Abstract
Rapamycin (RAPA) inhibits the mechanistic target of rapamycin (mTOR), a crucial immune system regulator. Dendritic cells (DC) generated in RAPA (RAPA-DC) enrich for CD4+ forkhead box p3 (FoxP3+) regulatory T cells and induce T cell apoptosis by an unknown mechanism. RAPA-DC also promote experimental allograft survival, yet paradoxically secrete increased IL-12, crucial for the generation of IFN-γ+ CD4+ T cells. However, IFN-γ is pro-apoptotic and IL-12-driven IFN-γ inhibits experimental graft-versus-host disease (GVHD). We hypothesized that IL-12hi RAPA-DC would facilitate IFN-γ-mediated apoptosis of alloreactive T cells and, unlike control (CTR)-DC, would reduce lethal GVHD. Following LPS stimulation, RAPA-DC exhibited decreased MHCII and co-stimulatory molecules and contained a significant population of CD86lo IL-12hi cells. Consistent with our hypothesis, both unstimulated and LPS-stimulated RAPA-DC enhanced alloreactive CD4+ T cell apoptosis in culture. Augmented T cell apoptosis was ablated by IFN-γ neutralization or using T cells lacking the IFN-γ receptor, and it was associated with increased expression of Fas and cleaved caspase 8. DC production or responses to IFN-γ were not important to increased apoptotic functions of RAPA-DC. LPS-stimulated IL-12p40−/− RAPA-DC induced lower levels of T cell apoptosis in culture, which was further decreased with addition of anti-IFN-γ. Finally, whereas CTR-DC accelerated mortality from GVHD, LPS-treated RAPA-DC significantly prolonged host survival. In conclusion, increased apoptosis of allogeneic CD4+ T cells induced by LPS-stimulated IL-12hi RAPA-DC is mediated in vitro through IFN-γ and in part by increased IL-12 expression. Enhanced production of IL-12, the predominant inducer of IFN-γ by immune cells, is a probable mechanism underlying the capacity of LPS-treated RAPA-DC to reduce GVHD.
Keywords: Rapamycin, Dendritic cells, IFN-γ, Apoptosis, T cells, Graft-versus-host disease
INTRODUCTION
Dendritic cells (DCs) are important bone marrow—derived professional antigen presenting cells that induce and regulate T cell responses [1–4]. This dichotomy reflects DC phenotypic and functional plasticity. Immature DCs (MHCIIloCD86lo) display inherent tolerogenicity, whereas mature DCs (MHCIIhi CD86hi), activated by various stimuli (in particular Toll-like receptor [TLR] or CD40 ligation and pro-inflammatory cytokines), are potent inducers of adaptive immunity. Numerous immunosuppressive and anti-inflammatory agents inhibit DC maturation and promote their tolerogenicity in vitro and in vivo [3,5].
Rapamycin (RAPA) inhibits the serine/threonine kinase mechanistic target of rapamycin (mTOR), a crucial immune response regulator that integrates environmental signals leading to cell growth and proliferation [6]. DCs generated in clinically relevant concentrations of RAPA (RAPA-DCs), either in vivo or in vitro, are immature and fail to mature phenotypically (fully up-regulate MHCII and CD86) [7–12]. They induce apoptosis of polyclonal allogeneic T cells (referred to hereafter as alloreactive T cells) and enrich for CD4+FoxP3+ T cells [9,12]. Alloantigen-presenting autologous RAPA-DCs promote experimental organ allograft survival, inhibit graft-versus-host disease (GVHD), and reduce dependence on post-transplant immunosuppression [9,13].
Paradoxically, mTOR inhibitors cause a dysregulation of cytokine production by DCs upon their exposure to pro-inflammatory stimuli [11,14,15]. Of particular significance, costimulatory molecule—low (CD86lo), lipopolysaccharide (LPS)-stimulated RAPA-DCs secrete increased IL-12 [11,16], a classical immunostimulatory cytokine that drives T helper type 1 cell responses. However, both IL-12p40 and the IL-12 receptor (R) have been shown to be integral to the de novo induction of regulatory Tcells (Treg) [11,17]. In addition, IFN-γ is required for activation-induced T cell death via caspase 8 [18], Fas-dependent apoptosis of donor CD4+ T cells in experimental GVHD [19], and the induction and function of Treg [20,21]. Significantly, administration of IL-12 inhibits experimental GVHD, dependent on IFN-γ—mediated donor T cell apoptosis, yet preserves important IFN-γ—dependent graft-versus-leukemia (GVL) effects [22–24]. Based on promising anti-tumor effects in experimental animal models, phase I and II trials of systemic IL-12 were undertaken in renal cell carcinoma, but excessive morbidity and mortality halted further investigation [25]. Thus, harnessing the therapeutic capacity of IL-12 for the prevention of GVHD [22–24] will require novel treatments without systemic, potentially toxic side effects.
Given our previous demonstration of an increased capacity of RAPA-DCs to support alloreactive T cell apoptosis [9,12], we aimed to determine if augmented IL-12 production by costimulatory molecule poor RAPA-DCs would mediate apoptosis of CD4+ T cells through IFN-γ. Further, based on the reported inhibitory effects of both RAPA-DCs [13] and IL-12 in experimental GVHD [22–24], we completed limited investigations into the therapeutic capacity of IL-12hi RAPA-DCs in an experimental model of GVHD, where we hypothesized they would have augmented capacity to reduce lethal GVHD.
METHODS
Animals
Wild-type C57BL/6J (B6; H2Kb) and BALB/c (H2Kd), B6.129S7-Ifngr1tm1Agt/J (IFN-γ-R−/−; H2Kb), CByJ.129S7(B6)-Ifngr1tm1Agt/J (IFN-γ-R−/−; H2Kd), and B6.129S1-Il12btm1Jm/J (IL-12p40−/−; H2Kb) mice (Jackson Laboratory; Bar Harbor, ME, US), 8 to 12 weeks of age, were used, with male mice used for in vitro experiments and female mice used for GVHD experiments. All animals were housed in specific pathogen-free conditions at the University of Pitts-burgh School of Medicine. Experiments were conducted in accordance with National Institutes of Health guidelines and under an Institutional Animal Care and Use Committee approved protocol.
DC Generation and Treatment
Conventional myeloid DCs were generated from B6, IFN-γ-R−/− (B6), IL-12p40−/− (B6), or BALB/c bone marrow in 7-day culture in murine recombinant granulocyte-macrophage colony-stimulating factor and recombinant IL-4 (R&D Systems, Minneapolis, MN) in the presence or absence of RAPA (beginning on day 2 of culture at 10 ng/mL; LC Laboratories, Woburn, ME) as described [9,26]. DCs remained unstimulated or were treated with bacterial LPS (TLR-grade LPS from Salmonella minnesota R595; Enzo Life Sciences, Farmingdale, NY) on day 7 (100 ng/mL) for an additional 18 hours. On day 8, nonadherent cells were harvested, and CD11c+ DCs were isolated using anti-CD11c immunomagnetic beads (Miltenyi Biotec; Auburn, CA, US) for positive selection (purity > 90%).
Mixed Leukocyte Reactions
BALB/c, B6, or IFN-γ-R−/− (BALB/c) bulk CD4+ T cells were isolated from spleens as described [10]. Briefly, splenic T cells were purified by negative selection of non-T cells using anti-CD11b, -TER-119, -Gr-1, -I-A/I-E, -B220, and -Gr-1 mAbs (BD PharMingen; San Jose, CA, US) and were removed via Mouse Depletion Dynabeads (Dynal Biotech, Grand Island, NY). B6, IFN-γ-R−/− (B6), or IL-12p40−/− (B6) CD11c+ DCs were used as stimulators of purified allogeneic CD4+ T cells in 5-day (unless otherwise indicated) mixed leukocyte reactions (MLRs) at a 1:10 ratio in 96-well, round-bottom plates, with or without anti-IFN-γ mAb added on day 1 (1.0 mg/mL, XMG1.2; eBiosciences; San Diego, CA, US).
Flow Cytometry
DC were analyzed for intracellular expression of IL-12 and for cell surface expression of CD11c, IAd, CD86, CD80, CD11b, CD8α, CD4, and B220 using fluorophore-conjugated mAbs (BD Biosciences or eBioscience). T cell apoptosis was quantified using an Annexin V-PE Apoptosis Detection kit (BD PharMingen). T cells were also examined for cell surface CD4 and Fas (CD95) and for intracellular IFN-γ expression using fluorophore-conjugated mAbs (BD Biosciences or eBioscience). Intracellular expression of cleaved caspase 8 was assessed using primary rabbit mAb (Cell Signaling, Danvers, ME; 8592S) followed by fluorophore-conjugated anti-rabbit mAb (A31573; Invitrogen, Grand Island, NY). Isotype-matched IgGs were used as controls. An LSR II or LSR Fortessa (BD Biosciences, San Jose, CA) was used for data acquisition and data analyzed using FlowJo (TreeStar, version 8.8.7, Ashland, OR).
Allogeneic Bone Marrow Transplantation and Induction of Lethal GVHD
The capacity of syngeneic DCs to limit GVHD was assessed in lethally irradiated (800 cGy) female BALB/c recipients reconstituted with 5 × 106 T cell-depleted B6 bone marrow cells on day 0. CD90.2 microbeads (Miltenyi Biotec) were used for T cell depletion according to the manufacturer’s recommendations. Recipient mice were given 1 × 106 CD11c+ BALB/c DC (control [CTR], RAPA, or RAPA-DCs exposed to LPS) and 1 × 106 B6 pan T cells on day 1. Pan T cells were isolated from spleens by negative selection using the Pan T Isolation Kit (Miltenyi Biotec) according to the manufacturer’s instructions. Mice in each group were allocated randomly to different cages to minimize cage-related effects. They received antibiotic water (trimeth-oprim-sulfamethoxazole; Hi-Tech Pharmacal, Amityville, NY) from day −7 through day +14.
Clinical GVHD Evaluation
Mice were assessed for GVHD morbidity using a standard scoring system [27] based on weight loss, posture, activity level, fur texture, and integrity of skin. The animals were monitored every other day (or more frequently if indicated), and mice with >20% body weight loss were killed.
Statistical Analyses
Results from pooled completed experiments are expressed as means (±standard deviation [SD] or standard error). The significances of differences between means were determined using Student’s t-test with P < .05 considered as significant. Survival curves were generated using GraphPad Prism 2.0C Software package (GraphPad Software, Inc., La Jolla, CA) with differences in survival determined by Kaplan-Meier analysis and the log-rank test.
RESULTS
Costimulatory Molecule-Low, LPS-Stimulated RAPA-DCs Express High Levels of IL-12 and Induce Apoptosis of Alloreactive CD4+ T Cells via an IFN-γ-Dependent Mechanism In Vitro
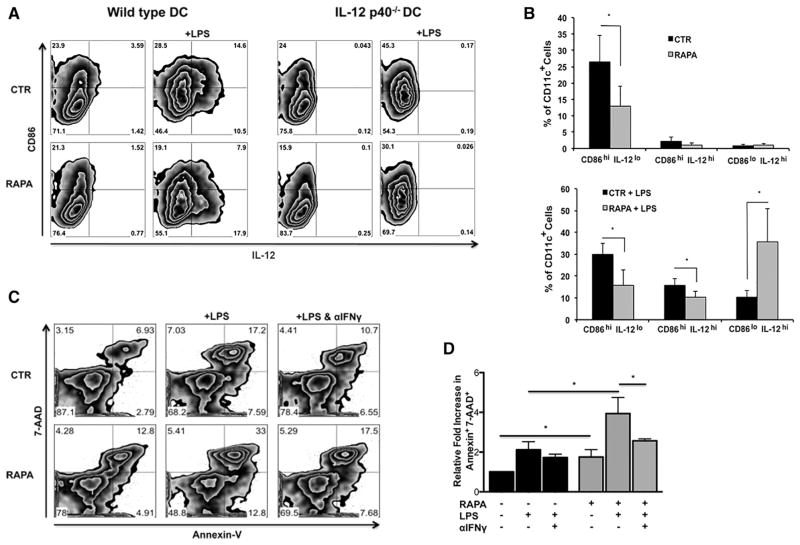
By conventional phenotyping, CD11c+ RAPA-DCs were very similar to CTR-DCs and displayed a phenotype consistent with conventional myeloid derived DCs (Supplemental Figure 1). Compatible with our previous data [11], RAPA-DCs displayed significantly reduced costimulatory molecule (CD86) expression (representative and aggregate data; Figure 1A,B) compared with CTR-DCs. RAPA-DCs also significantly down-regulated IAd and CD80 (Supplemental Figure 2). After LPS stimulation, CTR-DCs expressing IL-12p40 displayed high levels of CD86, where RAPA-DCs retained lower levels of CD86 but still up-regulated IL-12p40 expression (Figure 1A,B). Thus, RAPA-DCs after exposure to LPS displayed a dominant population of CD86loIL-12hi cells (Figure 1A,B). We demonstrated previously a direct correlation between intracellular IL-12 expression and secretion of IL-12 by mTOR-inhibited DCs [11,16]. RAPA-DCs from IL-12p40−/− mice also displayed reduced CD86 levels compared with CTR IL-12p40−/− DCs, both before and after LPS exposure. This suggests that an IL-12-independent regulatory mechanism suppresses CD86 expression on RAPA-DCs.
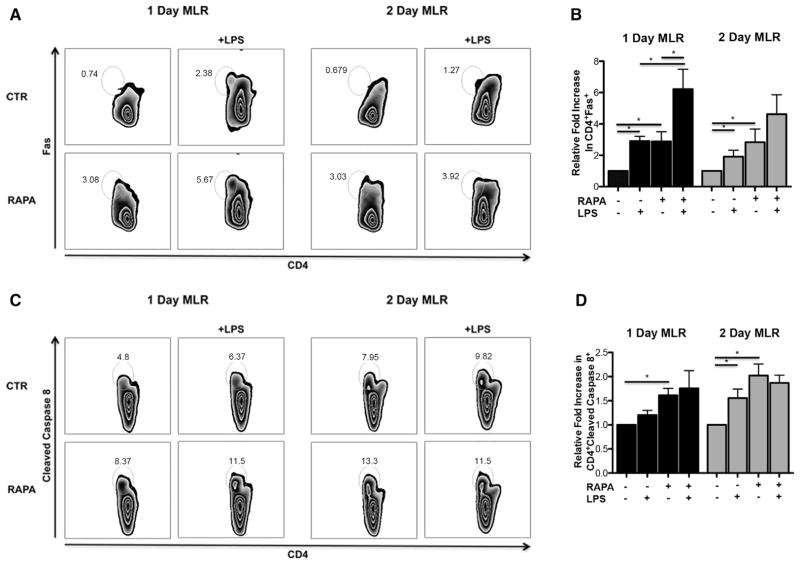
Figure 1.
LPS-stimulated RAPA-DCs express high IL-12 despite low costimulatory molecule expression and induce apoptosis of alloreactive CD4+ T cells in an IFN-γ-dependent manner. C57BL/6 (B6; H-2b) and IL-12p40−/− (B6) myeloid DCs (mDC) were generated in 7-day culture with granulocyte-macrophage colony-stimulating factor and IL-4 alone (CTR-DC) or in the presence of 10 ng/mL RAPA (RAPA-DC). CD11c-purified mDCs were incubated for 18 hours in media alone or with 100 ng/mL LPS and then analyzed for cell surface molecule expression by flow cytometry. B6 mDCs were then used in 5-day MLRs to stimulate BALB/c (H2d) CD4+ T cells. (A) RAPA-DCs, especially after LPS exposure, express low levels of CD86 and high levels of IL-12 compared with LPS-exposed CTR-DCs (CD86loIL-12hi population). IL-12p40−/− LPS-stimulated RAPA-DCs lack a high IL-12 population but have a similar CD86lo population compared with wild-type. (B) The percentage of CD86hi IL-12lo, CD86hi IL-12hi, and CD86lo IL-12hi from 7 independent experiments was averaged. Data depict mean + 1 SD. (C) Compared with CTR-DCs, especially after LPS stimulation, RAPA-DCs induced increased apoptosis (Annexin V+7-AAD+) of alloreactive CD4+ T cells (12.7% ± 2.3% versus 7.3% ± .4%; +LPS: 28.5% ± 4.4% versus 15.4% ± 2.9%; both P < .05). Blocking the activity of IFN-γ in LPS-treated RAPA-DC cultures decreased levels of apoptosis to those of LPS-treated CTR-DCs (18.7% ± 1.0% versus 15.4% ± 2.9%; NS). Plots are representative of 3 experiments performed. (D) Average fold increase in CD4+ Annexin V+ 7-AAD+ relative to CTR-DCs on day 5 of MLRs. Error bars indicate mean + 1 SD. n = 3. *P < .05.
We demonstrated previously [9] that unstimulated or CD40-ligated RAPA-DCs induce increased apoptosis (Annexin V+7-AAD+) of wild-type alloreactive CD4+ T cells in 5-day MLRs compared with CTR-DC counterparts. Our present data (Figure 1C,D) confirm the increased capacity of unstimulated RAPA-DCs to induce apoptosis when used as stimulators (12.7% ± 2.3% versus 7.3% ± .4%; P < .05). Importantly, we also now show that LPS-stimulated IL-12hi RAPA-DCs induce significantly more apoptosis compared with CTR-DCs (RAPA + LPS: 28.5% ± 4.4% versus 15.4% ± 2.9%; P < .05) (Figure 1C, representative data; Figure 1D, average across 3 experiments normalized to CTR). IL-12p70 is the principal inducer of IFN-γ production by immune cells, especially CD4+ T cells [28]. However, even while supporting T helper type 1 responses, IFN-γ is necessary for activation-induced cell death of T-cell receptor-stimulated T cells [18]. LPS-stimulated RAPA-DCs induced augmented apoptosis of allogeneic CD4+ T cells (Figure 1). Given that IL-12 released by antigen presenting cells stimulates IFN-γ production by CD4+ T cells [29], we investigated the role of IFN-γ in the pro-apoptotic function of RAPA-DCs using neutralizing Abs. The apoptosis of alloreactive CD4+ T cell induced by LPS-stimulated IL-12hi RAPA-DCs was decreased significantly when IFN-γ was blocked via anti-IFN-γ mAb (Figure 1C,D). In fact, with anti-IFN-γ mAb added to LPS-stimulated RAPA-DC cultures, the degree of apoptosis was reduced to levels similar to those induced by LPS-stimulated CTR-DCs (18.7% ± 1.0% versus 15.4% ± 2.9%; NS). Thus, apoptosis of alloreactive CD4+ T cells promoted by RAPA-DC in vitro is mediated, at least in part, by IFN-γ, a cytokine driven by IL-12 that is expressed at high levels by LPS-stimulated RAPA-DCs.
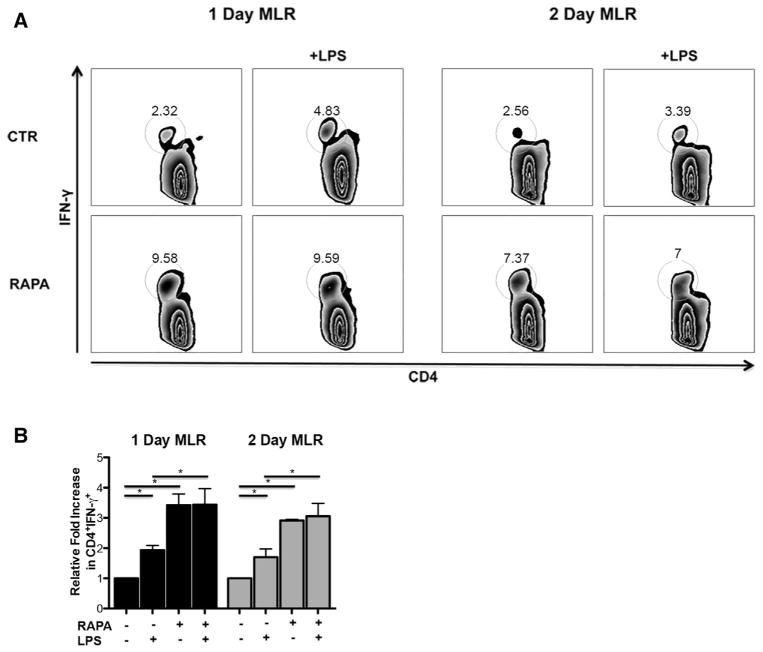
IL-12hi RAPA-DCs Induce Increased Production of IFN-γ by CD4+ T Cells
Given that apoptosis of CD4+ T cells driven by IL-12hi RAPA-DC is dependent on IFN-γ, we next sought to determine which cells were producing IFN-γ. IFN-γ expression was not detected in the supernatants of any DC groups by ELISA (CTR ± LPS or RAPA ± LPS; data not shown). As reported previously [11], production of IFN-γ by CD4+ T cells was not altered when assessed on day 5 of MLR with LPS-stimulated RAPA-DCs. Therefore, we analyzed IFN-γ production at earlier time points during MLRs (day 1 and day 2). As shown in Figure 2, both unstimulated RAPA-DCs and LPS-stimulated RAPA-DCs induced increased IFN-γ production by CD4+ T cells compared with CTR groups (day 1: 3.2± .8 versus 10.6 ± .9; LPS: 6.1 ± 1.2 versus 10.6 ± 2.1; P <.05; day 2: 3.6 ± 2.1 versus 10.5 ± 5.9; LPS: 6.0 ± 3.1 versus 10.1 ± 3.4; P < .05) (Figure 2A representative data, Figure 2B average across 3 experiments normalized to CTR). Therefore, IL-12hi RAPA-DC promote apoptosis of alloreactive CD4+ T cells via induction of IFN-γ by the T cells, not the DCs.
Figure 2.
IL-12hi RAPA-DCs induce increased production of IFN-γ by CD4+ T cells. B6 myeloid DCs (mDC) were generated as described in Figure 1 and then used in 1-or 2-day MLRs to stimulate BALB/c CD4+ T cells. Plots are representative of 3 independent experiments performed. (A) CD4+ T cells exposed to either RAPA-DCs or LPS-stimulated RAPA-DCs produce increased IFN-γ compared with CTR groups (day 1: 3.19± .8 versus 10.6 ± .9; LPS: 6.1 ± 1.2 versus 10.6 ± 2.1, P <.05; day 2: 3.62 ± 2.1 versus 10.5 ± 5.9; LPS: 6.0 ± 3.1 versus 10.1 ± 3.4, P <.05). (B) Average fold increase in CD4+ IFN-γ+ relative to CTR-DCs on days 1 or 2 of MLRs. Error bars indicate mean + 1 SD. n = 3. *P < .05.
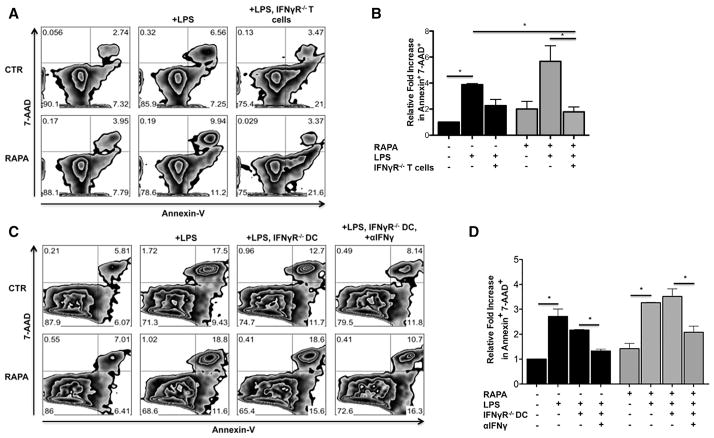
Apoptosis of Alloreactive CD4+ T Cells Induced by LPS-Exposed IL-12hi RAPA-DCs Depends on T Cell But Not DC Responses to IFN-γ
Using a neutralizing Ab, we determined that IFN-γ was an important mediator of the increased apoptosis of alloreactive CD4+ T cells induced by LPS-stimulated RAPA-DCs. We next performed studies to determine whether IFN-γ was acting in this capacity on DCs, CD4+ T cells, or both. When exposed to LPS-stimulated IL-12hi RAPA-DCs, IFN-γ-R−/− CD4+ T cells exhibited decreased apoptosis (Annexin V+7-AAD+) in 5-day MLRs when compared with wild-type CD4+ T cells (5.5% ± 1.9% versus IFN-γ-R−/− 6.4% ± 3.1%; +LPS: 15.6% ± 3.6% versus 4.9% ± 1.1%; P <.05 for LPS) (Figure 3A representative data; Figure 3B average across 2 experiments normalized to CTR). Unlike those of CD4+ T cells, DC responses to IFN-γ were not important in mediating the function of RAPA-DCs (Figure 3C representative data; Figure 3D average across 2 experiments normalized to CTR). There was no difference in the extent of apoptosis of alloreactive CD4+ T cells induced by IFN-γ-R−/− RAPA-DCs compared with wild-type RAPA-DCs (12.4% ± 7.6% versus 10.5% ± 7.6% respectively; +LPS: 27.2% ± 11.9% versus 30.1% ± 16.3%; both NS; Figure 3). The addition of anti-IFN-γ mAb to LPS-stimulated IFN-γ-R−/− RAPA-DCs significantly decreased levels of CD4+ T cell apoptosis similar to LPS-stimulated wild-type CTR-DCs (18.0% ± 10.3% versus 21.9% ± 6.3%; P = NS; Figure 3D). These data strongly support the conclusion that IFN-γ mediates increased apoptosis of alloreactive T cells directly and not through actions on DCs.
Figure 3.
Apoptosis induced by IL-12hi RAPA-DCs is dependent on T cell but not DC responses to IFN-γ. Wild type B6 or B6 IFN-γ-R−/− DC were generated then used in 5-day MLRs to stimulate wild-type BALB/c or IFN-γ-R−/−(H2d) CD4+ T cells. Plots are representative of 3 independent experiments performed. (A) IFN-γ-R−/− CD4+ T cells have decreased levels of apoptosis (Annexin V+7-AAD+) compared with wild-type T cells after exposure to LPS-treated IL-12hi RAPA-DCs (wild-type 5.5% ± 1.9% versus IFN-γ-R−/− 6.4% ± 3.1%; +LPS: 15.6% ± 3.6% versus 4.9% ± 1.1%; P <.05 for LPS). (B) Average fold increase in CD4+ Annexin V+ 7-AAD+ cells relative to CTR-DCs on day 5 of MLRs. Error bars indicate mean + 1 SD. n = 2. *P < .05. (C) IFN-γ-R−/− IL-12hi RAPA-DCs induce similar apoptosis (Annexin V+7-AAD+) of CD4+ T cells compared with wild-type (12.4% ± 7.6% versus 10.5% ± 7.6%, respectively; +LPS: 27.2% ± 11.9% versus 30.1% ± 16.3%; both NS). However, blocking IFN-γ significantly decreased apoptosis induced by LPS-stimulated IFN-γ-R−/− RAPA-DCs to levels similar to LPS-stimulated wild-type CTR-DC (18.0% ± 10.3% versus 21.9% ± 6.3%, P = NS). (D) Average fold increase in CD4+ Annexin V+ 7-AAD+ cells relative to wild-type CTR-DCs on day 5 of MLRs. Error bars indicate mean + 1 SD. n = 2. *P < .05.
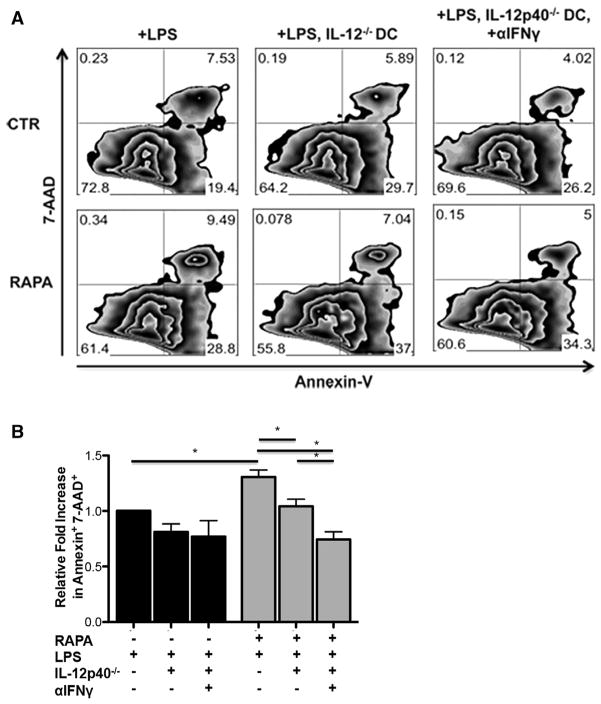
Induction of Apoptosis by LPS-Stimulated RAPA-DCs Depends on IL-12 Expression
Using IL-12p40−/− DCs, we next explored the role of DC IL-12 expression in the increased apoptosis of CD4+ T cells induced by RAPA-DC. As shown in Figure 4, LPS-stimulated IL-12p40−/− RAPA-DCs induced lower levels of apoptosis in 5-day MLRs, defined by Annexin V+ 7-AAD+ T cells, compared with wild-type DC (16.9% ± 8.9% versus 20.8% ± 10.0%; P < .05) (Figure 4A representative data; Figure 4B average across 3 experiments normalized to CTR LPS). The addition of anti-IFN-γ to MLRs further decreased apoptosis of alloreactive CD4+ T cells induced by LPS-stimulated IL-12p40−/− RAPA-DCs (12.0% ± 6.1% versus 16.9% ± 8.9%; P < .05).
Figure 4.
Apoptosis of alloreactive CD4+ T cells induced by IL-12hi RAPA-DCs is dependent on IL-12 expression. Wild-type B6 or B6 IL-12p40−/− myeloid DCs (mDC) were generated and then used in 5-day MLRs to stimulate BALB/c CD4+ T cells. Plots are representative of 3 independent experiments performed. (A) LPS-exposed IL-12p40−/− RAPA-DCs induce decreased apoptosis (Annexin V+7-AAD+) of alloreactive CD4+ T cells compared with wild-type (16.9% ± 8.9% versus 20.8% ± 10.0%; P < .05). Blocking IFN-γ further decreased apoptosis stimulated by LPS-exposed IL-12p40−/− RAPA-DCs (12.0% ± 6.1% versus 16.9% ± 8.9%, P < .05). (B) Average fold increase in CD4+ Annexin V+ 7-AAD+ cells relative to wild-type CTR-DCs on day 5 of MLRs. Error bars indicate mean + 1 SD. n = 3. *P < .05.
CD4+ T Cells Interacting with IL-12hi RAPA-DCs Exhibit Increased Fas and Cleaved Caspase 8
Based on the importance of the Fas pathway in IFN-γ-dependent activation-induced cell death and apoptosis of donor CD4+ T cells in experimental GVHD [18,19], we assessed if either the Fas receptor or the downstream pro-apoptotic effector caspase 8 is modulated on CD4+ T cells after 1 and 2 days co-culture with RAPA-DCs. Both unstimulated and LPS-stimulated RAPA-DCs enhanced the expression of Fas on CD4+ T cells compared with their respective CTR-DC counterparts, particularly after a 1-day exposure (day 1: 3.3± .8 versus 1.3 ± .6, LPS: 7.1 ± 1.4 versus 3.5 ± 1.0, P <.05; day 2: 4.1 ± 1.5 versus 1.9 ± 1.5, P < .05; LPS: 6.6 ± 2.4 versus 3.1 ± 1.6, P = .05) (Figure 5A representative data; Figure 5B average across 3 experiments normalized to CTR). Compared with CTR-DCs, RAPA-DCs stimulated increased expression of cleaved caspase 8 in CD4+ T cells, particularly after a 2-day co-culture (day 1: 14.2 ± 5.3 versus 9.2 ± 4.7, P <.05; LPS: 14.2 ± 2.4 versus 10.6 ± 4.0, P > .05; day 2: 11.4 ± 5.6 versus 6.2 ± 3.9, P <.05; LPS: 10.9 ± 6.6 versus 8.7 ± 4.2, P > .05) (Figure 5C representative data; Figure 5D average across experiments compared with CTR; n = 3 for day 1 and n = 4 for day 2). These data suggest that Fas—FasL interaction and cleavage of caspase 8 may be important mechanisms downstream of IFN-γ that promote apoptosis of CD4+ T cells by IL-12hi RAPA-DC.
Figure 5.
Apoptosis of CD4+ T cells by induced IL-12hi RAPA-DCs is dependent on the Fas/FasL pathway and caspase 8. B6 were generated and then used in 1- or 2-day MLRs to stimulate BALB/c CD4+ T cells. Plots are representative of 3 to 4 independent experiments performed (n = 4 for 2-day MLRs for caspase 8). (A) After exposure to IL-12hi RAPA-DCs, CD4+ T cells increased expression of Fas compared with LPS-stimulated CTR-DCs, particularly after a 1-day exposure (day 1: 7.1 ± 1.4 versus 3.5 ± 1.0, P < .05; day 2: 6.6 ± 2.4 versus 3.1 ± 1.6, P = .05). (B) Average fold increase in CD4+ Fas+ cells relative to wild-type CTR-DCs on day 1 or 2 of MLRs. Error bars indicate mean + 1 SD. n = 3. *P < .05. (C) CD4+ T cells also up-regulate cleaved caspase 8 expression after exposure to IL-12hi RAPA-DC compared with CTR-DCs, particularly after a 2-day MLR exposure (day 1: 14.2 ± 2.4 versus 9.2 ± 4.7, P = .05; day 2: 10.9 ± 6.6 versus 6.2 ± 3.9, P < .05). (D) Average fold increase in CD4+ cleaved caspase 8+ cells relative to wild-type CTR-DCs on day 1 or 2 of MLRs. Error bars indicate mean + 1 SD. n = 3 (1 day) or 4 (2 day). *P < .05.
IL-12hi RAPA-DCs Limit GVHD Lethality
Both RAPA-DCs [13] and IL-12 have been reported to inhibit experimental GVHD [22–24]; thus, we next investigated the therapeutic capacity of IL-12hi RAPA-DCs in an experimental model of GVHD. Previous work by Reichardt et al. [13] found that syngeneic DCs exposed to RAPA for 24 hours inhibited GVHD in a B6→BALB/c model of bone marrow transplantation. We sought to expand on this work using LPS-stimulated RAPA-DCs, which we hypothesized would have augmented ability to inhibit GVHD potentially through their increased IL-12 expression.
CTR- or RAPA-DCs were generated as described in Methods and either left unstimulated or stimulated with LPS to induce IL-12 expression. Given a known mTOR polymorphism in BALB/c compared with B6 mice [30], we verified that exposure to RAPA and LPS down-regulated CD86 and up-regulated IL-12 expression by BALB/c DCs similarly to B6 DCs [11,16]. Using flow cytometry, DCs were analyzed for cell surface costimulatory molecule expression (Figure 6A). As expected, RAPA-DCs, compared with CTR-DCs, exhibited decreased expression of MHC II (IAd), CD80, and CD86 compared with CTR-DC, both with and without LPS stimulation. As shown in Figure 6B, LPS-stimulated BALB/c RAPA-DCs also had profoundly up-regulated IL-12 compared with LPS-stimulated CTR-DCs. These findings confirmed that BALB/c DC exposed to RAPA and LPS down-regulate CD86 and up-regulate IL-12 similarly to B6 DCs, despite a polymorphism in mTOR of BALB/c mice that potentially could alter effects of RAPA [30].
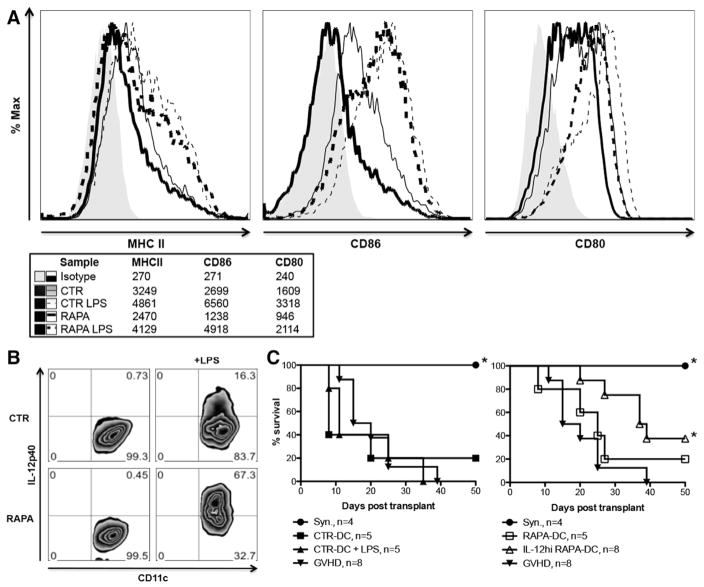
Figure 6.
Autologous IL-12hi RAPA-DC demonstrate low costimulatory molecule expression and inhibit acute GVHD. BALB/c CTR- and RAPA-DCs were generated and then analyzed for cell surface marker expression using flow cytometry. (A) Compared with equivalent CTR-DC groups, unstimulated RAPA- and LPS-exposed RAPA-DCs showed decreased surface expression (MFI) of IAd, CD86, and CD80. (B) BALB/c LPS-stimulated RAPA-DCs demonstrate increased IL-12 expression relative to LPS-stimulated CTR-DCs (CD11c+ IL-12hi population 67.3% versus 16.3%). (C) Lethally irradiated BALB/c recipients were given 5 × 106 B6 bone marrow cells plus 1 × 106 purified B6 T cells alone (GVHD control) or with 1 × 106 BALB/c DCs (CTR-DC ± LPS or RAPA-DC ± LPS). Irradiated BALB/c mice injected with syngeneic BALB/c bone marrow (syngeneic) are also indicated. Although mice receiving CTR-DCs had survival comparable with the control GVHD group (without DCs) (median survival in days: GVHD, 17.5 days; CTR, 8 days; CTR-LPS, 11 days; all P = NS), IL-12hi RAPA-DCs significantly and markedly prolonged survival from GVHD (median survival in days: 38 days, P =.007).
Autologous BALB/c DCs (CTR-DCs ± LPS or RAPA-DCs ± LPS) were administered as negative cellular vaccines at the time of bone marrow transplantation, in a fully MHC-mismatched model of acute lethal GVHD (B6→BALB/c). Host IL-12hi RAPA-DC significantly prolonged host survival (median survival in days: GVHD, 17.5 days; RAPA-DCs, 25 days, P = NS; IL-12hi RAPA-DCs, 38 days, P = .007; syngeneic control, >50 days, P = .002) (Figure 6C). In contrast, mice given CTR-DCs ± LPS had similar GVHD mortality to untreated control mice (CTR-DCs: 8 days; LPS—CTR-DCs: 11 days, both P = NS). These data demonstrate that syngeneic LPS-stimulated RAPA-DCs can prolong survival from GVHD when given as a negative cellular vaccine.
DISCUSSION
DCs are of critical importance in determining the balance between immunity and tolerance [31], and numerous studies have demonstrated the in vitro generation of tolerogenic (tol)DCs with potent modulating effects on autoimmunity [32] and allograft rejection [3,4,33,34]. TolDCs resemble quiescent or “immature” DCs in vivo, which under steady-state, noninflammatory conditions express low levels of surface MHC and costimulatory molecules (eg, CD40, CD80, CD86) [1,3]. Both tolDCs and immature DCs can induce unresponsiveness, or anergy, and apoptosis in naïve T cells, support Treg, and inactivate/delete memory and effector CD8+ T cells [3,4]. However, DC immaturity is not fixed, and the sensing of danger signals by DCs triggers signaling pathways that direct DC “maturation” [1]. Mature DCs have increased T cell-stimulatory capacity through augmented surface expression of MHC and costimulatory molecules, particularly CD86. Likewise, different stimuli trigger receptors on DCs to launch the appropriate production of cytokines needed to facilitate differentiation of naïve CD4+ T cells [35]. These receptors, including the well-described TLR family, detect local “danger,” including exogenous pathogens via recognition of pathogen-associated molecular patterns and tissue damage sensed via damage-associated molecular patterns. Damage-associated molecular pattern or pathogen-associated molecular pattern triggering of most TLRs, especially TLR4, results in DC secretion of IL-12p70, a cytokine needed for naïve CD4+ T cell differentiation to IFN-γ-secreting T helper type 1 cells [36].
Many signaling pathways activated by danger signals are integrated by mTOR, which functions in at least 2 intracellular complexes to correlate appropriate cell growth/proliferation and movement with signals arising from environmental stimuli (ie, growth factors, cytokines, TLR ligands, etc.) and internal cellular conditions (ie, levels of adenosine triphosphate, arachidonic acid, or oxygen) [6]. RAPA potently inhibits mTOR, and DCs exposed to RAPA have shown promise as tolDC, specifically promoting experimental organ allograft survival, inhibiting GVHD, and reducing the need for continued post-transplant immunosuppression [9,13]. Both in vitro and in vivo, exposure to RAPA suppresses DC generation, phenotypic maturation, and T cell stimulatory function [6,9–11,26]. Also consistent with tolerogenic functions, RAPA-DCs induce alloreactive Tcell anergy [26] and apoptosis [9,12] while enriching for Treg in vitro and in vivo [9,11,16]. However, when exposed to inflammatory stimuli, although RAPA-DCs fail to fully up-regulate costimulatory molecule (CD86) expression and remain poorly stimulatory, paradoxically, they exhibit dysregulation of cytokine production, including enhanced inflammatory IL-12 production and reduced IL-10 [11,14,37]. We have shown that this enhanced IL-12p70 production results from RAPA blocking mTOR inhibition of glycogen synthase kinase-B after TLR4 ligation [11].
Current knowledge suggests that IL-12hi DCs would act as potent generators of IFN-γ production by CD4+ cells [38]. Likewise, RAPA-enhanced IL-12 expression by DCs has been hypothesized to underlie recent reports of mTOR inhibition facilitating T cell responses to pathogens [39,40] and the occurrence, in a subset of RAPA-treated transplant patients, of inflammatory disorders, including interstitial pneumonitis [41] and glomerulonephritis [42]. However, post-transplant RAPA fails to enhance donor-specific T cell responses, even during concurrent infection [43]. Clinically, mTOR inhibition is typically associated with increased or maintained levels of in Foxp3+ T cells, unlike calcineurin inhibitors, which decrease Foxp3+ T cell incidence [44,45]. Likewise, as shown in Figure 1A, augmented IL-12p40 expression by LPS-exposed RAPA-DCs is dominated by a poorly stimulatory, costimulatory molecule—low (CD86lo) subset [11]. Importantly, we now establish that increased IL-12 production by RAPA-DCs supports IFN-γ-mediated apoptosis in vitro (Figure 4), and we have observed previously that IL-12p70 supports Treg induction [11].
In the present study, although IFN-γ was crucial to the increased apoptosis induced by RAPA-DCs relative to CTR-DCs after their exposure to LPS, we did not observe that blocking IFN-γ significantly modulated the incidence of Treg (data not shown). Given the growing use of mTOR inhibitors in both transplantation and other medical areas, it will be important to continue to establish how RAPA-mediated modulation of cytokines, such as IL-12 and IL-10, impact alloimmunity and transplant survival in relevant experimental models.
Our current findings also provide new insight into the mechanisms by which RAPA-DCs promote apoptosis of alloreactive CD4+ T cells [9]. Specifically, we reveal that RAPA-DC-mediated apoptosis of alloreactive CD4+ T cells is IFN-γ-dependent and that the IFN-γ-dependent effects are the result of IFN-γ produced by T cells that interact with RAPA-DCs. Our current studies also demonstrate that this effect is mediated through IFN-γ action on CD4+ T cells and involves their increased expression of Fas and cleaved caspase 8. However, we find that the ability of IFN-γ to drive apoptosis is not due to its capacity to increase DC expression of indoleamine 2,3-dioxygenase (data not shown), which causes local tryptophan depletion and release of kynurenines that cause caspase 8 cleavage and T cell apoptosis [46,47]. Further studies are required to determine whether the CD4+ T cell apoptosis induced by IL-12hi RAPA-DCs is also dependent on other cytokines dysregulated by mTOR inhibition or on the low costimulatory molecule expression by these cells.
Based on work by Sykes and colleagues [19,22–24] demonstrating inhibition of experimental GVHD with IL-12, we hypothesized that IL-12hi RAPA-DCs could provide a novel approach to harness the immunoregulatory properties of IL-12, without the morbidity and mortality associated with its systemic administration in clinical trials [25]. Although IL-12 has classically been described as an immunostimulatory cytokine that can mediate or intensify experimental GVHD [48,49], the timing and dosing of IL-12 is crucial in determining its effects. Thus, IL-12 inhibits GVHD when given early (1 hour before or 1 to 12 hours after bone marrow transplantation) but enhances GVHD when given late [50]. Our data suggest that RAPA-DCs are indeed an effective means to deliver IL-12 early after bone marrow transplantation and are compatible with the ability of IL-12 delivered with bone marrow transplantation to inhibit GVHD.
In the current studies, unstimulated RAPA-DCs failed to significantly prevent experimental GVHD. This is distinct from studies by Reichardt et al. [13], where splenic and bone marrow CD11c+ cells exposed to RAPA for 24 hours and infused with T cells after bone marrow transplantation were effective in prevention of GVHD. However, given the differences in preparation of RAPA-DCs between these studies and the lack of assessment by Reichardt et al. as to whether RAPA-DCs generated in this fashion overexpress IL-12, it is difficult to establish reasons for the differences in effectiveness between unstimulated RAPA-DCs in these two studies. Yet in these independent investigations, unstimulated syngeneic RAPA-DCs [13] or LPS-stimulated RAPA-DCs (the present report) delivered at the time of bone marrow transplantation have shown therapeutic benefit against GVHD. This is in contrast to CTR-DCs, which in both studies accelerated GVHD.
Previous work by Sykes and colleagues [22–24] demonstrated that GVHD protection through the administration of systemic IL-12 was IFN-γ dependent. In addition, IL-12 preserved important experimental GVL effects, also mediated through IFN-γ [23]. However, expansion to the clinical setting was halted because of excessive morbidity and mortality in phase I and II trials [25]. Both human and mouse DCs generated in or pulsed with RAPA express CCR7 at levels at or above CTR-DCs [9,13,51], and after their infusion, mouse RAPA-exposed DCs traffic to the secondary lymphoid tissue [13,26]. Thus, LPS-stimulated RAPA-DCs may enable targeted delivery of IL-12 to host lymphoid tissues without the toxic effects of IL-12 therapy as seen in clinical trials. We propose that increased IL-12 production may underlie the capacity of LPS-exposed RAPA-DCs to prevent GVHD, in addition to prolonging allograft survival. The present study does not include robust mechanistic in vivo studies to verify this hypothesis; therefore, future experiments are warranted to determine if IL-12p40−/− or IL-12p35−/− RAPA-DCs exposed to LPS retain the capacity to promote resistance to GVHD. These future studies will also provide the opportunity for precise histopathological assessment of GVHD target organs to further support the therapeutic benefit of RAPA-DCs secreting IL-12.
GVHD remains a significant cause of post-transplant morbidity and mortality and limits the broader application of hematopoietic stem cell transplantation, such as to patients with nonmalignant diseases or without a matched related donor. Given the known role of DCs in the pathogenesis of GVHD [4] and the failure of therapies that target effector T cells to adequately prevent or treat GVHD, approaches that target DCs or DC cellular therapies are an attractive emerging strategy [4]. DCs are also required for full GVL effects (host > donor DC) [52–54], and, importantly, modulation of donor DC subsets [55,56] and the administration of tolDCs [57] has been shown to preserve these crucial GVL effects while abrogating experimental GVHD. Clinical trials using cellular therapies for GVHD, including Treg and mesenchymal stem cells, are underway [4], and human tolDCs have been generated and characterized in vitro using clinical grade reagents and proven safe in initial phase I clinical testing in newly diagnosed cases of type 1 diabetes and rheumatoid arthritis [58–61]. It can be easily envisioned that these advances in DC cellular therapy will lead to future trials of tolDCs in GVHD. Further in vivo experimental studies of IL-12hi RAPA-DCs are clearly indicated to both elucidate the role of cellular IL-12 in their prevention of GVHD and directly assess if GVL effects are preserved or potentiated.
Supplementary Material
Acknowledgments
Financial disclosure: Supported by National Institutes of Health (NIH) grants (R01AI67541 and R01AI60994 to A.W.T., R01HL93716 and RO1GM63569 to M.Y.M., and K99/R00 HL97155 to H.R.T.) and the Pittsburgh Foundation (to M.Y.M.). B.R.R. is the recipient of an American Heart Association pre-doctoral fellowship (11PRE7070020) and a nonconcurrent NIH T32 (AI74490) research training predoctoral fellowship.
Footnotes
Conflict of interest statement: A.W.T. is co-inventor of US patents that concern generation of dendritic cells to promote transplant tolerance.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.bbmt.2013.11.007.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 3.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 4.Stenger EO, Turnquist HR, Mapara MY, Thomson AW. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood. 2012;119:5088–5103. doi: 10.1182/blood-2011-11-364091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 6.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackstein H, Taner T, Zahorchak AF, et al. Rapamycin inhibits IL-4—induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 8.Monti P, Mercalli A, Leone BE, et al. Rapamycin impairs antigen uptake of human dendritic cells. Transplantation. 2003;75:137–145. doi: 10.1097/00007890-200301150-00025. [DOI] [PubMed] [Google Scholar]
- 9.Turnquist HR, Raimondi G, Zahorchak AF, et al. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 10.Turnquist HR, Sumpter TL, Tsung A, et al. IL-1beta-driven ST2L expression promotes maturation resistance in rapamycin-conditioned dendritic cells. J Immunol. 2008;181:62–72. doi: 10.4049/jimmunol.181.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnquist HR, Cardinal J, Macedo C, et al. mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood. 2010;115:4758–4769. doi: 10.1182/blood-2009-10-251488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer RT, Turnquist HR, Wang Z, et al. Rapamycin-conditioned, alloantigen-pulsed myeloid dendritic cells present donor MHC class I/peptide via the semi-direct pathway and inhibit survival of antigen-specific CD8(+) T cells in vitro and in vivo. Transpl Immunol. 2011;25:20–26. doi: 10.1016/j.trim.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reichardt W, Durr C, von Elverfeldt D, et al. Impact of mammalian target of rapamycin inhibition on lymphoid homing and tolerogenic function of nanoparticle-labeled dendritic cells following allogeneic hematopoietic cell transplantation. J Immunol. 2008;181:4770–4779. doi: 10.4049/jimmunol.181.7.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weichhart T, Costantino G, Poglitsch M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Haidinger M, Poglitsch M, Geyeregger R, et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185:3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 16.Rosborough BR, Raich-Regue D, Matta BM, et al. Murine dendritic cell rapamycin-resistant and rictor-independent mTOR controls IL-10, B7-H1, and regulatory T-cell induction. Blood. 2013;121:3619–3630. doi: 10.1182/blood-2012-08-448290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Z, Yu S, Fitzgerald DC, et al. IL-12R beta 2 promotes the development of CD4+CD25+ regulatory T cells. J Immunol. 2008;181:3870–3876. doi: 10.4049/jimmunol.181.6.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dey BR, Yang YG, Szot GL, et al. Interleukin-12 inhibits graft-versus-host disease through an Fas-mediated mechanism associated with alterations in donor T-cell activation and expansion. Blood. 1998;91:3315–3322. [PubMed] [Google Scholar]
- 20.Wang Z, Hong J, Sun W, et al. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25− T cells to CD4+ Tregs. J Clin Invest. 2006;116:2434–2441. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood KJ, Sawitzki B. Interferon gamma: a crucial role in the function of induced regulatory T cells in vivo. Trends Immunol. 2006;27:183–187. doi: 10.1016/j.it.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Sykes M, Szot GL, Nguyen PL, Pearson DA. Interleukin-12 inhibits murine graft-versus-host disease. Blood. 1995;86:2429–2438. [PubMed] [Google Scholar]
- 23.Yang YG, Sergio JJ, Pearson DA, et al. Interleukin-12 preserves the graft-versus-leukemia effect of allogeneic CD8 T cells while inhibiting CD4-dependent graft-versus-host disease in mice. Blood. 1997;90:4651–4660. [PubMed] [Google Scholar]
- 24.Yang YG, Dey BR, Sergio JJ, et al. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest. 1998;102:2126–2135. doi: 10.1172/JCI4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose inter-leukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90:2541–2548. [PubMed] [Google Scholar]
- 26.Taner T, Hackstein H, Wang Z, et al. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce ag-specific T cell regulation and prolong graft survival. Am J Transplant. 2005;5:228–236. doi: 10.1046/j.1600-6143.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 27.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation. I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 28.Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh CS, Macatonia SE, Tripp CS, et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, Readinger JA, DuBois W, et al. Constitutive reductions in mTOR alter cell size, immune cell development, and antibody production. Blood. 2011;117:1228–1238. doi: 10.1182/blood-2010-05-287821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watowich SS, Liu YJ. Mechanisms regulating dendritic cell specification and development. Immunol Rev. 2010;238:76–92. doi: 10.1111/j.1600-065X.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilkens CM, Isaacs JD, Thomson AW. Development of dendritic cell-based immunotherapy for autoimmunity. Int Rev Immunol. 2010;29:156–183. doi: 10.3109/08830180903281193. [DOI] [PubMed] [Google Scholar]
- 33.Turnquist HR, Thomson AW. Taming the lions: manipulating dendritic cells for use as negative cellular vaccines in organ transplantation. Curr Opin Organ Transplant. 2008;13:350–357. doi: 10.1097/MOT.0b013e328306116c. [DOI] [PubMed] [Google Scholar]
- 34.Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol. 2011;23:252–263. doi: 10.1016/j.smim.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 36.Lotze MT, Zeh HJ, Rubartelli A, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 37.Ohtani M, Nagai S, Kondo S, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jagannath C, Lindsey DR, Dhandayuthapani S, et al. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 40.Turner AP, Shaffer VO, Araki K, et al. Sirolimus enhances the magnitude and quality of viral-specific CD8+ T-cell responses to vaccinia virus vaccination in rhesus macaques. Am J Transplant. 2011;11:613–618. doi: 10.1111/j.1600-6143.2010.03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singer SJ, Tiernan R, Sullivan EJ. Interstitial pneumonitis associated with sirolimus therapy in renal-transplant recipients. N Engl J Med. 2000;343:1815–1816. doi: 10.1056/nejm200012143432417. [DOI] [PubMed] [Google Scholar]
- 42.Dittrich E, Schmaldienst S, Soleiman A, et al. Rapamycin-associated post-transplantation glomerulonephritis and its remission after reintroduction of calcineurin-inhibitor therapy. Transplant Int. 2004;17:215–220. doi: 10.1007/s00147-004-0700-0. [DOI] [PubMed] [Google Scholar]
- 43.Ferrer IR, Wagener ME, Robertson JM, et al. Cutting edge: rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J Immunol. 2010;185:2004–2008. doi: 10.4049/jimmunol.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segundo DS, Ruiz JC, Izquierdo M, et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation. 2006;82:550–557. doi: 10.1097/01.tp.0000229473.95202.50. [DOI] [PubMed] [Google Scholar]
- 45.Brouard S, Puig-Pey I, Lozano JJ, et al. Comparative transcriptional and phenotypic peripheral blood analysis of kidney recipients under cyclosporin A or sirolimus monotherapy. Am J Transplant. 2010;10:2604–2614. doi: 10.1111/j.1600-6143.2010.03302.x. [DOI] [PubMed] [Google Scholar]
- 46.Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 47.Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 48.Williamson E, Garside P, Bradley JA, Mowat AM. IL-12 is a central mediator of acute graft-versus-host disease in mice. J Immunol. 1996;157:689–699. [PubMed] [Google Scholar]
- 49.Ordemann R, Hutchinson R, Friedman J, et al. Enhanced allo-stimulatory activity of host antigen-presenting cells in old mice intensifies acute graft-versus-host disease. J Clin Invest. 2002;109:1249–1256. doi: 10.1172/JCI14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sykes M, Pearson DA, Taylor PA, et al. Dose and timing of interleukin (IL)-12 and timing and type of total-body irradiation: effects on graft-vs.-host disease inhibition and toxicity of exogenous IL-12 in murine bone marrow transplant recipients. Biol Blood Marrow Transplant. 1999;5:277–284. doi: 10.1016/s1083-8791(99)70002-9. [DOI] [PubMed] [Google Scholar]
- 51.Sordi V, Bianchi G, Buracchi C, et al. Differential effects of immunosuppressive drugs on chemokine receptor CCR7 in human monocyte-derived dendritic cells: selective upregulation by rapamycin. Transplantation. 2006;82:826–834. doi: 10.1097/01.tp.0000235433.03554.4f. [DOI] [PubMed] [Google Scholar]
- 52.Matte CC, Liu J, Cormier J, et al. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10:987–992. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]
- 53.Reddy P, Maeda Y, Liu C, et al. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med. 2005;11:1244–1249. doi: 10.1038/nm1309. [DOI] [PubMed] [Google Scholar]
- 54.Chakraverty R, Eom HS, Sachs J, et al. Host MHC class II+ antigen-presenting cells and CD4 cells are required for CD8-mediated graft-versus-leukemia responses following delayed donor leukocyte infusions. Blood. 2006;108:2106–2113. doi: 10.1182/blood-2006-03-007427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li JM, Southerland LT, Lu Y, et al. Activation, immune polarization, and graft-versus-leukemia activity of donor T cells are regulated by specific subsets of donor bone marrow antigen-presenting cells in allogeneic hemopoietic stem cell transplantation. J Immunol. 2009;183:7799–7809. doi: 10.4049/jimmunol.0900155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Y, Giver CR, Sharma A, et al. IFN-gamma and indoleamine 2,3-dioxygenase signaling between donor dendritic cells and T cells regulates graft versus host and graft versus leukemia activity. Blood. 2012;119:1075–1085. doi: 10.1182/blood-2010-12-322891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chorny A, Gonzalez-Rey E, Fernandez-Martin A, et al. Vasoactive intestinal peptide induces regulatory dendritic cells that prevent acute graft-versus-host disease while maintaining the graft-versus-tumor response. Blood. 2006;107:3787–3794. doi: 10.1182/blood-2005-11-4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naranjo-Gomez M, Raich-Regue D, Onate C, et al. Comparative study of clinical grade human tolerogenic dendritic cells. J Transplant Med. 2011;9:89. doi: 10.1186/1479-5876-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raiotach-Regue D, Grau-Lopez L, Naranjo-Gomez M, et al. Stable antigen-specific T-cell hyporesponsiveness induced by tolerogenic dendritic cells from multiple sclerosis patients. Eur J Immunol. 2012;42:771–782. doi: 10.1002/eji.201141835. [DOI] [PubMed] [Google Scholar]
- 60.Giannoukakis N, Phillips B, Finegold D, et al. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026–2032. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas R. Dendritic cells and the promise of antigen-specific therapy in rheumatoid arthritis. Arthritis Res Ther. 2013;15:204. doi: 10.1186/ar4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.