SUMMARY
Objective
Maintenance of muscle mass is crucial to improving outcome and quality of life in cancer patients. Stimulating muscle protein synthesis is the metabolic basis for maintaining muscle mass, but in cancer patients normal dietary intake has minimal effects on muscle protein synthesis. Adding leucine to high protein supplements stimulates muscle protein synthesis in healthy older subjects. The objective was to determine if a specially formulated medical food, high in leucine and protein, stimulates muscle protein synthesis acutely in individuals with cancer to a greater extent than a conventional medical food.
Design
A randomized, controlled, double-blind, parallel-group design was used in 25 patients with radiographic evidence of cancer. Patients were studied before their cancer treatment was started or 4 weeks after their treatment was completed or halted. The fractional rate of muscle protein synthesis (FSR) was measured using the tracer incorporation technique with L-[ring-13C6]-phenylalanine. The experimental group (n = 13) received a medical food containing 40 g protein, based on casein and whey protein and enriched with 10% free leucine and other specific components, while the control group (n = 12) was given a conventionally used medical food based on casein protein alone (24 g). Blood and muscle samples were collected in the basal state and 5h hours after ingestion of the medical foods.
Results
The cancer patients were in an inflammatory state, as reflected by high levels of C-reactive protein (CRP), IL-1β and TNF-α, but were not insulin resistant (HOMA). After ingestion of the experimental medical food, plasma leucine increased to about 400 μM as compared to the peak value of 200 μM, after the control medical food (p < 0.001). Ingestion of the experimental medical food increased muscle protein FSR from 0.073 (SD: 0.023) to 0.097 (SD: 0.033) %/h (p = 0.0269). In contrast, ingestion of the control medical food did not increase muscle FSR; 0.073 (SD: 0.022) and 0.065 (SD: 0.028) %/h.
Conclusions
In cancer patients, conventional nutritional supplementation is ineffective in stimulating muscle protein synthesis. This anabolic resistance can be overcome with a specially formulated nutritional supplement.
Keywords: Muscle, Protein synthesis, Casein, Whey, Cancer, Leucine, Medical food
1. Introduction
Cancer is often associated with a constellation of responses which together lead to cachexia. The term cachexia has most recently been defined and refers to “…a complex metabolic syndrome characterized by loss of muscle…”.1 A crucial characteristic is that the muscle wasting is more rapid in cachexia than would be expected to occur due to decreased food intake alone, although anorexia is often one of several responses leading to cachexia. In addition to loss of appetite, metabolic changes occur in cancer patients that may amplify the loss of muscle. These metabolic responses may result from inflammation, insulin resistance, hypogonadism, or other causes.2 Cachexia and resulting muscle loss have been linked to poor outcomes in a variety of cancers,2 and therefore it can be hypothesized that a nutritional approach could help to minimize the effects of cachexia.
There have not been extensive studies of protein metabolism in cancer patients. Whole body protein turnover appears to be elevated in post-absorptive cancer patients compared to normal individuals without cancer.3–5 The response is not simply due to weight loss.6 Despite the accelerated whole body protein turnover in patients prior to significant weight loss, the rate of muscle protein synthesis has been reported to be reduced in patients with established cachexia.7 Muscle protein is generally diminished in cancer patients, with deleterious effects on clinical outcome.1,8 Increasing muscle mass is important as recurrence of cancer in treated patients is directly related to the extent of muscle loss.8
2. Are cancer patients resistant to stimulating protein synthesis?
In the presence of systemic inflammation, it appears to be extremely difficult to achieve whole body protein anabolism in cancer patients.9 It therefore seems that although food intake should be increased in cachectic cancer patients, gains in lean body mass are difficult to achieve unless specific metabolic abnormalities, like inflammation, are targeted.9 In cancer animal models, we observed a reduced postoperative increase of protein degradation, suggesting disturbed amino acid response capabilities in cancer.10–15 The metabolic basis for maintenance of lean body mass is that following a meal the stimulation of protein synthesis exceeds protein breakdown sufficiently to balance the net loss of protein in the post-absorptive or fasted state. Significant loss of lean body mass will occur if the protein synthetic response to anabolic stimuli such as amino acids is suppressed over a period of time. We therefore anticipate that the normal anabolic action of amino acids or protein on muscle protein synthesis is suppressed in cancer patients.
3. How to improve the muscle protein synthesis response?
3.1. Increasing protein and amino acid intake
The cachectic cancer patient usually has less than optimal nutrient intake.9 Therefore, amino acids released from the process of net muscle protein breakdown provide most of the precursors to synthesize the proteins involved in the inflammatory response. It is therefore to be expected that provision of extra essential amino acids, in the form of orally ingested protein, would provide essential precursors for the replacement of muscle protein and to reduce net muscle protein breakdown. Amino acids are the principal nutrient responsible for the stimulation of muscle protein synthesis.16 Further, there is an imbalance between the amino acid composition of skeletal muscle and acute phase proteins in inflammatory conditions,17 which may result in different amino acids than normal being limiting for muscle protein synthesis during inflammation.
Muscle protein synthesis in the elderly can be stimulated by continuous infusion of mixed amino acids in the post-absorptive state18 and also by a bolus ingestion of essential amino acids.19 Other studies show that whey protein has a superior effect on stimulating muscle protein synthesis, above the effect of the constituent essential amino acids.20 As muscle protein synthesis in elderly is less responsive to a small amount (7 gm) of essential amino acids21 and this diminished responsiveness to be likely in cancer patients as well, an effective medical food with higher protein content and a higher quality of protein potentially would be more effective in both cancer patients and elderly.
3.2. Increasing leucine
Animal studies have long indicated that the branched-chain amino acids22 and more specifically leucine are unique among the amino acids in the stimulation of muscle protein synthesis.23,24 Animal studies also indicate that the responsiveness of muscle protein synthesis to stimulation by leucine decreases with age,25 suggesting that more leucine might be required in older individuals to maximize the response of muscle protein synthesis. Therefore, raising the plasma concentration of leucine by the provision of a leucine-enriched medical food potentially can overcome impaired responsiveness of muscle protein synthesis. Consistent with the notion of leucine playing a regulatory role in the stimulation of muscle protein synthesis, it has recently been shown that supplementation with extra leucine improves the response of muscle protein synthesis in elderly subjects.26–28 In addition, we recently observed in a cancer-cachectic mouse model that muscle mass was increased more when the mice were fed a high protein, high leucine diet with additional fish oil.29
3.3. Does improving muscle FSR represent improved muscle function and mass?
In previous studies, we measured muscle protein synthesis in response to intake of nutritional supplements.30,31 Subsequent studies have shown that when acute intake of specific nutritional supplements increases muscle protein synthesis, intake of these nutritional supplements for longer periods is related to improved muscle function30,31 whereas increased muscle mass appears to be a more transient effect. Therefore, we anticipate that acute stimulation of muscle FSR would translate to a sustained improved muscle function when such a medical food is consumed for a longer period.
3.4. Aim of the study
The objective of the present study was to determine if intake of a specially formulated medical food, high in leucine and protein stimulates muscle protein synthesis acutely in patients with cancer to a greater extent than a conventional medical food. The experimental medical food is tailored for maximal stimulation by adding potential muscle protein synthesis stimulators whey protein and leucine. The results of our study clearly show that absence of improvement of muscle protein synthesis in catabolic cancer patients with involuntary weight loss is related to the composition of the medical food and not to the unresponsiveness of muscle in these cancer patients, per se.
4. Patients and methods
4.1. Patients
A total of 25 patients were enrolled in the study. One patient (817) did not undergo all 3 muscle biopsies due to extreme atrophy of the muscles. According to protocol, this patient was replaced to reach 2 × 12 study completers. All patients had radiographic evidence of cancer, were 40 years of age or more, and had the ability to sign the informed consent (Table 1). None had received treatment for their cancer for 4 weeks or less prior to the study. After providing informed consent, a thorough history and physical exam was performed on each prospective subject. Patients were excluded who had lost more than 10% of body weight over the six months prior to enrollment. The body mass index of all patients accepted into the study was between 20 and 30 kg/m2. Other exclusion criteria were; hemoglobin less than 9.0 g/dL, platelet count <100,000/mL, alterations in clotting, history of hypo- or hypercoagulation disorders including taking Coumadin, history of deep vein thrombosis or pulmonary embolism, PT with INR greater than 1.5, PTT greater than 40 s, uncontrolled hypertension, diagnosed type I diabetes, chronic use of insulin and untreated metabolic diseases including liver and renal disease. No patients were currently involved in a muscle strengthening program or using nutritional supplements enriched with branched chain amino acids. In addition, patients did not have unstable heart disease or recent myocardial infarction, and did not abuse alcohol (more than two servings per day) or drugs. Subjects had their diet standardized for 3 days prior to the experimental visit. Subjects received meals from our metabolic kitchen, or received instructions for diet standardization in case provision of study meals was not possible. Dietary analysis indicated no differences between study groups in protein intake prior to the experimental visit.
Table 1.
Tumor type and stage of studied patients.
| Subject ID | Agerace | Primary location | Stage | Body weight change |
|---|---|---|---|---|
| Control: Conventional medical food (n = 12) | ||||
| 818 | 62a | Colon, liver | III | −3.4% |
| 853 | 53a | Lung (NSCLC) | IIIB | 0.2% |
| 857 | 78a | Lung (NSCLC) | IA | −5.4% |
| 860 | 61a | Lung (NSCLC) | IIIB | −0.7% |
| 862 | 81a | Lung (NSCLC) | IIB | −3.2% |
| 864 | 71b | B-cell lymphoma | IIIB | −3.2% |
| 865 | 70a | Esophagus | II | 0% |
| 868 | 62a | Lung (NSCLC) | UK | −6.5% |
| 869 | 67c | Rectum | IIIB | 4.9% |
| 871 | 66a | Lung (NSCLC) | IV | −3.1% |
| 873 | 60b | Lung (SCLC) | IV | 5.3% |
| 951 | 68a | Colon | IV | −7.6% |
| EXP: Leucine-enriched high protein medical food (n=13) | ||||
| 817* | 75b | Colon - sigmoid | II | −1.2% |
| 851 | 59b | Colon, liver | UK | 0.6% |
| 854 | 69b | Colon | IV | −0.5% |
| 856 | 74a | Lung (NSCLC) | IIIA | −5.9% |
| 858 | 73a | Lung (NSCLC) | IV | −4.3% |
| 859 | 59a | Breast | IIB | −2.1% |
| 861 | 63b | Colon | IV | −4.4% |
| 863 | 71a | Lung (NSCLC) | IIIB | −2.5% |
| 866 | 68a | Lung (NSCLC) | UK | −2.6% |
| 867 | 65a | Rectum | IV | −3.7% |
| 870 | 74a | Lung (NSCLC) | IIB | −0.1% |
| 872 | 71a | Colon, rectum | IIIB | −6.3% |
| 952 | 79a | Colon | IIA | −2.9% |
All patients were male. Race:
Caucasian,
African-American,
Hispanic-American. The patients were studied before cancer treatment was started or at least 4 weeks after treatment was completed. Body weight change is change over last 4 weeks before start of the study.
Subject 817 was replaced according to protocol due to incomplete muscle biopsy procedure.
NSCLC = non-small cell lung cancer, UK = Unknown.
All subjects provided informed consent after being informed of the procedures involved and of all possible risks. The study was approved by the Institutional Review Boards of the University of Arkansas for Medical Sciences (UAMS) and the Little Rock Veteran’s Administration (VA) Hospital, Little Rock, Arkansas. The study protocol was also approved by the UAMS Cancer Center and the Clinical Research Center, and the VA Research and Development Committee. This trial is registered at ClinicalTrials.gov under NCT00446888.
4.2. Design
The general experimental protocol involved determining muscle protein synthesis in the basal state and over the five hours immediately following the ingestion of one of two liquid medical foods simulating a mixed meal. A randomized, controlled, double-blind, parallel-group design was utilized (Fig. 1). Both medical foods were packaged indistinguishably, except for the study code. The study staff was blind to the study code until completion of the study database and after all laboratory data were collected.
Fig. 1.
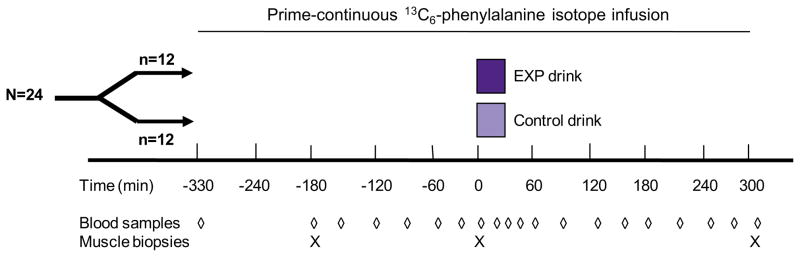
Study design. Stable isotope infusion was a primed-continuous L-[ring-13C6]phenylalanine infusion.
4.3. Study products
Both medical foods provided 640 kcal in 2 doses of 200 ml (Table 2). The control medical food (control) consisted of 15% of calories as intact protein (casein), 52% of calories as carbohydrate (sucrose and maltodextrin), and 33% of calories as fat (principally canola/sunflower blend). Several extra ingredients were added to the experimental medical food (EXP, FortiCare; with fish oil, high protein, leucine and specific oligosaccharides Nutricia Advanced Medical Nutrition, Zoetermeer, the Netherlands). EXP had 27% calories as total protein (intact protein: 24.2 g casein and 11.9 g whey, free amino acids: 4.16 g free leucine), 44% of calories as carbohydrate (sucrose, maltodextrin and trehalose), and 30% of calories as fat. In addition to the intact protein, 4.16 g of leucine as the free amino acid was included. The fat in EXP was a mixture of canola oil (4.03 g) and corn oil (7.94 g). In addition, fish oil (8.38 g) was added that contained 2.2 g of EPA and 1.1 g DHA. Minerals, trace elements and vitamins were added to both formulations in approximate comparable amounts (Table 2).
Table 2.
Composition of the medical foods.
| Component | Unit | Control medical food | Experimental medical food | |
|---|---|---|---|---|
| Energy | Kcal | 640 | 640 | |
| Protein | % | 15 | 26.7 | |
| Carbohydrates | % | 52.3 | 43.6 | |
| Fat | % | 32.6 | 29.8 | |
| Protein | Total | g | 24.0 | 40.1 |
| Intact protein | Casein | g | 24.00 | 24.2 |
| Whey | g | 0 | 11.9 | |
| Free amino acid | Leucine | g | 0 | 4.16 |
| Total leucine | g (%) | 2.0 (8.5) | 7.8 (19) | |
| Carbohydrates | Total | g | 83.6 | 69.7 |
| Sucrose | g | 27.3 | 16.8 | |
| Maltodextrin | g | 55.0 | 33.7 | |
| Trehalose | g | 0 | 16.8 | |
| Lactose | g | 0.05 | 2.36 | |
| Fat | Total | g | 23.2 | 21.2 |
| Fish Oil | g | 0 | 8.38 | |
| EPA | g | 0 | 2.2 | |
| DHA | g | 0 | 1.1 | |
| Canola/Sunflower blend | g | 5.75 | 4.03 | |
| Corn Oil | g | 0 | 7.94 | |
| Milk fat | g | 0.05 | 0 | |
| w 6/w 3 | g | 5.03 | 1.16 | |
| Fiber | Total | 0 | 8.24 | |
| Soluble | 6.80 | |||
| Insoluble | 1.44 | |||
| Minerals | Sodium | mg | 404 | 440 |
| Potassium | mg | 636 | 860 | |
| Choride | mg | 348 | 560 | |
| Calcium | mg | 364 | 600 | |
| Phosphorus | mg | 312 | 460 | |
| Magnesium | mg | 92 | 113 | |
| Trace elements | Iron | mg | 9.6 | 7.6 |
| Zinc | mg | 7.2 | 8.2 | |
| Copper | μg | 1080.0 | 1152.0 | |
| Manganese | mg | 2.0 | 2.72 | |
| Fluoride | mg | 0.6 | 0.64 | |
| Molydenum | μg | 60.0 | 64.0 | |
| Selenium | μg | 34.4 | 54.0 | |
| Chromium | μg | 40.0 | 44.0 | |
| Iodine | μg | 80.0 | 84.0 | |
| Vitamins | Vitamine A | μg re | 492.0 | 520.0 |
| Carotenoids | mg | 1.3 | 1.3 | |
| Vitamin D | μg | 4.4 | 4.4 | |
| Vitamin E | mg a-te | 7.6 | 12.80 | |
| Vitamin K | μg | 32.0 | 34.0 | |
| Vitamin B1 | mg | 0.9 | 0.96 | |
| Vitamin B2 | mg | 1.0 | 1.0 | |
| Niacin | mg ne | 10.8 | 11.6 | |
| Pantothenic Acid | mg | 3.2 | 3.4 | |
| Vitamin B6 | mg | 1.0 | 2.32 | |
| Folic Acid | μg | 160.0 | 212.0 | |
| Vitamin B12 | μg | 1.3 | 2.56 | |
| Biotin | μg | 24.0 | 23.6 | |
| Vitamin C | mg | 60.0 | 84.0 | |
| Choline | mg | 220.0 | 236.0 | |
| Extra additions | Taurine | mg | 0 | 52.0 |
| Carnitine | mg | 0 | 44.0 |
The patients consumed 400 ml of the medical foods within 30 min.
4.4. Isotope infusion protocol
Patients reported to the clinic in the morning after an overnight fast. On the morning of the study, an 18–22 gauge catheter was placed into veins of the right and left forearms for blood sampling tracer infusion. After obtaining a blood sample for background amino acid enrichment and fasting concentrations of blood glucose, CRP and cytokines, a priming dose (2 μmol/kg) of L-[ring-13C6] phenylalanine (Cambridge Isotope Labs, Andover, MA) was given. This was immediately followed by a continuous (0.07 μmol/kg/min) infusion of 13C6-phenylalanine and maintained throughout the experiment.
A muscle biopsy was performed 2 h after the start of isotope infusion and again at 5 h in order to determine the basal rate of muscle protein synthesis. Blood was taken from the sampling forearm catheter periodically for the determination of plasma phenylalanine enrichment (tracer/tracee ratio). Immediately following the second muscle biopsy, one dose of the medical food (200 ml) was ingested, followed by a second dose (200 ml) 20 min after the first sip of the first dose. Each dose was consumed within 10 min. Blood samples were then drawn over the next 5 h. A third muscle biopsy was taken 300 min after the first sip of the first dose of the medical food. All muscle biopsies were taken from the same muscle via the same incision.
The patients lay in bed throughout the study unless they had to use the bathroom. Muscle biopsies (50–100 mg) were taken under local anesthetic from the lateral portion of the vastus lateralis approximately 10–15 cm above the knee using a 5 mm Bergstrom biopsy needle (Stille, Stockholm, Sweden). Muscle samples were used to determine free intracellular and protein-bound phenylal-anine enrichment for the purpose of calculating the muscle protein fractional synthetic rate (FSR). Plasma samples were analyzed for phenylalanine enrichment, glucose, amino acid, and insulin concentrations.
4.5. Sample processing
Blood samples were collected in pre-chilled, heparinized tubes (Becton Dickinson Vacutainer system, Franklin Lakes, New Jersey, USA) and kept on ice to minimize enzymatic reactions. Plasma was obtained by centrifugation of whole blood at 4 °C for 10 min at 3120 g. All plasma samples were stored at −80 °C until use. After removing any visible fat and connective tissue, muscle samples were rinsed with ice-cold saline to remove any blood, blotted dry, and immediately frozen in liquid nitrogen before being stored at −80 °C. After thawing of the muscle samples, proteins were precipitated with 800 μl of 14% perchloroacetic acid. Tissue was homogenized, centrifuged, and tissue free amino acids (labeled phenylalanine) were extracted from the supernatant by cation exchange chromatography (Dowex AG 50W-8X, 100–200 mesh H+ form; Bio-Rad Laboratories, Richmond, CA), and dried under vacuum (Savant Industries, Farmingdale, NY). The remaining muscle pellet was washed and dried, hydrolyzed in 6N HCl at 50 °C for 24 h.
4.6. Antropometric measurements and biochemical analysis
Height, body weight, and body composition were determined on the day of enrollment. Body composition was determined by means of dual-energy x-ray absorptiometry (DEXA: QDR 4500W, Hologic, Inc., Bedford, MA.).
Muscle free and protein-bound L-[ring-13C6]phenylalanine enrichment were determined using the tert-butyldimethylsilyl derivative and GCMS (Agilent Technologies model 5973) with electron impact ionization and selective ion monitoring for ions 234 and 240. Glucose concentration was measured using an YSI glucose autoanalyzer. Amino acid concentrations in plasma were determined by liquid chromatography-mass spectrometry32 using the internal standard approach for each individual amino acid.33-C-reactive peptide and insulin concentrations were determined by ELISA assay. Cytokines (IL-1β, IL-6 and TNF-α) were measured using a commercial custom-made human Bio-Plex Cytokine bead immunoassay (Invitrogen, Merelbeke, Belgium) according to the manufacturer’s protocol.
4.7. Calculations
The tracer–tracee ratio (t/T) was calculated from the difference in the area counts of the mass fragments of 234 and 240. Enrichment or MPE (molar % excess) was calculated as (t/T)/(t/T+1). Whole body rate of appearance of phenylalanine (WbRaPHE) was calculated by dividing the infusion rate of L-[ring-13C6]phenylala-nine by the plasma t/T of phenyalanine.
The primary outcome variable was the fractional synthetic rate (FSR; %/h) of mixed muscle protein, which was calculated as , where ΔMPEp is the increment in the muscle protein-bound 13C6phenylalanine MPE between two biopsies. MPEic is the average intracellular 13C6phenylalanine MPE between the two biopsies during the steady-state in plasma L-[ring-13C6]phenylalanine MPE, and t is the time interval (min) between the biopsies. The factors 60 and 100 are used to express the FSR values in percent per hour. In addition, the FSR was calculated using the average plasma MPE between the two biopsies (area/time) and from the MPE difference between the first and last biopsy using both the intracellular and average plasma MPE. Because human muscle protein synthesis is modulated more by extracellular amino acid availability,34 we suggest that calculation with both intracellular and plasma enrichments should be done to be sure that the conclusions are not dependent on the choice of precursor enrichment.
4.8. Statistical analysis
A two-sample t-test was used to directly compare post-treatment FSR in the two groups. In addition, the post-treatment FSR means of the groups were compared after adjusting for basal FSR using analysis of covariance models. Nonparametric methods for the analysis of longitudinal data proposed by Brunner et al35 were used to compare the treatment groups with respect to glucose and insulin profiles. Other values were compared using t-tests or Wilcoxon rank sum tests. SAS® (SAS Institute, Cary, NC) and R (R Foundation for Statistical Computing, Vienna, Austria) software were used to perform these analyses. Statistical significance of these tests were defined as a two-tailed P < 0.05.
5. Results
5.1. Patients
All patients had a form of advanced cancer, mainly lung and colon cancer (Table 1). Descriptives and statistical analyses were performed on all 24 study completers (see Method section). No differences were present in body weight loss, body composition, demographics, and screening parameters between the groups (Table 3). The fat-free mass index (FFMI) of the patients was below the 10% percentile of a reference population of adult Caucasians.36
Table 3.
Patients’ characteristics, per-protocol population.
| Characteristic | Control n = 12 | EXP n = 12 | P value |
|---|---|---|---|
| Age (years) in mean ± SD | 66.6 ± 7.8 | 68.8 ± 6.2 | 0.461 |
| Race (number (%)): | |||
| –Caucasian | 9 (75%) | 9 (75%) | 0.549 |
| –African-American | 2 (16.7%) | 3 (25%) | |
| –Hispanic-American | 1 (8.3%) | 0 (0%) | |
| Body weight (kg) in mean ± SD | 83.9 ± 12.2 | 82.6 ± 12.1 | 0.790 |
| Lean weight (kg) in mean ± SD | 55.1 ± 8.0 | 55.2 ± 6.6 | 0.981 |
| LBM (%) in mean ± SD | 66.8 ± 3.4 | 69.6 ± 4.8 | 0.110 |
| Body Mass Index (kg/m2) in mean ± SD | 25.8 ± 2.1 | 25.1 ± 3.3 | 0.572 |
| FFMI (kg/m2) in mean ± SD | 17.7 ± 1.4 | 17.7 ± 1.6 | 0.958 |
| Body weight change (%) in mean ± SD | −1.9 ± 4.1 | −2.9 ± 2.2 | 0.459 |
| Cancer diagnosis (number (%)): | |||
| –Lung | 7 (58.3%) | 5 (41.7%) | 0.363 |
| –Colorectal | 3 (25%) | 6 (50%) | |
| –Breast | 0 (0%) | 1 (8.3%) | |
| –Oesophagus | 1 (8.3%) | 0 (0%) | |
| –B-cell lymphoma | 1 (8.3%) | 0 (0%) | |
| Stage (number (%)): | |||
| –I | 1 (8.3%) | 0 (0%) | 0.482 |
| –II A & B | 1 (8.3%) | 3 (25%) | |
| –III A & B | 6 (50%) | 3 (25%) | |
| –IV | 3 (25%) | 4 (33.3%) | |
| –Unknown | 1 (8.3%) | 2 (16.7%) | |
| ECOG peformance status (number (%)): | |||
| –0 | 1 (8.3%) | 3 (25%) | 0.531 |
| –1 | 8 (66.7%) | 7 (58.3%) | |
| –2 | 3 (25%) | 2 (16.7%) | |
| Pro-inflammatory markers | |||
| CRP (ng/mL) in mean ± SD | 22.2 ± 6.9 n = 9 | 28.7 ± 8.2 n = 11 | 0.076 |
| IL-1β (pg/mL) in median (min-max) | 15.9 (2.7–289.0) n = 9 | 12.9 (2.7–91.3) n = 11 | 0.909 |
| IL-6 (pg/mL) in median (min-max) | 43.6 (13.3–637.7) n = 9 | 27.1 (10.7–316.2) | 0.320 |
| TNF-α (pg/mL) in median (min-max) | 31.3 (1.7–86.0) n = 9 | 10.6 (1.7–57.4) | 0.721 |
| Glucose homeostasis | |||
| Fasting Glucose (mmol/L) in mean ± SD | 5.8 ± 0.4 n = 9 | 5.8 ± 1.0 | 0.921 |
| Fasting insulin (μU/mL) in median (min-max) | 3.1 (1.7–11.0) n = 9 | 3.0 (1.0–5.7) | 0.546 |
| HOMA in median (min-max) | 0.82 (0.41–2.94) n = 9 | 0.78 (0.21–1.93) | 0.522 |
| QUICKI in mean ± SD | 0.39 ± 0.03 n = 9 | 0.41 ± 0.05 | 0.307 |
| Heamotology | |||
| Systolic BP (mm Hg) in mean ± SD | 127.6 ± 10.7 | 130.8 ± 12.5 | 0.508 |
| Diastolic BP (mm Hg) in mean ± SD | 76.6 ± 11.3 | 83.8 ± 10.9 | 0.132 |
| Hemoglobin (g/dL) in mean ± SD | 14.0 ± 1.7 | 13.2 ± 1.9 | 0.328 |
| INR in median (min-max) | 1.1 (0.9–1.2) | 1.1 (1.0–1.2) | 0.570 |
| PTT (s) in mean ± SD | 29.0 ± 2.4 | 30.1 ± 2.0 | 0.250 |
| Platelets (/μL) in mean ± SD | 247.3 ± 42.7 | 249.4 ± 73.8 | 0.931 |
FFMI is fat-free mass index. Pro-inflammatory markers and glucose homeostasis could not be reported for all subjects due to blood sampling problems. Body weight change is change over last 4 weeks before start of the study. ANOVA was used to compare the baseline characteristics of the control group with the EXP group. For not normally distributed variables, the Mann–Whitney U test was used for this comparison and for the nominal variables, Pearson Chi-Square was used.
5.2. Plasma amino acids
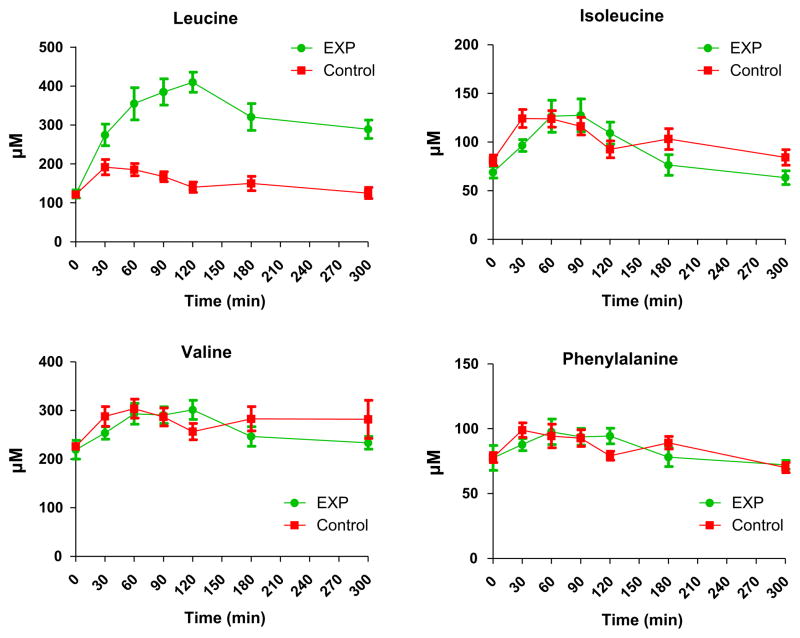
After intake of the 24 g protein in the control group and the 40 g (leucine-enriched) protein in the EXP group, a comparable increase was observed of the isoleucine, valine and phenylalanine plasma concentration (Fig. 2). However, the plasma leucine levels increased substantially more (P < 0.001) in the EXP group, most likely due to the addition of the 4 g free leucine and the difference in intact protein composition. Total leucine content was 2.0 g for Control and 7.8 g for EXP. For the other amino acids (not shown), we did not observe significant differences between EXP and control group for aspartate, glutamate, asparagine, glutamine, citrulline, serine, histidine, glycine, arginine, threonine, tyrosine, methionine, alanine, ornithine and lysine.
Fig. 2.
Plasma leucine, isoleucine, valine and phenylalanine levels after intake of the medical foods. A significant groups effect was observed with respect to leucine (P < 0.001), but not for isoleucine, valine or phenylalanine (P > 0.35 for all).
5.3. Enrichments of phenylalanine
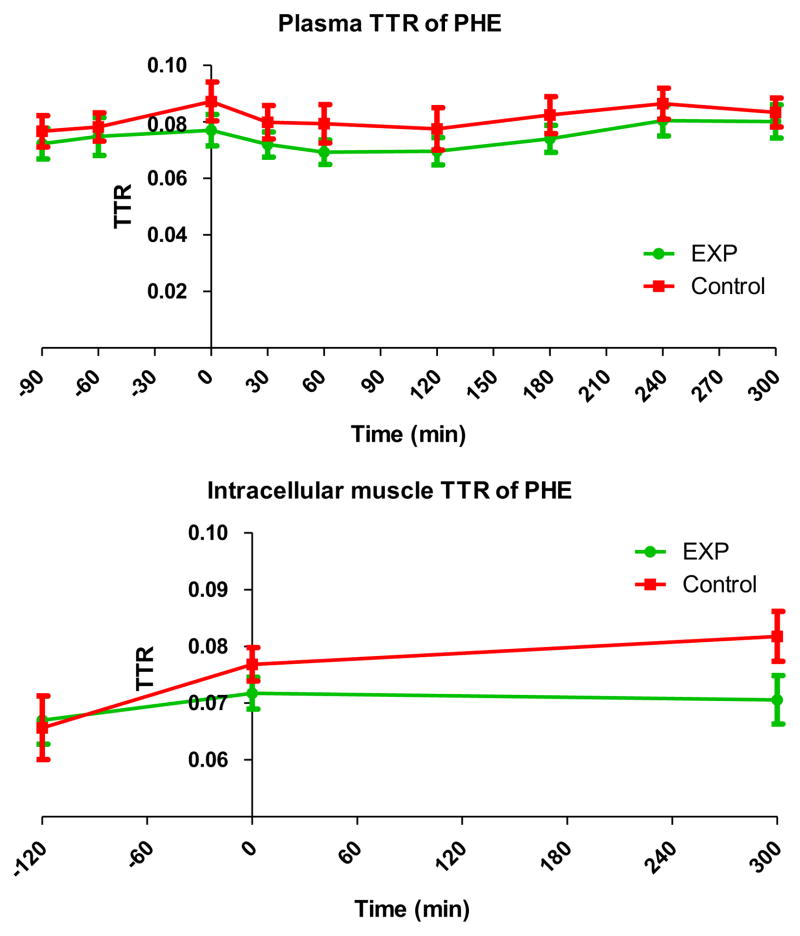
In both groups, the plasma t/T of phenylalanine was in steady state (Fig. 3) before intake of the medical foods. After intake of the medical foods, the plasma enrichment decreased and the WbRaPHE increased slightly in relation to the intake of phenylalanine, coming from the protein in the medical foods. The intracellular enrichment in muscle measured 2h before and just before the intake of the medical foods increased in both groups. 5 Hour after intake of the medical foods, the intracellular enrichment was comparable to the enrichment, just before intake.
Fig. 3.
Enrichment of L-[ring-13C6]phenylalanine in plasma (top panel) and muscle (middle panel) samples. The whole body rate of appearance of phenylalanine in the bottom panel.
5.4. Muscle protein fractional synthesis rate (FSR)
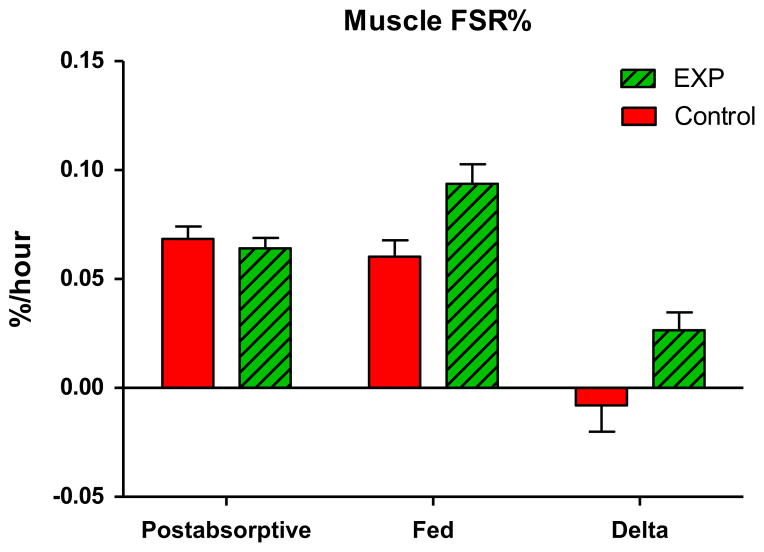
Muscle protein FSR was measured during 3 h before intake of the medical foods, representing the baseline, postabsorptive FSR and for 5 h after intake of the medical foods, representing the fed FSR. Table 4 shows the muscle FSR of each individual patient during the basal period and after intake of the medical foods. The groups were similar with respect to basal muscle FSR (P = 0.43). After feeding however, the mean FSR in the experimental group was found to be significantly greater than the control group (ANCOVA P = 0.023). Muscle FSR in the control group that received an amount of medical food, commonly used for supplementing patients, did not change after intake of the medical food (Fig. 4). However, muscle FSR increased by 40% in the EXP group (ANCOVA, P = 0.027).
Table 4.
Muscle enrichments (MPE) of L-[ring-13C6]Phenylalanine.
| Subject ID | FSR-basal in %/hour | FSR after taking medical foods in %/hour | Delta FSR in %/hour |
|---|---|---|---|
| Control: Conventional medical food n = 12 | |||
| 818 | 0.08102 | 0.11459 | 0.03356 |
| 853 | 0.12259 | 0.01175 | −0.11084 |
| 857 | 0.09541 | 0.02266 | −0.07276 |
| 860 | 0.08965 | 0.07114 | −0.01851 |
| 862 | 0.05432 | 0.07005 | 0.01573 |
| 864 | 0.06601 | 0.07002 | 0.00402 |
| 865 | 0.06226 | 0.09367 | 0.03141 |
| 868 | 0.07175 | 0.06589 | −0.00586 |
| 869 | 0.05597 | 0.06336 | 0.00739 |
| 871 | 0.04967 | 0.08169 | 0.03202 |
| 873 | 0.04924 | 0.05376 | 0.00453 |
| 951 | 0.07879 | 0.05747 | −0.02132 |
| Mean | 0.07306 | 0.06467 | −0.00839 |
| SD | 0.02185 | 0.02773 | 0.04374 |
| EXP: Specially formulated medical food n=12 | |||
| 851 | 0.04008 | 0.10064 | 0.06057 |
| 854 | 0.07729 | 0.10252 | 0.02523 |
| 856 | 0.07703 | 0.11804 | 0.04100 |
| 858 | 0.10664 | 0.17977 | 0.07313 |
| 859 | 0.06021 | 0.09729 | 0.03708 |
| 861 | 0.11810 | 0.010873 | −0.00937 |
| 863 | 0.04303 | 0.08437 | 0.04134 |
| 866 | 0.07158 | 0.05899 | −0.01259 |
| 867 | 0.06647 | 0.09336 | 0.02689 |
| 870 | 0.06303 | 0.06440 | 0.00137 |
| 872 | 0.08754 | 0.05786 | −0.02969 |
| 952 | 0.07010 | 0.09254 | 0.02244 |
| Mean | 0.07343 | 0.09654 | 0.02312 |
| SD | 0.02278 | 0.03262 | 0.03069 |
Fig. 4.
Muscle protein fractional synthetic rate (FSR) in cancer patients in the post-absorptive state and after intake of a medical food, calculated from the intracellular MPE of Phenylalanine M+6. The postabsorbative FSR means of the groups were not found to be significantly different (P = 0.43); however, mean FSR of the experimental group (N = 12) was found to be significantly greater than that of the control group (N = 12) (ANCOVA P = 0.023).
When the FSR was calculated from the average plasma enrichment, the delta FSR was 0.00146% (SD: 0.04189%) in the control group and 0.02553% (SD: 0.02851%) in the EXP group. In addition, FSR can be calculated between the first biopsy and last biopsy, indicating the overall difference in FSR. When using the intracellular enrichment as precursor; 0.07065 (SD: 0.01438%) and 0.09086 (SD: 0.02807) and when using the average plasma enrichment as precursor; 0.07085 (SD: 0.02932) and 0.08863 (SD: 0.03654). These data all confirm the difference between the control and experimental diet groups.
5.5. Plasma glucose and insulin
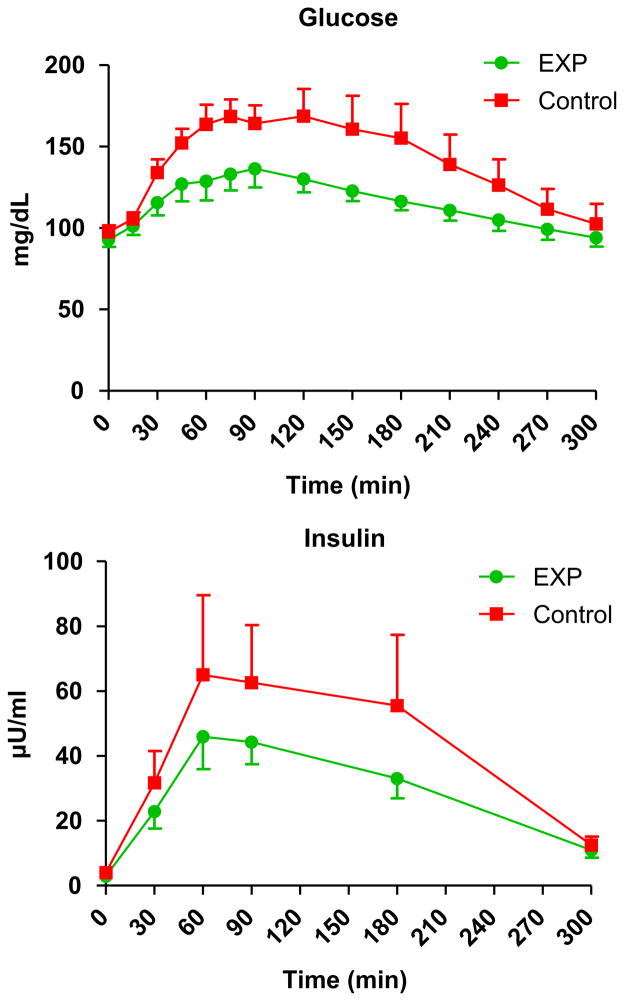
After intake of the medical foods, there was no difference between the groups in plasma glucose and insulin profiles (Group-by-Time Interaction P > 0.10; Fig. 5); however, the groups were found to be significantly different with respect to overall level of plasma glucose (Group Effect, P = 0.044).
Fig. 5.
Plasma glucose and insulin levels after intake of the medical food in the experimental and control groups. There was no significant group-by-time interaction for glucose and insulin levels (P > 0.10). There was significant group effect for glucose (p = 0.044), but not for insulin (P = 0.553). For both glucose and insulin, a signicant time effect was observed (P < 0.001).
5.6. Adverse events
We did not observe any adverse event for 1 week after the experiments that could be related to intake of the medical foods.
6. Discussion
Our results demonstrate that it is possible to stimulate muscle protein synthesis in catabolic cancer patients with involuntary weight loss with a specially formulated medical food, rich in leucine and protein. Cachexia is defined as a state in which muscle mass is lost at a rate greater than would be anticipated from reduced food intake alone.1 A corollary of this definition is that decreased responsiveness to the normal anabolic effect of a meal would be expected. Consistent with the expectation of decreased responsiveness in cachexia, our results show that a medical food with a composition in the same range as commercially available medical foods did not stimulate muscle protein synthesis. We term the condition of diminished responsiveness to nutrition, anabolic resistance without major weight loss as pre-cachexia,37 because if sustained over a period of time this condition will lead to progressive loss of muscle mass. When the medical food was optimized for its composition, muscle fractional protein synthesis rate was stimulated. The results of our study show that absence of improvement of muscle protein synthesis in catabolic cancer patients with involuntary weight loss is related to the composition of the medical food and not to the unresponsiveness of muscle in these cancer patients, per se. Further, our study also shows that true cachexia is preceded by a pre-cachexia state during which significant weight loss has yet to occur but normal responsiveness to nutrition is blunted. Specially formulated nutritional support is necessary and appropriate in patients with pre-cachexia in order to avoid the development of cachexia and resultant adverse responses.
6.1. Selection of experimental design
There were two possible options for selection of patients. We could have studied a very homogeneous group of patients. This approach would have presumably minimized variability, but would have restricted conclusions to that specific group of patients. Rather, we performed the study in patients with radiologically-confirmed cancer of any type. Non-small cell lung cancer was the most common form of cancer, but other forms were also included (Table 1). The fact that all patients reacted similarly within each treatment group enables us to generalize our results to most cancer patients, instead of being limited by very restrictive entrance criteria. Most patients had lost weight in the month before the study, but the weight loss did not lead to a lower than normal BMI. This suggests that the patients were not yet cachectic. In the post-absorptive cancer patients, both the observed WbRaPHE was higher (80 μmol/kg ffm/h vs 40 μmol/kg ffm/h)38,39 and the FSR in muscle26,40 was higher (0.073%/hour vs 0.055%/h), compared to comparable healthy subjects of the same age group. Previous studies have observed reduced7 or unchanged4,41 muscle FSR in cancer patients. Although the small increase of muscle FSR could be within the variation of the FSR measurement, our observations probably defined the studied pre-cachectic cancer patients.
6.2. How to improve a nutritional supplement for maximal stimulation of muscle protein synthesis
Commonly used medical foods have protein contents in the range of 15–18% of total energy, while high protein versions usually contain between 20–22 energy% protein. Our control medical food was therefore comparable to the most commonly used nutritional supplements that are recommended for patients with cancer. Our experimental medical food was formulated to create an optimal mixture that we hypothesized to be more effective to stimulate muscle protein synthesis than commonly-used nutritional support.
6.3. Why did we not see an increase in FSR with the control conventional medical food?
The control medical food contained 24 g of protein. We previously showed that 40 g of a balanced amino acid mixture increased muscle protein synthesis significantly in elderly healthy volunteers.42 Also, we observed that 15 g of whey protein or 7 g of essential amino acids stimulated muscle protein synthesis in healthy elderly.20 A key factor in these studies is the fact that amino acids or protein is given without any carbohydrates or fat. In contrast, our control medical food which contained 24 g of protein, 84 g of carbohydrates and 23 g of fat did not stimulate muscle protein synthesis. This observation is consistent with our previous work that showed that adding carbohydrates to an amino acid meal did not increase muscle protein synthesis in healthy elderly individuals, in contrast to the response to amino acids without carbohydrates.43 This phenomenon specifically is related to age as young healthy volunteers did have a stimulation of muscle protein synthesis after a meal with amino acid and carbohydrates. Also, it is clear that the change of composition of the experimental medical food has overcome the effects of carbohydrates on the attenuation of muscle protein synthesis. It was suggested that this is related to insulin resistance in elderly, but the exact mechanism was not delineated. Our patients were not insulin resistant, according to the measured QUICKI.44 An alternative explanation could be that the low glycemic index (GI = 40) of the experimental medical food (Table 2;45) resulted in a lower increase of glucose and insulin. This may have contributed to muscle protein synthesis. However, more studies need to be done as it is unknown whether there is a maximal amount of carbohydrates that can be added to medical food without affecting the muscle protein synthesis response.
6.4. Increased protein level in the experimental medical food
The amount of protein that was given via the experimental medical food was increased from 24 g to 40 g by adding whey protein to the casein protein, increasing the essential amino acid content. Recommended protein intake for cancer patients is between 1.2 and 2 g/kg bw/day.9 In relation to the body weight of the studied patients, between 96 and 160 g protein/day is advised. Therefore, we think that increasing the protein intake with 40 g by consumption of the experimental medical food is compatible with recommended protein intake of cancer patients.
Increased concentrations of essential amino acids after ingestion of a meal relate to the amount of protein taken. In general, the larger the amount of protein ingested, the higher the plasma concentration. We previously observed that plasma phenylalanine concentration increased about 10–20 μM in response to 20 g of casein or 20 g of whey protein in healthy young adults,46 comparable to the increase we observed in the phenylalanine plasma concentration after our control medical food (Fig. 2). Therefore, we expected but did not observe that adding 12 additional grams of whey protein would increase the concentration above the control levels. This was also observed in the concentrations of isoleucine and valine (Fig. 2). It is possible that the maximal protein absorption rate was already reached with the control medical food and that the extra amount of protein did not contribute to higher amino acids levels and thus greater stimulation of muscle protein synthesis.16 Therefore, it seems more likely that the altered composition of the experimental medical food was more important than the amount of protein in the medical food.
Branched chain amino acids valine and isoleucine did not decrease upon supplementation with high amounts of leucine. This shows that the high amounts of valine and isoleucine in the experimental medical food were sufficient to prevent compromised valine and isoleucine uptake due to BCAA antagonism.
6.5. The beneficial effects of the extra leucine
Ingestion of leucine-enriched amino acid solutions rapidly and potently activate the mammalian target of rapamycin signaling pathway and protein synthesis in human skeletal muscle.47 This most likely explains the enhanced muscle protein synthesis that is observed when increasing the percentage of leucine in a meal.48 In particular, when leucine is added to a protein meal in elderly, the ability of the meal to stimulate protein synthesis is improved.26
Recently, we observed in ovarian cancer patients with increased inflammatory parameters in muscle that 40 g of a balanced amino acid mixture still stimulated muscle protein synthesis, but to a lesser extent than in age-matched controls.41 No differences were observed in muscle protein breakdown in response to the meal. We concluded that in that study we probably did not use the optimal amino acid composition of the meal to stimulate muscle protein synthesis in cancer patients to a comparable rate as in health individuals. The addition of an extra amount of leucine to a balanced amino acid composition improved the response above the level of the control medical food as observed in the present study.
6.6. Are the added PUFA effective?
A substantial amount of EPA (2.2 g) and DHA (1.1 g) was added to the experimental medical food in order to conform to suggested optimal daily dosages.49,50 Polyunsaturated fatty acids (PUFAs) are known to be able to reduce inflammation. In addition, studies in mice with cachexia-causing implanted tumors have revealed that acute intake of an EPA/DHA mixture can reduce protein breakdown51,52 and modulate immune function.53,54 Also, we recently reported in a cancer-cachectic mouse model that a combination of EPA/DHA, high protein, leucine and oligosaccharides was required to obtain a synergistic effect, leading to a reduced inflammatory state and improved immune competence.55 Therefore, ingestion of an EPA/DHA mixture potentially is a new approach to improving muscle protein synthesis. A recent study has confirmed an improved muscle protein synthesis by 8 weeks treatment with EPA/DHA.56 However, no human studies are available that studied the acute effects of EPA/DHA on muscle protein synthesis.
We therefore have to speculate on the possible acute effects of EPA/DHA on muscle FSR in the present study. We believe the added EPA/DHA had no role because of the acute nature of our study. It is known that the peak absorption of EPA is at t = 4–6h after a meal57 and that it takes several hours before maximal plasma EPA levels are obtained. Using this information, it would appear that the maximal plasma EPA and DHA levels would not coincide with the peak increase of the plasma amino acids, such as isoleucine, valine and phenylalanine (Fig. 2), representing the changes in concentration of the amino acids that derive from the dietary proteins. Thus, although it is unlikely that EPA/DHA played a significant role in affecting the acute response of muscle protein synthesis in the current study, this point could only be definitely addressed by a study in which only the EPA/DHA content was altered.
6.7. Role of the other ingredients in the experimental medical food
The experimental medical food contained additional whey protein, more leucine added as free amino acid and PUFAs. In addition, the experimental medical food contained fiber, taurine, creatine, different types of carbohydrates and some different levels of trace elements and vitamins. These additional factors would be anticipated to have effects on various functions after several doses, but would not be expected to acutely affect the response of muscle protein synthesis.
7. Conclusions
Our results demonstrated that when a medical food is optimized for protein, leucine, fish oil components, carbohydrates and fiber, muscle fractional protein synthesis rate can be stimulated. Therefore, the results of our acute study clearly show that absence of improvement of muscle protein synthesis in catabolic cancer patients with involuntary weight loss is related to the composition of the medical food and not to the unresponsiveness of muscle in these cancer patients, per se. In cancer patients, a specially formulated medical food can overcome the anabolic resistance to a conventional nutritional supplement.
Acknowledgments
The authors wish to thank Mrs. Lulu Xu, PhD and Mr. John Thaden, PhD for analysis of the samples.
Study design and planning were done in conjunction with the study sponsor, Nutricia Advanced Medical Nutrition, Danone Research – Centre for Specialised Nutrition. The sponsor provided the study products and funding. Data collection and analysis by statistician (Mr. Spencer) were done at UAMS. All authors had full access to study data. The corresponding author and principal investigator Dr. Wolfe has final responsibility for the decision to submit the publication.
Dr. Deutz and Dr. Wolfe were involved in data analysis and writing of the manuscript. Dr. Wolfe, Dr. Safar, Mr. Memelink, Dr. Ferrando and Dr. van Helvoort and Mr. Schutzler were involved in the study design and data collection. Mr. Spencer was responsible for statistical analysis. All authors have reviewed and approved the manuscript. The project described was supported by Award Number 1UL1RR029884 from the National Center For Research Resources.
Footnotes
Supported by a grant from Nutricia Advanced Medical Nutrition.
Conflict of interest
Dr. Wolfe is a member of the Danone Research Advisory Board and received compensation. Mr. Memelink and Dr. van Helvoort are employed by Nutricia Advanced Medical Nutrition, Danone Research – Centre for Specialised Nutrition. Dr. Ferrando, Dr. Deutz, Mr. Schutzler and Mr. Spencer have no conflict of interest to declare.
References
- 1.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–9. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89(2):381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 3.Biolo G, Antonione R, Barazzoni R, Zanetti M, Guarnieri G. Mechanisms of altered protein turnover in chronic diseases: a review of human kinetic studies. Curr Opin Clin Nutr Metab Care. 2003;6(1):55–63. doi: 10.1097/00075197-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Shaw JH, Humberstone DA, Douglas RG, Koea J. Leucine kinetics in patients with benign disease, non-weight-losing cancer, and cancer cachexia: studies at the whole-body and tissue level and the response to nutritional support. Surgery. 1991;109(1):37–50. [PubMed] [Google Scholar]
- 5.De Blaauw I, Deutz NE, Von Meyenfeldt MF. Metabolic changes in cancer cachexia - first of two parts. Clin Nutr. 1997;16(4):169–76. doi: 10.1016/s0261-5614(97)80002-7. [DOI] [PubMed] [Google Scholar]
- 6.Fearon KC. The Sir David Cuthbertson Medal Lecture 1991. The mechanisms and treatment of weight loss in cancer. Proc Nutr Soc. 1992;51(2):251–65. doi: 10.1079/pns19920036. [DOI] [PubMed] [Google Scholar]
- 7.Emery PW, Edwards RH, Rennie MJ, Souhami RL, Halliday D. Protein synthesis in muscle measured in vivo in cachectic patients with cancer. Br Med J (Clin Res Ed) 1984;289(6445):584–6. doi: 10.1136/bmj.289.6445.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadar L, Albertsson M, Areberg J, Landberg T, Mattsson S. The prognostic value of body protein in patients with lung cancer. Ann New York Acad Sci. 2000;904:584–91. doi: 10.1111/j.1749-6632.2000.tb06520.x. [DOI] [PubMed] [Google Scholar]
- 9.Arends J, Bodoky G, Bozzetti F, Fearon K, Muscaritoli M, Selga G, et al. ESPEN Guidelines on Enteral nutrition: non-surgical oncology. Clin Nutr. 2006;25(2):245–59. doi: 10.1016/j.clnu.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 10.de Blaauw I, Heeneman S, Deutz NE, von Meyenfeldt MF. Increased whole-body protein and glutamine turnover in advanced cancer is not matched by an increased muscle protein and glutamine turnover. J Surg Res. 1997;68(1):44–55. doi: 10.1006/jsre.1997.5007. [DOI] [PubMed] [Google Scholar]
- 11.de Blaauw I, Deutz NE, von Meyenfeldt MF. Cancer reduces the metabolic response of muscle to surgical stress in the rat. J Surg Res. 1998;80(1):94–101. doi: 10.1006/jsre.1998.5406. [DOI] [PubMed] [Google Scholar]
- 12.de Blaauw I, Deutz NE, Hulsewe KW, von Meyenfeldt MF. Attenuated metabolic response to surgery in tumor-bearing rats. J Surg Res. 2003;110(2):371–7. doi: 10.1016/s0022-4804(03)00041-6. [DOI] [PubMed] [Google Scholar]
- 13.Vissers YL, von Meyenfeldt MF, Argiles JM, Luiking YC, Dejong CH, Deutz NE. Protein breakdown on whole-body and organ level in non-cachectic tumour-bearing mice undergoing surgery. Clin Nutr. 2007;26(4):483–90. doi: 10.1016/j.clnu.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Vissers YL, von Meyenfeldt MF, Luiking YC, Dejong CH, Buurman WA, Deutz NE. Presence of tumour inhibits the normal post-operative response in arginine and NO production in non-cachectic mice. Clin Sci (Lond) 2007;112(10):527–32. doi: 10.1042/CS20060340. [DOI] [PubMed] [Google Scholar]
- 15.Vissers YL, von Meyenfeldt MF, Luiking YC, Dejong CH, Deutz NE. Interorgan synthesis of arginine is down-regulated in tumor-bearing mice undergoing surgical trauma. Metabolism. 2008;57(7):896–902. doi: 10.1016/j.metabol.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe RR. Regulation of muscle protein by amino acids. J Nutr. 2002;132(10):3219S–24S. doi: 10.1093/jn/131.10.3219S. [DOI] [PubMed] [Google Scholar]
- 17.Reeds PJ, Fjeld CR, Jahoor F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J Nutr. 1994;124(6):906–10. doi: 10.1093/jn/124.6.906. [DOI] [PubMed] [Google Scholar]
- 18.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101(9):2000–7. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286(3):E321–8. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 20.Katsanos CS, Chinkes DL, Paddon-Jones D, Zhang XJ, Aarsland A, Wolfe RR. Whey protein ingestion in elderly persons results in greater muscle protein accrual than ingestion of its constituent essential amino acid content. Nutr Res. 2008;28(10):651–8. doi: 10.1016/j.nutres.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82(5):1065–73. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 22.Garlick PJ, Grant I. Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochem J. 1988;254(2):579–84. doi: 10.1042/bj2540579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buse MG, Reid SS. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975;56(5):1250–61. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong SO, Layman DK. Effects of leucine on in vitro protein synthesis and degradation in rat skeletal muscles. J Nutr. 1984;114(7):1204–12. doi: 10.1093/jn/114.7.1204. [DOI] [PubMed] [Google Scholar]
- 25.Dardevet D, Sornet C, Balage M, Grizard J. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr. 2000;130(11):2630–5. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- 26.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol. 2006;291(2):E381–7. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 27.Koopman R, Verdijk L, Manders RJ, Gijsen AP, Gorselink M, Pijpers E, et al. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr. 2006;84(3):623–32. doi: 10.1093/ajcn/84.3.623. [DOI] [PubMed] [Google Scholar]
- 28.Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, et al. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiology. 2006;575(Pt 1):305–15. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Norren K, Kegler D, Argiles JM, Luiking Y, Gorselink M, Laviano A, et al. Dietary supplementation with a specific combination of high protein, leucine, and fish oil improves muscle function and daily activity in tumour-bearing cachectic mice. Br J Cancer. 2009;100(5):713–22. doi: 10.1038/sj.bjc.6604905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, et al. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. 2009 doi: 10.1016/j.clnu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Borsheim E, Bui QU, Tissier S, Kobayashi H, Ferrando AA, Wolfe RR. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin Nutr. 2008;27(2):189–95. doi: 10.1016/j.clnu.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meesters RJ, Wolfe RR, Deutz NE. Application of liquid chromatography-tandem mass spectrometry (LC-MS/MS) for the analysis of stable isotope enrichments of phenylalanine and tyrosine. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(1–2):43–9. doi: 10.1016/j.jchromb.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Wolfe RR, Chinkes DL. Isotope tracers in metabolic. Res Principles Pract Kinetic Analysis. 2004:274. [Google Scholar]
- 34.Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiology. 2003;552(Pt 1):315–24. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunner E, Domhof S, Langer F. Nonparametric Anal Longitudinal Data Factorial Exp. 2001:288. [Google Scholar]
- 36.Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obes Relat Metab Disord. 2002;26(7):953–60. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- 37.Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29(2):154–9. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Luiking YC, Poeze M, Ramsay G, Deutz NE. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr. 2009;89(1):142–52. doi: 10.3945/ajcn.2007.25765. [DOI] [PubMed] [Google Scholar]
- 39.Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Altered interorgan response to feeding in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82(2):366–72. doi: 10.1093/ajcn.82.2.366. [DOI] [PubMed] [Google Scholar]
- 40.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontology. 2006;41(2):215–9. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Dillon EL, Volpi E, Wolfe RR, Sinha S, Sanford AP, Arrastia CD, et al. Amino acid metabolism and inflammatory burden in ovarian cancer patients undergoing intense oncological therapy. Clin Nutr. 2007;26(6):736–43. doi: 10.1016/j.clnu.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–8. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to Combined Hyperaminoacidemia and glucose-induced Hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85(12):4481–90. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 45.van Norren K, van Helvoort A, Frost GS, Lansink M. P123 A specific nutritional composition for cancer patients with a low glycemic index. Clin Nutr Supplements. 2009;4(2):77. [Google Scholar]
- 46.Tipton KD, Elliott TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc. 2004;36(12):2073–81. doi: 10.1249/01.mss.0000147582.99810.c5. [DOI] [PubMed] [Google Scholar]
- 47.Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care. 2008;11(3):222–6. doi: 10.1097/MCO.0b013e3282fa17fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12(1):86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sijben JW, Calder PC. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc Nutr Soc. 2007;66(2):237–59. doi: 10.1017/S0029665107005472. [DOI] [PubMed] [Google Scholar]
- 50.Calder PC. The 2008 ESPEN Sir David Cuthbertson lecture: fatty acids and inflammation - From the membrane to the nucleus and from the laboratory bench to the clinic. Clin Nutr. 2010;29(1):5–12. doi: 10.1016/j.clnu.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Smith HJ, Khal J, Tisdale MJ. Downregulation of ubiquitin-dependent protein degradation in murine myotubes during hyperthermia by eicosapentaenoic acid. Biochem Biophys Res Commun. 2005;332(1):83–8. doi: 10.1016/j.bbrc.2005.04.097. [DOI] [PubMed] [Google Scholar]
- 52.Smith HJ, Greenberg NA, Tisdale MJ. Effect of eicosapentaenoic acid, protein and amino acids on protein synthesis and degradation in skeletal muscle of cachectic mice. Br J Cancer. 2004;91(2):408–12. doi: 10.1038/sj.bjc.6601981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barber MD, Fearon KC, Ross JA. Eicosapentaenoic acid modulates the immune response but has no effect on a mimic of antigen-specific responses. Nutrition. 2005;21(5):588–93. doi: 10.1016/j.nut.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 54.Mickleborough TD, Tecklenburg SL, Montgomery GS, Lindley MR. Eicosa-pentaenoic acid is more effective than docosahexaenoic acid in inhibiting proinflammatory mediator production and transcription from LPS-induced human asthmatic alveolar macrophage cells. Clin Nutr. 2009;28(1):71–7. doi: 10.1016/j.clnu.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 55.Faber J, Vos P, Kegler D, van Norren K, Argiles JM, Laviano A, et al. Beneficial immune modulatory effects of a specific nutritional combination in a murine model for cancer cachexia. Br J Cancer. 2008;99(12):2029–36. doi: 10.1038/sj.bjc.6604785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2010 doi: 10.3945/ajcn.110.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nordoy A, Barstad L, Connor WE, Hatcher L. Absorption of the n-3 eicosapentaenoic and docosahexaenoic acids as ethyl esters and triglycerides by humans. Am J Clin Nutr. 1991;53(5):1185–90. doi: 10.1093/ajcn/53.5.1185. [DOI] [PubMed] [Google Scholar]