Abstract
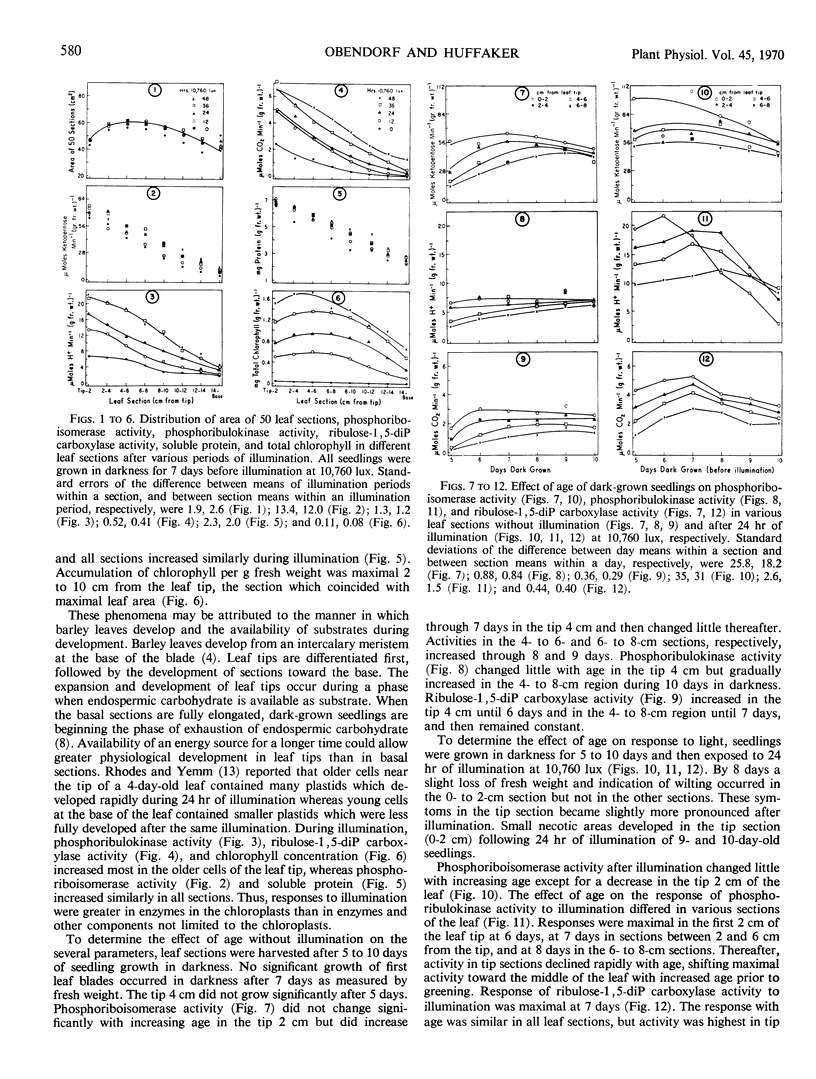
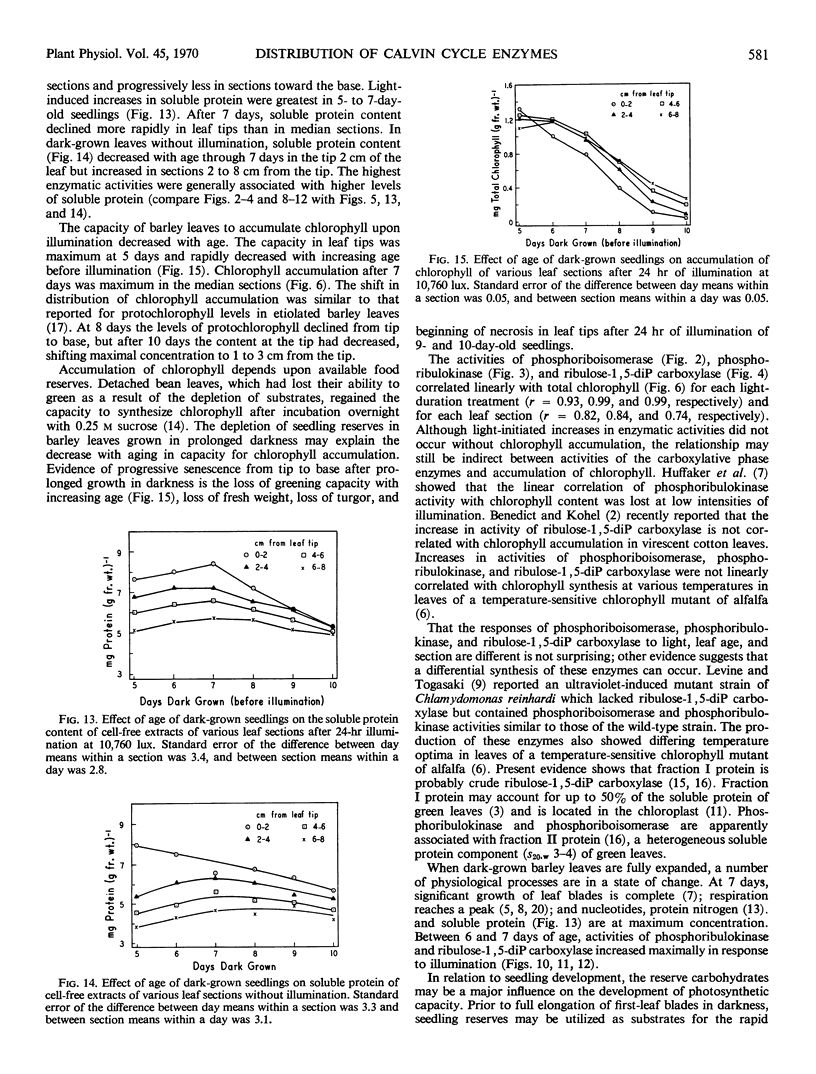
Activities of phosphoriboisomerase, phosphoribulokinase, and ribulose 1,5-diphosphate carboxylase, protein content, and chlorophyll accumulation in dark-grown barley seedlings were measured before and after illumination. Enzymatic activities, levels of soluble protein, and accumulation (upon illumination) of chlorophyll in leaves declined from tips toward the base. In response to increasing time of illumination, chlorophyll accumulation and activities of phosphoribulokinase and ribulose 1,5-diphosphate carboxylase (enzymes located in chloroplasts) increased most in tip portions whereas activity of phosphoriboisomerase and levels of soluble protein (constituents not confined to chloroplasts) increased similarly in all sections of the leaf. Maximum activity of phosphoribulokinase and maximum accumulation of chlorophyll shifted toward median portions of the leaf blade with increased age of seedling before illumination. Maximum activity of ribulose 1,5-diphosphate carboxylase and maximum level of soluble protein occurred in all leaf sections when the seedlings were 7 days of age before illumination.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C. R., Kohel R. J. The synthesis of ribulose-1,5-diphosphate carboxylase and chlorophyll in virescent cotton leaves. Plant Physiol. 1969 Apr;44(4):621–622. doi: 10.1104/pp.44.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGGMAN L., SINGER S. J., WILDMAN S. G. The proteins of green leaves. V. A cytoplasmic nucleoprotein from spinach and tobacco leaves. J Biol Chem. 1953 Dec;205(2):969–983. [PubMed] [Google Scholar]
- Huffaker R. C., Obendorf R. L., Keller C. J., Kleinkopf G. E. Effects of Light Intensity on Photosynthetic Carboxylative Phase Enzymes and Chlorophyll Synthesis in Greening Leaves of Hordeum vulgare L. Plant Physiol. 1966 Jun;41(6):913–918. doi: 10.1104/pp.41.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYTTLETON J. W., TS'O P. O. The localization of fraction I protein of green leaves in the chloroplasts. Arch Biochem Biophys. 1958 Jan;73(1):120–126. doi: 10.1016/0003-9861(58)90246-7. [DOI] [PubMed] [Google Scholar]
- Levine R. P., Togasaki R. K. A mutant strain of Chlamydomonas reinhardi lacking ribulose diphosphate carboxylase activity. Proc Natl Acad Sci U S A. 1965 May;53(5):987–990. doi: 10.1073/pnas.53.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODES M. J., YEMM E. W. DEVELOPMENT OF CHLOROPLASTS AND THE SYNTHESIS OF PROTEINS IN LEAVES. Nature. 1963 Dec 14;200:1077–1080. doi: 10.1038/2001077a0. [DOI] [PubMed] [Google Scholar]
- TROWN P. W. AN IMPROVED METHOD FOR THE ISOLATION OF CARBOXYDISMUTASE. PROBABLE IDENTITY WITH FRACTION I PROTEIN AND THE PROTEIN MOIETY OF PROTOCHLOROPHYLL HOLOCHROME. Biochemistry. 1965 May;4:908–918. doi: 10.1021/bi00881a018. [DOI] [PubMed] [Google Scholar]
- VON NOORT, WILDMAN S. G. PROTEINS OF GREEN LEAVES. IX. ENZYMATIC PROPERTIES OF FRACTION-I PROTEIN ISOLATED BY A SPECIFIC ANTIBODY. Biochim Biophys Acta. 1964 Aug 19;90:309–317. [PubMed] [Google Scholar]


