Mucosal Immunology of the Reproductive Tract
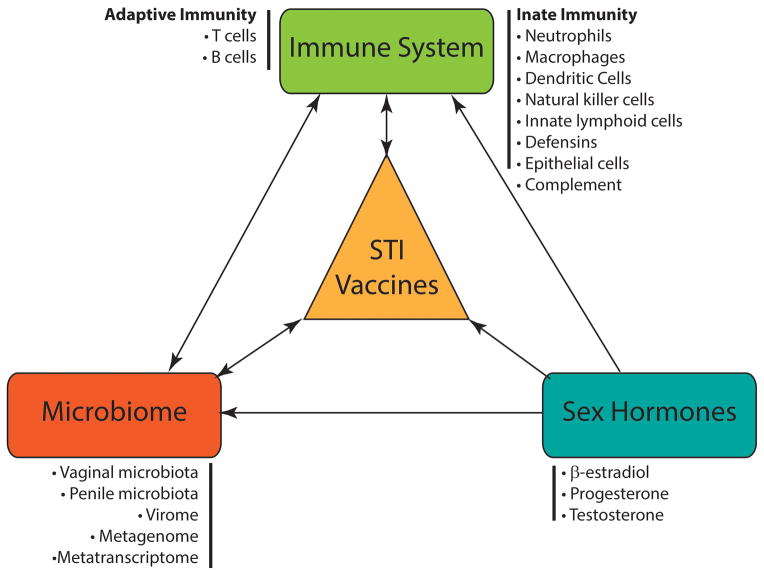
The female and male reproductive tracts are complex compartmentalized systems where immune cells, hormones, and microorganisms interact (Figure 1). The characteristics of the reproductive tract mucosa are distinct from other mucosal sites.[1] Unlike the gastrointestinal and respiratory mucosae, they lack inductive mucoepithelial sites (e.g. Peyer’s patches). As such, a significant proportion of IgG in genital secretions is derived from the local circulation. Sexually transmitted infections, especially chlamydia, can still elicit a strong local IgA and cell-mediated immune response.[2–4] Unlike most other mucosal sites (except the lower respiratory tract), the dominant immunoglobulin in genital secretions is IgG rather than IgA.[5]
Figure 1.
There are multiple potential interactions between the immune system, sex hormones, the microbiome and vaccine efficacy. Some of these interactions may be bidirectional. Vaccines against sexually transmitted pathogens should take into account all of these factors.
The Female Reproductive Tract
The female reproductive tract may be divided into two parts: the lower (vagina and ectocervix) and upper (endocervix, uterus, fallopian tubes) tracts. The lower tract epithelium consists of multiple cell layers of stratified squamous epithelial cells that lack tight junctions allowing the movement of small molecules between the cell lines. The upper tract epithelium consists of a single tightly bound layer of columnar cells. The transition or transformation zone between the two has been shown to be a major effector and inductive site for cell mediated immune responses.[6]
Innate Immunity
The epithelial surfaces of the female reproductive tract are covered with mucus which exhibits microbicidal activity.[7] The epithelial cells actively participate in the innate immune response.[8, 9] In addition to their barrier function, they express pattern recognition receptors (PRRs) that mediate secretion of cytokines, chemokines, and antimicrobial peptides. They are also involved in antigen presentation. Neutrophils are distributed throughout the female genital tract, with the highest numbers in the upper tract. They are involved in phagocytosis, and the production of cytokines and antimicrobial peptides.[10] Antimicrobial peptides, which include defensins, chemokines, antiproteases, and enzymes play an important role in innate responses.[11] Macrophages and dendritic cells are similarly present throughout the female reproductive tract, with higher concentrations in the upper tract.[12] They are involved in phagocytosis and antigen presentation. In addition to their role in antigen presentation, dendritic cells have been shown to be critical players in inducing homing of effector and memory lymphocytes to mucosal tissues and in activation of memory T-cells.[13, 14] These functions highlight their role as an important bridge between the innate and adaptive immune responses. Natural killer (NK) cells are widely distributed, but have a distinct phenotype from NK cells found in the systemic circulation.[15] They produce pro-inflammatory cytokines, promote macrophage activation, and cytotoxic T-cell generation. A newly described population of innate lymphoid cells (ILCs) play a role in regulating epithelial cell responses and maintaining local homeostasis. ILCs have been described in the skin, and in the intestinal and respiratory tracts (NK cells comprise a sub-group of ILCs).[16] Several studies have highlighted the role of commensal bacteria in regulating the development, maintenance, and function of ILCs.[17] Far less is known about ILCs in the reproductive tract.
Adaptive Immunity
The humoral (Th2) arm of the adaptive immune response in the genital tract consists mainly of IgG as well as secretory IgA (sIgA).[18] The ratio of these antibodies varies by site. sIgA is characterized by enhanced neutralizing activity [19, 20] and enhanced resistance to proteolysis [21]. Unlike IgG, sIgA does not activate complement. In addition to local production, there appears to be significant contribution of IgG from the systemic circulation to genital secretions.[22, 23] The uterus is an important source of immunoglobulins in cervicovaginal secretions. T-lymphocytes are found in the stroma of the upper and lower reproductive tract as well as within epithelial cells (intraepithelial lymphocytes).[24] CD8+ T-cells drive Th1 cell-mediated immunity that targets intracellular pathogens. CD4+ T-cells secrete IFN-γ and drive B-cell maturation. Th17 cells play a role in host defense against extracellular pathogens by mediating the recruitment of neutrophils and macrophages to infected tissues.[25, 26] The female reproductive tract restricts entry of activated T-cells in the absence of inflammation or infection.[27] Consequently, parenteral vaccines that rely on cellular immunity to prevent STIs have not been successful. Recently, vaccines that elicit tissue-resident memory T-cell responses have been shown to be feasible [28, 29] and may hold the key to a successful vaccination strategy against herpes simplex viruses and other sexually transmitted pathogens.
The Male Reproductive Tract
In the male reproductive tract, keratinized stratified squamous epithelial cells cover the external surface of the penis. The male urethral orifice consists of a non-keratinized stratified squamous epithelium that transitions in the penile shaft to a pseudostratified columnar epithelium. The urethral epithelium expresses several membrane-associated mucins that act as a first-line of defense.[30] The male reproductive tract is an immune privileged site. For example, tight junctions between Sertoli cells prevent entry of complement and immunoglobulins into the seminiferous tubules. This is referred to as the blood-testis barrier. This relative suppression of adaptive immunity is accompanied by an enhanced innate immune response against local infections. Far less is known about the mucosal immune system of the male reproductive tract than is known about the female tract.
Innate Immunity
Antimicrobial peptides are found in the testes, seminal vesicles, epididymis, and prostate.[31] As with the female reproductive tract, epithelial cells lining the male urethral tract express PRRs and are involved in antigen presentation.[32] Macrophages and dendritic cells are abundant in the prepuce and penile urethra and are found in the epididymis and prostate.[33] They are notably absent in the seminal vesicles. Neutrophils are present in the prepuce and variably present in the urethra, prostate, and epididymis. NK cells have been demonstrated in the prostate, testis, and prepuce.
Adaptive Immunity
IgG is the main immunoglobulin found in seminal plasma and it is serum-derived. IgA, mainly IgA1, is also present and is derived from serum and in situ production. B-cells that produce these antibodies are mainly found in the penile urethra and prostate. CD8+ T-cells and CD4+ T-cells are abundant in the penile urethra and also found in the vas deferens, epididymis, seminiferous tubules, and prepuce. It appears that the penile urethra, with the abundant distribution of immune cells, may be a major site of immune induction.[32]
The human microbiota
“Microbiota” represent an assemblage of microorganisms present in a defined environment. The overwhelming majority of microbial species (>99%) resist cultivation in the laboratory.[34, 35] The development of methods to detect fastidious or non-cultivable organisms through amplification and determination of the sequence of conserved genes, or culture-independent profiling, has precipitated a revolution in microbiology. It relies on amplification and sequencing of the marker genes (such as the 16S ribosomal RNA (rRNA) gene) and has greatly increased appreciation for the complexity, in even seemingly simple microbial consortia, including the genital microbiota.
Microbiota and Immune Responses
Researchers have begun to assert that the human microbiome should be considered in vaccine research.[36] Data is mounting that the gut microbiota plays a role in modulating immune response both locally and systemically.[37–39] Among participants in clinical trials testing the efficacy of oral vaccines against polio, rotavirus and cholera, there were disparities in host immune response outcomes based on geography (developing vs. developed countries). [36] It is hypothesized that the gut microbiota may have contributed to the diverse vaccine efficacy. Ferreira et al. [36] reviewed several studies of probiotic strains which were used for a short time frame, on the order of 1–5 weeks, and concluded that probiotics boosted antibody responses to oral vaccines against rotavirus,[40, 41] Salmonella,[42] poliovirus [43] and Vibrio cholera [44–46]. Among infants who were parenterally administered vaccines against diphtheria, tetanus, Haemophilus influenzae type B, and hepatitis B, probiotics proved beneficial in improving immune responses.[47–49] While these findings are exciting, the mechanism of interaction between the gut microbiota and host responses remains largely unknown. An even more unfamiliar territory is the role of the penile or vaginal microbiota in the context of STI vaccinations.
Vaginal Microbiota
Vaginal bacterial communities are thought to play an important role in preventing colonization by pathogenic organisms, including those responsible for sexually transmitted infections (STIs), vulvovaginal candidiasis, and urinary tract infections.[50, 51] Fundamental differences exist in the microbial diversity of vaginal communities present among reproductive-age women.[52, 53]
Molecular studies based on the 16S rRNA gene have identified over 265 microbial species in the vagina.[52, 54] Composition and relative abundance of these species varies dramatically between women and rapid fluctuations between Lactobacillus-dominated and non-dominated states are common.[52, 54] Lactobacillus spp. play a critical role in maintaining a healthy vagina. It is postulated that lactobacilli restrict the growth of non-indigenous organisms by acidifying the milieu and producing bacteriocins and lactic acid.[55]
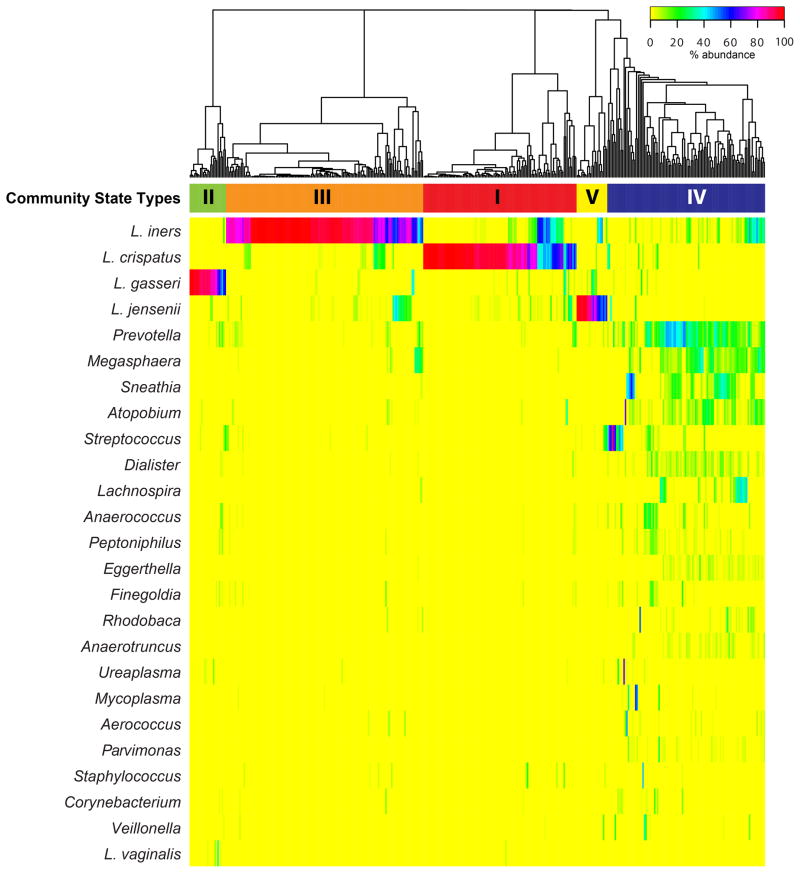
There are five consistent groupings, referred to by Ravel et al. as community state types (CSTs), into which the vaginal microbiota can be categorized (Figure 2).[52] These five CSTs are described as dominated by Lactobacillus crispatus (CST I), Lactobacillus gasseri (CST II), Lactobacillus iners (CST III), or Lactobacillus jensenii (CST V), whereas the fifth (CST IV) has lower proportions of Lactobacillus spp. and higher proportions of anaerobic organisms including BV-associated bacteria [53] such as Prevotella, Megasphaera, Sneathia, and Atopobium. The latter CST was recently split into two states termed CST IV-A and IV-B.[54] CST IV-A is characterized by various species of anaerobic bacteria belonging to the genera Anaerococcus, Peptoniphilus, Prevotella and Streptococcus, while CST IV-B is characterized with higher proportions of the genera Atopobium and Megasphaera among others. (Table 1)
Figure 2.
Heatmap showing the distribution of microbial taxa found in the vaginal microbial communities of 394 reproductive-age women. Adapted with permission from Proceedings of the National Academy of Sciences of the United States of America [52].
Table I.
Community State Types (CST) in the Vaginal Microbiota†
| CST | Dominant bacterial species |
|---|---|
| I | L. crispatus |
| II | L. gasseri |
| III | L. iners |
| IV-A* | Low-Lactobacillus |
| IV-B* | Low-Lactobacillus |
| V | L. jensenii |
CSTs reflect the clustering of samples based on bacterial composition and abundance. Gajer and Brotman et al. previously reported on these 6 CSTs among women in Baltimore, MD.54
CST IV-A is characterized by various species of anaerobic bacteria including Anaerococcus, Peptoniphilus and Prevotella spp., whereas CST IV-B had higher proportions of bacteria from the genera Atopobium and Megasphaera among others.
The human vagina and the bacterial communities that reside therein represent a finely balanced mutualistic association. Dysbiosis of the vaginal microbiology, such as observed in bacterial vaginosis (BV), have been linked to an approximate 2-fold increased risk for acquisition of STIs, including HIV, gonorrhea, chlamydia, trichomoniasis, herpes simplex virus (HSV) and human papillomaviruses (HPV).[56–61] Likewise, BV-associated bacteria have been shown to increase viral replication and vaginal shedding of HIV-1 and HSV-2.[62–67] Although the etiology of BV remains unknown, it is characterized by a relatively low abundance of Lactobacillus spp. and increased abundance of anaerobic bacteria, including G. vaginalis, Prevotella spp., Mobiluncus spp., and A. vaginae as well as other taxa of the order Clostridiales (BVAB1, BVAB2, BVAB3).[53] Enzymes and decarboxylases produced by anaerobic bacteria are thought to degrade proteins to odorific amines, which is characteristic of BV.[68]
The Nugent Gram stain scoring system has a relatively high sensitivity to the diagnosis of BV among symptomatic women.[69] There is also a strong association between CST and Nugent scoring. In Ravel et al.’s study of 394 women, among those who had high Nugent scores, 86.3% were in CST IV, although 13% were classified to L. iners- and 1% to L. gasseri-dominated communities.[52] None of the 105 women classified to L. crispatus-dominated communities had a high Nugent score. That 13% of L. iners dominated communities rank in the high Nugent scores may reflect difficulties in differentiating L. iners from G. vaginalis by Gram stain because of similarities in morphology between the two species.
BV is likely multifactorial in etiology.[70] Numerous epidemiologic investigations have identified factors that increase a woman’s risk to BV. Menstrual blood, a new sexual partner, the number of sex partners, vaginal douching, lack of condom use, and African American ethnicity appear to be among the strongest risk factors for BV.[71–75] The racial disparities may reflect specific host-microbe interactions. The distribution of CSTs also is different among various races/ethnicities (Figure 3), with a higher percentage of African-American and Hispanic women categorized as CST III (L. iners-dominated) or CST IV (low relative abundance of Lactobacillus spp.).[52] Other suspected causative factors for BV include smoking, vaginal lubricants, and the presence of bacteriophages that destroy Lactobacillus spp.[76, 77]
Figure 3.
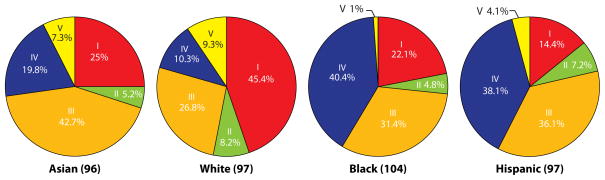
Percentage of vaginal bacterial community state types within each ethnic group of women. The number of women from each ethnic group is in parantheses. Reproduced with permission from Proceedings of the National Academy of Sciences of the United States of America [52].
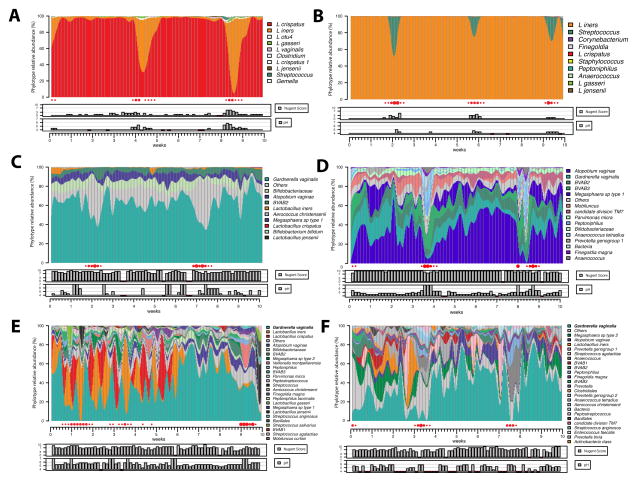
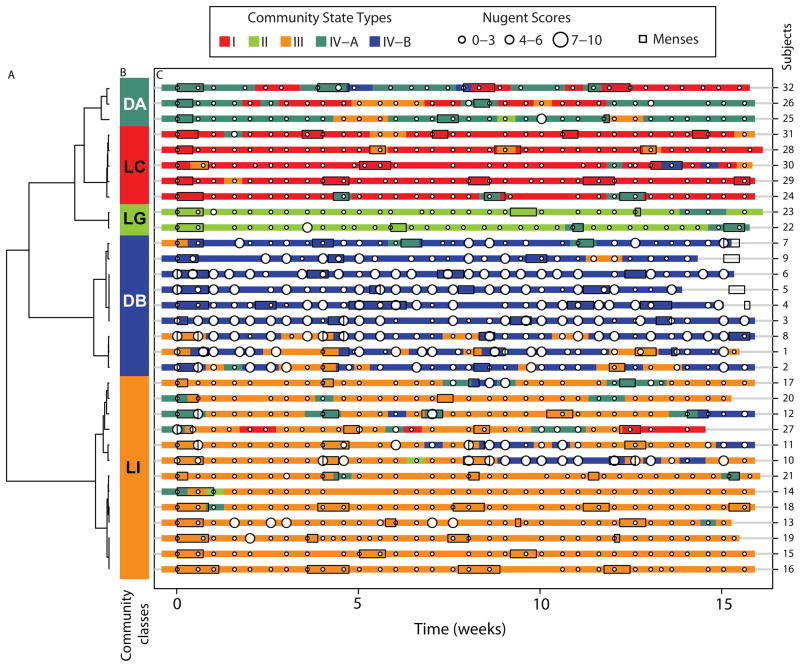
Evaluations of the longitudinal dynamics of bacterial communities has revealed that some communities change markedly over short time periods, whereas others are relatively stable.[54, 78] (Figure 4 and 5) The menstrual cycle is associated with a significant (negative) effect on the stability of the microbiota, but these effects are influenced by bacterial communities.[54] Sexual activity is also associated with lack of stability. Profiles of CSTs can be derived from time series of community samples and clustered into five cohorts, which Gajer and Brotman et al. referred to as community classes.[54] These classes reflect similarities in changes in community composition over time. Some classes were highly dynamic and reflected frequent switches between different CSTs. Classes dominated by L. crispatus and L. gasseri experienced the fewest fluctuations at the level of community composition, however, some communities that lacked significant number of Lactobacillus spp. also demonstrated stability (Figure 5). These communities were stable over time and were observed to have consistently high or intermediate Nugent scores. Vaginal communities dominated by L. iners demonstrated either a lack of constancy or notable stability. L. iners-dominated communities were often seen transitioning to CST IV, a low-Lactobacillus state. These findings are critical, as they highlight a novel concept- there may be intervals of susceptibility to STIs and risk could be established by the frequency and duration of these increased susceptibility events.
Figure 4.
Daily temporal dynamics of vaginal bacterial communities in six women over a 10-week period. The relative abudance of each phylotype is depicted as interpolated bar graphs. Phylotypes color codes are indicated on the right of each bar graph. Daily Nugent scores (range 0–10) and pH (range 4–7) are indicated below the graph. Red solid circles represent menstruation. Missing pH values are indicated by red box, otherwise pH is in line with a value of 4. Missing Nugent scores are also indicated by the red box, otherwise the score is in line with a score of 0. The figures show that the top four participants (A, B, C, D) carry highly stable communities dominated by L. crispatus (A), L. iners (B) and non-Lactobacillus dominated communities (C and D). Women E and F experienced very low stability communities with both high Nugent scores and pH. Unpublished data, personal communication from Ravel and Brotman.
Figure 5.
Profiles of community state types, Nugent scores and menses for 32 women over a 16 week period. (A) Dendogram of distances between proportions of the five communities state types identified and measured within a woman over time. (B) Color bar indicates community class designation and is defined by clusters of proportions of community state types within a woman over time. (C) Profiles of community state types in which Nugent scores have been superimposed. Menses for each woman are indicated by boxes. Each time point is represented by a color-coded community state type (color key on top). Reproduced with permission from Science Translational Medicine.[54]
The microbiome is thought to impact the cervicovaginal mucosal immune responses. Certain bacterial products, particularly from anaerobes, have been shown to result in induction of proinflammatory cytokine production through TLR stimulation [79], dendritic cell activation and maturation,[80] and immune cell migration, apoptosis, and phagocytosis through the production of specific short-chain fatty acids.[81] G. vaginalis, a facultative anaerobe, has been shown to produce sialidases, which are capable of inactivating local IgA.[82]
The vaginal microbiome plays a major role in women’s reproductive health. We are just beginning to understand the temporal dynamics of vaginal bacterial communities, how they shift from a healthy state to a BV-like state, and how the bacterial communities differ in terms of resistance and resilience to internally or externally imposed disturbances. Surprisingly little is known about the composition of vaginal bacteria across the lifespan, how the interactions of the microbiota with vaccines may vary by age, how they differ between individuals, or how we can harness the vaginal microbiome to protect against STIs. Finally, it is worth mentioning that chronic viral infections, our virome, are a stable part of our metagenome.[83] The virome may significantly influence the host’s physiological and immunological responses, adding an additional layer of complexity to these interactions.
Penile Microbiota
The penile microbiome has been less studied than the vaginal microbiota. The coronal sulcus (CS) and distal urethra have distinct bacterial communities.[84] The microbiota in the urine appears to reflect distal urethral microbiota.[85] The CS microbiota appears more stable than the urine microbiota and the composition of the CS microbiota is strongly influenced by circumcision.[84, 86] BV-associated taxa, including Atopobium, Megasphaera, Mobiluncus, Prevotella and Gemella, are detected in CS specimens from both sexually experienced and inexperienced participants.[84] Lactobacilli and streptococci are found in high relative abundance in urine but their abundance is inversely correlated. The penis and the urethra can be colonized by a variety of BV-associated bacteria that may be a result of sexual contact.[84]
Price et al. demonstrated a decrease in anaerobic bacteria of the penile coronoal sulci after medical male circumcision (MMC).[86] It is hypothesized that circumcision may reduce genital mucosal inflammation by altering microbial burden. Randomized controlled trials have shown MMC reduces the risk of HIV and STI acquisition, including HSV and HPV in men and HPV, BV and T. vaginalis in women.[87–89]
Sex Hormones and the Reproductive Tract
Sex Hormones and Immune Responses
The interaction between sex hormones and the immune system is complex. Most of the published data have focused on the female reproductive tract. Limited data exist for the male reproductive tract.
Immune responses in the female genital tract are regulated by sex hormones: Antigen presentation, cytokine production, immunoglobulin production and transport, and induction of tolerance have all been shown to be influenced by variations in the levels of sex hormones.[9, 90] In addition, the impact of sex hormones appears to differ between the lower and upper genital tract in women. Most cells in the reproductive tract express estradiol receptors (epithelial cells, macrophages, stromal cells, and lymphocytes). There appears to be some consistency in hormonal effects on lower genital tract immunity- namely, a dampening of cervicovaginal immune responses around the time- and for a short period of time following ovulation.[91] This is consistent with the body’s attempt to optimize the environment to promote successful fertilization and subsequent embryo development. Some investigators have defined the term “window of vulnerability” that begins shortly before ovulation (around day 12 of a normal menstrual cycle- the pre-ovulatory follicular phase at the time of the β-estradiol peak) and persists until around day 21 (mid luteal phase around the time of the progestational peak).[92] During this period, mucus of low viscosity is produced rather than the protective viscous mucus produced at other times (this helps to promote sperm motility, but also enhances access to pathogens). Interestingly, that is the period when the microbiota exhibit its highest level of stability.[54] Immunoglobulin and antimicrobial peptide levels in the lower tract are low (but antimicrobial peptide levels increase in the upper tract).[18, 93–95] Cell-mediated immunity is also affected by sex hormone levels.[90] In the upper tract, cellular immunity is high during the follicular phase, but declines during the luteal phase-most likely to optimize implantation. In the lower tract, cellular responses, particularly cytotoxic T-cell responses appear to be elevated throughout the menstrual cycle independent of hormonal stimulation.
The use of exogenous sex hormones, i.e. hormonal contraception (HC), by hundreds of millions of women worldwide, further complicates the picture. There has been a great deal of interest in studying the impact of sex hormones (both endogenous and exogenous) on susceptibility to STIs. Animal and cell-culture models have long suggested that sex hormones modify the risk of some lower genital infections, including HIV. Epidemiological studies in humans have yielded conflicting results.[96] Part of the inconsistency has been attributed to significant behavioral confounding factors in these studies. However, other biological explanations are possible- even probable. Most of the studies did not correlate systemic hormone levels to the measured outcome, and many did not take into account duration of exogenous hormone exposure.[96, 97] For example, duration of HC use has been shown to have a direct impact on susceptibility to infection and to be a critical factor in the development of immune responses to infection (see ‘Vaccines and Sex Hormones’, below).
Sex Hormones and the Microbiota
An intriguing study was conducted in 29 healthy women initiating oral contraception.[98] Gingival sulcus specimens were obtained prior to HC initiation (HC has been associated with increased risk of gingivitis in some studies), 10 days post initiation, and 3 weeks later. There was little change in the microbial communities between pre-HC and 10 days post HC but at 3 weeks post-HC, a striking increase in the number of Prevotella species was noted. This small study suggests that mucosal microbial communities are affected by sex hormones and that duration of exposure may be a critical variable. The impact of sex hormones on the vaginal microbiome has not yet been determined, but the estrogen stimulated accumulation of glycogen in the vaginal epithelium is thought to play a major role in maintaining a protective Lactobacillus-dominated microbiota.
Data from our group and others suggest that the use of certain types of hormonal contraceptives may decrease the risk of disruptions in the vaginal microbiota as defined by the clinical syndrome of BV.[99–103] HC may exert their effects on the vaginal microbiota in at least two different ways. As mentioned above, estrogen stimulates glycogen accumulation in vaginal epithelial cells, in turn, glycogen could serve as a carbohydrate source for Lactobacillus spp. [104] The end product, lactic acid, helps vaginal fluid maintain low pH and prevents the overgrowth of bacteria associated with BV.[55] Studies have also suggested an association between higher estrogen serum levels and reduced BV prevalence.[105] The other mechanism by which HC, especially progestin, may affect the vaginal microbiota is through its inhibitory effect on uterine bleeding. Menstruation has been positively correlated with low Lactobacillus vaginal microbiota.[54, 75] Data from cohorts of pregnant women also suggest stability of the microbiota during pregnancy.[106]
Vaccines and the Reproductive Tract
Parenteral vaccines against mucosal pathogens of the genital tract have been successful, particularly when they induce strong serum IgG levels that cross mucosal epithelia to provide local protection. The HPV vaccine is the most obvious example.[107].
There are only a few examples of mucosal vaccines (oral polio, cholera, and influenza). Several factors have hindered the development of effective mucosal vaccines. Mucosal immune responses are, to a certain extent, compartmentalized. While vaginal, intranasal, and sublingual immunizations have been found to elicit adequate genital mucosal immune responses- the intranasal route, oral and rectal routes of immunization have been less successful.[108] In rodent models, the combination of parenteral and intranasal routes of immunization yielded the best outcome when comparing combination approaches. Very few studies have been performed in humans. In one of the few studies conducted in women, vaginal immunization with the B subunit of cholera toxin resulted in higher cervicovaginal antibody responses compared to the oral and rectal immunization routes.[109] In men, parenteral and systemic immunizations resulted in the detection of IgG and IgA antibodies in semen. Intranasal and rectal routes of immunization have not been well explored in men.
Another challenge of mucosal vaccination is immunological tolerance.[110] Most mucosal sites tend to exhibit mucosal tolerance via induction of regulatory T-cells (Treg) that dampen immune responses following antigen exposure. To overcome this tendency for tolerance, mucosal vaccines must be potent. Potency may be enhanced by the use of live vaccines, whole cell vaccines that express one or more pathogen-associated molecular pattern (PAMP), and/or the use of adjuvants.
Vaccines and Sex Hormones
The impact of endogenous and exogenous sex hormones on mucosal immune responses must be considered when trying to optimize vaccine responses in the genital tract. The importance of this concept has been clearly demonstrated in animal models. Using a mouse model, the use of depot medroxyprogesterone acetate (DMPA) increased susceptibility to HSV-2 infection >100 fold.[111] A significant lowering of antibody responses to HSV-2 in DMPA-treated mice following immunization with a HSV-2 vaccine was also reported. Most intriguing was the incidental observation that the duration of DMPA use prior to HSV-2 challenge affected the immune response to future re-challenge. In an elegant study, mice immunized intravaginally with an attenuated strain of HSV-2 following longer (15 days) exposure to DMPA (DMPA-15 group) failed to show protection when challenged with wild-type HSV-2.[112] In contrast, mice that were immunized shortly after DMPA treatment (DMPA-5 group), were fully protected and showed no genital pathology after HSV-2 challenge. High viral replication titers, low levels of gamma interferon, dampening of TH1 responses, and poor specific antibody responses characterized the DMPA-15 group in contrast to the DMPA-5 group. These experiments demonstrate that duration of HC use may impact innate and acquired immune responses, thereby influencing the susceptibility to and course of the infection.
Far less is known about the impact of sex hormones on responses to vaccines in humans. A study by Johansson et al. highlights the potentially critical role of sex hormones: In 21 volunteers who received a mucosal vaccine containing cholera toxin B antigen, the investigators administered the vaccine either independently of the menstrual stage or on days 10 and 24 in the cycle in different groups of participants.[113] Vaginal and nasal vaccinations both resulted in significant IgA and IgG anti-cholera toxin B subunit responses in serum in the majority of the volunteers in the various vaccination groups. Only vaginal vaccination given on days 10 and 24 in the cycle induced strong specific antibody responses in the cervix. In another study, women who received the parenteral HPV vaccine had the highest levels of cervical IgG and IgA detected during the follicular phase of the cycle, and these levels decreased significantly around the time of ovulation.[114]
In an era where much of the hope of future STI control lies in vaccine development, the effects of endogenous and exogenous sex hormones on mucosal and systemic immune responses must be critically evaluated.
Vaccines and the Microbiome
There are no studies that evaluate the association between the vaginal microbiota and successful vaccination. These studies are critical and could lead to a novel dual approach to STI prevention which integrates (1) vaccines and (2) control of the microbiota. To achieve these goals, continued efforts to better understand bacterial community dynamics over time (inter-bacterial and bacterial-host) are necessary. Such studies would lead to the development of interventions to maintain a healthy microbiota. For example, the development of personalized pre-biotics that would maintain a healthy vaginal microbiota, preventing adverse ecological shifts, or of probiotic mixtures that could seed a microbial community to restore and/or maintain a healthy environment, may be envisionned. Numerous studies have already demonstrated the positive effect of probiotics (primarily through boosting Lactobacillus) on prevention of infections in the female reproductive tract. Unfortunately, there is little rationale for the selection of probiotic strains; none consider the differences in vaginal microbiota observed among women and there are few well-designed randomized placebo-controlled studies. The application of genomic technologies represent a major step towards achieving this goal. Personalized treatments could be geared toward a better appreciation of species-specific and temporal changes in microbiota.
Conclusions
The success of the HPV vaccine (reviewed by Schiller et al. [115]) has re-energized the field of STI vaccine research after earlier disappointing results with HSV [116, 117] and gonorrhea [118, 119] vaccines. There are currently several new candidate HSV and chlamydia vaccines in various stages of development and recent advances in the fields of immunology and vaccine design offer hope for the development of vaccines targeting gonorrhea and syphilis.[120] To optimize vaccine responses against STIs, in addition to optimizing antigen types, formulations, adjuvants, and delivery methods,[121–123] we need a clear understanding of the interactions taking place at the mucosal surfaces. Vaccine development must take into account the differences between the systemic and mucosal immune responses, the compartmentalization of the mucosal immune responses, the unique characteristics of the reproductive tract mucosae, the role of the microbiome, the impact of sex hormones, and the interactions among all of these factors. We are just beginning to decipher these complex relationships.
Highlights.
The reproductive tract is a unique mucosa that lacks inductive mucoepithelial sites
Reproductive tract mucosal immune responses are compartmentalized
Dampening of immune responses around the time of ovulation has been observed
Immune responses are affected by resident bacterial communities and sex hormones
Successful vaccines against STIs must take into account its unique environment
Acknowledgments
Sources of financial support: This study was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers K01-AI080974 (Brotman), U19-AI084044 (Ravel, Bavoil) and R01-AI089878 (Ghanem). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rebecca M. Brotman, Email: rbrotman@som.umaryland.edu.
Jacques Ravel, Email: jravel@som.umaryland.edu.
Patrik M. Bavoil, Email: PBavoil@umaryland.edu.
Patti E. Gravitt, Email: pgravitt@jhsph.edu.
Khalil G. Ghanem, Email: kghanem@jhmi.edu.
References
- 1.McDermott MR, Clark DA, Bienenstock J. Evidence for a common mucosal immunologic system. II. Influence of the estrous cycle on B immunoblast migration into genital and intestinal tissues. J Immunol. 1980;124:2536–9. [PubMed] [Google Scholar]
- 2.Terho P, Meurman O. Chlamydial serum IgG, IgA and local IgA antibodies in patients with genital-tract infections measured by solid-phase radioimmunoassay. Journal of Medical Microbiology. 1981;14:77–87. doi: 10.1099/00222615-14-1-77. [DOI] [PubMed] [Google Scholar]
- 3.Kelly KA, Rank RG. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infection and Immunity. 1997;65:5198–208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cain TK, Rank RG. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infection and Immunity. 1995;63:1784–9. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell MW, Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002;4:667–77. doi: 10.1016/s1286-4579(02)01585-x. [DOI] [PubMed] [Google Scholar]
- 6.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–63. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–5. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol. 2010;63:544–65. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wira CR, Ghosh M, Smith JM, Shen L, Connor RI, Sundstrom P, et al. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal immunology. 2011;4:335–42. doi: 10.1038/mi.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selsted ME, Ouellette AJ. Defensins in granules of phagocytic and non-phagocytic cells. Trends in cell biology. 1995;5:114–9. doi: 10.1016/s0962-8924(00)88961-8. [DOI] [PubMed] [Google Scholar]
- 11.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 12.Iijima N, Thompson JM, Iwasaki A. Dendritic cells and macrophages in the genitourinary tract. Mucosal immunology. 2008;1:451–9. doi: 10.1038/mi.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–7. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luci C, Hervouet C, Rousseau D, Holmgren J, Czerkinsky C, Anjuère F. Dendritic cell-mediated induction of mucosal cytotoxic responses following intravaginal immunization with the nontoxic B subunit of cholera toxin. J Immunol. 2006;176:2749–57. doi: 10.4049/jimmunol.176.5.2749. [DOI] [PubMed] [Google Scholar]
- 15.Mselle TF, Meadows SK, Eriksson M, Smith JM, Shen L, Wira CR, et al. Unique characteristics of NK cells throughout the human female reproductive tract. Clinical immunology (Orlando, Fla) 2007;124:69–76. doi: 10.1016/j.clim.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Sonnenberg GF, Artis D. Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity. 2012;37:601–10. doi: 10.1016/j.immuni.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veldhoen M, Withers DR. Immunology. Innate lymphoid cell relations. Science. 2010;330:594–5. doi: 10.1126/science.1198298. [DOI] [PubMed] [Google Scholar]
- 18.Mestecky J, Raska M, Novak J, Alexander RC, Moldoveanu Z. Antibody-mediated protection and the mucosal immune system of the genital tract: relevance to vaccine design. Journal of reproductive immunology. 2010;85:81–5. doi: 10.1016/j.jri.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renegar KB, Jackson GD, Mestecky J. In vitro comparison of the biologic activities of monoclonal monomeric IgA, polymeric IgA, and secretory IgA. J Immunol. 1998;160:1219–23. [PubMed] [Google Scholar]
- 20.Russell MW, Reinholdt J, Kilian M. Anti-inflammatory activity of human IgA antibodies and their Fab alpha fragments: inhibition of IgG-mediated complement activation. Eur J Immunol. 1989;19:2243–9. doi: 10.1002/eji.1830191210. [DOI] [PubMed] [Google Scholar]
- 21.Corthesy B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J Immunol. 2007;178:27–32. doi: 10.4049/jimmunol.178.1.27. [DOI] [PubMed] [Google Scholar]
- 22.Naz RK, Menge AC. Antisperm antibodies: origin, regulation, and sperm reactivity in human infertility. Fertil Steril. 1994;61:1001–13. doi: 10.1016/s0015-0282(16)56747-8. [DOI] [PubMed] [Google Scholar]
- 23.Kutteh WH, Moldoveanu Z, Mestecky J. Mucosal immunity in the female reproductive tract: correlation of immunoglobulins, cytokines, and reproductive hormones in human cervical mucus around the time of ovulation. AIDS Res Hum Retroviruses. 1998;14 (Suppl 1):S51–5. [PubMed] [Google Scholar]
- 24.Kamat BR, Isaacson PG. The immunocytochemical distribution of leukocytic subpopulations in human endometrium. The American journal of pathology. 1987;127:66–73. [PMC free article] [PubMed] [Google Scholar]
- 25.Feinen B, Jerse AE, Gaffen SL, Russell MW. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal immunology. 2010;3:312–21. doi: 10.1038/mi.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chege D, Chai Y, Huibner S, Kain T, Wachihi C, Kimani M, et al. Blunted IL17/IL22 and pro-inflammatory cytokine responses in the genital tract and blood of HIV-exposed, seronegative female sex workers in Kenya. PloS one. 2012;7:e43670. doi: 10.1371/journal.pone.0043670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–3. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuburu N, Graham BS, Buck CB, Kines RC, Pang YY, Day PM, et al. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J Clin Invest. 2012;122:4606–20. doi: 10.1172/JCI63287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–7. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russo CL, Spurr-Michaud S, Tisdale A, Pudney J, Anderson D, Gipson IK. Mucin gene expression in human male urogenital tract epithelia. Hum Reprod. 2006;21:2783–93. doi: 10.1093/humrep/del164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lievin-Le Mo V, Servin al AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315–37. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pudney J, Anderson DJ. Immunobiology of the human penile urethra. The American journal of pathology. 1995;147:155–65. [PMC free article] [PubMed] [Google Scholar]
- 33.Pudney J, Anderson D. Innate and acquired immunity in the human penile urethra. Journal of reproductive immunology. 2011;88:219–27. doi: 10.1016/j.jri.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bomar L, Maltz M, Colston S, Graf J. Directed Culturing of Microorganisms Using Metatranscriptomics. mBio. 2011;2:e00012-11-e-11. doi: 10.1128/mBio.00012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–69. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira RBR, Antunes LCM, Finlay BB. Should the Human Microbiome Be Considered When Developing Vaccines? PLoS pathogens. 2010;6:e1001190. doi: 10.1371/journal.ppat.1001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gill N, Finlay BB. The gut microbiota: challenging immunology. Nat Rev Immunol. 2011;11:636–7. doi: 10.1038/nri3061. [DOI] [PubMed] [Google Scholar]
- 38.Eloe-Fadrosh EA, McArthur MA, Seekatz AM, Drabek EF, Rasko DA, Sztein MB, et al. Impact of oral typhoid vaccination on the human gut microbiota and correlations with s. Typhi-specific immunological responses. PloS one. 2013;8:e62026. doi: 10.1371/journal.pone.0062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bjorksten B. Diverse microbial exposure - consequences for vaccine development. Vaccine. 2012;30:4336–40. doi: 10.1016/j.vaccine.2011.10.074. [DOI] [PubMed] [Google Scholar]
- 40.Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine. 1995;13:310–2. doi: 10.1016/0264-410x(95)93319-5. [DOI] [PubMed] [Google Scholar]
- 41.Azevedo MSP, Zhang W, Wen K, Gonzalez AM, Saif LJ, Yousef AE, et al. Lactobacillus acidophilus and Lactobacillus reuteri modulate cytokine responses in gnotobiotic pigs infected with human rotavirus. Beneficial Microbes. 2012;3:33–42. doi: 10.3920/BM2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang H, Elina T, Heikki A, Seppo S. Modulation of humoral immune response through probiotic intake. FEMS Immunology & Medical Microbiology. 2000;29:47–52. doi: 10.1111/j.1574-695X.2000.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 43.Vrese M, Rautenberg P, Laue C, Koopmans M, Herremans T, Schrezenmeir J. Probiotic bacteria stimulate virus–specific neutralizing antibodies following a booster polio vaccination. Eur J Nutr. 2004;44:406–13. doi: 10.1007/s00394-004-0541-8. [DOI] [PubMed] [Google Scholar]
- 44.Focareta A, Paton JC, Mo rona R, Cook J, Paton AW. A Recombinant Probiotic for Treatment and Prevention of Cholera. Gastroenterology. 2006;130:1688–95. doi: 10.1053/j.gastro.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Paineau D, Carcano D, Leyer G, Darquy S, Alyanakian M-A, Simoneau G, et al. Effects of seven potential probiotic strains on specific immune responses in healthy adults: a double-blind, randomized, controlled trial. FEMS Immunology & Medical Microbiology. 2008;53:107–13. doi: 10.1111/j.1574-695X.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- 46.Matsuda F, Chowdhury MI, Saha A, Asahara T, Nomoto K, Tarique AA, et al. Evaluation of a probiotics, Bifidobacterium breve BBG-01, for enhancement of immunogenicity of an oral inactivated cholera vaccine and safety: A randomized, double-blind, placebo-controlled trial in Bangladeshi children under 5 years of age. Vaccine. 2011;29:1855–8. doi: 10.1016/j.vaccine.2010.12.133. [DOI] [PubMed] [Google Scholar]
- 47.Kukkonen K, Nieminen T, Poussa T, Savilahti E, Kuitunen M. Effect of probiotics on vaccine antibody responses in infancy – a randomized placebo-controlled double-blind trial. Pediatr Allergy Immunol. 2006;17:416–21. doi: 10.1111/j.1399-3038.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 48.West CE, Gothefors L, Granström M, Käyhty H, Hammarström M-LKC, Hernell O. Effects of feeding probiotics during weaning on infections and antibody responses to diphtheria, tetanus and Hib vaccines. Pediatric Allergy and Immunology. 2007;19:53–60. doi: 10.1111/j.1399-3038.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 49.Soh SE, Ong DQR, Gerez I, Zhang X, Chollate P, Shek LP-C, et al. Effect of probiotic supplementation in the first 6 months of life on specific antibody responses to infant Hepatitis B vaccination. Vaccine. 2010;28:2577–9. doi: 10.1016/j.vaccine.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 50.Sl H. Normal vaginal flora. In: Holmes KkSPFMPALSMSWEPPWJM., editor. Sexually transmitted diseases. New York: McGraw-Hill; 2008. pp. 289–307. [Google Scholar]
- 51.Kirjavainen PV, Pautler S, Baroja ML, Anukam K, Crowley K, Carter K, et al. Abnormal immunological profile and vaginal microbiota in women prone to urinary tract infections. Clinical and vaccine immunology : CVI. 2009;16:29–36. doi: 10.1128/CVI.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108 (Suppl 1):4680–7. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. The New England journal of medicine. 2005;353:1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 54.Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UM, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Science translational medicine. 2012;4:132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Hanlon DE, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11:200. doi: 10.1186/1471-2334-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peipert JF, Lapane KL, Allsworth JE, Redding CA, Blume JD, Stein MD. Bacterial vaginosis, race, and sexually transmitted infections: does race modify the association? Sex TransmDis. 2008;35:363–7. doi: 10.1097/OLQ.0b013e31815e4179. [DOI] [PubMed] [Google Scholar]
- 57.Brotman RM, Klebanoff MA, Nansel TR, Yu KF, Andrews WW, Zhang J, et al. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis. 2010;202:1907–15. doi: 10.1086/657320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2003;37:319–25. doi: 10.1086/375819. [DOI] [PubMed] [Google Scholar]
- 59.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–8. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 60.King CC, Jamieson DJ, Wiener J, Cu-Uvin S, Klein RS, Rompalo AM, et al. Bacterial vaginosis and the natural history of human papillomavirus. Infectious diseases in obstetrics and gynecology. 2011;2011:319460. doi: 10.1155/2011/319460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myer L, Denny L, Telerant R, Souza M, Wright TC, Jr, Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. The Journal of infectious diseases. 2005;192:1372–80. doi: 10.1086/462427. [DOI] [PubMed] [Google Scholar]
- 62.Cu-Uvin S, Hogan JW, Caliendo AM, Harwell J, Mayer KH, Carpenter CC, et al. Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2001;33:894–6. doi: 10.1086/322613. [DOI] [PubMed] [Google Scholar]
- 63.Sha BE, Zariffard MR, Wang QJ, Chen HY, Bremer J, Cohen MH, et al. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Dis. 2005;191:25–32. doi: 10.1086/426394. [DOI] [PubMed] [Google Scholar]
- 64.Coleman JS, Hitti J, Bukusi EA, Mwachari C, Muliro A, Nguti R, et al. Infectious correlates of HIV-1 shedding in the female upper and lower genital tracts. AIDS (London, England) 2007;21:755–9. doi: 10.1097/QAD.0b013e328012b838. [DOI] [PubMed] [Google Scholar]
- 65.Mitchell C, Balkus JE, Fredricks D, Liu C, McKernan-Mullin J, Frenkel LM, et al. Interaction Between Lactobacilli, Bacterial Vaginosis-Associated Bacteria, and HIV Type 1 RNA and DNA Genital Shedding in U.S. and Kenyan Women. AIDS Res Hum Retroviruses. 2013;29:13–9. doi: 10.1089/aid.2012.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier SL. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B Streptococcus colonization. Clin Infect Dis. 2005;40:1422–8. doi: 10.1086/429622. [DOI] [PubMed] [Google Scholar]
- 67.Cohen CR, Lingappa JR, Baeten JM, Ngayo MO, Spiegel CA, Hong T, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med. 2012;9:e1001251. doi: 10.1371/journal.pmed.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sobel JD. Bacterial vaginosis. Annu Rev Med. 2000;51:349–56. doi: 10.1146/annurev.med.51.1.349. [DOI] [PubMed] [Google Scholar]
- 69.Schwebke JR, Hillier SL, Sobel JD, McGregor JA, Sweet RL. Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. Obstetrics and gynecology. 1996;88:573–6. doi: 10.1016/0029-7844(96)00233-5. [DOI] [PubMed] [Google Scholar]
- 70.Marrazzo JM. A persistent(ly) enigmatic ecological mystery: bacterial vaginosis. The Journal of infectious diseases. 2006;193:1475–7. doi: 10.1086/503783. [DOI] [PubMed] [Google Scholar]
- 71.Fethers KA, Fairley CK, Hocking JS, Gurrin LC, Bradshaw CS. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;47:1426–35. doi: 10.1086/592974. [DOI] [PubMed] [Google Scholar]
- 72.Brotman RM, Klebanoff MA, Nansel TR, Andrews WW, Schwebke JR, Zhang J, et al. A longitudinal study of vaginal douching and bacterial vaginosis--a marginal structural modeling analysis. Am J Epidemiol. 2008;168:188–96. doi: 10.1093/aje/kwn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwebke JR. Bacterial vaginosis: are we coming full circle? J Infect Dis. 2009;200:1633–5. doi: 10.1086/648093. [DOI] [PubMed] [Google Scholar]
- 74.Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex TransmDis. 2007;34:864–9. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 75.Eschenbach DA, Thwin SS, Patton DL, Hooton TM, Stapleton AE, Agnew K, et al. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000;30:901–7. doi: 10.1086/313818. [DOI] [PubMed] [Google Scholar]
- 76.Pavlova SI, Tao L. Induction of vaginal Lactobacillus phages by the cigarette smoke chemical benzo[a]pyrene diol epoxide. Mutat Res. 2000;466:57–62. doi: 10.1016/s1383-5718(00)00003-6. [DOI] [PubMed] [Google Scholar]
- 77.Marrazzo JM, Koutsky LA, Eschenbach DA, Agnew K, Stine K, Hillier SL. Characterization of vaginal flora and bacterial vaginosis in women who have sex with women. The Journal of infectious diseases. 2002;185:1307–13. doi: 10.1086/339884. [DOI] [PubMed] [Google Scholar]
- 78.Srinivasan S, Liu C, Mitchell CM, Fiedler TL, Thomas KK, Agnew KJ, et al. Temporal Variability of Human Vaginal Bacteria and Relationship with Bacterial Vaginosis. PLoS ONE. 2010;5:e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zariffard MR, Novak RM, Lurain N, Sha BE, Graham P, Spear GT. Induction of tumor necrosis factor- alpha secretion and toll-like receptor 2 and 4 mRNA expression by genital mucosal fluids from women with bacterial vaginosis. J Infect Dis. 2005;191:1913–21. doi: 10.1086/429922. [DOI] [PubMed] [Google Scholar]
- 80.St John EP, Martinson J, Simoes JA, Landay AL, Spear GT. Dendritic cell activation and maturation induced by mucosal fluid from women with bacterial vaginosis. Clinical immunology (Orlando, Fla) 2007;125:95–102. doi: 10.1016/j.clim.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mirmonsef P, Gilbert D, Zariffard MR, Hamaker BR, Kaur A, Landay AL, et al. The effects of commensal bacteria on innate immune responses in the female genital tract. Am J Reprod Immunol. 2011;65:190–5. doi: 10.1111/j.1600-0897.2010.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lewis WG, Robinson LS, Perry J, Bick JL, Peipert JF, Allsworth JE, et al. Hydrolysis of Secreted Sialoglycoprotein Immunoglobulin A (IgA) in ex Vivo and Biochemical Models of Bacterial Vaginosis. J Biol Chem. 2012;287:2079–89. doi: 10.1074/jbc.M111.278135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 84.Nelson DE, Dong Q, Van der Pol B, Toh E, Fan B, Katz BP, et al. Bacterial communities of the coronal sulcus and distal urethra of adolescent males. PloS one. 2012;7:e36298. doi: 10.1371/journal.pone.0036298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong Q, Nelson DE, Toh E, Diao L, Gao X, Fortenberry JD, et al. The microbial communities in male first catch urine are highly similar to those in paired urethral swab specimens. PLoS One. 2011;6:e19709. doi: 10.1371/journal.pone.0019709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Price LB, Liu CM, Johnson KE, Aziz M, Lau MK, Bowers J, et al. The effects of circumcision on the penis microbiome. PLoS One. 2010;5:e8422. doi: 10.1371/journal.pone.0008422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tobian AAR, Gray RH. The medical benefits of male circumcision. JAMA : the journal of the American Medical Association. 2011;306:1479–80. doi: 10.1001/jama.2011.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mehta SD, Gaydos C, MacLean I, Odoyo-June E, Moses S, Agunda L, et al. The effect of medical male circumcision on urogenital Mycoplasma genitalium among men in Kisumu, Kenya. Sexually Transmitted Diseases. 2012;39:276–80. doi: 10.1097/OLQ.0b013e318240189c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mehta SD, Moses S, Parker CB, Agot K, MacLean I, Bailey RC. Circumcision status and incident herpes simplex virus type 2 infection, genital ulcer disease, and HIV infection. AIDS (London, England) 2012;26:1141–9. doi: 10.1097/QAD.0b013e328352d116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunological reviews. 2005;206:306–35. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 91.Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65:196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–17. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kyongo JK, Jespers V, Goovaerts O, Michiels J, Menten J, Fichorova RN, et al. Searching for lower female genital tract soluble and cellular biomarkers: defining levels and predictors in a cohort of healthy Caucasian women. PLoS One. 2012;7:e43951. doi: 10.1371/journal.pone.0043951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumar R, Vicari M, Gori I, Achtari C, Fiche M, Surbeck I, et al. Compartmentalized secretory leukocyte protease inhibitor expression and hormone responses along the reproductive tract of postmenopausal women. Journal of reproductive immunology. 2011;92:88–96. doi: 10.1016/j.jri.2011.06.103. [DOI] [PubMed] [Google Scholar]
- 95.Fahey JV, Wira CR. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis. 2002;185:1606–13. doi: 10.1086/340512. [DOI] [PubMed] [Google Scholar]
- 96.Morrison CS, Turner AN, Jones LB. Highly effective contraception and acquisition of HIV and other sexually transmitted infections. Best practice & research Clinical obstetrics & gynaecology. 2009;23:263–84. doi: 10.1016/j.bpobgyn.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 97.Blish CA, Baeten JM. Hormonal contraception and HIV-1 transmission. American journal of reproductive immunology (New York, NY: 1989) 2011;65:302–7. doi: 10.1111/j.1600-0897.2010.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klinger G, Eick S, Klinger G, Pfister W, Graser T, Moore C, et al. Influence of hormonal contraceptives on microbial flora of gingival sulcus. Contraception. 1998;57:381–4. doi: 10.1016/s0010-7824(98)00044-4. [DOI] [PubMed] [Google Scholar]
- 99.Riggs M, Klebanoff M, Nansel T, Zhang J, Schwebke J, Andrews W. Longitudinal association between hormonal contraceptives and bacterial vaginosis in women of reproductive age. Sex TransmDis. 2007;34:954–9. [PubMed] [Google Scholar]
- 100.Rifkin SB, Smith MR, Brotman RM, Gindi RM, Erbelding EJ. Hormonal contraception and risk of bacterial vaginosis diagnosis in an observational study of women attending STD clinics in Baltimore, MD. Contraception. 2009;80:63–7. doi: 10.1016/j.contraception.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 101.McClelland RS, Richardson BA, Graham SM, Masese LN, Gitau R, Lavreys L, et al. A prospective study of risk factors for bacterial vaginosis in HIV-1-seronegative African women. Sexually transmitted diseases. 2008;35:617–23. doi: 10.1097/OLQ.0b013e31816907fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193:1478–86. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 103.Smart S, Singal A, Mindel A. Social and sexual risk factors for bacterial vaginosis. Sex Transm Infect. 2004;80:58–62. doi: 10.1136/sti.2003.004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cruickshank R. The conversion of the glycogen of the vagina into lactic acid. The Journal of Pathology and Bacteriology. 1934 [Google Scholar]
- 105.Preti G, Huggins G. The Human vagina. Amsterdam/New York: Elsevier North-Holland Biomedical Press; 1978. [Google Scholar]
- 106.Romero R. The vaginal microbiome during pregnancy and before preterm labor and delivery. 59th Annual Meeting of the Society for Gynecological Investigations; San Diego, CA. 2012. [Google Scholar]
- 107.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 108.Naz RK. Female genital tract immunity: distinct immunological challenges for vaccine development. Journal of reproductive immunology. 2012;93:1–8. doi: 10.1016/j.jri.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 109.Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun. 1997;65:1387–94. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Russell MW, Mestecky J. Tolerance and protection against infection in the genital tract. Immunological investigations. 2010;39:500–25. doi: 10.3109/08820131003674834. [DOI] [PubMed] [Google Scholar]
- 111.Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77:4558–65. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol. 2003;77:9845–51. doi: 10.1128/JVI.77.18.9845-9851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Johansson EL, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infection and immunity. 1998;66:514–20. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nardelli-Haefliger D, Wirthner D, Schiller JT, Lowy DR, Hildesheim A, Ponci F, et al. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J Natl Cancer Inst. 2003;95:1128–37. doi: 10.1093/jnci/djg018. [DOI] [PubMed] [Google Scholar]
- 115.Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Micro. 2012;10:681–92. doi: 10.1038/nrmicro2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. The New England journal of medicine. 2002;347:1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 117.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, et al. Efficacy results of a trial of a herpes simplex vaccine. The New England journal of medicine. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boslego JW, Tramont EC, Chung RC, McChesney DG, Ciak J, Sadoff JC, et al. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine. 1991;9:154–62. doi: 10.1016/0264-410x(91)90147-x. [DOI] [PubMed] [Google Scholar]
- 119.Greenberg L, Diena BB, Ashton FA, Wallace R, Kenny CP, Znamirowski R, et al. Gonococcal vaccine studies in Inuvik. Can J Public Health. 1974;65:29–33. [PubMed] [Google Scholar]
- 120.Huston WM, Harvie M, Mittal A, Timms P, Beagley KW. Vaccination to protect against infection of the female reproductive tract. Expert Rev Clin Immunol. 2012;8:81–94. doi: 10.1586/eci.11.80. [DOI] [PubMed] [Google Scholar]
- 121.Fukuyama Y, Tokuhara D, Kataoka K, Gilbert RS, McGhee JR, Yuki Y, et al. Novel vaccine development strategies for inducing mucosal immunity. Expert Rev Vaccines. 2012;11:367–79. doi: 10.1586/erv.11.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gebril A, Alsaadi M, Acevedo R, Mullen AB, Ferro VA. Optimizing efficacy of mucosal vaccines. Expert Rev Vaccines. 2012;11:1139–55. doi: 10.1586/erv.12.81. [DOI] [PubMed] [Google Scholar]
- 123.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]