Abstract
Chronic administration of L-methionine leads to memory impairment, which is attributed to increase in the level of oxidative stress in the brain. On the other hand, metformin is a commonly used antidiabetic drug with strong antioxidant properties. In the current study, we tested if chronic metformin administration prevents memory impairment induced by administration of L-methionine. In addition, a number of molecules related to the action of metformin on cognitive functions were examined. Both metformin and L-methionine were administered to animals by oral gavage. Testing of spatial learning and memory was carried out using radial arm water maze (RAWM). Additionally, hippocampal levels or activities of catalase, thiobarbituric acid reactive substances (TBARs), glutathione peroxidase (GPx), glutathione (GSH), oxidized glutathione (GSSG) and GSH/GSSG ratio were determined. Results showed that chronic L-methionine administration resulted in both short- and long- term memory impairment, whereas metformin treatment prevented such effect. Additionally, L-methionine treatment induced significant elevation in GSSG and TBARs, along with reduction in GSH/GSSG ratio and activities of catalase, and GPx. These effects were shown to be restored by metformin treatment. In conclusion, L-methionine induced memory impairment, and treatment with metformin prevented this impairment probably by normalizing oxidative stress in the hippocampus.
Keywords: Memory, learning, hippocampus, maze, oxidative stress, L-methionine, metformin.
INTRODUCTION
L-methionine is an essential, non-polar amino acid that is usually obtained from outside sources such as sesame oil, meat, and fish [1]. L-methionine is considered as intermediate substrate for the synthesis of other amino acids such as homocysteine, which is formed by demethylation of L-methionine [2, 3].
Homocysteine is a non-essential amino acid that is already present in the blood. It has been implicated in oxidative stress and cognitive dysfunction [4]. Recent studies on animals have shown that chronic administration of L-methionine results in hyperhomocysteinemia condition [4, 5] and the subsequent development of cerebrovascular diseases including stroke [6]; atherosclerosis [7, 8], and vascular dementia [9]. In addition, hyperhomocysteinemia is associated with increased levels of oxidative stress and lipid peroxidation that interfere with memory formation [5, 10-13]. Hyperhomocysteinemia also contributes to the increased risk of dysfunction in endothelial cells that may interfere with blood supply to brain and the subsequent cognitive decline [13].
Metformin is an oral anti-diabetic agent from the biguanid group. Metformin is considered a first line therapy for patients with type 2 diabetes. It works by increasing insulin receptors at peripheral tissues and inhibiting glucose synthesis from the liver [14]. Studies showed that metformin possesses an antioxidant activity that is beneficial for restoring cognitive function in conditions other than hyperhomocystienemia such as high-fat diet ingestion [15] and obesity [16]. In the current study, we determined if metformin prevents memory impairment induced by chronic administration of L-methionine probably through neutralizing the oxidative stress in the brain.
MATERIAL AND METHODS
In all experiments, adult male Wistar rat that weight 200 to 250g were assessed. The animals were housed in plastic cages (maximum four rats per cage) under hygienic conditions and maintained at normal room temperature with free access to food and water. Animals were housed on a 12 h light/dark cycle (light on 7 am) at 25°C. All experimental procedures were performed during the light cycle and were approved by animal care and use committee (ACUC) of the Jordan University of Science and Technology.
Animals were randomly assigned to four groups: control (Control), L-methionine (L-Meth, 1.7g/kg/day, suspended in 0.5% w/v Carboxyl Methyl Cellulose (CMC)), metformin (Met, 30mg/kg/day), L-methionine with metformin (L-Meth+Met). L-methionine was purchased from Sigma Chemical Co. (Saint Loius, MO, USA) while metformin was a generous gift from United Drug Manufacturing Co, Amman, Jordan. Both L-methionine and metformin were administered by oral gavage for 4 weeks. Animals in the control group were administered the vehicle via oral gavage (0.5% w/v CMC). All manipulations including administration of L-methionine, metformin and vehicle were started on the same day, and continued for 4 weeks. The RAWM training was carried out immediately after treatment. Drugs administration was continued throughout the radial arm water maze (RAWM) testing days.
BEHAVIORAL TEST: RADIAL ARM WATER MAZE (RAWM)
RAWM was used to test spatial learning and memory among all four groups of animals [17-20]. The RAWM contains six swim paths (stainless steel arms) extending out of black central area tube with a hidden platform located at the end of one arm (the goal arm). The water was maintained at 24±1°C. The experiments were carried out in a room that was dimly lit with two different pictures on the walls which served as cues for the rats. The animals must find a platform that was hidden on the goal arm, which was not changed for a particular rat. Rats were given six successive trials separated by 5 min rest, then another six consequences trials (acquisition phase), followed by 30 min short-term memory and 5 h and 24 h long-term memory tests. The RAWM procedure was carried out as described in [21, 22].
ANIMALS SUBTOTAL EXSANGUINATIONS AND BRAIN DESICCATION
The animals were anesthetized using 40mg/Kg of thiopental administered Intraperitonially (IP), thereafter; animals were killed using subtotal exsanguinations. Then, the brain was immediately dissected out; the hippocampus was removed and immediately frozen using liquid nitrogen.
CALORIMETRIC IMMUNOASSAYS
Hippocampus was homogenized manually using small pestle in lysis buffer (137 mM NaCl, 20 mM Tris–HCl pH 8.0, 1% NP-40, 10% glycerol, 0.5 mM sodium vanadate, 1 mM polymethane sulfonyl floride (PMSF), and proteases inhibitor cocktail (Sigma-Aldrich Corp, MI, USA). Homogenates were centrifuged to remove insoluble material (14,000xg for 5 min, 4 ◦C). Total protein concentrations were estimated using commercially available kit (BioRAD, Hercules, CA, USA). To quantify GSH, tissues were homogenized manually using small pestle in 5% 5-Sulfosalicylic Acid (SSA). Homogenates were centrifuged to remove insoluble material (10,000 xg for 10min, 4°C), then the samples were assayed for total GSH/GSSG according to manufacture instructions (Glutathione Assay Kit, Sigma-Aldrich Corp, Mi, USA). In brief, 10μl of 1M 2- vinylpyridine (Glutathione Assay Kit, Sigma-Aldrich Corp, Mi, USA) was added per 1ml of supernatant to quantify GSSG. GSH was calculated by subtracting total glutathione species value from GSSG value.
Assay of glutathione peroxidase (GPx) was performed using commercially available kit (Glutathione Peroxidase Cellular Activity Assay Kit, Sigma-Aldrich, MI, USA). Samples were kinetically quantified at 340nm using spectrophotometry (UV-VIS spectrophotometer, UV-1800, Shimadzu, Japan). Catalase activity was measured calorimetrically using commercially available kit (Cayman Chemical, Ann Arbor, MI, USA) and ELISA reader. Similarly, thiobarbituric acid reactive substance (TBARS) levels were measured calorimetrically using commercially available kits according to manufacturer’s instructions (Cayman Chem, Ann Arbor, MI, USA). ELISA plates were read at the wave lengths specified in the kit using an automated plate reader (ELx800, Bio-tek instruments, plate reader, Highland Park, Winooski, USA).
Homocysteine level in serum was measured by using rat homocysteine Kit from Cusabio Biotech Co (CSB-E13376r, USA). Briefly, 100μl Biotin-antibody working solution was added to 100µl tissue homogenates. The mixture was incubated for 60 min at 37 ◦C, and then it was washed. Thereafter, 100μl of HRP-avidin working solution was added and incubated for 60 min at 37 ◦C. After that, 90μl of TMB substrate was added and it was incubated for 30 min at 37 ◦C. Finally, 50μl of stop solution was added and plates were read at 450 nm using the automated reader.
STATISTICAL ANALYSIS
The statistical analysis was done using GraphPad Prism (4.0) for Windows. The numbers of errors made by animals were compared using two-way AVOVA; succeeded by Bonferroni posttest. Repeated measures factor was time and between-subjects factor was the groups, and they were treated as independent variables. Oxidative stress biomarkers were compared using one-way AVOVA; succeeded by Bonferroni posttest. Significance level was set at P <0.05. The mean ± SEM was used to represent all values.
RESULTS
The Effect of L-methionine, and Metformin on the Homocysteine Serum Levels
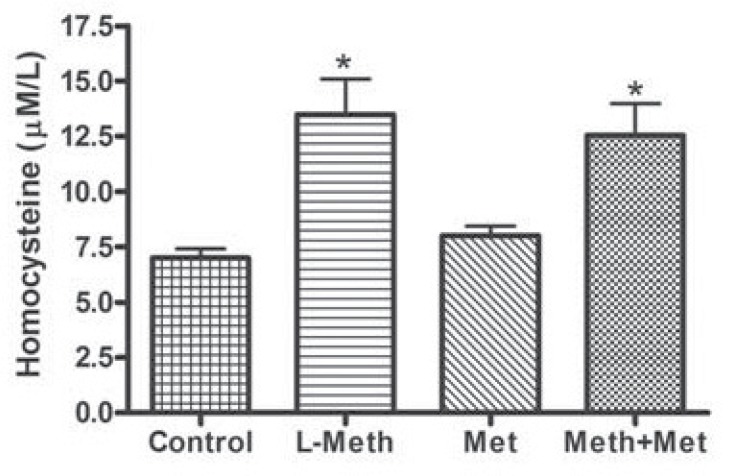
Serum levels of homosysteine were significantly higher in both the L-Meth, and the L-Meth+Met groups compared to that of control and Met groups. Thus, as expected L-methionine treatment was associated with significant increase in the level of homocysteine in Animals’ serum, whereas metformin did not affect serum homocysteine levels (Fig. 1).
Fig. (1).
Plasma levels of homocysteine. Levels of homocystiene were significantly increased in the L-metioninine (L-Meth) and Lmethionine and metformin treated (L-Meth+Met) groups compared to the control and metformin treated (Met) groups. Data are expressed as mean ± SEM from 10-12 animals. * indicates significant difference (P < 0.05) from other groups.
THE INTERACTIVE EFFECT OF METFORMIN AND L-METHIONINE ON LEARNING AND MEMORY
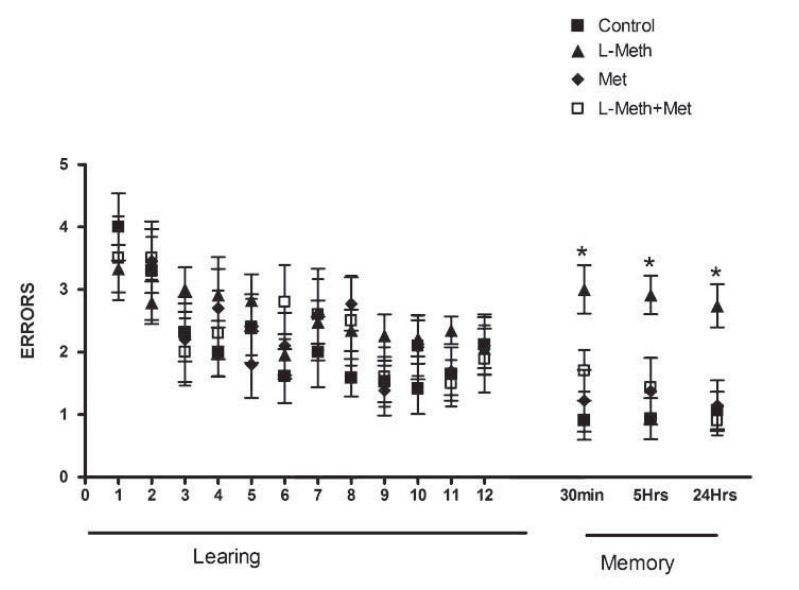
During the RAWM training, all animals started with higher number of errors at the beginning of the training day. After that, number of errors tended to decline as animals had more training trials (the acquisition phase), with no significant difference among experimental groups in the number of errors committed at each trial in all acquisition trials (Fig. 2).
Fig. (2).
Performance of animal groups during the RAWM. In all groups, the number of errors declined as the learning proceeded learning (trials 1-12), with no significant change among groups. In the memory tests performed after 30min, 5hr, and 24hr after the end of the acquisition, the number of errors committed by L-Meth group was significantly higher than that of L-Meth+Met, Met, and control groups. Data are expressed as mean ± SEM from 10-12 animals. * indicates significant difference (P < 0.05) from other groups.
In the short-term memory test, rats in the control, Met and L-Meth+Met groups made similar number of errors to find the hidden platform. In contrast, animals in the Meth group made significantly more errors to find the hidden platform than other experimental groups (Fig. 2). In the long-term memory tests, which were carried out 5-hr and 24 hr after the end of 12th trial, animals in the Meth group made significantly more errors in finding the hidden platform than all other experimental groups. On the other hand, animals in the control, Met and L-Meth+Met groups made similar numbers of errors (Figs. 2). These findings indicate that metformin treatment prevented short- and long- term memory impairments induced by chronic L-methionine administration in rats.
THE EFFECT OF L-METHIONINE AND METFORMIN ON HIPPOCAMPUS OXIDATIVE STRESS MARKERS
Changes in Catalase Activity
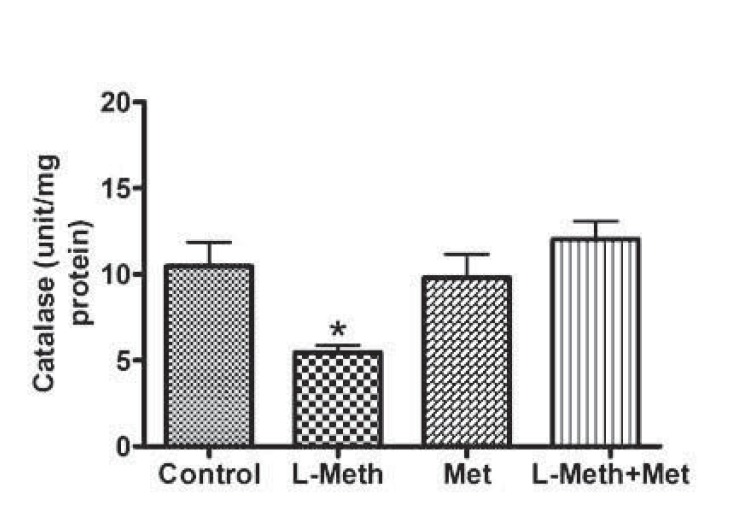
L-methionine treatment was associated with significant decrease in hippocampus catalase activity (Fig. 3). On the other hand, treatment with metformin normalized L-methionine induced reduction in catalase activity.
Fig. (3).
Hippocampal Catalase Activity. Catalase activity was significantly reduced in L-Meth group compared to L-Meth+Met, Met, and control groups. Data are expressed as mean ± SEM from 10-12 animals. * indicates significant difference (P < 0.05) from other groups.
Level of the GSH, GSSG and GSH/GSSG Ratio
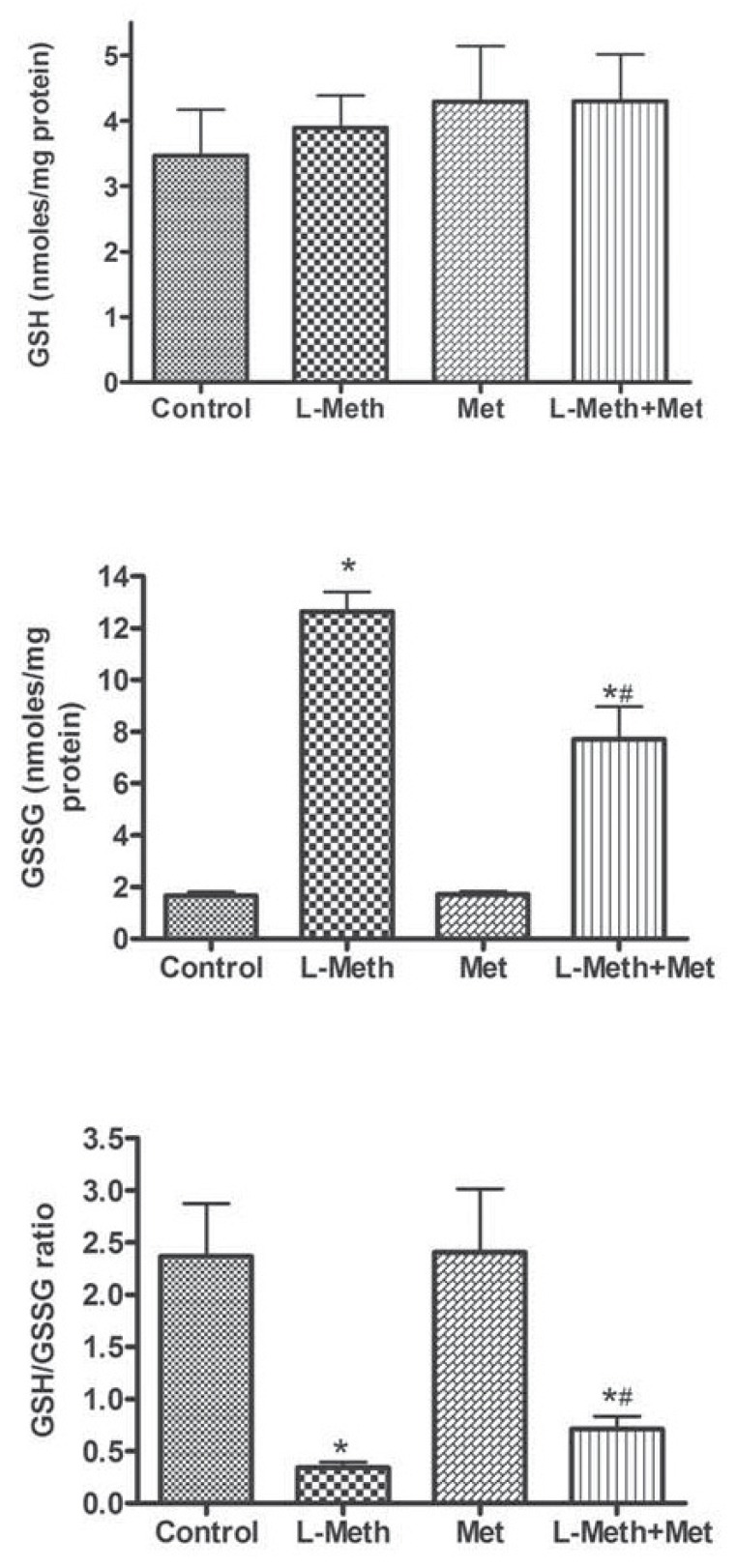
Whereas no change was observed at the level of GSH (Fig. 4A), the level of GSSG was significantly increased in association with L-methionine. This increase was partially prevented by the treatment with metformin (Fig. 4B). Concerning the ratio GSH/GSSG, which represent an important indicator of oxidative stress level [23], it was markedly reduced by L-methionine treatment (Fig. 4C), and it was partially normalized by metformin treatment (Fig. 4).
Fig. (4).
Hippocampal levels of different forms of glutathione. No change was observed in the GSH levels among different groups (A). In the L-Meth group, the levels of GSSG (B), and GSH/GSSG ratio (B) were significantly elevated compared to other groups. However, levels of GSSG and GSH/GSGG ratio were significantly higher in L-Meth+Met group compared to Met and control groups, indicating that metformin treatment only partially restored GSSG and GSH/GSSG ratio. Data are expressed as mean ± SEM from 10- 12 animals. * indicates significant difference (P < 0.05) from other groups.
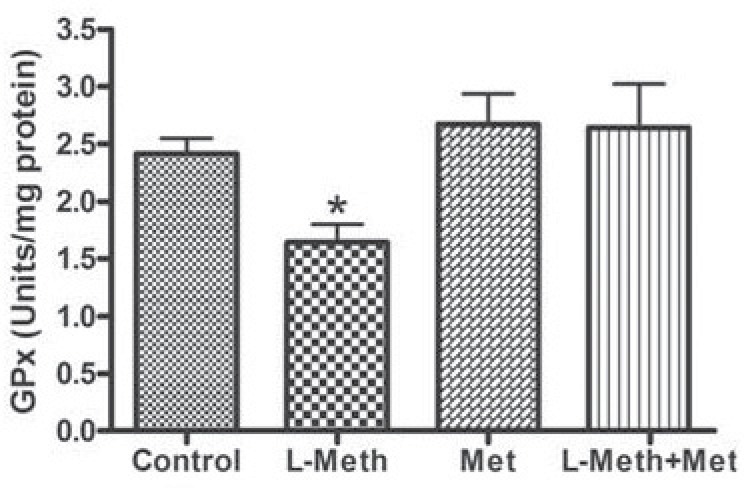
Changes in the Activity of GPx
In the hippocampus, the activity of GPx was reduced by chronic treatment with L-methionine (L-Meth group), this reduction was normalized by metformin treatment (Fig. 5).
Fig. (5).
Hippocampal GPx Activity. L-Meth group showed significant reduction in GPx activity compared to L-Meth+Met, Met, and control groups. Each point is the mean ± SEM of 10-12 rats. * indicates significant difference compared to all other groups, (P < 0.05).
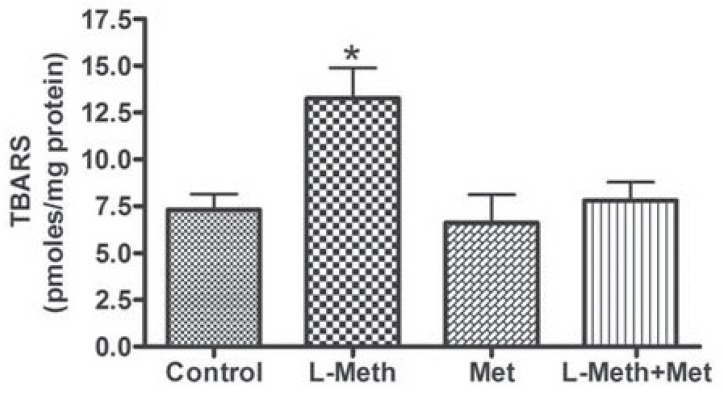
Level of TBARs
L-methionine treatment induced significant increase in TBARs, which was prevented by the treatment with metformin (Fig. 6).
Fig. (6).
Levels of TBARS in the hippocampus. The levels of TBARS were elevated in the L-Meth group compared Met, and control groups. Metformin treatment prevented this elevation in TBARS levels (L-Meth+Met group). Data are expressed as mean ± SEM from 10-12 animals. * indicates significant difference (P < 0.05) from other groups.
DISCUSSION
In this study, we showed that metformin treatment prevents impairment of both short- and long- term memory associated with chronic administration of L-methionine. This could be achieved through metformin relieving effect on L-methionine-induced oxidative stress.
Results of the present study showed that chronic L-methionine administration induced hyperhomocysteinemia and impaired short- and long- term memory. These findings are in confirmation of previous studies showing that excessive L-methionine administration results in memory impairment in animals [13, 24-26], and deteriorates cognitive functions in humans, through the induction of hyperhomocysteinemia [4, 27].
The major mechanism by which L-methionine induced memory impairment may be related to increased oxidative stress levels in the brain due to development of hyper-homocystenemia [10-13]. It is believed that hyper-homocystenemia in cells leads to auto-oxidation of thiol groups that generates hydrogen peroxide and the reactive radical oxygen species, superoxide and hydroxyl radical, and, thus, leads to oxidative stress [28-31]. For instance, it was shown that chronic L-methionine treatment impairs memory along with elevating oxidative stress in the brain as indicated by increase in TBARS, and reduction in GSH [13]. Additionally, memory impairment induced by L-methionine treatment was prevented by treatment with major antioxidants including vitamins E and C [12], melatonin [11], and resveratrol [10]. In confirmation, results of the current study showed that chronic L-methionine treatment induced both memory impairment and oxidative stress in the hippocampus as evidenced by increased TBARS, reduced GSH/GSSG ration, and reduced antioxidant enzymes activities such as catalase and GPx, which further substantiate evidence indicating the involvement of oxidative stress in L-methionine ingestion-induced memory impairment.
On the other hand, current results showed that chronic metformin treatment prevented short- and long- term memory impairment induced by chronic L-methionine administration. In accordance, previous report showed that administration of metformin for 3 weeks to insulin resistant high-fat diet fed animals completely reversed cognitive impairment [15]. In addition, metformin was shown to attenuate Alzheimer's disease-like changes including impaired cognitive functions in obese, leptin-resistant mice [16].
The observed beneficial effects of metformin in protecting memory impairment has been related to its antioxidant effect [15, 32, 33]. Current results show that chronic metformin treatment normalized changes in hippocampal levels of catalase, GPx, GSH/GSSG ratio and TBARS, induced by L-methionine treatment. In accordance, a previous study showed that metformin protected type 2 diabetic rats from oxidative stress through preventing elevation in GPx activity, reducing the levels of TBARs, and increasing GSH in the whole brain [33]. Another study showed that meformin treatment restored elevation in brain TBARS induced by chronic ingestion of high-fat diet that led to insulin resistance [15]. Therefore, it is likely that metformin restores L-methionine induced memory impairment through normalizing oxidative stress level in the hippocampus.
Other mechanisms for the general cognition protective effect of metformin were suggested. For example, metformin has been recently shown to activate an atypical protein kinase C-CBP pathway leading to the induction of neurogenesis in adult mouse brain [34]. However, this effect was observed in the cortex at metformin dose that is several folds higher than the dose used in the current study. Thus, it is unlikely that neurogensis induced by metformin to be a contributing factor in the effects observed in the current study.
The hippocampus, a bilateral limbic structure that lies beneath the cerebral cortex, is known to be involved in learning and memory [35-37]. New information is temporarily stored in the hippocampus before being transferred to the cerebral cortex for long-term memory storage [38]. Hyperhomocystenemia has been shown to directly target the hippocampus [11, 39-41], which is especially sensitive for oxidative stress [11, 42-44]. Metformin, on the other hand, was previously shown to act on memory impairments associated with hippocampal structure [16, 45]. Thus, in here, the hippocampus was majorly studied. Future work could target other areas in the brain.
Collectively, metformin was shown to protect against short- and long- term memory impairment induced by chronic L-methionine administration, probably, through metformin’s ability to restore normal oxidative level in the hippocampus.
ACKNOWLEDGEMENTS
Support for this project was via a grant (No 57/2010) from the Jordan University of Science and Technology.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Kalhan SC, Marczewski SE. Methionine, homocysteine one carbon metabolism and fetal growth. Rev. Endocr. Metab. Disord. 2012;13(2):109–119. doi: 10.1007/s11154-012-9215-7. [DOI] [PubMed] [Google Scholar]
- 2.Lee BC, Gladyshev VN. The biological significance of methionine sulfoxide stereochemistry. Free Radic. Biol. Med . 2011;50(2):221–227. doi: 10.1016/j.freeradbiomed.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui ZJ, Han ZQ, Li ZY. Modulating protein activity and cellular function by methionine residue oxidation. Amino Acids. 2012;43(2):505–517. doi: 10.1007/s00726-011-1175-9. [DOI] [PubMed] [Google Scholar]
- 4.Miller AL. The methionine-homocysteine cycle and its effects on cognitive diseases. Altern. Med. Rev . 2003;8(1):7–19. [PubMed] [Google Scholar]
- 5.Obeid R, Herrmann W. Mechanisms of homocysteine neuro- toxicity in neurodegenerative diseases with special reference to dementia. FEBS lett. 2006;580(13):2994–3005. doi: 10.1016/j.febslet.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 6.Wu XQ, Ding J, Ge AY, Liu FF, Wang X, Fan W. Acute phase homocysteine related to severity and outcome of atherothrombotic stroke. Eur. J. Intern. Med. 2013;24(4):362–367. doi: 10.1016/j.ejim.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Cacciapuoti F. Lowering homocysteine levels with folic acid and B-vitamins do not reduce early atherosclerosis, but could interfere with cognitive decline and Alzheimer's disease. J. Thromb. Thrombolysis . 2013;36(3):258–262. doi: 10.1007/s11239-012-0856-x. [DOI] [PubMed] [Google Scholar]
- 8.Aronow WS. Homocysteine.The association with atherosclerotic vascular disease in older persons. Geriatrics. 2003;58(9):22–24, 27-28. [PubMed] [Google Scholar]
- 9.Sudduth TL, Powell DK, Smith CD, Greenstein A, Wilcock DM. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J. Cereb. Blood Flow Metab. 2013;33(5):708–715. doi: 10.1038/jcbfm.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koz ST, Etem EO, Baydas G, Yuce H, Ozercan HI, Kuloglu T, Koz S, Etem A, Demir N. Effects of resveratrol on blood homocysteine level, on homocysteine induced oxidative stress, apoptosis and cognitive dysfunctions in rats. Brain Res. 2012;1484:29–38. doi: 10.1016/j.brainres.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Baydas G, Ozer M, Yasar A, Tuzcu M, Koz ST. Melatonin improves learning and memory performances impaired by hyperhomocysteinemia in rats. Brain Res . 2005;1046(1-2):187–194. doi: 10.1016/j.brainres.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Reis EA, Zugno AI, Franzon R, Tagliari B, Matte C, Lammers ML, Netto CA, Wyse AT. Pretreatment with vitamins E and C prevent the impairment of memory caused by homocysteine administration in rats. Metab. Brain Dis. 2002;17(3):211–217. doi: 10.1023/a:1019982223034. [DOI] [PubMed] [Google Scholar]
- 13.Koladiya RU, Jaggi AS, Singh N, Sharma BK. Ameliorative role of Atorvastatin and Pitavastatin in L-Methionine induced vascular dementia in rats. BMC Pharmacol. 2008;8:14. doi: 10.1186/1471-2210-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin. Sci. (Lond) . 2012;122(6):253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pintana H, Apaijai N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci. 2012;91(11-12):409–414. doi: 10.1016/j.lfs.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Deng J, Sheng W, Zuo Z. Metformin attenuates Alzheimer's disease-like neuropathology in obese leptin-resistant mice. Pharmacol. Biochem. Behav. 2012;101(4):564–574. doi: 10.1016/j.pbb.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alzoubi KH, Srivareerat M, Tran TT, Alkadhi KA. Role of alpha7- and alpha4beta2-nAChRs in the neuroprotective effect of nicotine in stress-induced impairment of hippocampus-dependent memory. Int. J. Neuropsychopharmacol. 2013;16(5):1105–1113. doi: 10.1017/S1461145712001046. [DOI] [PubMed] [Google Scholar]
- 18.Alzoubi KH, Aleisa AM, Gerges NZ, Alkadhi KA. Nicotine reverses adult-onset hypothyroidism-induced impairment of learning and memory: Behavioral and electrophysiological studies. J. Neurosci. Res . 2006;84(5):944–953. doi: 10.1002/jnr.21014. [DOI] [PubMed] [Google Scholar]
- 19.Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9(5):542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Alzoubi KH, Abdul-Razzak KK, Khabour OF, Al-Tuweiq GM, Alzubi MA, Alkadhi KA. Adverse effect of combination of chronic psychosocial stress and high fat diet on hippocampus-dependent memory in rats. Behav. Brain Res . 2009; 204(1):117–123. doi: 10.1016/j.bbr.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Alzoubi KH, Khabour OF, Salah HA, Hasan Z. Vitamin E prevents high-fat high-carbohydrates diet-induced memory impairment the role of oxidative stress. Physiol Behav. . 2013;119:72–78. doi: 10.1016/j.physbeh.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Alzoubi KH, Abdul-Razzak KK, Khabour OF, Al-Tuweiq GM, Alzubi MA, Alkadhi KA. Caffeine prevents cognitive impairment induced by chronic psychosocial stress and/or high fat-high carbohydrate diet. Behav. Brain Res . 2013;237:7–14. doi: 10.1016/j.bbr.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J, Trnkova L, Kruseova J, Eckschlager T, Kizek R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett . 2012;4(6):1247–1253. doi: 10.3892/ol.2012.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudduth TL, Powell DK, Smith CD, Greenstein A, Wilcock DM. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J. Cereb Blood Flow Metab . 2013;33:708–15. doi: 10.1038/jcbfm.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baydas G, Koz ST, Tuzcu M, Nedzvetsky VS, Etem E. Effects of maternal hyperhomocysteinemia induced by high methionine diet on the learning and memory performance in offspring. Int. J. Dev. Neurosci . 2007;25(3):133–139. doi: 10.1016/j.ijdevneu.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Vuaden FC, Savio LE, Piato AL, Pereira TC, Vianna MR, Bogo MR, Bonan CD, Wyse AT. Long-term methionine exposure induces memory impairment on inhibitory avoidance task and alters acetylcholinesterase activity and expression in zebrafish (Danio rerio). Neurochem. Res . 2012;37(7):1545–1553. doi: 10.1007/s11064-012-0749-6. [DOI] [PubMed] [Google Scholar]
- 27.Obeid R, Herrmann W. Mechanisms of homocysteine neuro- toxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett . 2006;580(13):2994–3005. doi: 10.1016/j.febslet.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 28.McCully KS. Chemical pathology of homocysteine.IV. Excitotoxicity oxidative stress endothelial dysfunction and inflammation. Ann. Clin. Lab. Sci . 2009;39(3):219–232. [PubMed] [Google Scholar]
- 29.Matthias D, Becker CH, Riezler R, Kindling PH. Homocysteine induced arteriosclerosis-like alterations of the aorta in normotensive and hypertensive rats following application of high doses of methionine. Atherosclerosis. 1996;122(2): 201–216. doi: 10.1016/0021-9150(95)05740-4. [DOI] [PubMed] [Google Scholar]
- 30.Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J. Clin. Invest. 1996;98(1):5–7. doi: 10.1172/JCI118776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinecke JW, Rosen H, Suzuki LA, Chait A. The role of sulfur-containing amino acids in superoxide production and modification of low density lipoprotein by arterial smooth muscle cells. J. Biol. Chem. 1987;262(21):10098–10103. [PubMed] [Google Scholar]
- 32.Majithiya JB, Balaraman R. Metformin reduces blood pressure and restores endothelial function in aorta of streptozotocin-induced diabetic rats. Life Sci . 2006;78(22):2615–2624. doi: 10.1016/j.lfs.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Correia S, Carvalho C, Santos MS, Proenca T, Nunes E, Duarte AI, Monteiro P, Seica R, Oliveira CR, Moreira PI. Metformin protects the brain against the oxidative imbalance promoted by type 2 diabetes. Med. Chem. 2008;4(4):358–364. doi: 10.2174/157340608784872299. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Gallagher D, DeVito LM, Cancino GI, Tsui D, He L, Keller GM, Frankland PW, Kaplan DR, Miller FD. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell . 2012;11(1):23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 36.Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17(6):669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- 37.Olton DS. Spatial memory. Sci. Am. 1977;236(6):82–84 89-94 96 98. doi: 10.1038/scientificamerican0677-82. [DOI] [PubMed] [Google Scholar]
- 38.Ivanco TL, Racine RJ. Long-term potentiation in the reciprocal corticohippocampal and corticocortical pathways in the chronically implanted freely moving rat. Hippocampus. 2000;10(2):143–152. doi: 10.1002/(SICI)1098-1063(2000)10:2<143::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 39.Matte C, Pereira LO, Dos Santos TM, Mackedanz V, Cunha AA, Netto CA, Wyse AT. Acute homocysteine administration impairs memory consolidation on inhibitory avoidance task and decreases hippocampal brain-derived neurotrophic factor immuno- content: prevention by folic acid treatment. Neuroscience . 2009;163(4):1039–1045. doi: 10.1016/j.neuroscience.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 40.Wei W, Liu YH, Zhang CE, Wang Q, Wei Z, Mousseau DD, Wang JZ, Tian Q, Liu GP. Folate/vitamin-B12 prevents chronic hyperhomocysteinemia-induced tau hyperphosphorylation and memory deficits in aged rats. J. Alzheimers Dis. 2011;27(3):639–650. doi: 10.3233/JAD-2011-110770. [DOI] [PubMed] [Google Scholar]
- 41.Troen AM, Shea-Budgell M, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. B-vitamin deficiency causes hyper- homocysteinemia and vascular cognitive impairment in mice Proc. Natl. Acad. Sci. U. S. A . 2008;105(34):12474–12479. doi: 10.1073/pnas.0805350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alzoubi KH, Khabour OF, Tashtoush NH, Al-Azzam SI, Mhaidat NM. Evaluation of the effect of pentoxifylline on sleep-deprivation induced memory impairment. Hippocampus. 2013;23(9):812–819. doi: 10.1002/hipo.22135. [DOI] [PubMed] [Google Scholar]
- 43.Avila-Costa MR, Colin-Barenque L, Fortoul TI, Machado-Salas P, Espinosa-Villanueva J, Rugerio-Vargas C, Rivas-Arancibia S. Memory deterioration in an oxidative stress model and its correlation with cytological changes on rat hippocampus CA1. Neurosci. Lett. 1999;270(2):107–109. doi: 10.1016/s0304-3940(99)00458-9. [DOI] [PubMed] [Google Scholar]
- 44.Alzoubi KH, Khabour OF, Rashid BA, Damaj IM, Salah HA. The neuroprotective effect of vitamin E on chronic sleep deprivation-induced memory impairment: the role of oxidative stress. Behav. Brain Res . 2012;226(1): 205–210. doi: 10.1016/j.bbr.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Bhutada P, Mundhada Y, Bansod K, Tawari S, Patil S, Dixit P, Umathe S, Mundhada D. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav. Brain Res. 2011;220(1):30–41. doi: 10.1016/j.bbr.2011.01.022. [DOI] [PubMed] [Google Scholar]