Abstract
There is growing evidence that the imbalance between oxidative stress and the antioxidant defense system may be associated with the development neuropsychiatric disorders, such as depression and anxiety. Major depression and anxiety are presently correlated with a lowered total antioxidant state and by an activated oxidative stress (OS) pathway. The classical antidepressants may produce therapeutic effects other than regulation of monoamines by increasing the antioxidant levels and normalizing the damage caused by OS processes. This chapter provides an overview of recent work on oxidative stress markers in the animal models of depression and anxiety, as well as patients with the aforementioned mood disorders. It is well documented that antioxidants can remove the reactive oxygen species (ROS) and reactive nitrogen species (RNS) through scavenging radicals and suppressing the OS pathway, which further protect against neuronal damage caused oxidative or nitrosative stress sources in the brain, hopefully resulting in remission of depression or anxiety symptoms. The functional understanding of the relationship between oxidative stress and depression and anxiety may pave the way for discovery of novel targets for treatment of neuropsychiatric disorders.
Keywords: Oxidative stress, Antioxidants, Depression and anxiety, Oxidative stress pathway, Antidepressants.
INTRODUCTION
Depressive and anxiety disorders are among the most common mental health conditions around the world with a lifetime prevalence of 16% and 10%, respectively. They are also highly associated with substantial co-morbidity and mortality [1]. Depression and anxiety are considered to be complex brain traits that result from the interplay of multiple genetic and environmental determinants. Under the current antidepressant monotherapy, the arrival of newer drugs has not improved efficacy; only half of patients improve with their first prescription and only one-third ever achieve remission [2]. Therefore, there is a clear need to better understand the mechanisms of action of classical antidepressants, as those insights may lead to improved strategies in the development of more efficacious treatments with different therapeutic targets.
It has been debated that brain oxidative stress disturbances might be a plausible pathogenesis and risk factor for several specific diseases of the nervous system including behavioral disturbances and disorders [3]. Recent developments suggesting that oxidative stress-induced neuroinflammatory response, mitochondrial dysfunction, neuroplastic deficits and intracellular signaling pathways may help to elucidate the interrelationship between oxidative stress and depressive and anxiety disorders, providing a novel avenue for treatment [4,5]. The clinical and preclinical studies on oxidative stress and the antioxidant effects of antidepressants and anxiolytic agents suggest that they can remove reactive oxygen species (ROS) and reactive nitrogen species (RNS) through scavenging radicals and suppressing the oxidative stress (OS) pathway, which further protect against oxidative stress-induced neuronal damage and may result in the remission and functional recovery of depression or anxiety symptoms. We propose an innovative therapy focusing on the relationship between oxidative stress and depression and anxiety on systemic, cellular and molecular levels, which may bring crucial insights to improve strategies in antidepressant or anxiolytic drug development.
Environmental and Endogenous Factors of Oxidative Stress in Psychiatric Disorders
Oxidative phosphorylation, which takes place in the mitochondria of the cell, is the major source of ATP in aerobic organisms. The downside of this important process is that it often produces free radicals, commonly resulting in elevated levels of ROS and RNS. Though typically described as a harmful component of cellular respiration, they do have important beneficial effects as well. At low or moderate concentrations, they take part in normal physiological processes such as cellular responses to injury or infection, signaling, and mitosis [6]. However, oxidative stress results when the balance is shifted between the pro-oxidant substances that are produced during aerobic metabolism and the antioxidant defense system that performs the function of neutralization. This is indicative of the dysfunction within a series of protective mechanisms, both enzymatic and non-enzymatic. Enzymatic system components regularly discussed include superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px). In non-enzymatic system, the most discussed components are molecular in size and include compounds such as glutathione, alpha-tocopherol (vitamin E), ascorbic acid (vitamin C), flavonoids, polyphenol compounds and minerals (e.g. zinc, copper, and selenium) [7]. Under normal conditions the ROS and RNS are tightly regulated by an orchestral flow of all these species. Some proteins (e.g. albumin, transferrin, haptoglobin and ceruloplasmin) also work as radical scavengers which function as antioxidants by binding to ROS and RNS. Under critical conditions, the oxidative stress resulting from impaired oxidative defense mechanisms can evoke pathological changes such as neuropathies. The result of a prolonged or elevated oxidative stress state is damage to major groups of cellular macro-molecules (proteins, lipids, carbohydrates, and nucleic acids), which ultimately result in apoptosis [8,9]. Given that the brain has one of the highest mass-specific oxygen consumption rates in the body [10], even the smallest imbalances in antioxidant defense mechanisms and oxidative stress can be deleterious to neurons [11]. Oxidative stress-related molecular mechanisms may represent one factor influencing the etiology of depressive and anxiety disorders. An imbalance between the generation of ROS by endogenous/exogenous pro-oxidants and the defense mechanism against ROS by antioxidants has been found in these disorders [12]. However, information at large about how oxidative stress affects the processes of mental disorders is still unclear.
Oxidative Stress and Neuroinflammation
Oxidative stress is a major upstream component in the signaling cascade involved in the activation of redox-sensitive transcription factors and pro-inflammatory gene expression leading to inflammatory response [13]. Inflammation is a condition characterized by dysfunction of cytokine cascades and cellular immune responses, as well as increased levels of acute phase proteins and complement factors. Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) are the primary inflammatory mediators that activate nuclear factor-κB (NF-κB), which in turn activates the production of cytokines, such as IL-6 and IL-8, and T-cell derived cytokines including interferon (IFN) [14]. IL-1β, IL-6 and TNF-α, in turn, induce the production of acute phase proteins, such as haptoglobin and C-reactive protein (CRP) [15]. Increasing evidence suggests that chronic mild inflammatory processes both in the periphery and the brain, termed neuroinflammation, are involved in the pathophysiology of depression and anxiety. Clinical studies suggest that there are moderate increased plasma levels of pro-inflammatory cytokines, such as IL-6 and TNF-α; and increased IL-1β and NF-κB protein and mRNA levels in post-mortem frontal cortex of bipolar patients [16]. NF-κB is a heterodimer of two subunits, usually p65 and p50 [17]. Interestingly, mice lacking p50 have deficits in specific cognitive tasks and have a noted decrease in anxiety-like behaviors compared to wild-type mice [18]. Moreover, chronic NF-κB blocking by systemic administration of pyrrolidine dithiocarbamate (PDTC) attenuates the higher levels of cytosolic and mitochondrial ROS generation in the kidneys of spontaneously hypertensive rats when compared to treated wild type rats [19]. These findings suggest that NF-κB activation may induce oxidative stress and inflammation that leads to neuronal damage, which may be related to the depressive- and anxiety-like behaviors.
Inflammation and oxidative stress are inextricably connected in physiological as well as pathological conditions; they have even been termed “essential partners” in certain diseases [20]. Under normal physiological status, oxidative stress and immune system activation are generally short-lived due to intrinsic negative feedback mechanisms, such as increased production of antioxidant compounds or anti-inflammatory cytokines. In certain chronic disease states, however, both of these systems remain activated and may form a positive self-sustaining feedback loop, or a “co-activation” state [21]. Such co-activation over time may lead to a higher risk of disease as well as increased severity [20, 22, 23]. Consistent with experimental animal study, elevation in the activation of inflammatory pathways has been also observed in patients with anxiety disorders and major depression [24, 25]. The increased levels of pro-inflammatory cytokines also seem to be involved in mild emotional disruptions, as increasing activation of inflammation-related transcription factors, such as NF-κB and CREB, begin to play a role in the processes of mood disorders. These transcription factors also regulate the expression level of several inflammation-related enzymes, including NOS, COX2, and NADPH oxidase, that enhance production of ROS [26, 27]. It is worth noting that the infusion of pro-inflammatory cytokines may be the best experimental human model of depression because the elevated cytokines are known to be associated with depression and anxiety symptoms based on the clinical investigations [28-30]. These direct effects of inflammation on intracellular and extracellular signaling are essential to the understanding of how the immunological system interacts with depression and anxiety.
Oxidative Stress and Mitochondrial Dysfunction
Mitochondria are the main intracellular sites of ROS generation and are also prime target for oxidative damage, which induce further mitochondrial dysfunction and ROS generation. Increasing evidence suggests that mitochondrial dysfunction leads to oxidative stress and increases in intra-cellular Ca2+ levels, damage or deletions of mitochondrial DNA, alterations in mitochondrial morphology and mitochondrial fission or fusion, all which may result in neuronal death [31, 32]. These permutations in mitochondrial function likely play an important role in various aspects of mood disorders [31-33]. Comorbidity of mitochondrial diseases and psychiatric disorders have also been reported. Particularly, many patients that manifest psychiatric presentations also have been diagnosed with some form of mitochondrial disease [34]. Stabilizing mitochondrial function may represent one of the critical components for the treatment of psychiatric disorders [35].
Several bioenergetics that have demonstrated to improve mitochondrial function, namely creatine, coenzyme Q10, nicotinamide, riboflavin and lipoic acid, have also been confirmed to have neuroprotective efficacy in neuro-degenerative and neuropsychiatric disorders [5, 36, 37]. Furthermore, several genetic studies, both in humans and in rodents, also have provided evidence for the involvement of mitochondrial dysfunction in neuropsychiatric diseases. Patients with certain mitochondrial diseases, such as progressive external ophthalmoplegia (PEO) and mitochondrial recessive ataxia syndrome (MIRAS), have psychiatric symptoms, including anxiety and depression [38] Both PEO and MIRAS can be caused by mutations in the nuclear encoded mitochondrial polymerase gamma (POLG) gene. Mutations in POLG, which is responsible for mitochondrial DNA replication, result in randomly distributed mtDNA point mutations. Interestingly, transgenic mice expressing mutant POLG specifically in forebrain neurons show a mood disorder-like phenotype [39]. Studies of another transgenic model with the Y955C POLG mutation have suggested that one of the pathological mechanisms may be oxidative damage to mtDNA [40]. The effect of mtDNA damage to the forebrain neurons of mice and its consequences on behavioral phenotypes has also been studied in a transgenic mouse model that have been designed specifically to result in the induction of a high number of apyrimidinic sites to the mitochondrial genome [41]. On a behavioral level, the transgenic mice have elevated locomotor activity, but decrease cognitive ability and anxiety-like behaviors. In addition, evidence for the involvement of mitochondrial variations on anxiety-like behaviors in mice have been obtained from a model in which C57BL/6J mice with substituted mitochondria from FVN/NJ mice show increased anxiety-like behaviors compared to C57BL/6J mice with either AKR/J mtDNA or their own mtDNA [42].
In humans, decreased mitochondrial ATP production rate and deletions of mtDNA have been observed in patients with major depressive disorder comorbid with somatic illnesses. A post-mortem gene expression study of the dorsolateral prefrontal cortex of post-traumatic stress disorder (PTSD) patients and controls using mitochondria focused micro-arrays has found that the majority of the differentially expressed transcripts were related to mitochondrial dysfunction and oxidative phosphorylation [43], conditions which may be associated with excessive ROS production. It seems that a mitochondrial deficit is sufficient to trigger different psychiatric disorders; however, it remains to be determined whether the mitochondrial dysfunctions contribute to the disease development or are epiphenomena, i.e. how nonspecific mitochondrial dysfunction may cause specific symptom.
Oxidative Stress, Neuroplasticity and the Related Signaling
The term “neuroplasticity” refers to an array of important processes of brain response and adaptation to stimuli, both internal and external. These modifications include alterations in dendritic function, synaptic remodeling, long-term potentiation (LTP), axonal sprouting, neurite extension, synaptogenesis, and neurogenesis. Although little evidence suggests neuronal loss in depression and anxiety, mild oxidative stress in these disorders may further modify various molecules associated with intracellular signaling, giving an overall decrease in brain activity similar to that of cell death. Oxidative modifications of these synaptic molecules have been found in depressive and anxiety disorders and may be affecting neurotransmission and impairing neuroplasticity in key brain regions associated with mood disorders [44, 45]. A recent genome-wide association study of bipolar disorders (BD), which is another chronic major mental illness composed of recurrent episodic mood disturbances ranging from mania to severe depression, revealed that most of the highly significant associations were implicated in signaling cascades of plasticity [46]. It has been proposed that abnormalities in neuroplasticity lead to maladaptive developments in neural circuits, affecting the information processing that mediates various facets of BD symptomatology [47, 48]. Another study using peripheral biomarkers suggested that BD patients show increased oxidative stress and decreased neuron-specific endolase, a neuronal glycolytic enzyme known to mediate neuroplastic pathways and cell survival [49]. Futhermore, an animal study showed that oxidative stress is correlated with BDNF reductions, as well as reductions in cyclic AMP responsive element binding protein (CREB) and synapsin I molecules that are involved in cellular plasticity cascades [50]. Such research suggests that oxidative stress may play a role in the abnormal neuroplastic processes found in the patho-physiology of depressive and anxiety disorders.
Optimal brain health is promoted by intermittent challenges to neurons, such as exercise and cognitive stimulation that result in expression of synaptic proteins related to neuronal proliferation, differentiation and survival. Such proteins include those that modulate mitochondrial biogenesis, and neuronal resistance to oxidative stress, such as antioxidant enzymes, DNA repair enzymes and those involved in BDNF signaling [10]. One of the mechanisms by which synaptic proteins and BDNF signaling exert neuroprotective effects is via the modulation of synaptic plasticity by regulating dendritic length, numbers, and spine density. However, these protective proteins are suppressed under conditions of chronic adverse stress and disease states, such as neurodegenerative and psychiatric disorders. Moreover, excessive formation of ROS in the brain under disease states may lead to dysfunction of genes and proteins involved in neuroplasticity and neurogenesis, which result in neuronal apoptosis [51].
More evidence demonstrated that N-methyl-D-aspartic acid (NMDA) receptor allows the passage of calcium and sodium into the cell, thereby promoting the activation of p38, JNK, and p53 that are generally proapoptotic [52, 53]. NMDA receptor activation triggers the activation of Ca2+/calmodulin-dependent protein kinase IV (CaMKIV), present in mitochondrial external membrane, which may activate nitric oxide synthases (NOS1 and NOS3), thereby leading to increased nitric oxide production [54]. Activation of NMDA receptors also increases intracellular Ca2+ levels which associated with oxidative stress may trigger endoplasmic reticulum stress [55]; mitochondrial Ca2+ uptake in combination with NO production may trigger the collapse of mitochondrial membrane potential, culminating in delayed cell death and release of free radicals [54]. A growing body of evidence has pointed to the ionotropic glutamate NMDA receptor as an important player in the etiology of psychopathologies, including anxiety and major depression. Clinical findings suggest that ketamine which has been used clinically as a dissociative anesthetic since the 1960s and is regarded as a noncompetitive glutamate NMDA receptor antagonist may be useful for the treatment of major depression [56, 57]. The possible mechanism may be involved in NMDA receptor-mediated oxidative stress and the downstream cGMP dependent pathway.
Recently, the Wnt signaling pathway, a network of proteins that helps in the communication of external stimuli to the nucleus and cell at large, was found as a novel treatment target for anti-psychiatric disorders because of the aforementioned pathway. It’s believed that through careful manipulation of this pathway, pharmaceutical intervention may result in the expression of various target genes resulting in a return to status quo. Wnt genes encode a family of secreted signaling glycoproteins that play a vital role during neural development and in an assistant role in the developed nervous system. Wnt signaling has been well characterized as a pathway that helps regulates cell fate determination, cell proliferation and cell polarization during growth and development of multi-cellular organisms [58-63]. More recently, there have also been suggestions that Wnt signaling may play a role in synaptic modulation and plasticity in later stages of development making them excellent candidates for therapeutic targeting. It has recently been shown that Wnt7a can regulate axonal remodeling and synaptic differentiation and induce the clustering of the synaptic protein synapsin I at the pre-synaptic terminal. These findings demonstrate that Wnt signaling may play an important role in psychiatric disorders later as more is understood about how the various component of Wnt are modulated.
Although there is no direct evidence that abnormalities of the Wnt pathway can be induced by oxidative stress and in turn lead to the development of psychiatric disorders, some studies suggest a possible involvement of ROS-induced changes in this signaling in the brain of aged mice and Alzheimer’s disease [64]. Recent reports have even demonstrated the interconnection between Wnt signaling and oxidative response mechanisms. One key example is nitric oxide, a diffusible free radical gas that mediates cell-to-cell communication, can increase expression of a member of the Wnt signaling pathway known as Wnt-induced secreted protein-1 [65], suggesting that an involvement of the Wnt signaling in the regulation of many events that hallmark cellular disruption in mood disorders, such as alterations in cell cycle progression and cell growth [66]. Other findings also suggest the existence of a neuroprotective effect of Wnt through interaction with BDNF and a possible complex feedback loop between Wnt and mediators of inflammation and oxidative stress. Therefore, it seems reasonable to assume that therapies that act in these pathways may have a beneficial effect on psychiatric disorders.
There is also emerging data that supports that neurotrophic effects are key aspects to lithium and valproate efficacy, drugs known for having little conscience toward their mechanism of action [67]. Since lithium and valproate are known, at least in part, to affect Wnt signaling through the activities of GSK-3β and that Wnt signaling molecules are abundant at synaptic regions, this pathway seems to be a good candidate for an investigation into activity of these mood stabilizing agents [68, 69]. Additionally, direct inhibition of GSK-3β seems to have antidepressant-like activity by enhancing overall serotonergic activity [70]. Genetic studies have also implicated polymorphisms of GSK-3β gene in both the vulnerability to development of mood disorders and overall severity of said disorders [71]. These studies provide further evidence that the genetic variants in the GSK-3β could partially be involved in some mood disorders, such as major depression and bipolar disorders.
There is a large body of evidence that antidepressants can increase activity of signaling pathways related to antioxidant effects; neuroplasticity by upregulation of cAMP/PKA/CREB cascade, regulation of CaMKII activity and upregulation of the MAPK cascade are choice examples [72]. The hypothesis that long-term antidepressant treatment enhances neuro-plasticity is based on upregulation of stress-responsive neuro-trophic factors, namely BDNF signaling, in the hippocampus and the prefrontal cortex [73]. Chronic antidepressant administration has been demonstrated to increase the number of new neurons in the dentate gyrus of the hippocampus, a change not found with other classes of psychotropic drugs. Reduced cellular resilience refers to several processes causing neurons, especially in the hippocampus, to become more vulnerable to insults, such as ischemia and excitotoxicity, as a result of excessive stress. This is noteworthy as there is increasing evidence that cAMP/PKA/pCREB and cGMP/PKG/pCREB pathways are related to neuroplasticity, cellular resilience, anti-inflammation and anti-oxidative stress factors. These then become important targets in the treatment of depression and anxiety for a multifaceted approach [74-77].
The Role of Antioxidants in Depression: Preclinical and Clinical Evidences
Oxidative damage to macromolecules such as lipid, protein and nucleic acids as a result of excessive ROS [78] lead to neuronal dysfunction that is associated with the development of depression disorder [79, 80]. It is extensively reported that chronic unpredictable chronic stress impairs the antioxidant status of brain tissue, presumably by generating excessive ROS and increasing oxidative stress. Although the brain metabolizes 20% of the total oxygen in the body, it has a limited amount of antioxidant capacity, so it is particularly vulnerable to the production of ROS [81]. Some brain regions, especially those in the limbic system (e.g. hippocampus and frontal cortex), act in concert to mediate the symptoms of depression and anxiety [82, 83], and seem to be strongly affected by the deleterious effects of an oxidative insult [84], making them excellent candidates to observe antioxidant factors. Cu/Zn SOD is a key antioxidant enzyme involved in superoxide detoxification in cellular metabolism and is widely expressed in different neuronal populations, such as hippocampal pyramidal and granule neurons [85]). Decreased expression of Cu/Zn SOD in the hippocampus is often seen in stressed animals [86]. Chronic treatment with antidepressants restores Cu/Zn SOD expression in a dose dependent manner [85-88].
Oxidative stress markers and antioxidant concentrations have been evaluated in numerous animal studies to investigate the role of drugs in the treatment of depression and anxiety. Despite the fact that antidepressants ameliorate the oxidative status, their mechanism of action is still not fully elucidated. The classic hypothesis was formulated around the idea that the antidepressants restore noradrenergic and serotoninergic neurotransmitter systems to normal levels as a primary or direct effect, while the antioxidant efforts are becoming a noted secondary effect. Recently, a new concept of antidepressants action has been proposed based on growing evidence indicating the immediate antioxidant effects of antidepressants in the treatment of major depressive disorder (MDD). Some evidence has shown that there is co-existence of increased oxidative stress with symptoms of depression, as evidenced by enhanced lipid peroxidation [89-91]. Examples include studies in which chronic mild stress (CMS)-induced depression in rodents lowered concentrations of brain glutathione [92] and total glutathione concentrations in the cortex [93-96]. In addition, unpredictable chronic mild stress (UCMS) induced a lowered total antioxidant capacity (TAC) and GSH content, in addition to decreased SOD and CAT activities [97]. Additionally, recent evidence from behavioral tests suggests that metabolic oxidative stress and key subunits of NADPH oxidase, particularly p47phox and p67phox, mediate depressive-like behaviors in sociability and forced swimming tests in mice [98]. The subsequent study suggests that inhibition of NADPH oxidase produces beneficial antidepressant-like effects. A growing body of data has reported that antioxidants (e.g. vitamin C, rutin, caffeic acid and rosmarinic acid) possess antidepressant activity with relatively lower doses than commonly used antidepressants such as imipramine or fluoxetine [99,69]. The mechanism of action of antioxidants on the central nervous system is not well elucidated; however, it has been demonstrated that rutin exerts its antidepressant activity similarly to conventional antidepressants by increasing the availability of serotonin and norepinephrine in the synaptic cleft [99].
The oxidative stress pathway provides an intriguing target for pharmacological intervention. Though there is a plethora of evidence from animal models suggesting that treatment with antioxidants can reduce oxidative stress and ameliorate depressive-like behaviors, there are very few human clinical trials of antioxidants that have been published on depressive disorders [100]. The limited investigation from the clinical trials suggests that the implemented therapies are based on supplementation with antioxidants or on reinforcement of endogenous antioxidant pathways with compounds such as N-acetylcysteine (NAC) [9]. NAC is a substrate for the glutamate/cysteine antiporter of glial cells, which can be converted to cysteine in the brain [101]. The glial cysteine uptake causes export of glutamate and modulation of glutamatergic neurotransmission in brain regions related to emotional function. The increased cellular cysteine can also reinforce GSH-related antioxidant defenses, as it is the rate-limiting component of GSH synthesis [9]. Traditional bipolar treatments, such as valproate and lithium, also have antioxidant properties through increased GSH levels [102]. A double blind, placebo controlled trial of NAC in trichotillomania, a condition related to obsessive-compulsive disorder (OCD), reports significant improvement of symptoms observable after 9 weeks of active use.
Case studies have further reported symptom reduction by NAC in OCD [101], trichotillomania, and pathological nail biting and skin picking [103]. Depressive symptoms in bipolar disorder were significantly reduced by 24-week adjunction of NAC to usual medication in another double blind, placebo controlled trial [9]. In major depressed patients, subchronic treatment with fluoxetine and citalopram partially reversed the depression-related increased in serum SOD and malondialdehyde (MDA) and decreased in plasma ascorbic acid [104, 105]. Additionally, selective serotonin reuptake inhibitors (SSRIs) significantly decreased MDA levels and activities of peripheral antioxidant activity. In another study [106], the combined treatment of depressed patients with fluoxetine alone and combined with acetylsalicylic acid during 3 months was examined. The combined treatment significantly reduced SOD, catalases and GPX, and the MDA levels, suggesting that this treatment improves lipid peroxidation and may normalize disturbed antioxidant levels. Sarandol et al. [107] investigated 96 patients with major depression and determined their plasma MDA, the susceptibility of red blood cells to oxidation, plasma vitamin E and C, TAC, SOD and whole blood GPX activities after antidepressants treatment. They found that a 6-week treatment with antidepressants did not change the disordered levels in depression. However, the ex vivo effects of desipramine, imipramine, maprotiline and mirtazapine on the mRNA levels of SOD isoforms, GPX, catalase, gamma-glutamylcysteine synthetase, glutathion-S-transferase and glutathion reductase were examined and found that the short-term treatment with antidepressants for 2.5 hours decreased antioxidant mRNA levels, whereas long-term treatment of 24 hours significantly increased the mRNA levels of these antioxidants [108]. The contradictory findings prompt further study for how the antidepressants affect the redox balance in the body.
It is well known that oxidative stress is associated with DNA damage, endothelial dysfunction, and telomere shortening. Notably, patients with bipolar disorders have a marked increase of DNA damage in white blood cells and more importantly, this DNA damage is highly correlated with the severity of symptoms [109]. However, the degree of redox imbalance in these patients is not correlated to the degree or severity of the depressive symptoms. Due to depression most likely resulting from irregularities in a host of different cellular networks, only focusing on a single system may prove fruitless in the efforts to treat depression at large. Moreover, even with proper care and treatment, a third of bipolar patients taking classic antidepressant treatment continue to suffer from depressive behaviors which alters daily function and leading to a lessened quality of life. This could be partially explained by the contradictory results, since several antidepressants have little to no change oxidative stress markers. To further this point, it has been shown that some parameters of oxidative stress can also be normalized by mood stabilizing treatment [49, 110, 111] This is in accordance with preclinical studies showing that lithium and valproate exert antioxidant effects in vitro and in vivo [111]. This may help demonstrate a rationale for why the adjunct treatments with mood stabilizers and antidepressants have such high efficacy in the treatment of bipolar disorder. A recent trial assessing the potential application of the antioxidant N-acetylcysteine has shown promising results, which suggest that it may ameliorate depressive symptoms within two weeks compared to the standard one month outlook of current SSRI treatments [112].
Mitochondrion is a well-known target of oxidative stress, a point that is rarely debated. Inhibition of the mitochondrial MAO is likely the most studied effect of antidepressants on mitochondrial functions. An early observation of iproniazid, an irreversible MAO inhibitor, having antidepressant effects [113] lead to the pursuit and discovery of other compounds with similar mechanisms of action. Generally, selective inhibitors of MAO-A and nonselective MAO inhibitors seem to be effective in the treatment of patients with depression, panic disorder, and other anxiety disorders [114]. Antidepressants, which act primarily as serotonin and/or norepinephrine reuptake inhibitors, show inhibitory activity towards MAO as well bringing more validity to the concept of antioxidant action in the treatment of depression and anxiety.
The Role of Antioxidants in Anxiety: Preclinical and Clinical Evidences
Several studies have mentioned that oxidative stress can cause anxiogenic behavior, but the relationship between them is indirect. Desrumaux et al. [115] showed that vitamin E deficiency in the mouse brain significantly caused oxidative stress, resulting in increased anxiogenic behaviors without abnormalities in the locomotor performance. Berry et al. [116] reported that the mice develop the anxiety-like behaviors during aging, likely due to the accumulation of oxidative damage, which is a characteristic of normal aging processes. A subsequent study in rats by Souza et al. [117] revealed that the consumption of a highly palatable diet enriched with sucrose leads to an obese phenotype, increased protein oxidation of the frontal cortex and an induction in anxiety-like behaviors in the dark/light choice test without altering locomotion in an open field test.
Recent evidence suggests a direct correlation between oxidative stress and anxiety. For example, Masood et al. (2008) reported that oxidative stress induced by L-buthionine-(S,R)-sulfoximine (BSO) in the hypothalamus and amygdala occurs in parallel with anxiety-like behavioral patterns in mice. Another study in outbred Swiss mice by Bouayed et al. [118, 119] indicated that increased anxiety-like behavior is positively correlated with increases in reactive oxygen species in granulocytes. Since there is a strong relationship between stress, neonatal handling and feeding behaviors, the influences of these three factors on behavioral parameters and oxidative stress in key brain regions have been investigated. These studies suggest that these factors are closely involved in behavioral activity, such as anxiety and locomotion, as well as redox components, such as ROS/SOD and NADPH levels [120].
The advent of anxiety can result from a multitude of different avenues, such as pharmacological side effect of various treatments (e.g. methyl-β-carboline-3-carboxylate), stressful situations (e.g. immobilization stress) or natural conditions (e.g. aging process). Although the link between oxidative stress disturbances and anxiety is not being disputed, it’s whether oxidative stress is a side effect of emotional stress or disorders, or if oxidative stress is the genesis of the condition remains unclear; a chicken versus egg dilemma of physiological and behavioral aspects. Could prevention of oxidative stress attenuate oxidative stress-induced anxiety-like behavior or is it a bandage about to a wound? To answer this question, one study employed a rat model in which oxidative stress was induced by L-buthionine-(S,R)-sulfoximine (BSO) for 3 or 7 days and the net change in anxiety-like behaviors was assessed [100]. In the study, two different treatment options to prevent oxidative stess was applied: antioxidant tempol supplementation as a pharmacological intervention or the use of a moderate treadmill exercise regimen The findings suggested that BSO- induced anxiety-like behavior of rats was prevented by both antioxidant tempol supplementation and moderate treadmill exercise in rats. This was one of the first studies that provided a direct causal role of oxidative stress in anxiety-like behavior in rats. A follow-up study replicated these findings using another oxidative stress inducer, xanthine plus xanthine oxidase (X+XO), which led to increased anxiety-like behaviors in rats [87]. These studies support, at least in part, the interconnection between oxidative stress and anxiety-like behaviors. However, it is important to note that this relationship is not perfectly correlated. Results from Masood et al. [118] showed that the well-known anxiolytic diazepam did not fully reverse the anxiety generated by BSO treatment, suggesting that ROS could be one of many factors causing spontaneous anxiety.
Although psychopharmacological studies present antioxidants as a potential new strategy for the treatment of anxiety, the use of these substances has to be with used caution. Several studies are required to investigate the toxicity of antioxidants at nonnutritional doses. At high doses, it has been discussed that antioxidants could enact deleterious effects on health. An example, it has been demonstrated that epigallocatechin gallate (EGCG) polyphenols at pharmacological doses (30 and 60 mg/kg) abolishes anxiety in mice; however, at 150 mg/kg this tea polyphenol lead to death in mice with 100% mortality in less than 24 hours. Despite the fact that antioxidants given at higher doses could be toxic, they are presumed safe in food due to their presence at low doses levels, instead working through a combined effect through many avenues of antioxidant activity [121]. Therefore, antioxidants from a normal diet could play a role in the prevention anxiety. For example, Viggiano and co-workers [122] demonstrated that the symptoms of aged rats with anxiety fed for 10 weeks with a standard diet supplemented with fresh fruit were significantly ameliorated than aged rats fed with the standard diet sans the fruit. The decrease of anxiety was not associated with a change in TAC but a reduction of overall oxidative stress was found. Additionally, it has been suggested that the anti-anxiety effect of sustained intake of fruits, olive oil and honey may be due to the diet containing complex mixtures of nutrients, including vitamines, flavonoids, phenolic acids, several carotenoids, acting on a synergistic or additive manner.
The connection between anxiety and oxidative stress in humans has been studied as well but to a much lesser extent than that of other animals. Yasunari and his colleagues [123] found a significant relationship between trait anxiety and ROS formation in monocytes of hypertensive individuals. The efficacy of natural remedies, most of which have antioxidant properties, in treatment of anxiety disorders was recently reviewed. The findings suggested that various herbal remedies, such as passionflower, may play some role in the alleviation of anxiety [124]. Some target synthetic compounds focusing on depression and anxiety, such as phosphodiesterase 5 inhibitors, have also been proven to be effective in neutralizing radical oxygen species, which may result in the treatment of anxiety-related disorders, such as the irritable bowel syndrome [125].
Taken together, findings from all animals, from humans to mice, studies clearly support involvement of altered oxidative stress-related mechanisms in anxiety disorders, but to what extent and how their representation of various symptoms or trait markers have not yet been conclusively resolved. Some studies have addressed the effect of antidepressant treatment on oxidative stress markers in patients with anxiety or depressive disorders [126, 127]. However, in the absence of healthy controls receiving treatment or patient groups receiving placebo, the inter-pretation of these results is difficult and nigh mute.
SUMMARY & FUTURE DIRECTIONS
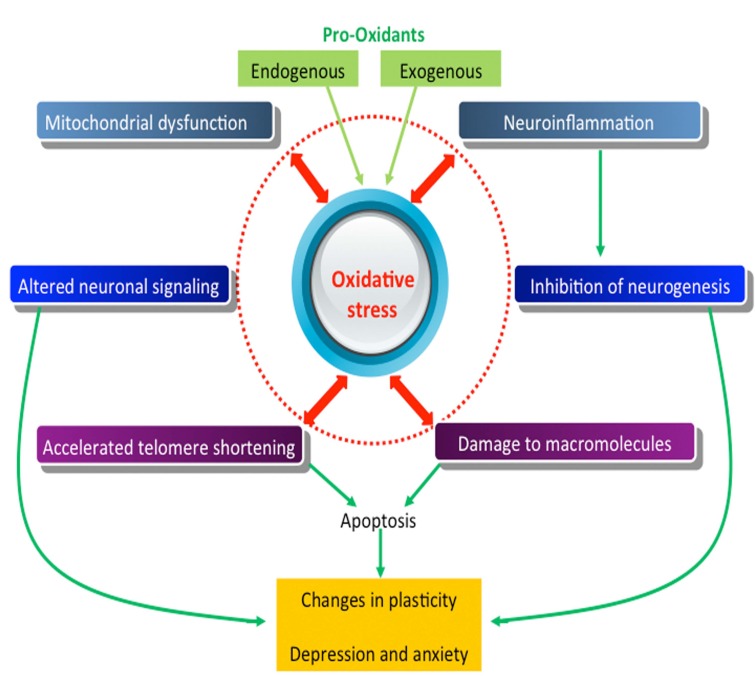
The oxidative stress hypothesis of depression and anxiety is slowly gaining merit. The information reviewed in this chapter reveals the influence of oxidative stress in the function, inflammation, plasticity, and signaling of neurons, as well as mitochondrial dysfunction in the pathogenesis of depressive and anxiety disorders (Fig. 1). Identification of new molecular pathways provides promise, as they are potentially amenable to pharmacological intervention. Increased interest in exploring novel pathways involved in the oxidative stress is good news for the study of depressive and anxiety disorders, an area that has stagnated in novel treatment approaches.
Fig. (1).
Mechanism of oxidative stress-induced neuronal damage.
Cyclic AMP (cAMP) and cyclic GMP (cGMP), critical second messengers, play an important role in regulating central nervous system’s functions, such as emotion-related learning and memory in addition to nerve growth and regeneration [128]. Cyclic nucleotide phosphodiesterases (PDEs) are a super-family of enzymes that are involved in the regulation of the intracellular second messengers cAMP and cGMP, by controlling their rates of degradation. Since PDEs have been demonstrated to play distinct roles in the process of emotion as well as learning and memory, selective PDE inhibitors could also help modulate mood disorders. The effects of specific PDE inhibitors as pharmacological agents on depression and anxiety are a field of high research interest. It has found that PDE2 inhibition is able to inhibit oxidative stress-induced anxiety in mice [77]. A noted anxiolytic effect of two PDE2 inhibitors, Bay 60-7550 and ND7001, was observed in the elevated plus-maze and open field test in stressed mice. The related mechanism is demonstrated by primary neuronal cultures, which suggest that Bay 60-7550 and ND7001 decrease reactive oxygen species generation and improve the total antioxidant capacity in cerebral cortical neurons. These behavioral and neuro-chemical results support the notion that PDE2 may be a pharmacological target for treatment of neuropsychiatric diseases, which is involved in their antioxidant actions.
Another phosphodiesterase subtype, PDE4, has also been implicated in various disorders such as depression, anxiety, and inflammation-related disorders ten years ago. However, causal links among these actions with antioxidant effects by rolipram, a non-selective PDE4 inhibitor, have not been established. To date, a growing body of evidence suggests that PDE4 inhibitors alleviate the depressive-like behaviors, which is at least in part mediated by modulating oxidative stress status in animal brains [4]. However, there remain large gaps in our understanding of major contributions of PDE4 inhibitors on depression and anxiety by regulation of oxidative/antioxidant parameters. The reason for this lack of understanding is that the same as the lack of knowledge about the major aspects of oxidative stress and its overall contributions to emotional disorder.
To fully elucidate the role of oxidative stress in the development of mood disorders like depression and anxiety, there must be several questions answered:
What are the molecular mechanism and the relationship between oxidative stress and neuroinflammatory changes in mood disorders?
How do specific second messengers, such as cyclic nucleotides, kinases and transcription factors, affect depression and anxiety?
What are the mechanisms and roles for oxidative stress in the development and plasticity of neuronal circuitry?
Can neuronal dysfunction and degeneration be prevented by interventions that sustain mitochondrial function?
Can synaptic plasticity and depressive- and anxiety-like behaviors be reversed by interventions that target oxidative stress?
How do changes in oxidative stress mediate the effects of environmental factors that either improve (exercise, dietary energy restriction) or worsen (diabetes, overeating and a sedentary lifestyle) brain health?
As these questions become answered, the prospect of novel research strategies into the treatment of depression and anxiety may come to fruition, a welcome relief to all those presently suffering who may feel abandoned without suitable treatments options. The advent of the aforementioned knowledge may take years to identify but a journey with high rewards at its conclusion.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Kessler RC, Adler LA, Berglund P, Green JG, McLaughlin KA, Fayyad J, Russo LJ, Sampson NA, Shahly V, Zaslavsky AM. The effects of temporally secondary co-morbid mental disorders on the associations of DSM-IV ADHD with adverse outcomes in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A). Psychol. Med. 2013;8:1–14. doi: 10.1017/S0033291713002419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skolnick P. Beyond monoamine-based therapies: clues to new approaches. J. Clin. Psychiatry. 2002;63:19–23. [PubMed] [Google Scholar]
- 3.Moylan S, Eyre HA, Maes M, Baune BT, Jacka FN, Berk M. Exercising the worry away: How inflammation, oxidative and nitrogen stress mediates the beneficial effect of physical activity on anxiety disorder symptoms and behaviours. Neurosci. Biobehav. Rev. 2013;37:573–584. doi: 10.1016/j.neubiorev.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Jindal A, Mahesh R, Bhatt S. Etazolate, a phosphodiesterase 4 inhibitor reverses chronic unpredictable mild stress-induced depression-like behavior and brain oxidative damage. Pharmacol. Biochem Behav. 2013;105C:63–70. doi: 10.1016/j.pbb.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Aboul-Fotouh S. Coenzyme Q10 displays antidepressant-like activity with reduction of hippocampal oxidative/nitrosative DNA damage in chronically stressed rats. Pharmacol. Biochem. Behav. 2013;104:105–112. doi: 10.1016/j.pbb.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Halliwell B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007;35(Pt 5):1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 8.Filomeni G, Ciriolo MR. Redox control of apoptosis: an update. Antioxid. Redox Signal. 2006;8(11-12):2187–2192. doi: 10.1089/ars.2006.8.2187. [DOI] [PubMed] [Google Scholar]
- 9.Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Bush AI. N-acetyl cysteine for depressive symptoms in bipolar disorder--a double-blind randomized placebo-controlled trial. Biol. Psychiatry. 2008;64(6):468–475. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Rothman SM, Mattson MP. Activity-dependent, stress-responsive BDNF signaling and the quest for optimal brain health and resilience throughout the lifespan. Neuroscience. 2012;In press doi: 10.1016/j.neuroscience.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasote DM, Hegde MV, Katyare SS. Mitochondrial dysfunction in psychiatric and neurological diseases: Cause(s): consequence(s): and implications of antioxidant therapy. Biofactors. doi: 10.1002/biof.1093. [DOI] [PubMed] [Google Scholar]
- 12.Mällo T, Matrov D, Kõiv K, Harro J. Effect of chronic stress on behavior and cerebral oxidative metabolism in rats with high or low positive affect. Neuroscience. 2009;164:963–74. doi: 10.1016/j.neuroscience.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 13.Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010;48(9):1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Maes M. A review on the acute phase response in major depression. Rev. Neurosci. 1993;4(4):407–416. doi: 10.1515/revneuro.1993.4.4.407. [DOI] [PubMed] [Google Scholar]
- 16.Drexhage RC, Knijff EM, Padmos RC, Heul-Nieuwenhuijzen Lv, Beumer W, Versnel MA, Drexhage HA. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev. Neurother. 2010;10(1):59–76. doi: 10.1586/ern.09.144. [DOI] [PubMed] [Google Scholar]
- 17.Liou HC, Baltimore D. Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Curr. Opin. Cell Biol. 1993;5(3):477–87. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- 18.Kassed CA, Herkenham M. NF-kappaB p50-deficient mice show reduced anxiety-like behaviors in tests of exploratory drive and anxiety. Behav. Brain Res. 2004;154(2):577–584. doi: 10.1016/j.bbr.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Elks CM, Mariappan N, Haque M, Guggilam A, Majid DS, Francis J. Chronic NF-{kappa}B blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am. J. Physiol. Renal Physiol. 2009;296(2):F298–305. doi: 10.1152/ajprenal.90628.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambade A, Mandrekar P. Oxidative stress and inflammation: essential partners in alcoholic liver disease. Int. J. Hepatol. 2012;2012:853175. doi: 10.1155/2012/853175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jesmin J, Rashid MS, Jamil H, Hontecillas R, Bassaganya-R J. Gene regulatory network reveals oxidative stress as the underlying molecular mechanism of type 2 diabetes and hypertension. BMC Med. Genomics. 2010;3:45. doi: 10.1186/1755-8794-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotani K, Taniguchi N. Correlation Between High-sensitivity C-reactive Protein and Reactive Oxygen Metabolites During A One-year Period Among Asymptomatic Subjects. J. Clin. Med. Res. 2012;4(1):52–55. doi: 10.4021/jocmr755w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terlecky SR, Terlecky LJ, Giordano CR. Peroxisomes, oxidative stress, and inflammation. World J. Biol. Chem. 2012;3(5):93–97. doi: 10.4331/wjbc.v3.i5.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haeri S, Baker AM, Ruano R. Do pregnant women with depression have a pro-inflammatory profile?. J. Obstet. Gynaecol. Res. 2013 doi: 10.1111/jog.12017. [DOI] [PubMed] [Google Scholar]
- 25.Duivis HE, Vogelzangs N, Kupper N, de Jonge P, Penninx BW. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: Findings from the Netherlands Study of Depression and Anxiety (NESDA). Psycho-neuroendocrinology. Feb 8. 2013 doi: 10.1016/j.psyneuen.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Kelley KW, O'Connor JC, Lawson MA, Dantzer R, Rodriguez-Zas SL, McCusker RH. Aging leads to prolonged duration of inflammation-induced depression-like behavior caused by Bacillus Calmette-Guérin. Brain Behav. Immun. 2013;pii:S0889–1591(13)00125-6. doi: 10.1016/j.bbi.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Song Y, Yang J, Zhang Y, Zhao P, Zhu XJ, Su HC. The contribution of TNF-a in the amygdala to anxiety in mice with persistent inflammatory pain. Neurosci. Lett. 2013;13 doi: 10.1016/j.neulet.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Wadee AA, Kuschke RH, Wood LA, Berk M, Ichim L, Maes M. Serological observations in patients suffering from acute manic episodes. Hum. Psychopharmacol. 2002;17(4):175–179. doi: 10.1002/hup.390. [DOI] [PubMed] [Google Scholar]
- 29.Karson A, Demirtas T, Bayramgürler D, Balci F, Utkan T. Chronic Administration of Infliximab (TNF-a Inhibitor) Decreases Depression and Anxiety-like Behaviour in Rat Model of Chronic Mild Stress. Basic Clin Pharmacol Toxicol Nov 20. 2012 doi: 10.1111/bcpt.12037. [DOI] [PubMed] [Google Scholar]
- 30.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;In press doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao X, Zheng CY, Qin GW, Tang XC, Zhang HY. S-52 a novel nootropic compound, protects against ß-amyloid induced neuronal injury by attenuating mitochondrial dysfunction. J. Neurosci. Res. 2012;90(10):1981–1988. doi: 10.1002/jnr.23086. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi M, Miyata R, Tanuma N. Oxidative stress in developmental brain disorders. Adv. Exp. Med. Biol. 2012;724:278–290. doi: 10.1007/978-1-4614-0653-2_21. [DOI] [PubMed] [Google Scholar]
- 33.Quiroz JA, Gray NA, Kato T, Manji HK. Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33(11):2551–2565. doi: 10.1038/sj.npp.1301671. [DOI] [PubMed] [Google Scholar]
- 34.Fattal O, Budur K, Vaughan AJ, Franco K. Review of the literature on major mental disorders in adult patients with mitochondrial diseases. Psychosomatics. 2006;47(1):1–7. doi: 10.1176/appi.psy.47.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Jou SH, Chiu NY, Liu CS. Mitochondrial dysfunction and psychiatric disorders. Chang Gung. Med. J. 2009;32(4):370–379. [PubMed] [Google Scholar]
- 36.Chaturvedi RK, Beal MF. Mitochondrial approaches for neuro- protection. Ann. N. Y. Acad. Sci. 2008;1147:395–412. doi: 10.1196/annals.1427.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beal MF. Therapeutic approaches to mitochondrial dysfunction in Parkinson's disease. Parkinsonism. Relat. Disord. 2009;(Suppl 3 ):S189–194. doi: 10.1016/S1353-8020(09)70812-0. [DOI] [PubMed] [Google Scholar]
- 38.Hakonen AH, Heiskanen S, Juvonen V, Lappalainen I, Luoma PT, Rantamaki M, Goethem GV, Lofgren A, Hackman P, Paetau A, Kaakkola S, Majamaa K, Varilo T, Udd B, Kaariainen H, Bindoff LA, Suomalainen A. Mitochondrial DNA polymerase W748S mutation a common cause of autosomal recessive ataxia with ancient European origin. Am. J. Hum. Genet. 2005;77(3):430–441. doi: 10.1086/444548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kasahara T, Kubota M, Miyauchi T, Noda Y, Mouri A, Nabeshima T, Kato T. Mice with neuron-specific accumulation of mitochondrial DNA mutations show mood disorder-like phenotypes. Mol. Psychiatry. 2006;11(6):577–93. doi: 10.1038/sj.mp.4001824. [DOI] [PubMed] [Google Scholar]
- 40.Graziewicz MA, Bienstock RJ, Copeland WC. The DNA polymerase gamma Y955C disease variant associated with PEO and parkinsonism mediates the incorporation and translesion synthesis opposite 7 8-dihydro-8-oxo-2'-deoxyguanosine. Hum Mol. Genet. 2007;16(22):2729–2739. doi: 10.1093/hmg/ddm227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lauritzen KH, Moldestad O, Eide L, Carlsen H, Nesse G, Storm JF, Mansuy IM, Bergersen LH, Klungland A. Mitochondrial DNA toxicity in forebrain neurons causes apoptosis neurodegeneration and impaired behavior. Mol. Cell Biol. 2010;30(6):1357–1367. doi: 10.1128/MCB.01149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gimsa U, Kanitz E, Otten W, Ibrahim SM. Behavior and stress reactivity in mouse strains with mitochondrial DNA variations. Ann. N. Y. Acad. Sci. 2009;1153:131–138. doi: 10.1111/j.1749-6632.2008.03960.x. [DOI] [PubMed] [Google Scholar]
- 43.Su YA, Wu J, Zhang L, Zhang Q, SuDM He, P Wang, B.D LiH, Webster MJ. Traumatic Stress Brain Study Group Rennert O.. Ursano R.J. Dysregulated mitochondrial genes and networks with drug targets in postmortem brain of patients with posttraumatic stress disorder (Pt SD) revealed by human mitochondria-focused cDNA microarrays. Int. . J. Biol. Sci. 2008;4(4):223–235. doi: 10.7150/ijbs.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 2012;36(2):764–785. doi: 10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Brocardo PS, Boehm F, Patten A, Cox A, Gil-Mohapel J, Christie BR. Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: Protective effects of voluntary physical exercise. Neuropharmacology. 2012;62(4):1607–1618. doi: 10.1016/j.neuropharm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Baum AE, Hamshere M, Green E, Cichon S, Rietschel M, Noethen MM, Craddock N, McMahon FJ. Meta-analysis of two genome-wide association studies of bipolar disorder reveals important points ofagreement. Mol. Psychiatry. 2008;13(5):466–467. doi: 10.1038/mp.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schloesser RJ, Huang J, Klein PS, Manji HK. Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33(1):110–133. doi: 10.1038/sj.npp.1301575. [DOI] [PubMed] [Google Scholar]
- 48.Einat H, Manji HK. Cellular plasticity cascades: genes-to-behavior pathways in animal models of bipolar disorder. Biol. Psychiatry. 2006;59(12):1160–1171. doi: 10.1016/j.biopsych.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Machado-Vieira R, Andreazza AC, Viale CI, Zanatto V, Cereser V Jr, da Silva V R, Kapczinski F, Portela LV, Souza DO, Salvador M, Gentil V. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. Neurosci. Lett. 2007;421(1):33–36. doi: 10.1016/j.neulet.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 50.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur. J. Neurosci. 2004;19(7):1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- 51.Madathil KS, Karuppagounder SS, Haobam R, Varghese M, Rajamma U, Mohanakumar KP. Nitric oxide synthase inhibitors protect against rotenone-induced, oxidative stress mediated parkinsonism in rats. Neurochem. Int. 2013 doi: 10.1016/j.neuint.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Rev. Mol. Med. 2004;6(21):1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
- 53.McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation the MAP kinase signaling pathways. Antioxid. Redox Signal. 2006;8(9-10):1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 54.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J. Physiol. 2000;529 Pt 1:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carter CJ. Multiple genes and factors associated with bipolar disorder converge on growth factor and stress activated kinase pathways controlling translation initiation: implications for oligodendrocyte viability. Neurochem. Int. 2007;50(3):461–490. doi: 10.1016/j.neuint.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charne DS, Manji HK. Arandomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63(8):856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 57.da Silva FC, do Carmo de Oliveira, Cito M, da Silva MI, Moura BA, de Aquino Neto, M.R. Feitosa, M.L. de Castro, Chaves R, Macedo DS, de Vasconcelos SM, de França F MM, de Sousa FC. Behavioral alterations and pro-oxidant effect of a single ketamine administration to mice. Brain Res. Bull. 2010;83(1-2):9–15. doi: 10.1016/j.brainresbull.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 58.Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cell Signal. 2010;22(5):717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 59.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 60.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132(20):4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 61.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 62.Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech. Dev. 1999;83(1-2):27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 63.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–4680. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 64.Stranahan AM, Lee K, Becker KG, Zhang Y, Maudsley S, Martin B, Cutler RG, Mattson MP. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol. Aging. 2010;31:1937–49. doi: 10.1016/j.neurobiolaging.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang H, Zhang R, Wen S, McCafferty DM, Beck PL, MacNuaghton WK. Nitric oxide increases\Wnt-induced secreted protein-1 (WSIP-1/CCN4) expression and functionin colitis. J Mol Med. 2009;87:435–445. doi: 10.1007/s00109-009-0445-4. [DOI] [PubMed] [Google Scholar]
- 66.Jin T, George FI, Sun J. Wnt and beyond Wnt multiple mechanisms control the transcriptional property of beta-catenin. Cell Signal. 2008;20:1697–704. doi: 10.1016/j.cellsig.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 67.Grassi-Oliveira R, Brietzke E, Pezzi JC, Lopes RP, Teixeira AL, Bauer ME. Increased soluble tumor necrosis factor-alpha receptors in patients with major depressive disorder. Psychiatry Clin. Neurosci. 2009;63(2):202–208. doi: 10.1111/j.1440-1819.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- 68.Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, Hernandez F, Anderton B, Rosenblum K, Bliss T, Cooke SF, Avila J, Lucas JJ, Giese KP, Stephenson J, Lovestone S. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur. J. Neurosci. 2007;25:81–86. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 69.Takeichi M, Abe K. Synaptic contact dynamics controlled by cadherin and catenins.Takeda H Tsuji M Inazu M. Egashira T. Matsumiya T. Rosmarinic acid and caffeic acid produce antidepressive-like effect in the forced swimming test in mice. Eur. J. Pharmacol. 2002;449(3):261–7. doi: 10.1016/s0014-2999(02)02037-x. [DOI] [PubMed] [Google Scholar]
- 70.Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol. Psychiatry. 2004;55:781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 71.Saus E, Soria V, Escaramís G, Crespo JM, Valero J, Gutiérrez-Zotes A, Martorell L, Vilella E, Menchón JM, Estivill X, Gratacòs M, Urretavizcaya M. A haplotype of glycogen synthase kinase 3ß is associated with early onset of unipolar major depression. Genes Brain Behav. 2010;9(7):799–807. doi: 10.1111/j.1601-183X.2010.00617.x. [DOI] [PubMed] [Google Scholar]
- 72.Pittenger C, Duman RS. Stress depression and neuroplasticity a convergence of mechanisms. Neuropsychopharmacology. 2008;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 73.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 74.Zhang LL, Wang JJ, Liu Y, Lu XB, Kuang Y, Wan YH, Chen Y, Yan HM, Fei J, Wang ZG. GPR26-deficient mice display increased anxiety- and depression-like behaviors accompanied by reduced phosphorylated cyclic AMP responsive element-binding protein level in central amygdala. Neuroscience. 2011;196:203–214. doi: 10.1016/j.neuroscience.2011.08.069. [DOI] [PubMed] [Google Scholar]
- 75.Wang C, Yang XM, Zhuo YY, Zhou H, Lin HB, Cheng YF, Xu JP, Zhang HT. The phosphodiesterase-4 inhibitor rolipram reverses Aß-induced cognitive impairment and neuro- inflammatory and apoptotic responses in rats. Int. J. Neuro-psychopharmacol. 2012;15(6):7497–7466. doi: 10.1017/S1461145711000836. [DOI] [PubMed] [Google Scholar]
- 76.Anbrin M, Ahmed N S, Jamal M, James M. O’Donnell.Reversal of Oxidative Stress-Induced Anxiety by Inhibition of Phosphodiesterase-2 in Mice. J. Pharmacol. Exp. Ther. 2008;326(2):369–379. doi: 10.1124/jpet.108.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anbrin M, Ying H, Hassan H, Lan X, Hao L, Wei W, Adel H, Chang-Guo Z, James M. O'Donnell.Anxiolytic Effects of Phosphodiesterase-2 Inhibitors Associated with Increased cGMP Signaling. J Pharmacol. Exper. 2009;331(2):690–699. doi: 10.1124/jpet.109.156729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao C, Deng W, Gage F H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 79.Atmaca M, Tezca E, Kuloglu M, Ustundag B, Tunckol H. Antioxidant enzyme and malondialdehyde values in social phobia before and after citalopram treatment. Eur. Arch. Psychiatry Clin. Neurosci. 2004;254:231–235. doi: 10.1007/s00406-004-0484-3. [DOI] [PubMed] [Google Scholar]
- 80.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 81.Barry H. Reactive Species and Antioxidants.Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006;141(2):312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nikolai V, Malykhin R C, Peter S, Nicholas J. Coupland.Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. J. Psychiatry Neurosci. 2010;35(5):337–343. doi: 10.1503/jpn.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Christopher B, Stephen B, McHugh Rolf S, Peter H, Seeburg J, Nicholas P, Rawlins DMB. Hippocampal NMDA receptors and anxiety At the interface between cognition and emotion. Eur. J. Pharmacol. 2010;626(1):49–56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yi C, Jun-Feng WL, Shao L, Trevor Y. Oxidative damage to RNA but not DNA in the hippocampus of patients with major mental illness. J. Psychiatry Neurosci. 2010;35(5):296–302. doi: 10.1503/jpn.090083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peluffo H, Acarin L, Faiz M, Castellano B, Gonzalez B. Cu/Zn superoxide dismutase expression in the postnatal rat brain following an excitotoxic injury. J. Neuroinflamm. 2005;2:12. doi: 10.1186/1742-2094-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu S, Zhou Q, Chen C. Antioxidant enzyme activities and lipid peroxidation in earthworm Eisenia fetida exposed to 1 3 46 7,8-hexahydro-4,6,6,7,8,8-hexamethyl-cyclopenta-?-2-benzopyran. Environ. Toxicol. 2012;27:472–9. doi: 10.1002/tox.20661. [DOI] [PubMed] [Google Scholar]
- 87.Samina S, Mohammad A, Manish T, Iiris H, Gaurav C, Craig V, Anthony Vu. Potential Contribution of Oxidative Stress and Inflammation to Anxiety and Hypertension. Brain Res. 2011;1404:63–71. doi: 10.1016/j.brainres.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Owen M, Wolkowitz S H, Mellon E S, Epel J L, Firdaus S Dhabhar, Yali S, Victor I R, Rebecca R, Heather M, Burke E K, Mariana C. Leukocyte Telomere Length in Major Depression: Correlations with Chronicity, Inflammation and Oxidative Stress - Preliminary Findings. J. Craig Nelson Elizabeth H. Blackburn. PLoS One. 2011;6(3):e17837. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maes M, De Vos N, Pioli R, Demedts P, Wauters A, Neels H, Christophe A. Lower serum vitamin E concentrations in major depression.Another marker of lowered antioxidant defenses in that illness. J. Affect. Disord. 2000;58(3):241–246. doi: 10.1016/s0165-0327(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 90.Tsuboi H, Tatsumi A, Yamamoto K, Kobayashi F, Shimoi K, Kinae N. Possible connections among job stress, depressive symptoms lipid modulation and antioxidants. J. Affect. Disord. 2006;91(1):63–70. doi: 10.1016/j.jad.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 91.Sarandol A, Kirli S, Akkaya C, Ocak N, Eroz E, Sarandol E. Coronary artery disease risk factors in patients with schizophrenia: effects of short term antipsychotic treatment. J. Psychopharmacol. 2007;21(8):857–863. doi: 10.1177/0269881107077609. [DOI] [PubMed] [Google Scholar]
- 92.Bisgaard CF, Bak S, Christensen T, Jensen ON, Enghild JJ, Wiborg O. Vesicular signalling and immune modulation as hedonic fingerprints: proteomic profiling in the chronic mild stress depression model. J. Psychopharmacol. 2012;26(12):1569–1583. doi: 10.1177/0269881112460110. [DOI] [PubMed] [Google Scholar]
- 93.Zafir A, Ara A, Banu N. Invivo antioxidant status: a putative target of antidepressant action. Prog. Neuropsychopharmacol. Biol. Psychatry. 2009;33(2):220–228. doi: 10.1016/j.pnpbp.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 94.Kour K, Sharma N, Chandan BK, Koul S, Sangwan PL, Bani S. Protective effect of Labisia pumila on stress-induced behavioral, biochemical, and immunological alterations. Planta Med. 2010;76(14):1497–1505. doi: 10.1055/s-0029-1240953. [DOI] [PubMed] [Google Scholar]
- 95.Eren I, Naziroglu M, Demirdas A, Celik O, Uguz AC, Altunbasak A, Ozmen I, Uz E. Venlafaxine modulates depression-induced oxidative stress in brain and medulla of rat. Neurochem. Res. 2007;a32(3):497–505. doi: 10.1007/s11064-006-9258-9. [DOI] [PubMed] [Google Scholar]
- 96.Eren I, Naziroglu M, Demirdas A. Protective effects of lamotrigine, aripiprazole and escitalopram on depression-induced oxidative stress in rat brain. Neurochem. Res. 2007;32(7):1188–1195. doi: 10.1007/s11064-007-9289-x. [DOI] [PubMed] [Google Scholar]
- 97.Zhang D, Wen XS, Wang XY, Shi M, Zhao Y. Antidepressant effect of Shudihuang on mice exposed to unpredictable chronic mild stress. J. Ethnopharmacol. 2009;123(1):55–60. doi: 10.1016/j.jep.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 98.Seo JS, Park JY, Choi J, Kim TK, Shin JH, Lee JK, Han PL. NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J. Neurosci. 2012;32:9690–9. doi: 10.1523/JNEUROSCI.0794-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bouayed J, Bohn T. Exogenous antioxidants - Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev. 2010;3(4):228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav. Brain Res. 2010;208:545–552. doi: 10.1016/j.bbr.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 101.Lafleur DL, Pittenger C, Kelmendi B, Gardner T, Wasylink S, Malison RT, Sanacora G, Krystal JH, Coric V. N-acetylcysteine augmentation in serotonin reuptake inhibitor refractory obsessive-compulsive disorder. Psychopharmacology (Berl). 2006;184(2): 254–256. doi: 10.1007/s00213-005-0246-6. [DOI] [PubMed] [Google Scholar]
- 102.Cui J, Shao L, Young LT, Wang JF. Role of glutathione in neuroprotective effects of mood stabilizing drugs lithium and valproate. Neuroscience. 2007;144(4):1447–1453. doi: 10.1016/j.neuroscience.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 103.Berk M, Jeavons S, Dean OM, Dodd S, Moss K, Gama CS, Malhi GS. Nail-biting stuff?.The effect of N-acetyl cysteine on nail-biting. CNS Spectr. 2009;14(7):357–60. doi: 10.1017/s1092852900023002. [DOI] [PubMed] [Google Scholar]
- 104.Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003;8(6):365–370. doi: 10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- 105.Ozcan ME, Gulec M, Ozerol E, Polat R, Akyol O. Antioxidant enzyme activities and oxidative stress in affective disorders. Int. Clin. Psychopharmacol. 2004;19(2):89–95. doi: 10.1097/00004850-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 106.Galecki P, Szemraj J, Zboralski K, Florkowski A, Lewinski A. Relation between functional polymorphism of catalase gene (-262C>T) and recurrent depressive disorder. Neuro. Endocrinol. Lett. 2009;30(3):357–62. [PubMed] [Google Scholar]
- 107.Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum. Psychopharmacol. 2007;22(2):67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- 108.Schmidt AJ, Heiser P, Hemmeter UM, Krieg JC, Vedder H. Effects of antidepressants on mRNA levels of antioxidant enzymes in human monocytic U-937 cells. Prog. Neuropsychopharmacol Biol. Psychiatry. 2008;32(6):1567–73. doi: 10.1016/j.pnpbp.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 109.Andreazza AC, Frey BN, Erdtmann B, Salvador M, Rombaldi F, Santin A, Gonçalves CA, Kapczinski F. DNA damage in bipolar disorder. Psychiatry Res. 2007;153(1):27–32. doi: 10.1016/j.psychres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 110.Frey BN, Andreazza AC, Kunz M, Gomes FA, Quevedo J, Salvador M, Gonçalves CA, Kapczinski F. Increased oxidative stress and DNA damage in bipolar disorder: a twin-case report. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(1):283–285. doi: 10.1016/j.pnpbp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 111.Shao L, Young LT, Wang JF. Chronic treatment with mood stabilizers lithium and valproate prevents excitotoxicity by inhibiting oxidative stress in rat cerebral cortical cells. Biol. Psychiatry. 2005;58(11):879–884. doi: 10.1016/j.biopsych.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 112.Berk BC. Novel approaches to treat oxidative stress and cardiovascular diseases. Trans. Am. Clin. Climatol. Assoc. 2007;118:209–214. [PMC free article] [PubMed] [Google Scholar]
- 113.Fagervall I, Ross SB. Inhibition of monoamine oxidase in monoaminergic neurones in the rat brain by irreversible inhibitors. Biochem Pharmacol. 1986;35(8):1381–1387. doi: 10.1016/0006-2952(86)90285-6. [DOI] [PubMed] [Google Scholar]
- 114.Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855–870. doi: 10.1017/s1092852900016965. [DOI] [PubMed] [Google Scholar]
- 115.Desrumaux C, Risold PY, Schroeder H, Deckert V, Masson D, Athias A, Laplanche H, Le Guern N, Blache D, Jiang XC, Tall AR, Desor D, Lagrost L. Phospholipid transfer protein (PLTP) deficiency reduces brain vitamin E content and increases anxiety in mice. FASEB J. 2005;19 (2):296–297. doi: 10.1096/fj.04-2400fje. [DOI] [PubMed] [Google Scholar]
- 116.Berry A, Capone F, Giorgio M, Pelicci PG, de Kloet ER, Alleva E, Minghetti L, Cirulli F. Deletion of the life span determinant p66Shc prevents age-dependent increases in emotionality and pain sensitivity in mice. Exp. Gerontol. 2007;42:37–45. doi: 10.1016/j.exger.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 117.Souza CG, Moreira JD, Siqueira IR, Pereira AG, Rieger DK, Souza DO, Souza TM, Portela LV, Perry ML. Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life Sci. 2007;81:198–203. doi: 10.1016/j.lfs.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 118.Masood A, Nadeem A, Mustafa SJ, O'Donnell JM. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther. 2008;326(2):369–79. doi: 10.1124/jpet.108.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bouayed J, Rammal H, Younos C, Soulimani R. Positive correlation between peripheral blood granulocyte oxidative status and level of anxiety in mice. Eur. J. Pharmacol. 2007;564(1-3):146–149. doi: 10.1016/j.ejphar.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 120.Marcolin Mde L, Benitz AN, Arcego DM, Noschang C, Krolow R, Dalmaz C. Effects of early life interventions and palatable diet on anxiety and on oxidative stress in young rats. Physiol. Behav. 2012;106(4):491–498. doi: 10.1016/j.physbeh.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 121.Bouayed J. Polyphenols: a potential new strategy for the prevention and treatment of anxiety and depression. Curr Nutr Food Sci. 2010;6:13–18. [Google Scholar]
- 122.Viggiano A, Viggiano A, Monda M, Turco I, Incarnato L, Vinno V , Viggiano E, Baccari ME, De Luca B. Annurca, apple-rich diet restores long-term potentiation and induces behavioral modifications in aged rats. Exp. Neurol. 2006;199(2):354–361. doi: 10.1016/j.expneurol.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 123.Yasunari K, Matsui T, Maeda K, Nakamura M, Watanabe T, Kiriike N. Anxiety-induced plasma norepinephrine augmentation increases reactive oxygen species formation by monocytes in essential hypertension. Am. J. Hypertens. 2006;19(6):573–578. doi: 10.1016/j.amjhyper.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 124.Kinrys G, Coleman E, Rothstein E. Natural remedies for anxiety disorders: potential use and clinical applications. Depress Anxiety. 2009;26(3):259–265 . doi: 10.1002/da.20460. [DOI] [PubMed] [Google Scholar]
- 125.Gonçalves de Medeiros MT, de Oliveira RB, dos Santos AA, de Leopoldino DM, Lima MC, Nobre RA, Nobree SMÂ. The effects of sildenafil on rectal sensitivity and tone in patients with the irritable bowel syndrome. Aliment Pharmacol. Ther. 2012;35:577–86. doi: 10.1111/j.1365-2036.2011.04977.x. [DOI] [PubMed] [Google Scholar]
- 126.Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M, Kap O, Yumru M, Savas HA, Akyol O. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch. Med. Res. 2007;38(2):247–252. doi: 10.1016/j.arcmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 127.Ersoy MA, Selek S, Celik H, Erel O, Kaya MC, Savas HA, Herken H. Role of oxidative and antioxidative parameters in etiopathogenesis and prognosis of panic disorder. Int. J. Neurosci. 2008;118(7):1025–1037. doi: 10.1080/00207450701769026. [DOI] [PubMed] [Google Scholar]
- 128.Tanis KQ, Duman RS. Intracellular signaling pathways pave roads to recovery for mood disorders. Ann. Med. 2007;39(7):531–544. doi: 10.1080/07853890701483270. [DOI] [PubMed] [Google Scholar]