Abstract
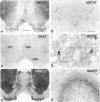
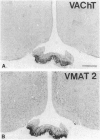
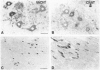
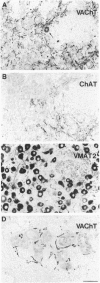
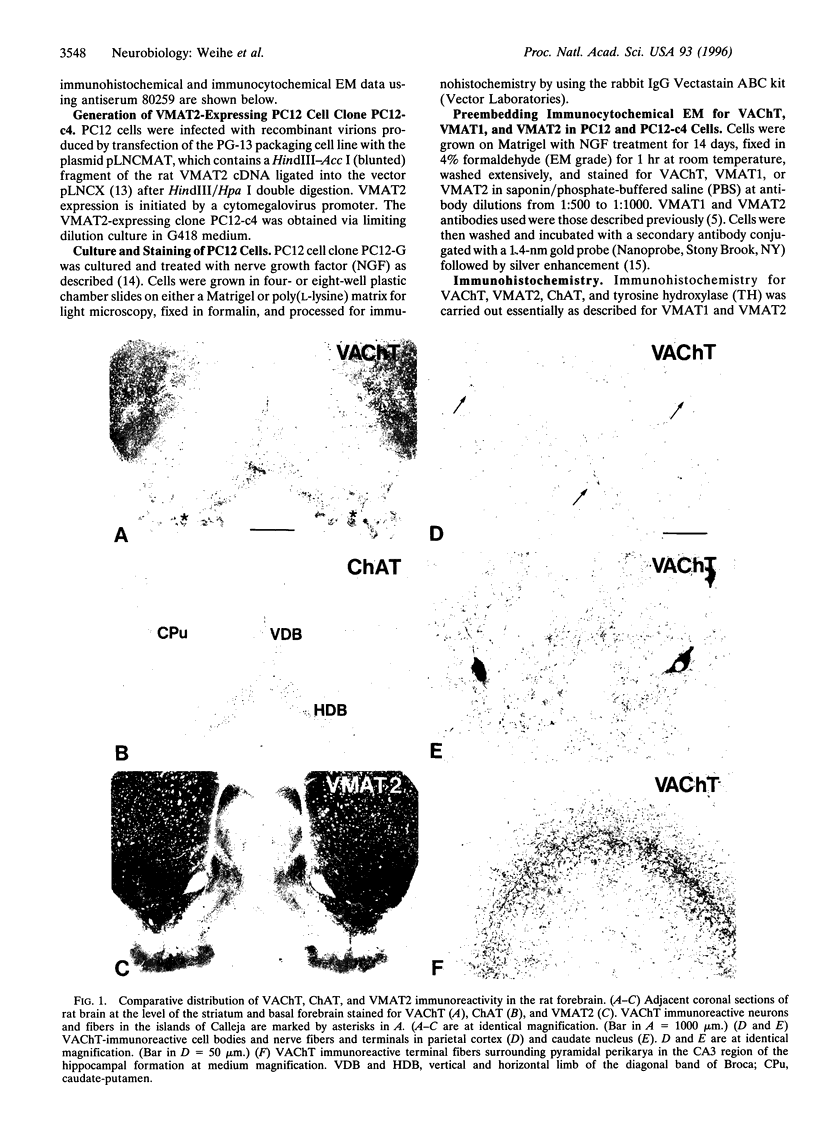
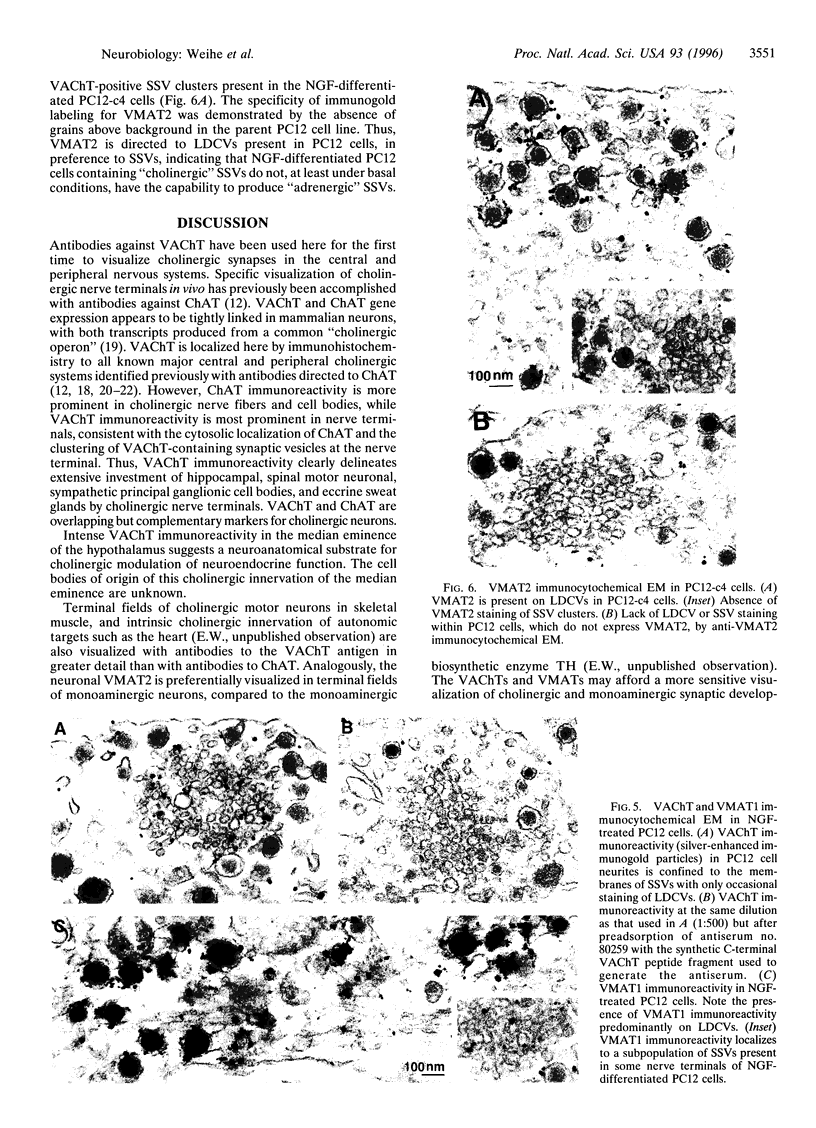
Immunohistochemical visualization of the rat vesicular acetylcholine transporter (VAChT) in cholinergic neurons and nerve terminals has been compared to that for choline acetyltransferase (ChAT), heretofore the most specific marker for cholinergic neurons. VAChT-positive cell bodies were visualized in cerebral cortex, basal forebrain, medial habenula, striatum, brain stem, and spinal cord by using a polyclonal anti-VAChT antiserum. VAChT-immuno-reactive fibers and terminals were also visualized in these regions and in hippocampus, at neuromuscular junctions within skeletal muscle, and in sympathetic and parasympathetic autonomic ganglia and target tissues. Cholinergic nerve terminals contain more VAChT than ChAT immunoreactivity after routine fixation, consistent with a concentration of VAChT within terminal neuronal arborizations in which secretory vesicles are clustered. These include VAChT-positive terminals of the median eminence or the hypothalamus, not observed with ChAT antiserum after routine fixation. Subcellular localization of VAChT in specific organelles in neuronal cells was examined by immunoelectron microscopy in a rat neuronal cell line (PC 12-c4) expressing VAChT as well as the endocrine and neuronal forms of the vesicular monoamine transporters (VMAT1 and VMAT2). VAChT is targeted to small synaptic vesicles, while VMAT1 is found mainly but not exclusively on large dense-core vesicles. VMAT2 is found on large dense-core vesicles but not on the small synaptic vesicles that contain VAChT in PC12-c4 cells, despite the presence of VMAT2 immunoreactivity in central and peripheral nerve terminals known to contain monoamines in small synaptic vesicles. Thus, VAChT and VMAT2 may be specific markers for "cholinergic" and "adrenergic" small synaptic vesicles, with the latter not expressed in nonstimulated neuronally differentiated PC12-c4 cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agoston D. V., Conlon J. M., Whittaker V. P. Selective depletion of the acetylcholine and vasoactive intestinal polypeptide of the guinea-pig myenteric plexus by differential mobilization of distinct transmitter pools. Exp Brain Res. 1988;72(3):535–542. doi: 10.1007/BF00250599. [DOI] [PubMed] [Google Scholar]
- Alfonso A., Grundahl K., Duerr J. S., Han H. P., Rand J. B. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science. 1993 Jul 30;261(5121):617–619. doi: 10.1126/science.8342028. [DOI] [PubMed] [Google Scholar]
- Bauerfeind R., Jelinek R., Hellwig A., Huttner W. B. Neurosecretory vesicles can be hybrids of synaptic vesicles and secretory granules. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7342–7346. doi: 10.1073/pnas.92.16.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerfeind R., Régnier-Vigouroux A., Flatmark T., Huttner W. B. Selective storage of acetylcholine, but not catecholamines, in neuroendocrine synaptic-like microvesicles of early endosomal origin. Neuron. 1993 Jul;11(1):105–121. doi: 10.1016/0896-6273(93)90275-v. [DOI] [PubMed] [Google Scholar]
- Erickson J. D., Eiden L. E. Functional identification and molecular cloning of a human brain vesicle monoamine transporter. J Neurochem. 1993 Dec;61(6):2314–2317. doi: 10.1111/j.1471-4159.1993.tb07476.x. [DOI] [PubMed] [Google Scholar]
- Erickson J. D., Eiden L. E., Hoffman B. J. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10993–10997. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. D., Varoqui H., Schäfer M. K., Modi W., Diebler M. F., Weihe E., Rand J., Eiden L. E., Bonner T. I., Usdin T. B. Functional identification of a vesicular acetylcholine transporter and its expression from a "cholinergic" gene locus. J Biol Chem. 1994 Sep 2;269(35):21929–21932. [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Peter D., Roghani A., Schuldiner S., Privé G. G., Eisenberg D., Brecha N., Edwards R. H. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992 Aug 21;70(4):539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- Liu Y., Schweitzer E. S., Nirenberg M. J., Pickel V. M., Evans C. J., Edwards R. H. Preferential localization of a vesicular monoamine transporter to dense core vesicles in PC12 cells. J Cell Biol. 1994 Dec;127(5):1419–1433. doi: 10.1083/jcb.127.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M. A., Holmberg K., Xu Z. Q., Cozzari C., Hartman B. K., Emson P., Goldstein M., Elfvin L. G., Hökfelt T. Localization of choline acetyltransferase in rat peripheral sympathetic neurons and its coexistence with nitric oxide synthase and neuropeptides. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11819–11823. doi: 10.1073/pnas.92.25.11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg M. J., Liu Y., Peter D., Edwards R. H., Pickel V. M. The vesicular monoamine transporter 2 is present in small synaptic vesicles and preferentially localizes to large dense core vesicles in rat solitary tract nuclei. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8773–8777. doi: 10.1073/pnas.92.19.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter D., Jimenez J., Liu Y., Kim J., Edwards R. H. The chromaffin granule and synaptic vesicle amine transporters differ in substrate recognition and sensitivity to inhibitors. J Biol Chem. 1994 Mar 11;269(10):7231–7237. [PubMed] [Google Scholar]
- Peter D., Liu Y., Sternini C., de Giorgio R., Brecha N., Edwards R. H. Differential expression of two vesicular monoamine transporters. J Neurosci. 1995 Sep;15(9):6179–6188. doi: 10.1523/JNEUROSCI.15-09-06179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch D. M., Iacangelo A. L., Eiden L. E. Glucocorticoid- and nerve growth factor-induced changes in chromogranin A expression define two different neuronal phenotypes in PC12 cells. Mol Endocrinol. 1988 Oct;2(10):921–927. doi: 10.1210/mend-2-10-921. [DOI] [PubMed] [Google Scholar]
- Roghani A., Feldman J., Kohan S. A., Shirzadi A., Gundersen C. B., Brecha N., Edwards R. H. Molecular cloning of a putative vesicular transporter for acetylcholine. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10620–10624. doi: 10.1073/pnas.91.22.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo H. E., Fink T., Yanaihara N., Weihe E. Distribution and relative proportions of neuropeptide Y- and proenkephalin-containing noradrenergic neurones in rat superior cervical ganglion: separate projections to submaxillary lymph nodes. Peptides. 1994;15(8):1479–1487. doi: 10.1016/0196-9781(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Régnier-Vigouroux A., Huttner W. B. Biogenesis of small synaptic vesicles and synaptic-like microvesicles. Neurochem Res. 1993 Jan;18(1):59–64. doi: 10.1007/BF00966923. [DOI] [PubMed] [Google Scholar]
- Sann H., McCarthy P. W., Schemann M., Jurzak M., Poethke R., Pierau F. K. Choline acetyltransferase-immunoreactive neurones in a prevertebral sympathetic ganglion, the inferior mesenteric ganglion. J Auton Nerv Syst. 1995 Sep 5;54(3):195–205. doi: 10.1016/0165-1838(95)00019-t. [DOI] [PubMed] [Google Scholar]
- Schäfer M. K., Weihe E., Varoqui H., Eiden L. E., Erickson J. D. Distribution of the vesicular acetylcholine transporter (VAChT) in the central and peripheral nervous systems of the rat. J Mol Neurosci. 1994 Spring;5(1):1–26. doi: 10.1007/BF02736691. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng J. H., Dosemeci A., Bressler J. P., Brightman M. W., Simpson D. L. Characterization of synaptic vesicles and related neuronal features in nerve growth factor and ras oncogene differentiated PC12 cells. J Neurosci Res. 1995 Oct 15;42(3):323–334. doi: 10.1002/jnr.490420306. [DOI] [PubMed] [Google Scholar]
- Tischler A. S., Greene L. A. Morphologic and cytochemical properties of a clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Lab Invest. 1978 Aug;39(2):77–89. [PubMed] [Google Scholar]
- Usdin T. B., Eiden L. E., Bonner T. I., Erickson J. D. Molecular biology of the vesicular ACh transporter. Trends Neurosci. 1995 May;18(5):218–224. doi: 10.1016/0166-2236(95)93906-e. [DOI] [PubMed] [Google Scholar]
- Varoqui H., Diebler M. F., Meunier F. M., Rand J. B., Usdin T. B., Bonner T. I., Eiden L. E., Erickson J. D. Cloning and expression of the vesamicol binding protein from the marine ray Torpedo. Homology with the putative vesicular acetylcholine transporter UNC-17 from Caenorhabditis elegans. FEBS Lett. 1994 Mar 28;342(1):97–102. doi: 10.1016/0014-5793(94)80592-x. [DOI] [PubMed] [Google Scholar]
- Weihe E., Schäfer M. K., Erickson J. D., Eiden L. E. Localization of vesicular monoamine transporter isoforms (VMAT1 and VMAT2) to endocrine cells and neurons in rat. J Mol Neurosci. 1994 Fall;5(3):149–164. doi: 10.1007/BF02736730. [DOI] [PubMed] [Google Scholar]