Abstract
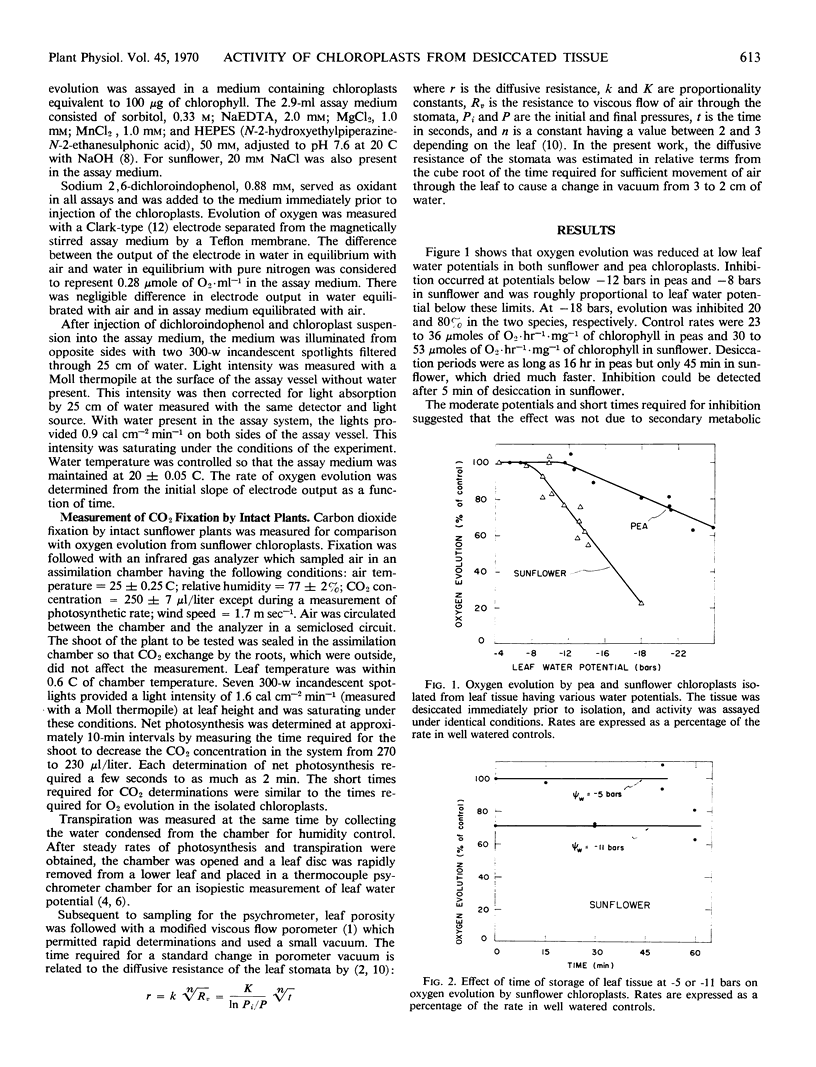
Chloroplasts were isolated from pea and sunflower leaves having various water potentials. Oxygen evolution by the chloroplasts was measured under identical conditions for all treatments with saturating light and with dichloroindophenol as oxidant. Evolution was inhibited when leaf water potentials were below -12 bars in pea and -8 bars in sunflower and the inhibition was proportional to leaf water potential below these limits. Inhibition was more severe in sunflower than in pea chloroplasts. In sunflower, it could be detected after 5 minutes of leaf desiccation, and, up to 1 hour, the effect was independent of the duration of low leaf water potential.
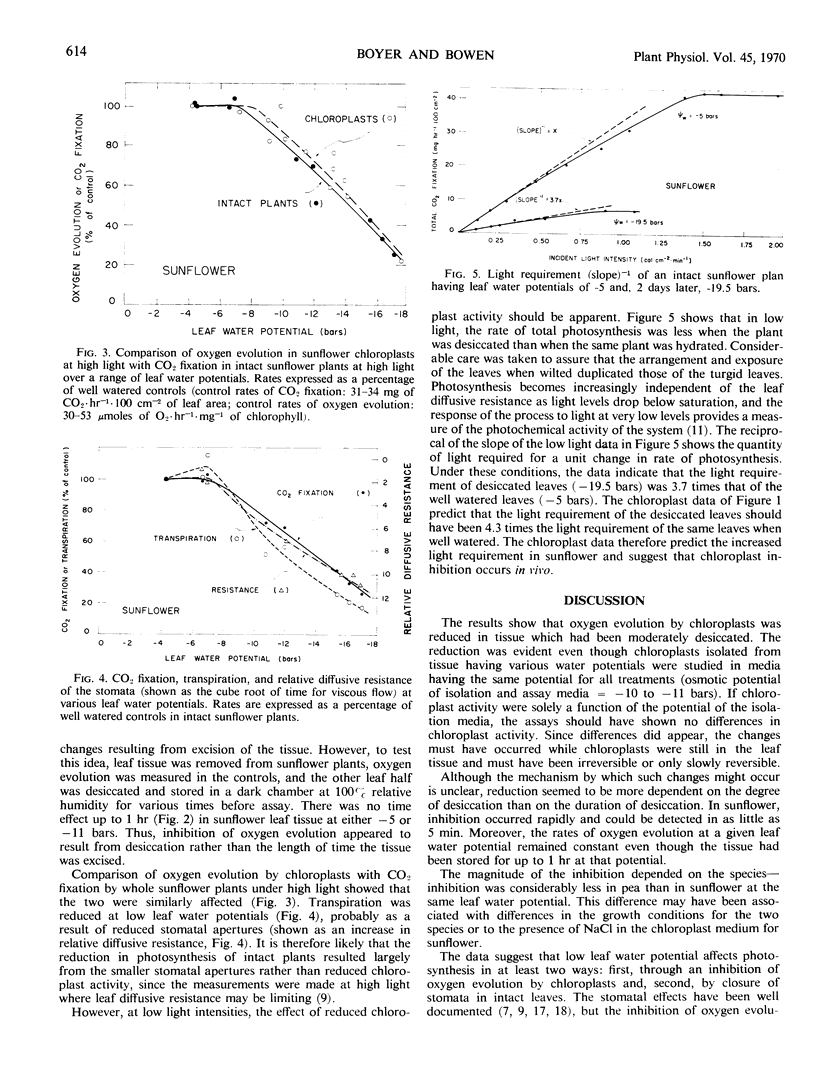
In high light, the reduction in activity of sunflower chloroplasts paralleled the reduction in CO2 fixation by intact sunflower plants having low leaf water potentials. Stomatal apertures and transpiration rates were also reduced under these conditions and were probably limiting. In low light, intact sunflowers required more light per unit of CO2 fixed when leaf water potentials were low than when they were high. This increased light requirement in the intact system was of a magnitude which could be predicted from the reduced oxygen evolution by the isolated chloroplasts. It was concluded that moderately low leaf water potential affects photosynthesis in at least two ways: first, through an inhibition of oxygen evolution by chloroplasts and, second, by closure of stomata in intact leaves.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer J. S. Effects of Osmotic Water Stress on Metabolic Rates of Cotton Plants with Open Stomata. Plant Physiol. 1965 Mar;40(2):229–234. doi: 10.1104/pp.40.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Isopiestic technique: measurement of accurate leaf water potentials. Science. 1966 Dec 16;154(3755):1459–1460. doi: 10.1126/science.154.3755.1459. [DOI] [PubMed] [Google Scholar]
- Boyer J. S. Leaf water potentials measured with a pressure chamber. Plant Physiol. 1967 Jan;42(1):133–137. doi: 10.1104/pp.42.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. The isolation of spinach chloroplasts in pyrophosphate media. Plant Physiol. 1968 Sep;43(9):1415–1418. doi: 10.1104/pp.43.9.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel P. S. Light-Induced Chloroplast Shrinkage in vivo Detectable After Rapid Isolation of Chloroplasts From Pisum sativum. Plant Physiol. 1968 May;43(5):781–787. doi: 10.1104/pp.43.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshi D. Effect of Soil Moisture and Phenylmercuric Acetate upon Stomatal Aperture, Transpiration, and Photosynthesis. Plant Physiol. 1963 Nov;38(6):713–721. doi: 10.1104/pp.38.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twente J. W., Twente J. A. Regulation of hibernating periods by temperature. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1044–1051. [PMC free article] [PubMed] [Google Scholar]


