Abstract
Metastable aldehydes produced by lipid peroxidation act as 'toxic second messengers' that extend the injurious potential of free radicals. 4-hydroxy 2-nonenal (HNE), a highly toxic and most abundant stable end product of lipid peroxidation, has been implicated in the tissue damage, dysfunction, injury associated with aging and other pathological states such as cancer, Alzheimer, diabetes, cardiovascular and inflammatory complications. Further, HNE has been considered as a oxidative stress marker and it act as a secondary signaling molecule to regulates a number of cell signaling pathways. Biological activity of HNE depends on its intracellular concentration, which can differentially modulate cell death, growth and differentiation. Therefore, the mechanisms responsible for maintaining the intracellular levels of HNE are most important, not only in the defense against oxidative stress but also in the pathophysiology of a number of disease processes. In this review, we discusse the significance of HNE in mediating various disease processes and how regulation of its metabolism could be therapeutically effective.
Keywords: 4-hydroxy 2-nonenal, oxidative stress, cancer, cataract, Alzheimer
1. INTRODUCTION
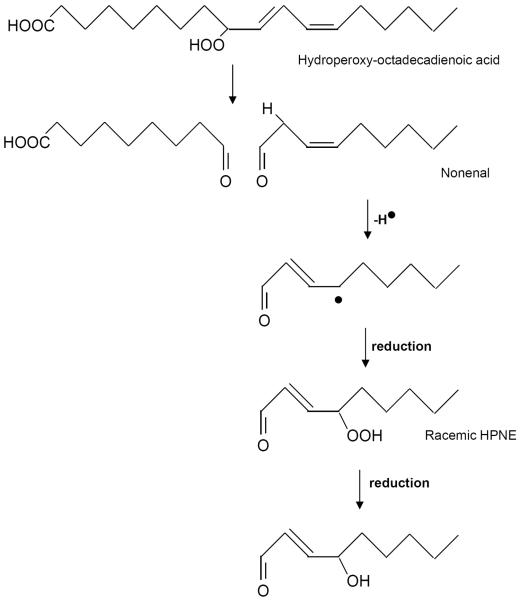
Free radicals such as superoxide anion and hydroxyl radicals have been suggested to stimulate tissue injury related to several disease states and the degenerative processes of senescence. However, the mechanism (s) of free radical-induced injury remains poorly understood [1, 2]. Due to their high reactivity, the toxicity of free radicals is limited to the site of their generation [3]. The injury may be extended by the metastable products of free radical reactions, such as aldehydes which can act as “toxic second messengers” [4]. One of the most abundant and cytotoxic lipid -derived aldehyde is 4-hydroxy 2-nonenal (HNE). The HNE is formed by the oxidation of ω-6 polyunsaturated fatty acids [5; Figure-1]. During autoxidation, fatty acids form alkoxyl radicals [6] that undergo beta-scission leading to the formation of several saturated and unsaturated oxo-compounds of which HNE is one of the most reactive and under some conditions represents 95 % of the generated aldehydes [7]. Currently, HNE is considered an important marker of oxidative stress, a possible contributory agent to several diseases such as Alzheimer and a stimulant of prominent pathobiochemical pathways such as inflammation, indicating a potential contribution of the aldehyde to the pathogenesis of several chronic diseases [8–10]. The biological occurrence of this molecule appears within the range of 0.1–1 uM [5]. Steady-state concentration of HNE can easily reach 5 uM to 5 mM or more within membranes during various pathological conditions [11, 12]. HNE has been shown to have high toxicity to mammalian cells, can inactivate various enzymes and also inhibit DNA and protein synthesis [13].
Fig.1.
Formation of HNE from linoleic acid.
2. BIOCHEMICAL PROPERTIES OF HNE
HNE is a tremendously reactive [14–16] and is considered to be the most toxic aldehyde because of the presence of α, β-double bond at C-2 position, carbonyl group at C-1 and hydroxyl group at C-4 position [17, 18]. This aldehyde can readily react with molecules containing thiol and amino groups (Figure-2). Amino acids such as cysteine, histidine and lysine are the primary reactants with HNE [18–19]. Because of the presence of C=C double bond, HNE can react with nucleophiles, such as cysteine or glutathione and form Michael adducts [20, 21], also known as primary reaction. However, primary reaction velocity is greatly enhanced if the reaction is catalysed by enzyme glutathione-S-transferases (GSTs) [22, 23]. Once this primary reaction occurrs leading to free rotation at C2–C3 bond secondary reaction takes place which involves the carbonyl and the hydroxyl groups in which primary amines may alternatively react with the carbonyl group to form Schiff bases [18]. Interestingly, thiol or amino groups react primarily at C-3 position and secondarily at the carbonyl C-1 due to a partial positive charge at C-3 because of the presence of C=C double bond and carbonyl group (C=O) [18]. Hydroxyl group at C-4 also offers inductive effect which further increases the partial positive charge [18, 24].
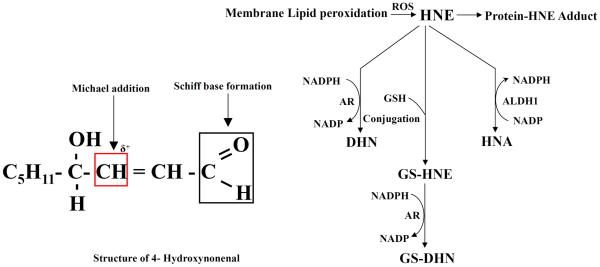
Fig.2.
HNE and its metabolism.
HNE is an extraordinary lipid aldehyde generated during peroxidation of unsaturated fatty acyl residues esterified in phospholipids [25–27]. It has been considered that degradation of hydroperoxides leads to the formation of aldehydic products such as HNE, malonaldehyde (MDA) etc. Spiteller et al. reported that decomposition of 13-hydroperoxy-9, 11-octadecadienoic acid (13-HPODE) generates these aldehydic products [27]. These toxic lipid aldehydes (HNE and MDA) could be generated by the oxidation of linoleic acid and arachidonic acid in vitro [28, 29]. Furthermore, metals-mediated generation of ROS via Fenton-like reactions in the cell membrane also produces hydroxyl radicals which accelerate lipid peroxidation. Metals also participate in the formation of lipid peroxidation end-products, such as HNE. In addition, the peroxidation of fatty acids, particularly arachidonic acid, leads to the formation of a number of cytotoxic aldehydes including HNE [30, 31]. There are three main pathways associated with the metabolism of HNE: The HNE could be reduced to DHN by aldose reductase (AR) or oxidized to HNA by ALDH1. Also, HNE could conjugate with proteins, and more readily with glutathione (GSH) catalyzed by the glutathione S-transferases (GSTs) such as hGSTA4-4 and hGST5.8 to form GS-HNE [32–34]. The GS-HNE could be reduced by AR to GS-DHN [35]. Both GS-HNE and GS-DHN are actively transported out by multidrug resistance associated protein (MRP) and Ral-binding protein (RLIP76). Recent studies indicate that RLIP76 is responsible for significant (70%) transport of the GS-conjugates of HNE in cultured cells [36].
3. BIOLOGICAL FUNCTIONS OF HNE
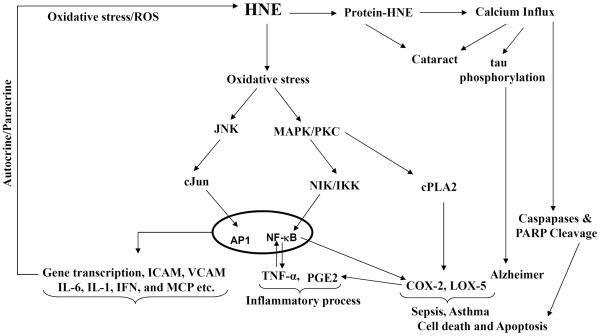
HNE exerts physiologically beneficial effects depending on its intracellular concentration [13]. Lower intracellular concentrations (< 2 uM) of HNE seems to be beneficial to cells as they promote cell survival and proliferation [37, 38]. Higher concentrations of HNE (10 to 60 μM) have genotoxic effects since they lead to sister chromatid exchange [41], micronuclei formation and DNA fragmentation [39–42]. At concentrations > 100 μM, HNE and related aldehydes cause lethal toxicity, and at these concentrations, inhibition of glycolytic enzymes, mitochondrial respiration, DNA and protein synthesis also occurs [42]. Low concentrations of HNE have been shown to disturb cellular calcium homeostasis [43]. Thus, the observations that large amounts of lipid aldehydes accumulate under pathological conditions, and that they affect cellular metabolism at relatively low concentrations, certainly substantiate the view that these aldehydes act as 'toxic second messengers'. Further, HNE at micromolar concentrations has consistently been shown to up-regulate the expression of transcription factors such as Nuclear Factor-Kappa B (NF-kB) which regulate the expression of a variety of genes involved in cell proliferation and differentiation the activation of protein kinase C (PKC), mitogen activated protein kinase (MAPK) [44, 35, 36], and while at higher concentrations HNE has been shown to inhibit the formation of NF-kB [45]. Higher concentration of HNE increases AP1 and other apoptotic related proteins. Poli et al [22] have demonstrated that inhibitors of PKC can significantly prevent HNE-induced AP-1 nuclear binding. Since HNE can react with proteins and enzymes, resulting in their modification and inactivation. Out of several major toxic effects of HNE produced during oxidative stress also cause damage to cellular components such as proteins and DNAs. However, several studies have demonstrated that HNE at nontoxic levels can potently activate stress response pathways such as MAPK and nuclear translocation of a redox-sensitive transcriptional factor, Nrf2 [46]. HNE also increases the expression and synthesis of the main fibrogenic cytokine, the transforming growth factor β1 (TGF β1), by macrophages. Besides up-regulation of the inflammatory and TGF β 1, HNE has recently been reported to induce the expression and synthesis of monocyte chemotactic protein-1 (MCP1), which plays a major role in atherosclerosis [47, 48]. Further, recent studies demonstrate that GS-conjugates of HNE could play a key role in the inflammatory signaling [49, 50; Figure-3]. HNE and GS-HNE but not AR-catalyzed reduced form of GS-HNE, GS-DHN, has been shown to cause cell death or growth via activation of PKC, MAPK and NF-KB in colon cancer cells, vascular smooth muscle cells and macrophages. These studies suggest that GS-conjugates of HNE could act as oxidative stress-induced signaling intermediates that could increase or decrease the inflammation based on their concentration as well as type of cells where they generated and acted upon.
Fig.3.
Role of HNE in human diseases.
4. INVOLVEMENT OF HNE IN DISEASE PROCESSES
The involvement of lipid peroxidation product HNE has been demonstrated in important neurodegenerative diseases such as Alzheimer's disease (AD) [51], Parkinson's desease (PD) [52], multiple sclerosis [53] and other diseases such as cancer [54], diabetes [55], inflammatory complications [56], atherosclerosis [57], osteoporosis [58], cataract and age-related macular degeneration [59–61]. A number of studies have identified increased HNE and HNE-modified proteins in human diseases.
5. NEURODEGENERATIVE DISEASES
AD is a neurodegenerative condition in which nerve tissue in the brain breaks down which gradually reduces the ability to learn, think, and memorize [62]. Recent studies have established the relationship between oxidative stress-generated toxic aldehydes and possible causes of AD [63, 64]. HNE was found to be neurotoxic since elevated levels of HNE have been reported in the brain tissues and ventricular fluid of AD patients which is correlated with increased neuronal apoptosis [65–67]. Further, beta-amyloid was found to be positive for HNE and its modification could contribute to the toxicity associated with the amyloid deposits [68]. Antibodies against HNE-histidine were found to be reactive with neurofibrillary tangles and beta-amyloid core of senile plaques [69]. HNE at concentrations less than 10 μM has been shown to impair Na+/K+-ATPase activity, covalent modification of the glucose transporter GLUT3 which impairs glucose transport, increase in neuronal vulnerability that leads to excitotoxicity and impairs mitochondrial function in neurons and synaptosomes of AD patients [71, 72]. Further, HNE by directly binding to GTP-binding protein Gq11 has been shown to impair coupling of muscarinic cholinergic receptors to Gq11 in cortical neuron cultures which is significantly related to AD [73]. Parkinson disease (PD) is another age-related neurodegenerative disease clinically characterized by tremors at rest, muscular rigidity, bradycinesia and dementia [74]. Increased levels of HNE and HNE-protein adducts have been found in neurons of PD patients [68, 75, 76]. Castellani et al have shown that HNE is present in Lewy bodies in PD. A number of antioxidants including glutathione that alter the intracellular concentrations of HNE have been shown to prevent AD and PD [77].
6. CANCER
HNE is considered to contribute to the mutagenic and carcinogenic effects associated with oxidative stress-induced lipid peroxidation [78–81]. HNE and or its related bioactive metabolites can damage DNA, leading to formation of pro-mutagenic lesions in inflammation-driven cancers [82]. Several studies have shown that formation of protein-HNE adducts in renal and colon cancer cells and tissues are related to growth and progression of kidney and colon cancers [83–86]. Increased HNE or protein-HNE levels were observed in renal proximal tubules in a rat model of renal adenocarcinoma [87]. Further, increased HNE has been shown to be associated with hepatocarcinogenesis initiation in long-evans cinnamon (LEC) rats and these studies indicate that protein modification by HNE could be a potential mechanism of cellular disturbances leading to liver cancer initiation [88, 89]. Normal plasma concentration of HNE was found to be 0.65 μM in healthy human subjects [90, 91]. Our recent studies indicate that AR catalyzed reduced form of GS-HNE; GS-DHN could mediate carcinogenic signals in human colon cancer cells [92–94]. Further, inhibition of AR that effectively reduces HNE and GS-HNE (Km 10–30 uM) has been shown to prevent colon cancer growth and metastasis [95]. Overexpression of GSTs that conjugate HNE to GS-HNE has been shown to protect cells from UV induced cytotoxicity in K562 leukemia cells [96]. Recent studies also indicate that the RLIP76, a protein that transports and maintains the cellular levels of HNE, GS-HNE and GS-DHN could be used to regulate the tumorigenesis process [97].
7. CATARACT AND AMD
Cataractogenesis is a multi-factorial process in which progressive loss of light transmission by the lens is associated with profound changes in its structure, physiology and metabolism [98–101]. Overwhelming experimental evidence supports the view that oxidative stress plays a central role in cataract formation [102]. Although the lens is exposed to low oxygen tension (<30 mm Hg), and is rich in antioxidants such as glutathione (GSH), constant exposure to light imposes continuous oxidative stress [103]. Moreover, the concentration of H2O2, which causes lipid peroxidation and generates HNE, has been shown to be increased in patients with cataracts [104, 105]. Further, HNE has been shown to induce lens opacification and increased formation of protein-HNE adducts in cell membranes of epithelial cells [104, 106]. Downstream of protein-HNE adducts intercalate cell membranes and cause membrane fluidity changes that leads to calcium influx and activation of caspases inducing apoptosis that contributes to cataractogenesis 107, 108]. Therefore, the strategies that decrease the intracellular concentrations of HNE could be anti-cataractogenic. Indeed, we have recently shown that ablation of ALDH1 by ALDH1-specific siRNA resulted in increased lens opacification in rat lenses accompanied by increased formation of HNE-protein adducts [106]. Moreover, metal chelation up-regulates ALDH1 thereby increases HNE-detoxification and oxidation-associated toxicity [60]. Further, over-expression of GST has been shown to prevent cytotoxic effects of HNE in ocular cells [107–109]. In age-related macular degeneration (AMD), the retina is particularly susceptible to oxidative stress because of its high concentration of easily oxidized PUFAs and the presence of retinal pigments that generate ROS when exposed to light. Lipid peroxidation- derived lipid aldehydes such as HNE and malondialdehyde have been shown to be significantly increased in the retina of AMD eyes as well as in patient's plasma [110, 111].
8. CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD)
Smoking and environmental pollutants such as air pollutants and industrial dust, are main risk factors for COPD, a fifth leading cause of death worldwide [112, 113]. Cigarette smoke is one of the major sources of ROS [114]. The inflammatory response to cigarette smoke is augmented due to increased release of ROS in neutrophils and macrophages. Indeed, increased oxidative stress and elevated levels of protein-HNE adducts has been observed in the lungs of COPD patients [112]. Furthermore, exposure of cigarette smoke to mice has been shown to increase HNE adducts in alveolar macrophages and bronchiole epithelial cells indicating involvement of HNE in cigarette smoke –induced COPD [113].
9. ALCOHOLIC LIVER DISEASE (ALD)
Alcoholic liver disease (ALD) is one of the major cause of death in United States and is linked with period and magnitude of alcohol consumed [115]. More than 90% of individuals consuming alcohol on a daily basis develop fatty liver (steatosis). Recent studies indicate that HNE and other lipid peroxidation products react with cellular proteins and the aldehyde-modified proteins serve as a biomarker for alcohol-induced oxidative stress [116]. Moreover, several repots have identified HNE- or MDA-conjugated proteins in liver [117, 118]. These reports demonstrate that despite the highly effective antioxidant systems, the chronic alcohol ingestion generates a pro-oxidative environment in the liver resulting in lipid peroxidation and the covalent modification of hepatic proteins which are linked to the pathophysiology of ALD [116].
10. INFLAMMATORY DISEASES
Increased formation of HNE and protein-HNE has been shown in many inflammatory diseases, such as endotoxemia, ischaemia-reperfusion injury [120], atherosclerosis [121], and rheumatoid arthritis [122]. Recent studies indicate that glutathione-conjugate of HNE induces vascular smooth muscle cell proliferation as well as death depending on its intra-cellular concentrations [36, 109, 123]. These studies indicate that GS-aldehyde conjugates could act as cellular signaling intermediates. Further, a recent study by Spite et al indicates that GS-HNE is a more potent inducer of inflammation than nonconjugated HNE [124]. Infact, GS-HNE has been shown to directly activate human neutrophils to generate superoxide anions, which in turn may facilitate HNE generation in a feedback cycle. In isolated neutrophils from mice, GS-HNE increases the cell surface expression of the CD11b/CD18 which could increase neutrophil influx into the peritoneal cavity [125]. Further, HNE and GS-HNE, but not GS-DHN has been shown to activate NF-kB in macrophages, indicating that GS-DHN formed by the reduction of GS-HNE by AR could be a novel signaling intermediate [126].
Most of the recent studies indicate that HNE and HNE-protein adducts could act as biomarkers for a number of human diseases since their formation is significantly enhanced in most of the human pathologies [127–129]. Recent studies indicate that regulation of proteins that maintain the cellular homeostasis of HNE could alter fate of these pathologies and serve as potential therapeutic targets [130]. Additional studies are required to clearly investigate how these enzymes/proteins regulate the formation of this important molecule, HNE, and disease progression [131]. Additionally, further, studies are necessary to understand how GS-HNE metabolites could act as signaling intermediates by regulating various transcription factors that express inflammatory markers and initiate inflammatory pathologies.
CONCLUSIONS and FUTURE PERSPECTIVES
Recent studies indicate without doubt that lipid aldehydes and glutathione-lipid aldehydes can act as signaling intermediates or toxic messengers of oxidative stress. Increased formation HNE and its protein adducts have been observed in number disease processes in humans. HNE has been shown to alter the cellular redox homeostasis responsible for cell growth, death and differentiation. Further, recent studies indicate that glutathiolation of HNE regulates its toxic effects. Despite multiple studies showing the connection between oxidative stress-generated lipid aldehydes (specifically HNE) and pathological consequences leading to a number of disease processes, the mechanisms by which varying concentrations of HNE detects cells fate towards death or growth is not clearly known. It is still not clear, how HNE and its glutathione conjugates regulate activation of redox transcription factors such as NF-kB and AP1? Moreover the role of HNE and its glutathione conjugates in regulation of innate and adoptive immune responses is not known. This is an important area, which needs to be investigated to understand the role of lipid aldehydes in the pathophysiology of inflammatory disorders. Investigations on how HNE regulates inflammasome activation will provide clues to identify the possible mechanism by which HNE mediates innate immune response. Regulation of enzyme activities of GST, AR and ALDH1 which are involved in the metabolism of HNE and maintaining the in situ concentration of HNE, could also control the pathophysiology of disease progression.
Acknowledgements
This work in the author's laboratory was supported by NIH grants DK36116 and CA129383 (SKS).
Footnotes
Disclosures: None.
REFERENCES
- [1].Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- [2].Poli G, Schaur J. 4-Hydroxynonenal in the pathomechanisms of oxidative stress. IUBMB Life. 2000;50:315–321. doi: 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- [3].Uchida K, Stadtman ER. Modification of histidine residues in proteins by reaction with 4-hydroxinonenal. Proc. Natl. Acad. Sci. USA. 1992;89:4544–4589. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Carletto C, Nicolaÿ JF, Courbebaisse Y. Oxidative stress and cutaneous ageing: the 'toxic second messengers' concept and an interesting family of products, 'pseudodipeptides'. Int J Cosmet Sci. 2000;22:361–70. doi: 10.1046/j.1467-2494.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- [5].Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Rad Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- [6].Harold W. Gardner. Oxygen radical chemistry of polyunsaturated fatty acids. Free Rad Biol Med. 1989;7:65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- [7].Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–45. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- [8].Awasthi YC, Sharma R, Sharma A, Yadav S, Singhal SS, Chaudhary P, Awasthi S. Self-regulatory role of 4-hydroxynonenal in signaling for stress-induced programmed cell death. Free Radic Biol Med. 2008;45:111–8. doi: 10.1016/j.freeradbiomed.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Uchida K, Kumagai T. 4-Hydroxy-2-nonenal as a COX-2 inducer. Molecular Aspects of Medicine. 2003;24:213–218. doi: 10.1016/s0098-2997(03)00016-5. [DOI] [PubMed] [Google Scholar]
- [10].Zarkovic K. 4-Hydroxynonenal and neurodegenerative diseases. Molecular Aspects of Medicine. 2003;24:293–303. doi: 10.1016/s0098-2997(03)00024-4. [DOI] [PubMed] [Google Scholar]
- [11].Poli G, Dianzani MU, Cheeseman KH, Slater TF, Lang J, Esterbauer H. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem. J. 1985;227:629–638. doi: 10.1042/bj2270629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Koster JF, Slee RG, Montfoort A, Lang J, Esterbauer H. Comparison of the inactivation of glucose-6-phosphatas e by in situ lipid peroxidation-derive d 4-hydroxynonena l and exogenous 4-hydroxynonenal. Free Radic. Res. 1986:273–287. doi: 10.3109/10715768609051637. [DOI] [PubMed] [Google Scholar]
- [13].Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [14].Guichardant M, Taibi-Tronche P, Fay LB, Lagarde M. Covalent modifications of aminophospholipids by 4-hydroxynonenal. Free Radic Biol Med. 1998;9:1049–56. doi: 10.1016/s0891-5849(98)00149-x. [DOI] [PubMed] [Google Scholar]
- [15].Vazdar M, Jurkiewicz P, Hof M, Jungwirth P, Cwiklik L. Behavior of 4 hydroxynonenal in phospholipid membranes. J Phys Chem B. 2012;116:6411–5. doi: 10.1021/jp3044219. [DOI] [PubMed] [Google Scholar]
- [16].Hammer A, Ferro M, Tillian HM, Tatzber F, Zollner H, Schauenstein E, Schaur RJ. Effect of oxidative stress by iron on 4-hydroxynonenal formation and proliferative activity in hepatomas of different degrees of differentiation. Free Radic Biol Med. 1997;23:26–33. doi: 10.1016/s0891-5849(96)00630-2. [DOI] [PubMed] [Google Scholar]
- [17].Poli G, Schaur RJ. 4-Hydroxynonenal in the Pathomechanisms of Oxidative Stress. IUBMB. Life. 2000;50:315–321. doi: 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- [18].Schaur RJ. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol. Aspects Med. 2003;24:149–159. doi: 10.1016/s0098-2997(03)00009-8. [DOI] [PubMed] [Google Scholar]
- [19].Poli G, Biasi F, Leonarduzzi G. 4-Hydroxynonenal-protein adducts: A reliable biomarker of lipid oxidation in liver diseases. Mol Aspects Med. 2008;29:67–71. doi: 10.1016/j.mam.2007.09.016. [DOI] [PubMed] [Google Scholar]
- [20].Wakita C, Maeshima T, Yamazaki A, Shibata T, Ito S, Akagawa M, Ojika M, Yodoi J, Uchida K. Stereochemical configuration of 4-hydroxy-2-nonenal-cysteine adducts and their stereoselective formation in a redox-regulated protein. J Biol Chem. 2009;284:28810–22. doi: 10.1074/jbc.M109.019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].LoPachin RM, Gavin T, Petersen DR, Barber DS. Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation. Chem Res Toxicol. 2009;22:1499–508. doi: 10.1021/tx900147g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Poli G, Schaur RJ. 4-Hydroxynonenal in the Pathomechanisms of Oxidative Stress. IUBMB Life. 2000;50:315–321. doi: 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- [23].Engle MR, Singh SP, Czernik PJ, Gaddy D, Montague DC, Ceci JD, Yang Y, Awasthi S, Awasthi YC, Zimniak P. Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxynonenal: generation and analysis of mGsta4 null mouse. Toxicol Appl Pharmacol. 2004;194:296–308. doi: 10.1016/j.taap.2003.10.001. [DOI] [PubMed] [Google Scholar]
- [24].Witz G. Biological interactions of α,β-unsaturated aldehydes. Free Radic Biol Med. 1989;7:333–349. doi: 10.1016/0891-5849(89)90137-8. [DOI] [PubMed] [Google Scholar]
- [25].Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- [26].Parola M, Bellomo G, Robino G, Barrera G, Dianzani MU. 4-Hydroxynonenal as a biological signal: molecular basis and pathophysiological implications. Antioxid. Redox Signal. 1999;1:255–284. doi: 10.1089/ars.1999.1.3-255. [DOI] [PubMed] [Google Scholar]
- [27].Spiteller P, Kern W, Reiner J, Spiteller G. Aldehydic lipid peroxidation products derived from linoleic acid. Biochim. Biophys. Acta. 2001;1531:188–208. doi: 10.1016/s1388-1981(01)00100-7. [DOI] [PubMed] [Google Scholar]
- [28].Michiels C, Remacle J. Cytotoxicity of linoleic acid peroxide, malondialdehyde and 4-hydroxynonenal towards human fibroblasts. Toxicology. 1991;66:225–34. doi: 10.1016/0300-483x(91)90221-l. [DOI] [PubMed] [Google Scholar]
- [29].Griesser M, Boeglin WE, Suzuki T, Schneider C. Convergence of the 5-LOX and COX-2 pathways: heme-catalyzed cleavage of the 5S-HETE-derived diendoperoxide into aldehyde fragments. J Lipid Res. 2009;50:2455–62. doi: 10.1194/jlr.M900181-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- [31].Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- [32].Zhang M, Shoeb M, Goswamy J, Liu P, Xiao TL, Hogan D, Campbell GA, Ansari NH. Overexpression of aldehyde dehydrogenase 1A1 reduces oxidation-induced toxicity in SH-SY5Y neuroblastoma cells. J Neurosci Res. 2010;88:686–94. doi: 10.1002/jnr.22230. [DOI] [PubMed] [Google Scholar]
- [33].Xiao T, Shoeb M, Siddiqui MS, Zhang M, Ramana KV, Srivastava SK, Vasiliou V, Ansari NH. Molecular cloning and oxidative modification of human lens ALDH1A1: implication in impaired detoxification of lipid aldehydes. J Toxicol Environ Health A. 2009;72:577–84. doi: 10.1080/15287390802706371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Engle MR, Singh SP, Czernik PJ, Gaddy D, Montague DC, Ceci JD, Yang Y, Awasthi S, Awasthi YC, Zimniak P. Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxynonenal: generation and analysis of mGsta4 null mouse. Toxicol Appl Pharmacol. 2004;194:296–308. doi: 10.1016/j.taap.2003.10.001. [DOI] [PubMed] [Google Scholar]
- [35].Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006;281:33019–29. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- [36].Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, Srivastava SK. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J Biol Chem. 2006;281:17652–60. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- [37].Vatsyayan R, Lelsani PC, Chaudhary P, Kumar S, Awasthi S, Awasthi YC. The expression and function of vascular endothelial growth factor in retinal pigment epithelial (RPE) cells is regulated by 4-hydroxynonenal (HNE) and glutathione S-transferaseA4-4. Biochem Biophys Res Commun. 2012;417:346–51. doi: 10.1016/j.bbrc.2011.11.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem. 2009;284:27438–48. doi: 10.1074/jbc.M109.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Singh SP, Chen T, Chen L, Mei N, McLain E, Samokyszyn V, Thaden JJ, Moore MM, Zimniak P. Mutagenic effects of 4-hydroxynonenal triacetate, a chemically protected form of the lipid peroxidation product 4-hydroxynonenal, as assayed in L5178Y/Tk+/− mouse lymphoma cells. J Pharmacol Exp Ther. 2005;313:855–61. doi: 10.1124/jpet.104.080754. [DOI] [PubMed] [Google Scholar]
- [40].Manini P, Napolitano A, Camera E, Caserta T, Picardo M, Palumbo A, d'Ischia M. Ni2+ enhances Fe2+/peroxide-induced oxidation of arachidonic acid and formation of geno/cytotoxic 4-hydroxynonenal: a possible contributory mechanism in nickel toxicity and allergenicity. Biochim Biophys Acta. 2003;1621:9–16. doi: 10.1016/s0304-4165(03)00010-2. [DOI] [PubMed] [Google Scholar]
- [41].Brambilla G, Sciabà L, Faggin P, Maura A, Marinari UM, Ferro M, Esterbauer H. Cytotoxicity, DNA fragmentation and sister-chromatid exchange in Chinese hamster ovary cells exposed to the lipid peroxidation product 4-hydroxynonenal and homologous aldehydes. Mutat Res. 1986;171:169–76. doi: 10.1016/0165-1218(86)90051-0. [DOI] [PubMed] [Google Scholar]
- [42].Chandra A, Srivastava SK. A synthesis of 4-hydroxy-2- trans -nonenal and 4-(3H) 4-hydroxy-2- trans –nonenal. Lipids. 1997;32:779–782. doi: 10.1007/s11745-997-0100-6. [DOI] [PubMed] [Google Scholar]
- [43].Vigh L, Smith RG, Soós J, Engelhardt JI, Appel SH, Siklós L. Sublethal dose of 4-hydroxynonenal reduces intracellular calcium in surviving motor neurons in vivo. Acta Neuropathol. 2005;109:567–75. doi: 10.1007/s00401-004-0977-1. [DOI] [PubMed] [Google Scholar]
- [44].Poli G, Leonarduzzi F, Biasi E. Chiarpotto. Oxidative stress and cell signalling. Curr. Med. Chem. 2004;11:1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- [45].Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J. 2002;367:729–40. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Siow RC, Ishii T, Mann GE. Modulation of antioxidant gene expression by 4-hydroxynonenal: atheroprotective role of the Nrf2/ARE transcription pathway. Redox Rep. 2007;12:11–5. doi: 10.1179/135100007X162167. [DOI] [PubMed] [Google Scholar]
- [47].Yehualaeshet T, O'Connor R, Green-Johnson J, Mai S, Silverstein R, Murphy-Ullrich JE, Khalil N. Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am J Pathol. 1999;155:841–51. doi: 10.1016/s0002-9440(10)65183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Leonarduzzi G, Chiarpotto E, Biasi F, Poli G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol Nutr Food Res. 2005;49:1044–9. doi: 10.1002/mnfr.200500090. [DOI] [PubMed] [Google Scholar]
- [49].Yadav UC, Srivastava SK, Ramana KV. Understanding the role of aldose reductase in ocular inflammation. Curr Mol Med. 2010;10:540–9. doi: 10.2174/1566524011009060540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ramana KV, Srivastava SK. Aldose reductase: a novel therapeutic target for inflammatory pathologies. Int J Biochem Cell Biol. 2010;42:17–20. doi: 10.1016/j.biocel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zarkovic K. 4-hydroxynonenal and neurodegenerative diseases. Mol Aspects Med. 2003;24:293–303. doi: 10.1016/s0098-2997(03)00024-4. [DOI] [PubMed] [Google Scholar]
- [52].Selley ML. (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson's disease. Free Radic Biol Med. 1998;25:169–74. doi: 10.1016/s0891-5849(98)00021-5. [DOI] [PubMed] [Google Scholar]
- [53].Newcombe J, Li H, Cuzner ML. Low density lipoprotein uptake by macrophages in multiple sclerosis plaques: implications for pathogenesis. Neuropathol Appl Neurobiol. 1994;20:152–62. doi: 10.1111/j.1365-2990.1994.tb01174.x. [DOI] [PubMed] [Google Scholar]
- [54].Shiota M, Song Y, Takeuchi A, Yokomizo A, Kashiwagi E, Kuroiwa K, Tatsugami K, Uchiumi T, Oda Y, Naito S. Antioxidant therapy alleviates oxidative stress by androgen deprivation and prevents conversion from androgen dependent to castration resistant prostate cancer. J Urol. 2012;187:707–14. doi: 10.1016/j.juro.2011.09.147. [DOI] [PubMed] [Google Scholar]
- [55].Akude E, Zherebitskaya E, Roy Chowdhury SK, Girling K, Fernyhough P. 4-Hydroxy-2-nonenal induces mitochondrial dysfunction and aberrant axonal outgrowth in adult sensory neurons that mimics features of diabetic neuropathy. Neurotox Res. 2010;17:28–38. doi: 10.1007/s12640-009-9074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mukhopadhyay P, Horváth B, Kechrid M, Tanchian G, Rajesh M, Naura AS, Boulares AH, Pacher P. Poly(ADP-ribose) polymerase-1 is a key mediator of cisplatin-induced kidney inflammation and injury. Free Radic Biol Med. 2011;51:1774–88. doi: 10.1016/j.freeradbiomed.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kumano-Kuramochi M, Shimozu Y, Wakita C, Ohnishi-Kameyama M, Shibata T, Matsunaga S, Takano-Ishikawa Y, Watanabe J, Goto M, Xie Q, Komba S, Uchida K, Machida S. Identification of 4-hydroxy-2-nonenal-histidine adducts that serve as ligands for human lectin-like oxidized LDL receptor-1. Biochem J. 2012;442:171–80. doi: 10.1042/BJ20111029. [DOI] [PubMed] [Google Scholar]
- [58].Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem. 2009;284:27438–48. doi: 10.1074/jbc.M109.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Srivastava SK, Yadav UC, Reddy AB, Saxena A, Tammali R, Shoeb M, Ansari NH, Bhatnagar A, Petrash MJ, Srivastava S, Ramana KV. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem Biol Interact. 2011;191:330–8. doi: 10.1016/j.cbi.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang M, Shoeb M, Liu P, Xiao T, Hogan D, Wong IG, Campbell GA, Ansari NH. Topical metal chelation therapy ameliorates oxidation-induced toxicity in diabetic cataract. J Toxicol Environ Health A. 2011;74:380–91. doi: 10.1080/15287394.2011.538835. [DOI] [PubMed] [Google Scholar]
- [61].Xiao T, Shoeb M, Siddiqui MS, Zhang M, Ramana KV, Srivastava SK, Vasiliou V, Ansari NH. Molecular cloning and oxidative modification of human lens ALDH1A1: implication in impaired detoxification of lipid aldehydes. J Toxicol Environ Health A. 2009;72:577–84. doi: 10.1080/15287390802706371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Finkel LH. Neuroengineering models of brain disease. Annu Rev Biomed Eng. 2000;2:577–606. doi: 10.1146/annurev.bioeng.2.1.577. [DOI] [PubMed] [Google Scholar]
- [63].Hayashi T, Shishido N, Nakayama K, Nunomura A, Smith MA, Perry G, Nakamura M. Lipid peroxidation and 4-hydroxy-2-nonenal formation by copper ion bound to amyloid-beta peptide. Free Radic Biol Med. 2007;43:1552–9. doi: 10.1016/j.freeradbiomed.2007.08.013. [DOI] [PubMed] [Google Scholar]
- [64].Lee HP, Pancholi N, Esposito L, Previll LA, Wang X, Zhu X, Smith MA, Lee HG. Early induction of oxidative stress in mouse model of Alzheimer disease with reduced mitochondrial superoxide dismutase activity. PLoS One. 2012;7:e28033. doi: 10.1371/journal.pone.0028033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer's disease. Neurobiol Aging. 1998;19:33–6. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- [66].Lovell MA, Ehmann WD, Mattson MP, Markesbery WR. Elevated 4-hydroxynonenal in ventricular fluid in Alzheimer's disease. Neurobiol Aging. 1997;18:457–61. doi: 10.1016/s0197-4580(97)00108-5. [DOI] [PubMed] [Google Scholar]
- [67].Ando Y, Brannstrom T, Uchida K, Nyhlin N, Näsman B, Suhr O, Yamashita T, Olsson T, El Salhy M, Uchino M, Ando M. Histochemical detection of 4-hydroxynonenal protein in Alzheimer amyloid. J Neurol Sci. 1998;156:172–6. doi: 10.1016/s0022-510x(98)00042-2. [DOI] [PubMed] [Google Scholar]
- [68].Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- [69].Kawaguchi-Niida M, Shibata N, Morikawa S, Uchida K, Yamamoto T, Sawada T, Kobayashi M. Crotonaldehyde accumulates in glial cells of Alzheimer's disease brain. Acta Neuropathol. 2006;111:422–9. doi: 10.1007/s00401-006-0044-1. [DOI] [PubMed] [Google Scholar]
- [70].Siems WG, Hapner SJ, van Kuijk FJ. 4-hydroxynonenal inhibits Na(+)-K(+)-ATPase. Free Radic Biol Med. 1996;20:215–23. doi: 10.1016/0891-5849(95)02041-1. [DOI] [PubMed] [Google Scholar]
- [71].Mattson MP, Chan SL. Neuronal and glial calcium signaling in Alzheimer's disease. Cell Calcium. 2003;34:385–97. doi: 10.1016/s0143-4160(03)00128-3. [DOI] [PubMed] [Google Scholar]
- [72].Lu C, Chan SL, Haughey N, Lee WT, Mattson MP. Selective and biphasic effect of the membrane lipid peroxidation product 4-hydroxy-2,3-nonenal on N-methyl-D-aspartate channels. J Neurochem. 2001;78:577–89. doi: 10.1046/j.1471-4159.2001.00431.x. [DOI] [PubMed] [Google Scholar]
- [73].Kelly JF, Storie K, Skamra C, Bienias J, Beck T, Bennett DA. Relationship between Alzheimer's disease clinical stage and Gq/11 in subcellular fractions of frontal cortex. J Neural Transm. 2005;112:1049–56. doi: 10.1007/s00702-004-0243-7. [DOI] [PubMed] [Google Scholar]
- [74].Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- [75].Coppedè F, Armani C, Bidia DD, Petrozzi L, Bonuccelli U, Migliore L. Molecular implications of the human glutathione transferase A-4 gene (hGSTA4) polymorphisms in neurodegenerative diseases. Mutat Res. 2005;579:107–14. doi: 10.1016/j.mrfmmm.2005.02.020. [DOI] [PubMed] [Google Scholar]
- [76].Qin Z, Hu D, Han S, Reaney SH, Di Monte DA, Fink AL. Effect of 4-hydroxy-2-nonenal modification on alpha-synuclein aggregation. J Biol Chem. 2007;282:5862–70. doi: 10.1074/jbc.M608126200. [DOI] [PubMed] [Google Scholar]
- [77].Vatassery GT. Vitamin E and other endogenous antioxidants in the central nervous system. Geriatrics. 1998;53(Suppl 1):S25–7. [PubMed] [Google Scholar]
- [78].Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annual Review of Pharmacology and Toxicology. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- [79].Marnett LJ. Lipid peroxidation—DNA damage by malondialdehyde. Mutation Research. 1999;424:83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- [80].Marnett LJ. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 2002;181–182:219–222. doi: 10.1016/s0300-483x(02)00448-1. [DOI] [PubMed] [Google Scholar]
- [81].Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic Biol and Med. 2007;43:1109–1120. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- [82].Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- [83].Oberley TD, Toyokuni S, Szweda LI. Localization of hydroxynonenal protein adducts in normal human kidney and selected human kidney cancers. Free Radic Biol Med. 1999;27:695–703. doi: 10.1016/s0891-5849(99)00117-3. [DOI] [PubMed] [Google Scholar]
- [84].Zanetti D, Poli G, Vizio B, Zingaro B, Chiarpotto E, Biasi F. 4-hydroxynonenal and transforming growth factor-beta1 expression in colon cancer. Mol Aspects Med. 2003;24:273–80. doi: 10.1016/s0098-2997(03)00022-0. [DOI] [PubMed] [Google Scholar]
- [85].Skrzydlewska E, Sulkowski S, Koda M, Zalewski B, Kanczuga-Koda L, Sulkowska M. Lipid peroxidation and antioxidant status in colorectal cancer. World J Gastroenterol. 2005;11:403–6. doi: 10.3748/wjg.v11.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Skrzydlewska E, Stankiewicz A, Sulkowska M, Sulkowski S, Kasacka I. Antioxidant status and lipid peroxidation in colorectal cancer. J Toxicol Environ Health A. 2001;64:213–22. doi: 10.1080/15287390152543690. [DOI] [PubMed] [Google Scholar]
- [87].Toyokuni S, Uchida K, Okamoto K, Hattori-Nakakuki Y, Hiai H, Stadtman ER. Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate. Proc Natl Acad Sci U S A. 1994;91:2616–20. doi: 10.1073/pnas.91.7.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Marquez-Quiñones A, Cipak A, Zarkovic K, Fattel-Fazenda S, Villa-Treviño S, Waeg G, Zarkovic N, Guéraud F. HNE-protein adducts formation in different pre-carcinogenic stages of hepatitis in LEC rats. Free Radic Res. 2010;44:119–27. doi: 10.3109/10715760903338071. [DOI] [PubMed] [Google Scholar]
- [89].Chang B, Nishikawa M, Nishiguchi S, Inoue M. L-carnitine inhibits hepatocarcinogenesis via protection of mitochondria. Int J Cancer. 2005;113:719–29. doi: 10.1002/ijc.20636. [DOI] [PubMed] [Google Scholar]
- [90].Aldini G, Gamberoni L, Orioli M, Beretta G, Regazzoni L, Maffei Facino R, Carini M. Mass spectrometric characterization of covalent modification of human serum albumin by 4-hydroxy-trans-2-nonenal. J Mass Spectrom. 2006;41:1149–61. doi: 10.1002/jms.1067. [DOI] [PubMed] [Google Scholar]
- [91].Spies-Martin D, Sommerburg O, Langhans CD, Leichsenring M. Measurement of 4-hydroxynonenal in small volume blood plasma samples: modification of a gas chromatographic-mass spectrometric method for clinical settings. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;774:231–9. doi: 10.1016/s1570-0232(02)00242-8. [DOI] [PubMed] [Google Scholar]
- [92].Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res. 2006;66:9705–13. doi: 10.1158/0008-5472.CAN-06-2105. [DOI] [PubMed] [Google Scholar]
- [93].Tammali R, Ramana KV, Srivastava SK. Aldose reductase regulates TNF-alpha-induced PGE2 production in human colon cancer cells. Cancer Lett. 2007;252:299–306. doi: 10.1016/j.canlet.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ramana KV, Tammali R, Srivastava SK. Inhibition of aldose reductase prevents growth factor-induced G1-S phase transition through the AKT/phosphoinositide 3-kinase/E2F-1 pathway in human colon cancer cells. Mol Cancer Ther. 2010;9:813–24. doi: 10.1158/1535-7163.MCT-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Tammali R, Reddy AB, Saxena A, Rychahou PG, Evers BM, Qiu S, Awasthi S, Ramana KV, Srivastava SK. Inhibition of aldose reductase prevents colon cancer metastasis. Carcinogenesis. 2011;32:1259–67. doi: 10.1093/carcin/bgr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Cheng JZ, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, Singh SV, Zimniak P, Awasthi S, Awasthi YC. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J Biol Chem. 2001;276:41213–23. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]
- [97].Leake K, Singhal J, Nagaprashantha LD, Awasthi S, Singhal SS. RLIP76 regulates PI3K/Akt signaling and chemo-radiotherapy resistance in pancreatic cancer. PLoS One. 2012;7:e34582. doi: 10.1371/journal.pone.0034582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Hejtmancik JF. Congenital cataracts and their molecular genetics. Semin Cell Dev Biol. 2008;19:134–49. doi: 10.1016/j.semcdb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Bron AJ, Brown NA. Perinuclear lens retrodots: a role for ascorbate in cataractogenesis. Br J Ophthalmol. 1987;71:86–95. doi: 10.1136/bjo.71.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gerido DA, Sellitto C, Li L, White TW. Genetic background influences cataractogenesis, but not lens growth deficiency, in Cx50-knockout mice. Invest Ophthalmol Vis Sci. 2003;44:2669–74. doi: 10.1167/iovs.02-1311. [DOI] [PubMed] [Google Scholar]
- [101].Aydin B, Yagci R, Yilmaz FM, Erdurmus M, Karadağ R, Keskin U, Durmus M, Yigitoglu R. Prevention of selenite-induced cataractogenesis by N-acetylcysteine in rats. Curr Eye Res. 2009;34:196–201. doi: 10.1080/02713680802676885. [DOI] [PubMed] [Google Scholar]
- [102].Cekić S, Zlatanović G, Cvetković T, Petrović B. Oxidative stress in cataractogenesis. Bosn J Basic Med Sci. 2010;10:265–9. doi: 10.17305/bjbms.2010.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Spector A, Wang GM, Wang RR. Photochemically induced cataracts in rat lenses can be prevented by AL-3823A, a glutathione peroxidase mimic. Proc Natl Acad Sci U S A. 1993;90:7485–9. doi: 10.1073/pnas.90.16.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Srivastata SK, Awasthi S, Wang L, Bhatnagar A, Awasthi YC, Ansari NH. Attenuation of 4-hydroxynonenal-induced cataractogenesis in rat lens by butylated hydroxytoluene. Curr Eye Res. 1996;15:749–54. doi: 10.3109/02713689609003458. [DOI] [PubMed] [Google Scholar]
- [105].Ansari NH, Wang L, Srivastava SK. Role of lipid aldehydes in cataractogenesis: 4-hydroxynonenal-induced cataract. Biochem Mol Med. 1996;58:25–30. doi: 10.1006/bmme.1996.0028. [DOI] [PubMed] [Google Scholar]
- [106].Choudhary S, Xiao T, Vergara LA, Srivastava S, Nees D, Piatigorsky J, Ansari NH. Role of aldehyde dehydrogenase isozymes in the defense of rat lens and human lens epithelial cells against oxidative stress. Invest Ophthalmol Vis Sci. 2005;46:259–67. doi: 10.1167/iovs.04-0120. [DOI] [PubMed] [Google Scholar]
- [107].Subramaniam R, Roediger F, Jordan B, Mattson MP, Keller JN, Waeg G, Butterfield DA. The lipid peroxidation product, 4-hydroxy-2-trans-nonenal, alters the conformation of cortical synaptosomal membrane proteins. J Neurochem. 1997;69:1161–9. doi: 10.1046/j.1471-4159.1997.69031161.x. [DOI] [PubMed] [Google Scholar]
- [108].Fleuranceau-Morel P, Barrier L, Fauconneau B, Piriou A, Huguet F. Origin of 4-hydroxynonenal incubation-induced inhibition of dopamine transporter and Na+/K+ adenosine triphosphate in rat striatal synaptosomes. Neurosci Lett. 1999;277:91–4. doi: 10.1016/s0304-3940(99)00652-7. [DOI] [PubMed] [Google Scholar]
- [109].Yadav UC, Ramana KV, Awasthi YC, Srivastava SK. Glutathione level regulates HNE-induced genotoxicity in human erythroleukemia cells. Toxicol Appl Pharmacol. 2008;227:257–64. doi: 10.1016/j.taap.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ethen CM, Reilly C, Feng X, Olsen TW, Ferrington DA. Age-related macular degeneration and retinal protein modification by 4-hydroxy-2-nonenal. Invest Ophthalmol Vis Sci. 2007;48:3469–79. doi: 10.1167/iovs.06-1058. [DOI] [PubMed] [Google Scholar]
- [111].Schutt F, Bergmann M, Holz FG, Kopitz J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:3663–8. doi: 10.1167/iovs.03-0172. [DOI] [PubMed] [Google Scholar]
- [112].Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:490–5. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- [113].Takimoto T, Yoshida M, Hirata H, Kashiwa Y, Takeda Y, Goya S, Kijima T, Kumagai T, Tachibana I, Kawase I. 4-Hydroxy-2-nonenal induces chronic obstructive pulmonary disease-like histopathologic changes in mice. Biochem Biophys Res Commun. 2012;420:84–90. doi: 10.1016/j.bbrc.2012.02.119. [DOI] [PubMed] [Google Scholar]
- [114].Hsieh MM, Hegde V, Kelley MR, Deutsch WA. Activation of APE/Ref-1 redox activity is mediated by reactive oxygen species and PKC phosphorylation. Nucleic Acids Res. 2001;29:3116–22. doi: 10.1093/nar/29.14.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–69. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- [116].Smathers RL, Galligan JJ, Stewart BJ, Petersen DR. Overview of lipid peroxidation products and hepatic protein modification in alcoholic liver disease. Chem Biol Interact. 2011;192:107–12. doi: 10.1016/j.cbi.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kono H, Arteel GE, Rusyn I, Sies H, Thurman RG. Ebselen prevents early alcohol-induced liver injury in rats. Free Radic Biol Med. 2001;30:403–11. doi: 10.1016/s0891-5849(00)00490-1. [DOI] [PubMed] [Google Scholar]
- [118].Tsukamoto H, Horne W, Kamimura S, Niemelä O, Parkkila S, Ylä-Herttuala S, Brittenham GM. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995;96:620–30. doi: 10.1172/JCI118077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Jaworek J, Leja-Szpak A, Nawrot-Porabka K, Bonior J, Szklarczyk J, Kot M, Konturek SJ, Tomaszewska R, Pawlik WW. Effect of neonatal endotoxemia on the pancreas of adult rats. J Physiol Pharmacol. 2008;59:87–102. [PubMed] [Google Scholar]
- [120].He L, Liu B, Dai Z, Zhang HF, Zhang YS, Luo XJ, Ma QL, Peng J. Alpha lipoic acid protects heart against myocardial ischemia-reperfusion injury through a mechanism involving aldehyde dehydrogenase 2 activation. Eur J Pharmacol. 2012;678:32–8. doi: 10.1016/j.ejphar.2011.12.042. [DOI] [PubMed] [Google Scholar]
- [121].Vindis C, Escargueil-Blanc I, Uchida K, Elbaz M, Salvayre R, Negre-Salvayre A. Lipid oxidation products and oxidized low-density lipoproteins impair platelet-derived growth factor receptor activity in smooth muscle cells: implication in atherosclerosis. Redox Rep. 2007;12:96–100. doi: 10.1179/135100007X162248. [DOI] [PubMed] [Google Scholar]
- [122].Biniecka M, Kennedy A, Ng CT, Chang TC, Balogh E, Fox E, Veale DJ, Fearon U, O'Sullivan JN. Successful tumour necrosis factor (TNF) blocking therapy suppresses oxidative stress and hypoxia-induced mitochondrial mutagenesis in inflammatory arthritis. Arthritis Res Ther. 2011;13:R121. doi: 10.1186/ar3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Tammali R, Saxena A, Srivastava SK, Ramana KV. Aldose reductase regulates vascular smooth muscle cell proliferation by modulating G1/S phase transition of cell cycle. Endocrinology. 2010;151:2140–50. doi: 10.1210/en.2010-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Spite M, Summers L, Porter TF, Srivastava S, Bhatnagar A, Serhan CN. Resolvin D1 controls inflammation initiated by glutathione-lipid conjugates formed during oxidative stress. Br J Pharmacol. 2009;158:1062–73. doi: 10.1111/j.1476-5381.2009.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Filep JG. Lipid mediator interplay: resolvin D1 attenuates inflammation evoked by glutathione-conjugated lipid peroxidation products. Br J Pharmacol. 2009;158:1059–61. doi: 10.1111/j.1476-5381.2009.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Tammali R, Saxena A, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents hypoxia-induced increase in hypoxia-inducible factor-1alpha (HIF-1alpha) and vascular endothelial growth factor (VEGF) by regulating 26 S proteasome-mediated protein degradation in human colon cancer cells. J Biol Chem. 2011;286:24089–100. doi: 10.1074/jbc.M111.219733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Carini M, Aldini G, Facino RM. Mass spectrometry for detection of 4-hydroxy-trans-2-nonenal (HNE) adducts with peptides and proteins. Mass Spectrom Rev. 2004;23:281–305. doi: 10.1002/mas.10076. [DOI] [PubMed] [Google Scholar]
- [128].Ohhira M, Ohtake T, Matsumoto A, Saito H, Ikuta K, Fujimoto Y, Ono M, Toyokuni S, Kohgo Y. Immunohistochemical detection of 4-hydroxy-2-nonenal-modified-protein adducts in human alcoholic liver diseases. Alcohol Clin Exp Res. 1998;22:145S–149S. doi: 10.1111/acer.1998.22.s3_part1.145s. [DOI] [PubMed] [Google Scholar]
- [129].Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–23. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- [130].Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–74. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ramana KV. What is the role of lipid peroxidation product 4-hydroxynonenal in inflammation? Biochem Anal Biochem. 2012 doi: 10.4172/2161-1009.1000e105. [Google Scholar]