor directly relates to the surface coverage by Ru-O/CN-O in that cysteine is oxidized neither at bare GC nor at ZrO2 alone. Electrochemical deposition of Ru-O/CN-O onto ZrO2 results in the formation of nanoparticles (Fig. 3c), and these nanoparticles occur as agglomerates that are separated with ZrO2 by ca. 200 – 500 nm.
To determine whether it is possible for these agglomerates of Ru-O/CN-O to act as independent nanoscale electrodes, the diffusion layer thickness, δ, was calculated assuming hemispherical diffusion to these islands using the relationship, δ ≅ (Dtexpt)1/2. As previously reported [45,46], texpt = 10r2D−1 for cyclic voltammetry under hemispherical diffusion control of the current. Assuming r ≅ 50 nm from Fig. 3c and D ≅ 5 × 10−6 cm2s−1, δ is ca. 10−5 cm. Calculating δ on the assumption of linear diffusion where texpt = RT/vF [47] yields a value of ca. 10−3 cm. In both cases, the diffusion layers of adjacent islands of Ru-O/CN-O would overlap. When the diffusion fields around nanoscale centers of electrochemical activity overlap, the result is that linear diffusion control of the current occurs and the geometric area applies in the Randles-Sevcik Equation [48]. Hence, the influence of n on the currents in Fig. 5 cannot be explained by considering the Ru-O/CN-O domains of this LbL assembly as ideal nanoscale electrodes. Further work is in progress to interpret these results. A factor under consideration is that the exposed ZrO2 separating the Ru-O/CN-O agglomerates (see Fig. 3c) acts as a barrier to diffusion between the islands of catalyst, perhaps by extending above the plane defined by the Ru-O/CN-O surfaces. One conclusion that can be drawn from the results in Fig. 5 is that ZrO2 is highly porous so facile transport of cysteine through the LbL assembly occurs.
3.2 Layer-by-layer modification of the 50-nm pores in a templated ormosil film on GC
Our previous study [37] showed that the method used herein to coat a GC electrode yielded an organically modified silica (ormosil) film that was electrochemically inert except for the area at the base of the templated 50-nm pores. That is, the ormosil phase is an insulator when the sol-gel processing is with hydrogen ion as the catalyst [49]. Under the electrochemically assisted deposition conditions described in the Experimental section, the resulting electrode, which is designated as GC | ormosil (50-nm pores), had an electrochemically active area, which is comprised of the total area of the bases of the pores, equal to 40% of the geometric area of the base electrode. In that study [37], the catalyst that was adsorbed to the base of the pores, dirhodium phosphomolybdate on gold nanoparticles, did not yield a well-defined peak for the oxidation of cysteine prior to the oxidation of water at pH 2. Here, modification of the pore volume with (ZrO2 | Ru-O/CN-O)n to serve as the oxidation catalyst was investigated.
Initially, the voltammetric deposition of Ru-O/CN-O on GC | ormosil (50-nm pores) was performed to compare the deposit to that obtained at bare GC. In this experiment, ZrO2 was not present on the surfaces, so an alternative procedure for depositing Ru-O/CN-O was employed, namely scanning the electrode forty times over the range −0.2 to 1.2 V at 50 mV s−1 in 2 mmol dm−3 RuCl3, 2 mmol dm−3 K4Ru(CN)6, 0.5 mol dm−3− KCl at pH 2.0. The growth of this deposit on GC | ormosil (50-nm pores) is illustrated in Fig. 6. The general shape of the voltammogram agreed with that seen on bare GC. For example, anodic peaks were developed at 0.88 V and 1.01 V whereas they appeared at 0.85 and 0.98 V at ormosil-free GC. However, the ratio of the former to the latter peak was lower in GC | ormosil (50-nm) than at bare GC. In agreement with its behavior as a film on bare glassy carbon electrodes, Ru-O/CN-O deposited in GC | ormosil (50-nm pores) exhibits voltammetry that is not perturbed by ohmic loss of voltage. For example, the anodic peak potential near 1.0 V does not depend on the peak current; this potential was 1.015 ± 0.005 V over the range of the currents developed in the experiments illustrated in Fig. 6. The systematic increase in ipa at 1.015 V with the cycle number suggests that the pore geometry can be approximated as cylindrical rather than spherical. That the surface-bound PSS causes formation of a cylindrical pore is consistent with previous reports that with electrochemically assisted sol-gel deposition of films the pore is a “shadow” of the portion of the base electrode that is blocked. Evaporation-induced self-assembly of surfactants results in formation of cylindrical pores that reflect the diameter of these surface structures when a sol-gel film is formed by electrochemically assisted processing [50,51]. Analogously, adsorption of a sub-monolayer of generation-4 poly(amidoamine) dendrimer provided a template for ca. 10-nm pores in a sol-gel film on an electrode [17].
Fig. 6.
Cyclic voltammetric monitoring of the growth of Ru-O/CN-O in the pores of GC | ormosil (50-nm pores). Solution, 2.0 mmol dm−3 RuCl3, 2.0 mmol dm−3 K4RuCl6, with 0.5 mol dm−3 KCl at pH 2.0; forty cycles at 50 mVs−1 are displayed.
The LbL assembly of (ZrO2 | Ru-O/CN-O)n in GC | ormosil (50-nm pores) was performed using deposition conditions that were previously described (Fig. 1). As in the deposition of Ru-O/CN-O, the voltammetry was the same as that with the LbL assembly on bare GC, including the systematic increase in current with n. When two bilayers of Ru-O/CN-O were present, the current at 0.98 V increased by a factor of 2.1 relative to that at n = 1.
The GC | ormosil (50-nm pores) electrode that was modified with (ZrO2 | Ru-O/CN-O)n was tested on the oxidations of AsIII and of cysteine. Neither of these species is electroactive at GC. The oxidation of 1.0 mmol dm−3 cysteine in a 0.25 mol dm−3 K2SO4 electrolyte adjusted to pH 2.0 with H2SO4 (Fig. 7) yielded a diffusion-limited current over the v-range, 10 – 200 mV s−1. Linear least squares analysis of a plot of log ipa (baseline corrected) vs. log v where the current was measured at 1.0 V had R2 = 0.999 and a slope of 0.50, which agrees with the theoretical value for a current limited by linear diffusion. The shape of the peak for the oxidation of cysteine near 1.0 V is slightly different from that when the LbL assembly was on bare GC. This is related to the voltammetry of the Ru-O/CN-O mediator (solid line, Fig. 7; broken line, Fig. 4). The previously discussed mixed process in that potential region due to some incorporation of hexacyanoruthenate is probably responsible for this small difference; less may have been incorporated in the film deposited in GC | ormosil (50-nm), perhaps because the population of this species in the limited volume of the pores results in its partial depletion. Evidence is that the peak at 0.85 V is less prominent in Fig. 7 and that the pore structure somewhat influenced the ratio of the peaks for Ru-O/CN-O near 1.0 V, as described above. The experiment was repeated except that 1.0 mmol dm−3 AsIII was the test species. Linear least squares analysis of the plot had R2 = 0.999 and a slope of 0.54. It is noteworthy that in these experiments the current was not limited by hemispherical diffusion (or a combination of these two processes), which would be expected if diffusion to the cross-section of the pore at the ormosil-sample phase boundary were the limiting process, especially at the lower end of the v range. Instead, diffusion within the pores to the outer surface of the LbL assembly apparently is the current-limiting step.
Fig. 7.
Oxidation of 1.0 mmol dm−3 cysteine at a GC | ormosil (50-nm) electrode (broken line) and GC | ormosil (50-nm), (ZrO2, (Ru-O/CN-O)2 electrode (solid line). The dashed line is a voltammogram of GC | ormosil (50-nm), (ZrO2, (Ru-O/CN-O)2 in supporting electrolyte (0.25 mol dm−3 K2SO4 at pH 2.0); v, 50 mV s−1).
4. Conclusions
Layer-by-layer formation of a composite of ZrO2 and Ru-O/CN-O was demonstrated on a GC electrode and on a GC electrode modified with an ormosil film with pores templated to have a 50-nm diameter. Voltammetric measurements show that in both cases the LbL assemblies are highly conductive due to facile charge transport through Ru-O/CN-O by hydrogen and potassium ions. In that ZrO2 is not a good conductor, this result demonstrates that there is a high degree of disorder in each bilayer; that is, each layer contains some Ru-O/CN-O rather that existing in the form of a single-component system. This inter-mixing of the layers is consistent with Decher’s description of these deposits as “fuzzy nanoassemblies” [25]. The hypothesis that these composites retain the known conductivity of Ru-O/CN-O when ZrO2 is a component because of disorder of the bilayers is supported by SEM imaging. The ZrO2 increases the currents for electrocatalytic oxidations at Ru-O/CN-O, and the currents increase with the number of bilayers even though the electrode processes are diffusion limited. Possibly the ZrO2 increases the number of active sites of the Ru-O/CN-O by causing its deposition as nanoparticles. The favorable characteristics of these LbL composites are retained when they are formed in the pores of an ormosil film. Such modified electrodes have been shown to resist passivation by macromolecules, essentially providing a size-exclusion, electrocatalytic electrode. This attribute currently is being explored with the ZrO2 | Ru-O/CN-O composite as an oxidation catalyst for biological compounds.
Acknowledgments
The work was supported by the U.S. National Institutes of Health through grant R15GM087662-01 to J.A.C. and by Maestro Project 2012/04/A/ST4/00287 awarded by the National Science Center, Poland, to P.J.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cox JA, Kulesza PJ. Electrocatalytic oxidation and determination of arsenic(III) on a glassy carbon electrode modified with a thin film of mixed-valent ruthenium(III, II) cyanide. Anal Chem. 1984;56:1021–1025. [Google Scholar]
- 2.Kulesza PJ. A polynuclear mixed-valent ruthenium oxide/cyanoruthenate composite that yields thin coatings on a glassy carbon electrode with high catalytic activity toward methanol oxidation. J Electroanal Chem. 1987;220:295–309. [Google Scholar]
- 3.Kulesza PJ, Bandoch M. Preparation and electrochemical characterization of oxygen-transfer catalytic centers in thin films of mixed-valence ruthenium oxide cross-linked with cyanide. J Electroanal Chem. 1992;323:131–147. [Google Scholar]
- 4.Cataldi TRI, Centonze D, Guerrieri A. Mixed-valent ruthenium oxide-ruthenium cyanide inorganic film on glassy carbon electrodes as an amperometric sensor of aliphatic alcohols. Anal Chem. 1995;67:101–107. [Google Scholar]
- 5.Cataldi TRI, Silvia AM, Centonze D, Sabatini L. Voltammetric and XPS investigations of polynuclear ruthenium-containing cyanometalate film electrodes. J Electroanal Chem. 1996;406:91–99. [Google Scholar]
- 6.Crean FM, Schug K. Decomposition of aqueous hexacyanoruthenate(III) ions. Inorg Chem. 1984;23:853–857. [Google Scholar]
- 7.Tess ME, Cox JA. Chemical and biochemical sensors based on advances in materials chemistry. J Pharm Biomed Anal. 1999;19:55–68. doi: 10.1016/s0731-7085(98)00129-0. [DOI] [PubMed] [Google Scholar]
- 8.Cataldi TRI, Centonze D, Desimoni E, Forastiero V. Electrocatalysis and amperometric detection of ethanol at ruthenium-based inorganic films with improved response stability. Anal Chim Acta. 1995;310:357–262. [Google Scholar]
- 9.Chen SM, Hsueh SH. Preparation, characterization and electrocatalytic properties of polynuclear mixed-valent ruthenium oxide/hexacyanoruthenate film modified electrodes. J Electroanal Chem. 2004;566:291–303. [Google Scholar]
- 10.Cox JA, Gray TJ, Kulkarni KR. Stable modified electrodes for flow injection amperometry: application to the determination of thiocyanate. Anal Chem. 1988;60:1710–1713. [Google Scholar]
- 11.Cox JA, Gray TJ. Flow-injection amperometry of cysteine and glutathione at an electrode modified with a ruthenium-containing inorganic film. Electroanalysis. 1990;2:107–111. [Google Scholar]
- 12.Cox JA, Gray TJ. Controlled potential electrolysis of bulk solutions at a modified electrode: Application to oxidations of cysteine, cystine, methionine and thiocyanate. Anal Chem. 1990;62:2742–2744. doi: 10.1021/ac00223a018. [DOI] [PubMed] [Google Scholar]
- 13.Kumar AS, Zen J-M. Organic redox probes for the key oxidation states in mixed valence ruthenium oxide/cyanometallate (ruthenium prussian blue analogue) catalysts. Electroanalysis. 2004;16:1211–1220. [Google Scholar]
- 14.Holmstrom SD, Sandlin ZD, Steinecker WH, Cox JA. Mediated oxidation and determination of gaseous monomethyl hydrazine in a solid-state voltammetric cell employing a sol-gel electrolyte. Electroanalysis. 2000;12:262–266. [Google Scholar]
- 15.Cataldi TRI, Campa C, Centonze D. Electrocatalytic oxidation and amperometric detection of aliphatic and furanic aldehydes at a mixed-valent ruthenium oxide – ruthenium cyanide film on glassy carbon electrodes. Anal Chem. 1995;67:3740–3745. [Google Scholar]
- 16.Gorski W, Cox JA. Amperometric determination of N-nitrosamines in aqueous solution at an electrode coated with a ruthenium-based inorganic polymer. Anal Chem. 1994;66:2771–2774. [Google Scholar]
- 17.Cox JA, Alber KS, Brockway CA, Tess ME, Gorski W. Solid phase extraction in conjunction with solution or solid state voltammetry as a strategy for the determination of neutral organic compounds. Anal Chem. 1995;67:993–998. [Google Scholar]
- 18.Cox JA, Gray TJ. Flow injection determination of insulin based upon its oxidation at a modified electrode. Anal Chem. 1989;61:2462–1464. doi: 10.1021/ac00196a027. [DOI] [PubMed] [Google Scholar]
- 19.Gorski W, Kakey CA, Jonathan RT, Kennedy RT. Ruthenium catalyst for amperometric determination of insulin at physiological pH. J Electroanal Chem. 1997;425:191–199. [Google Scholar]
- 20.Loetanantawong B, Suracheep C, Somasundrum M, Surareungchai W. Electrocatalytic tetracycline oxidation at a mixed-valent ruthenium oxide – ruthenium cyanide – modified glassy carbon electrode and determination of tetracyclines by liquid chromatography with electrochemical detection. Anal Chem. 2004;76:2266–2272. doi: 10.1021/ac035085b. [DOI] [PubMed] [Google Scholar]
- 21.Kawagoe KT, Johnson DC. electrocatalysis of anodic oxygen-transfer reactions. Oxidation of phenol and benzene at bismuth-doped lead dioxide electrode in acidic solutions. J Electrochem Soc. 1994;141:3404–3409. [Google Scholar]
- 22.Popovic ND, Cox JA, Johnson DC. Electrocatalytic function of Bi(V) sites in heavily –doped PbO2film electrodes applied for anodic detection of selected sulfur compounds. J Electroanal Chem. 1998;455:153–160. [Google Scholar]
- 23.Kulesza PJ, Pieta IS, Rutkowska IA, Wadas A, Marks D, Klak K, Stobinski L, Cox JA. Electrocatalytic oxidation of small organic molecules in acid medium: Enhancement of activity of noble metal nanoparticles and their alloys by supporting or modifying them with metal oxides. Electrochim Acta. doi: 10.1016/j.electacta.2013.06.052. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camara OR, Trasatti S. Surface electrochemical properties of Ti/(RuO2 + ZrO2) electrodes. Electrochim Acta. 1996;41:419–427. [Google Scholar]
- 25.Decher G. Fuzzy nanoassemblies: Toward layered polymeric nanocomposites. Science. 1997;277:1232–1237. [Google Scholar]
- 26.Garćia M, Carfumán K, Díaz C, Garrido C, Osorio-Román I, Jesús Aguirre M, Isaacs M. Multimetallic porphyrins/polyoxotungstate modified electrodes by layer-by-layer method: Electrochemical, spectroscopic and morphological characterization. Electrochim Acta. 2012;80:390–398. [Google Scholar]
- 27.Azevedo CMN, Araki K, Angnes L, Toma HE. Electrostatically assembled films for improving the properties of tetraruthenated porphyrin-modified electrodes. Electroanalysis. 1998;10:467–471. [Google Scholar]
- 28.Man KYK, Wong HL, Chan WK. Use of a ruthenium-containing conjugated polymer as a photosensitizer in photovoltaic devices fabricated by a layer-by-layer deposition process. Langmuir. 2006;22:3368–3375. doi: 10.1021/la052655p. [DOI] [PubMed] [Google Scholar]
- 29.Salimi A Korani, Hallaj R, Soltanian S, Hadadzadeh H. Deposition of α-SiMo12O4-40-[Ru(bipyridine)(terpyridine)Cl]+ multilayer film on single wall carbon nanotube modified glassy carbon electrode: Improvement of the electrochemical properties and chemical stability. Thin Solid Films. 2010;518:5304–5310. [Google Scholar]
- 30.Pacey GE, Puckett SD, Cheng L, Khatib-Shahidi S, Cox JA. Detection of DNA damaging agents using layer-by-layer assembly. Anal Chim Acta. 2005;533:135–139. [Google Scholar]
- 31.Cox JA, Wiaderek K, Ernst EZ, Kulesza PJ. Fabrication of a composite of conducting polymers, nanoparticles, and an enzyme for bio-electrocatalysis of oxygen and hydrogen peroxide reduction. World J Eng. 2010;7(Supplement 2):441–442. [Google Scholar]
- 32.Aradilla D, Estrany F, Alemán C. Symmetric supercapacitors based on multilayers of conducting polymers. J Phys Chem C. 2011;115:8430–8438. [Google Scholar]
- 33.Endrödi B, Bíró A, Janáky C, Visy C. Layer by layer growth of electroactive conducting polymer/magnetite hybrid assemblies. Synthetic Metals. 2013;171:62–68. [Google Scholar]
- 34.Gan T, Hu C, Chen Z, Hu S. Novel electrocatalytic system for the oxidation of methyl jasmonate based on layer-by-layer assembling of montmorillonite and phosphotungstic acid nanohybrid on graphite electrode. Electrochim Acta. 2011;56:4512–4517. [Google Scholar]
- 35.Brunet E, Alonso M, Cerro C, Juanes O, Rodríguez-Ubis JC, Kaifer AE. A luminescence and electrochemical study of photoinduced electron transfer within the layers of zirconium phosphate. Adv Funct Mater. 2007;17:1603–1610. [Google Scholar]
- 36.Wang Q, Yu D, Wang Y, Sun J, Shen J. Incorporation of water-soluble and water-insoluble ruthenium complexes into zirconium phosphate films fabricated by the layer-by-layer adsorption and reaction method. Langmuir. 2008;24:11684–11690. doi: 10.1021/la802364z. [DOI] [PubMed] [Google Scholar]
- 37.Mehdi BL, Rutkowska IA, Kulesza PJ, Cox JA. Electrochemically assisted fabrication of size-exclusion films of organically modified silica and application to the voltammetry of phospholipids. J Solid State Electrochem. 2013;17:1581–1590. doi: 10.1007/s10008-013-2077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shacham R, Avnir D, Mandler D. Electrodeposition of methylated sol-gel films on conducting surfaces. Adv Mater. 1999;11:384–388. [Google Scholar]
- 39.Shacham R, Avnir D, Mandler D. Electrodeposition of dye-doped titania thin films. J Sol-Gel Sci Technol. 2004;31:329–334. [Google Scholar]
- 40.Shacham R, Mandler D, Avnir D. Electrochemically induced sol-gel deposition of zirconia thin films. Chem Eur J. 2004;10:1936–1943. doi: 10.1002/chem.200305469. [DOI] [PubMed] [Google Scholar]
- 41.Cox JA. Modification of electrodes with catalytic, size-exclusion films. J Solid State Electrochem. 2011;15:1495–1507. [Google Scholar]
- 42.Kulesza PJ, Grzybowska B, Malik MA, Chojak M, Miecznikowski K. Oxidation of methanol at an electrocatalytic film containing platinum and polynuclear oxocyanoruthenium microcenters dispersed within tungsten oxide matrix. J Electroanal Chem. 2001;512:110–118. [Google Scholar]
- 43.Trasatti S. Oxide/aqueous solution interfaces. Interplay of surface chemistry and electrocatalysis. Mat Chem Phys. 1987;16:157–174. [Google Scholar]
- 44.Trasatti S. Electrocatalysis by oxides - attempt at a unifying approach. J Electroanal Chem. 1980;111:125–131. [Google Scholar]
- 45.Shao Y, Mirkin MV, Fish G, Kokotov S, Palanker D, Lewis A. Nanometer-Sized Electrochemical Sensors. Anal Chem. 1997;69:1627–1634. [Google Scholar]
- 46.Shoup D, Szabo A. Chronoamperometric current at finite disk electrodes. J Electroanal Chem. 1982;140:237–245. [Google Scholar]
- 47.Bard AJ, Faulkner LR. Electrochemical Methods. 2. John Wiley and Sons; New York: 2001. [Google Scholar]
- 48.Ca DV, Sun L, Cox JA. Optimization of the dispersion of gold and platinum nanoparticles on indium tin oxide for the electrocatalytic oxidation of cysteine and arsenite. Electrochim Acta. 2006;51:2188–2194. [Google Scholar]
- 49.Cox JA, Wiaderek KM, Mehdi BL, Gudorf BP, Ranganathan D, Zamponi S, Berrettoni M. Influence of silanization on voltammetry at electrodes modified with silica films of controlled porosity formed by electrochemically initiated sol-gel processing. J Solid State Electrochem. 2011;15:2409–2417. [Google Scholar]
- 50.Miyata H, Kawashima Y, Itoh M, Watanabe M. Preparation of a mesoporous silica film with a strictly aligned porous structure through a sol-gel process. Chem Mater. 2005;17:5323–5327. [Google Scholar]
- 51.Ranganathan D, Zamponi S, Berrettoni M, Mehdi BL, Cox JA. Oxidation and flow-injection amperometric determination of 5-hydroxytryptophan at an electrode modified by electrochemically assisted deposition of a sol-gel film with templated nanoscale pores. Talanta. 2010;82:1149–1155. doi: 10.1016/j.talanta.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]