Abstract
This study was undertaken to investigate the mechanism by which phenethyl isothiocyanate (PEITC), a natural compound from cruciferous vegetables, exhibits antitumor effect on prostate cancer cells. Cell proliferation, cell cycle, western blot, gene transfer and reporter assays were used to test the effects of PEITC on the growth and IL6/JAK/STAT3 pathway in prostate cancer. The result showed that PEITC significantly inhibited DU145 cell proliferation in a dose-dependent manner and induced the cell arrest at G2-M phase. PEITC inhibited both constitutive and interleukin 6 (IL-6)-induced STAT3 activity in DU145 cells. IL-6-stimulated phosphorylation of JAK2, an STAT3 upstream kinase, was also attenuated by PEITC. Moreover, an antioxidant reagent, N-acetyl-l-cysteine (NAC) which suppresses reactive oxygen species (ROS) generation, reversed the early inhibitory effects of PEITC on cell proliferation, constitutive or IL-6-mediated JAK-STAT3 phosphorylation in PCa cells. Taken together, our data demonstrated that PEITC can inhibit the activation of the JAK-STAT3 signal-cascade in prostate cancer cells and the underlying mechanism may be partially involved with blocking cellular ROS production during the early stage of the signaling activation by IL-6.
Keywords: Phenethyl isothiocyanate, IL-6, STAT3, Androgen independent growth
INTRODUCTION
Prostate cancer (PCa) is a common cause of death in men, especially in Western countries. It still remains incurable in the androgen-refractory phase. Signal transducer and activator of transcription 3 (STAT3), a member of Janus kinase (JAK)/STAT signaling pathway, is a latent transcription factor which can be activated by many cytokines and growth factors (1). Activation of the JAK/STAT3 pathway via interleukin 6 (IL-6) has been linked to prostate cancer progression. Upon IL-6 binding to the IL-6 receptor, which consists of a ligand recognition subunit and a gp130 signal transducer, the signals are transduced exclusively by the cytoplasmic domain of gp130 to which Janus tyrosine kinases (JAKs) are constitutively attached (2). Activated JAKs can phosphorylate STAT3 at Tyr705, which induces formation of tail-to-tail STAT3 dimers. Finally, the dimerized STAT3 translocated into nucleus where they bind to specific DNA responsive elements within the promoters of target genes and thus activate transcription of associated genes which are important for the PCa proliferation (3). It has been shown that IL-6 neutralizing antibodies or STAT3 antisense nucleotides can inhibit cell growth and cause apoptosis in PCa cells (4, 5).
Clinical observations revealed the correlation between high level of serum IL-6 and androgen-refractory prostate tumors. In vitro studies also demonstrated that IL-6-dependent activation of the JAK/STAT3 pathway is accompanied by transition from androgen-dependent to androgen-independent prostate cancer cell growth (6). The hypothesis that STAT3 is involved in the development of hormone-refractory prostate cancer is further supported by the observation that levels of activated STAT3 are significantly higher in androgen receptor (AR) -negative cells (DU145 and PC3) than in AR-positive cells (LNCaP) (7). STAT3 activation could act to promote cell growth and survival in androgen-refractory prostate cancer independent of the AR. Recently Tam et al., investigated both the expression levels and activation of the IL-6/JAK/STAT3 pathway in matched hormone-sensitive and hormone-refractory tumors from the same patient, the results also showed that STAT3 is crucial for the transition to androgen-refractory prostate cancer (8). Moreover, STAT3 has been demonstrated to play a critical role in facilitating immune evasion by negatively regulating cellular and innate immune responses (9); it can induce the expression of CD46, one of the complement-regulatory proteins, and protects prostate cancer cells from complement-dependent cytotoxicity (10). All of these studies suggested that IL-6/JAK/STAT3 could be a potential therapeutic target for prostate cancer therapy.
Epidemiologic studies continue to support the premise that dietary intake of cruciferous vegetables may be protective against the risk of prostate cancer (11). Anticarcinogenic effect of cruciferous vegetables is attributed to organic isothiocyanates (ITCs) that occur naturally as thioglucoside conjugates (glucosinolates) in a variety of cruciferous vegetables, such as broccoli, watercress, and cabbage. Organic ITCs are generated due to hydrolysis of corresponding glucosinolates through catalytic mediation of myrosinase, which is released on damage of the plant cells during processing of cruciferous vegetables (11). Phenethyl-ITC (PEITC) is one of the most extensively studied ITCs, and is a hydrolyzed product of gluconasturtiin, a glucosinolate, typically found in watercress, radish and turnip (12). Studies have revealed that PEITC suppressed the growth of human prostate cancer cells in culture as well as in vivo in xenograft assays. Many potential mechanisms have been proposed for anti-PCa effects of PEITC including inhibition of androgen receptor, apoptosis induction (13, 14). It has been shown recently that PEITC can inhibit the cap-dependent translation and angiogenesis in androgen-refractory prostate cancer PC-3 cells (15, 16).
In this study we investigated the potential mechanisms of PEITC on human PCa by observing the effects of PEITC on IL-6-induced JAK/STAT3 pathway activation in PCa cells in vitro. We found that PEITC significantly inhibited PCa proliferation, increased cell arrest at G2-M phase, and repressed constitutive and IL-6-stimulated JAK/STAT3 activation and IL-6-induced AR transcriptional activity. Moreover, we showed that an antioxidant reagent, N-acetyl-l-cysteine (NAC) coul reversed the inhibitory effects of PEITC on PCa cell proliferation, constitutive or IL-6-mediated JAK/STAT3 phosphorylation and IL-6- induced AR transcriptional activity. Thus, our data suggest that PEITC can inhibit prostate cancer cell growth via repression of the JAK/STAT3 signal-cascade, a process that maybe associated with cellular redox reactions.
MATERIALS AND METHODS
Cells and reagents
Human PCa cell lines, DU145 and LNCaP, were from the American Type Culture Collection (Manassas, VA) and were routinely maintained in RPMI 1640 with 5% fetal bovine serum (FBS, Biofluids, Rockville, MD) at 37°C under a humidified atmosphere of 5% CO2. Recombinant human IL-6 (Leinco Technologies, Inc, MO) was used at 25 ng/ml as indicated in each experiment. PEITC and phenyl isothiocyanate (PITC) (LKT Laboratories, Inc., St. Paul, MN) were dissolved in dimethyl sulfoxide (DMSO), which also was used as a control vehicle.
Cell proliferation assay and cell cycle distribution analysis
The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium (MTS) assay was used to detect the cell proliferation. Cells were cultured in 96-well plates at a density of 1000 cells per well with 100 μl of culture medium. After incubation for 40 h, cells were treated with PEITC or DMSO as a control for 72h, then 20 μl of MTS (Promega, Madison, WI) was added. After another 2 hour incubation, 100 μl soluble formazan containing media were used to measure the absorbance at 490 nanometers (nm). The control without cells was used to blank the background.
To test the effect of ITCs on the cell cycle, DU145 cells were plated in a 10 cm-diameter dishes treated with PEITC or PITC or vehicle (DMSO) for 20 h. Cells were trypsinized, washed twice with PBS, and fixed in ice-cold 40% ethanol. Fixed cells were incubated with 10 μg/ml RNase (Roche Diagnostics Corporation, Indianapolis, IN) at 37 °C for 15 min, stained with 10 μg/ml propidium iodide (Sigma) at room temperature for 15 min and analyzed on a FACScan flow cytometer. The percentage of cells in different phases of the cell cycle was determined using ModFit cell cycle analysis software.
Western blot analysis
Cells were seeded in 24 well plates at 2.5 × 104 cells/well and cultured for 2 days. Cultured cells were then treated with PEITC or PITC or DMSO (for control) at indicated concentration. For the IL-6 stimulation experiment, cells were starved with serum free medium for 2-3 h in order to remove constitutive IL-6 generation and then add 25 ng/ml IL-6 combine with PEITC or PITC or DMSO (for control) at indicated concentration. Cell lysates were prepared in SDS sample buffer at 5, 15, 25, 40 min or 20 h. After heated at 90°C for 10 min, samples were subjected to Western blot analysis with the primary antibodies against pTyr-STAT3, STAT3, pTyr JAK2, JAK2 as indicated and horseradish peroxidase-conjugated secondary antibodies and visualized by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ). Total STAT3 and JAK2 were blotted to assure equal loading.
Cell transfection and luciferase reporter assays
Transfections were performed using the Lipofectamine reagent (Invitrogen, Camarillo, CA) for DU145 cells and genefactor (VennNova, LLC., Pompano Beach, FL) for LNCaP cells. Briefly, cells (2-3×104 cells/well) were seeded in 24-well plates, when cells grew to 70-80% confluence, 250 ng of the reporter constructs and 50 ng of SV40- β-gal were transfected. After 6 h incubation, the medium was changed to 1% CSS RPMI medium and cells were treated for an additional 6 h with or without IL-6 (25 ng/ml), in combination with PEITC or PITC. Whole cell extracts were prepared by extraction with 100 μl of lysis buffer (Promega, Madison, WI). Then luciferase activity was measured by using a luciferase assay system (Promega) and Turners Design Luminometer TD 20/20. Finally, luciferase activity was normalized with the β-galactosidase (β-gal) activity assayed by using the substrate, o-nitrophenyl-β-D-galactopyranoside (ONPG).
Statistical analysis
All values were given as means ± SDs. Means of groups were compared with the Student’s t test and p < 0.05 was used as the level of significance.
RESULTS
PEITC Inhibits cell growth and induces G2-M phase cell cycle arrest in PCa cells
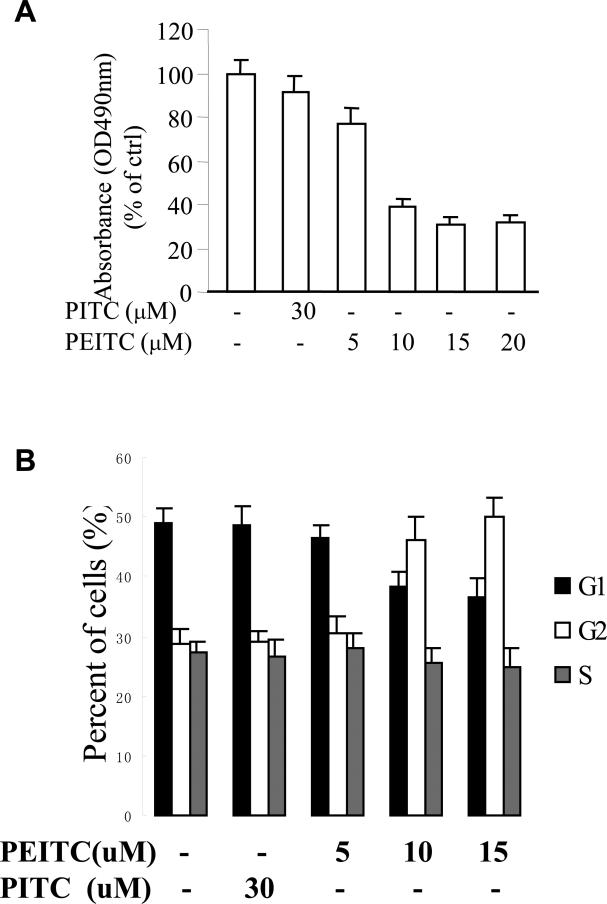
To evaluate the effects of ITCs on cell proliferation of human prostate cancer cell line DU145, we treated cells with PEITC or PITC at various concentrations for 72 h. Cell proliferation was estimated by MTS assay. Fig.1A shows that PEITC inhibited cell proliferation. The proliferation of DU145 cell was reduced by 25% after treatment with PEITC at 5 μM, and was further reduced by 68% at 20 μM. In contrast, when cells were treated with PITC, which is a structural analogue of PEITC but lacks a -CH2 spacer that links the aromatic ring to the -N=C=S group, no significant cell growth inhibition was observed. The cell-cycle study (Fig.1B) showed that G2-M population was significantly increased after treatment with 10 μM of PEITC in DU145 cells for 24 h. In contrast, little effect was observed when the cells were treated with PITC. These data suggest that PEITC, but not PITC, can repress human PCa cell growth and this inhibitory effect maybe associated with G2-M cell cycle arrest.
Fig. 1.
Effects of PEITC and PITC on the proliferation of androgen-refractory PCa DU145 cells, (A) DU145 cells were treated with various amounts of PEITC or PITC for 72 h. MTS assay was performed. The average of MTS measurements of the group without treatment was presented as 100% (n = 4–6). * P < 0.05 compared with the group of no treatment; (B) After treated with or without PEITC and PITC for 24 h, flow cytometer was used to analyze the DU145 cell cycle from G1 to S phase. * P < 0.05 compared with the group of no treatment.
PEITC inhibits constitutive and IL-6-induced STAT3 activation in PCa cells
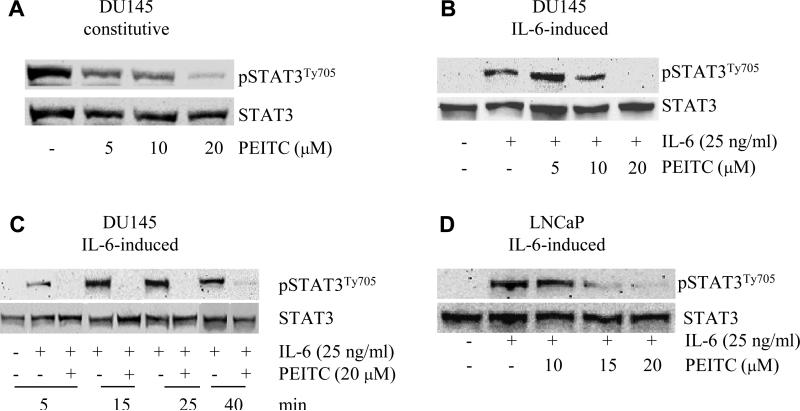
The activation of STAT3 signaling pathway is critical for the growth of PCa cells. STAT3 is constitutively activated in highly malignant PCa cell lines, such as DU145 and TSU cells, but not in LNCaP cells. To examine if the constitutively activated STAT3 signaling transduction pathway can be affected by ITCs, DU145 cells were exposed to PITC or PEITC for 20 h. Total and phosphorylated STAT3 expression levels were detected by western blot analysis. As shown in Fig. 2A, an activation of STAT3 via phosphorylation at tyrosine site 705 was detected in DU145 cells, consistent with previous reports that STAT3 is constitutively activated in DU145 cells (17). After treatment with PEITC at 5, 10, 20 μM, the phosphorylated STAT3 protein level in cells were decreased in a dose-depended manner. In contrast, the total STAT3 showed no change (Fig. 2A).
Fig. 2.
Effects of PEITC on the constitutive or IL-6-induced STAT3 activation in PCa cells. LNCaP and DU145 were treated with PEITC or IL-6, pSTAT3Ty705 protein levels were studied by Western blot, STAT3 levels was used to be an internal control. (A) DU145 cells were treated with various amounts of PEITC for 20 h. (B) DU145 cells were rinsed with and incubated with serum free medium for 2-3 h, then treated with 25 ng/ml of IL-6 and various amounts of PEITC for 40 min. (C). DU145 cells were treated with 20 μM PEITC for different times from 5 to 40 min. (D) LNCaP cells were treated with 25 ng/ml of IL-6 and various amounts of PEITC for 40 min.
To study if PEITC can inhibit IL-6-induced STAT3 activation in DU145 cells, DU145 cells were starved for 2-3 h in serum free medium, and then treated with 25 ng/ml IL-6 and PEITC and control group received the same amount of vehicles. The results from western blot showed that IL-6 can induce STAT3 activation which is consistent with the previous report. The important finding is PEITC inhibited the IL-6-induced STAT3 activation in a dose-depended manner. STAT3 phosphorylation was totally blocked by PEITC at a concentration of 20 μM. In order to test how fast PEITC can inhibit IL-6-induced STAT3 phosphorylation, DU145 cells were treated with IL-6 with or without PEITC for 5, 15, 25, 40 min. As shown in Fig. 2C, PEITC inhibited IL-6 activated STAT3 phosphorylation as early as 5 min after treatment. Similarly, IL-6-induced STAT3 phosphorylation and inhibition by PEITC were also found in LNCaP cells as shown in Fig. 2D.
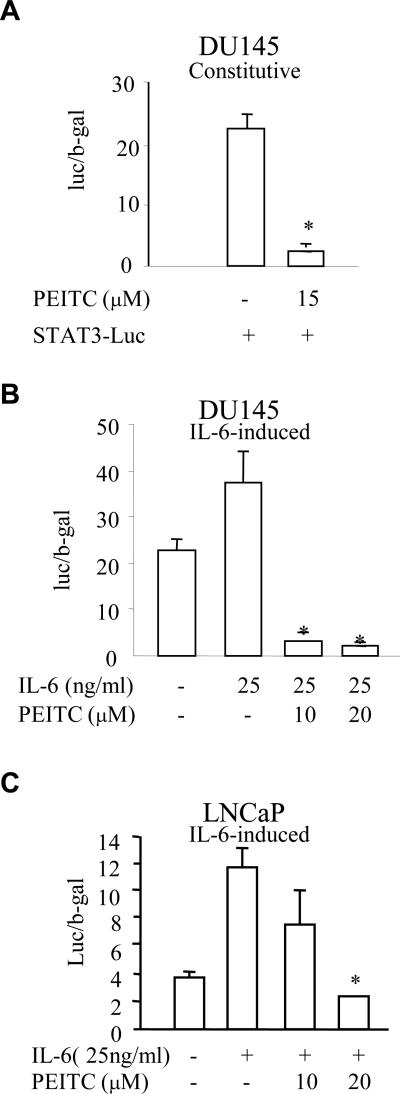
To further address the effects of PEITC on STAT3 activation in PCa cells, a luciferase reporter construct containing STAT3-binding sites was transfected into DU145 and LNCaP cells. After transfection, cells were treated with or without PEITC at various concentrations in the presence or absence of IL-6. Again, a constitutive STAT3 transactivation was detected in DU145 cells (Fig. 3A). A significant stimulatory effect of exogenous IL-6 on STAT3 transcriptional activity was observed in both DU145 (Fig. 3B) and LNCaP cells (Fig. 3C). PEITC inhibited the constitutive (Fig. 3A) and IL-6-induced STAT3 reporter activities in DU145 cells (Fig. 3B) and LNCaP cells (Fig. 3C). Taken together, these data indicated that PEITC can repress IL-6-induced STAT3 activation in both DU145 and LNCaP cells.
Fig. 3.
PEITC inhibits STAT3-mediated transactivation in PCa cells. A luciferase reporter construct containing STAT3 binding elements (STAT3-luc vector) was transfected into DU145 or LNCaP cells. Cells were then treated with PEITC or IL-6 for 6 h and STAT3-dependent luciferase activity was measured by a reporter gene assay. β-gal was used to be an internal control for normalizing the luciferase activity. (A) DU145 cells were transfeced with STAT3-luc vector and treated with 15 μM PEITC for 6 h. * p < 0.05 when compared to no PEITC treatment. (B) DU145 cells were transfected with STAT3-luc vector and treated with 25 ng/ml of IL-6 and various amounts of PEITC for 6 h. * p < 0.05 when compared to only IL-6 treatment. (C). LNCaP cells were transfeced with STAT3-luc vector and treated with 25 ng/ml of IL-6 and various amounts of PEITC for 6 h. * p < 0.05 when compared to only IL-6 treatment,
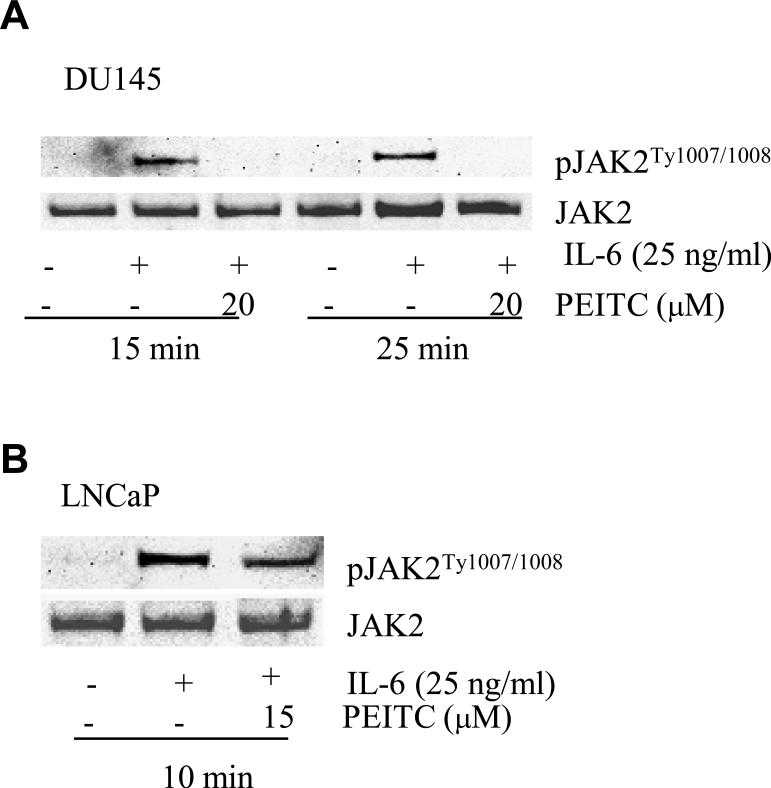
PEITC inhibits IL-6-induced JAK2 phosphorylation
IL-6 induces STAT3 activation by phosphorylation of JAK2 at the tyrosine 1007 and 1008 (18). To test whether the PEITC affects IL-6-induced activation of STAT3 via JAK2 in PCa cells, we studied the effects of PEITC on IL-6 stimulated JAK2 tyrosine phosphorylation in DU145 and LNCaP cells. Tyrosine phosphorylation of JAK was assessed by Western analysis in both cell lines after treatment with IL-6 and PEITC. IL-6-induced phosphorylation of JAK2 was totally blocked by PEITC in DU145 cells (Fig. 4A) and partially blocked in LNCaP cells (Fig. 4B), further supporting that PEITC inhibits IL-6-induced activation of the JAK/STAT3 pathway in PCa cells.
Fig. 4.
PEITC inhibits IL-6-induced JAK2 phosphorylation in PCa cells. (A) DU145 cells were treated with 25 ng/ml of IL-6 and 20 μM PEITC for 15 or 20 min. JAK phosphorylation at the tyrosine 1007 and 1008 was assessed by Western blot. JAK2 protein levels were used to be an internal control. (B) LNCaP cells were treated with 25 ng/ml of IL-6 and 15 μM PEITC for 10 min and JAK phosphorylation at the tyrosine 1007 and 1008 was studied by western blot. Total levels of JAKs were used as the internal controls.
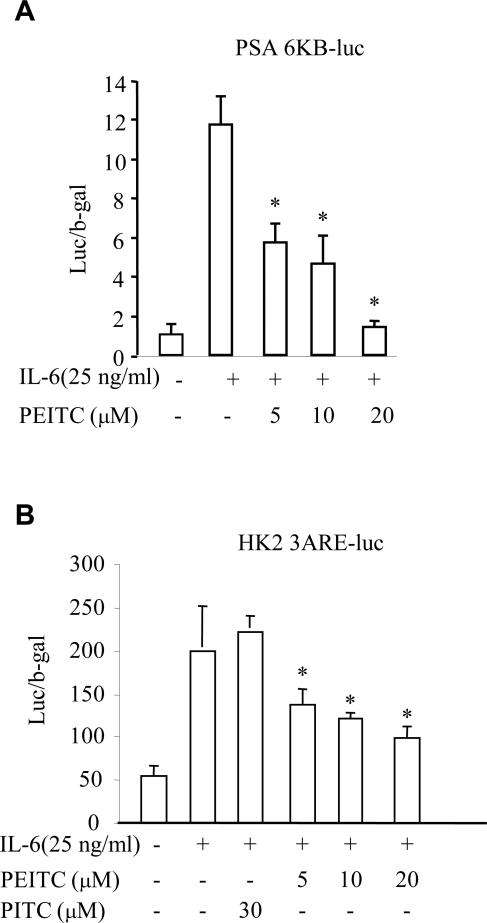
PEITC inhibits IL-6-induced AR transcriptional activity in LNCaP cells
The JAK/STAT3 pathways were reported to cross-talk with the AR signaling pathway (19). Activation of STAT3 has been implicated in the activation of AR induced by IL-6 in an androgen independent manner in LNCaP cells (19). To examine whether inhibition of PEITC on IL-6-induced activation of the JAK/STAT3 pathway can further affect AR-signaling in PCa cells, we tested the effects of PEITC on IL-6-associated activation of AR-signaling. PSA-6kb and hK2-3ARE luciferase reporter gene expression vectors, representing two commonly used AR activated gene promoters, were transfected into LNCaP cells. Transfected cells were then treated with or without IL-6 in the presence or absence PEITC at concentration of 5, 10, 20 μM or PITC at 30 μM (only in Fig. 5B) for 6 h followed by luciferase analysis. The results showed that luciferase activity in both PSA-6kb (Fig. 5A) and hK2-3ARE (Fig. 5B) induced by IL-6 in PCa cells was significantly inhibited by PEITC. This is consistent with the results in figure 3C and 4B that PEITC suppresses IL-6-mediated JAK/STAT3 activation in LNCaP cells.
Fig.5.
PEITC inhibits IL-6 induced AR transcriptional activity in LNCaP cells. (A) The luciferase expression vector containing PSA-6kb promoter was transfected into LNCaP cells, then treated the transfected cells with 25 ng/ml of IL-6 and variant amounts of PEITC for 6 h without androgens. The luciferase activity was measured by reporter gene assay and β-gal was used to be an internal control for normalizing the luciferase activity. (B) The luciferase expression vector containing hK2-3ARE promoter was transfected into LNCaP cells, then treated the transfected cells with 25 ng/ml of IL-6 and variant amounts of PEITC or 30 μM PITC for 6 h without androgens. The luciferase activity was measured by reporter gene assay, and β-gal was used to be an internal control for normalizing the luciferase activity. * p < 0.05 when compared to only IL-6 treatment.
N-acetyl-L-cysteine (NAC) can affect PEITC action
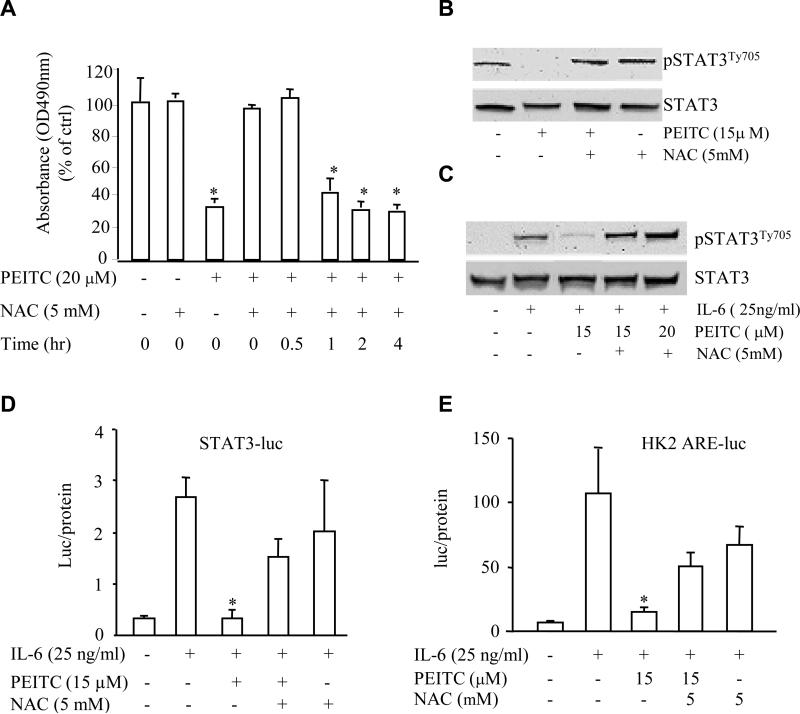
ROS can affect cell growth and cellular signaling pathway (20) and it has been reported that ITCs can increase intracellular ROS generation (21-23) in several systems including prostate cancer cells. To test whether ROS is involved in PEITC-associated inhibition of cell growth in PCa cells, we used N-acetyl-L-cysteine (NAC), an antioxidant that suppresses ROS generation, to test the role for ROS in PEITC-associated inhibition of cell growth and STAT3 activation in DU145 cells. We found that NAC at a dose of 5 mM completely reversed the inhibitory effect of PEITC on DU145 cell growth (Fig. 6A). Interestingly, this reversal effect of NAC was only observed at 0 h and 0.5 h (Fig. 6A) after its administration, after 1 h incubation with PEITC, NAC can not reverse the inhibitory effect of PEITC, suggesting that ROS or some kind of redox reactions occurred at the early phase of PEITC action on DU145 cells. Consistently, addition of NAC dramatically attenuated PEITC-repressed constitutive (Fig. 6B) and IL-6-induced (Fig. 6C) STAT3 protein phosphorylation and STAT3 transcriptional activity (Fig. 6D). Furthermore, inhibitory effect of PEITC on IL-6-stimulated AR transcriptional activity in LNCaP cell was also reversed by NAC administration (Fig. 6E). These results suggest that ROS generation or unidentified redox reaction may, at least in part, account for the effect of PEITC on STAT3 pathway repression and consequent growth inhibition at the early phase.
Fig.6.
NAC reverses the PEITC function in PCa cells. (A) NAC reversed the inhibition of PEITC on the proliferation of DU145 cells at 0 and 0.5 h after PEITC treatment by MTS assay. (B) NAC blocked the inhibition of PEITC on the constitutive (B) or IL-6-induced (C) STAT3 activation in DU145 cells by Western blot assay with protein samples harvested at 20 h or 30 min, respectively. (D) NAC reversed the inhibition of PEITC on the IL-6-induced STAT3 transcriptional activity by reporter gene assay. * p < 0.05 when compared to only IL-6 treatment. (E) NAC blocked the inhibition of PEITC on the IL-6-induced AR transcriptional activity in LNCaP cells in the absence of androgens by reporter gene assay. * p < 0.05 when compared to only IL-6 treatment.
DISCUSSION
As one of the most prevalent types of malignancy in the United States and many other countries, prostate carcinogenesis has been viewed as a multistage and complex process consisting of initiation, promotion, and progression (24). Although PCa patients can benefit from androgen ablation, eventually most of them become poorly responsive as the disease progresses to the androgen-refractory state (25). Recent studies implicate that IL-6 may function as an autocrine or paracrine growth factor since it stimulates proliferation of various cancer cells, including PCa cells (26-28). It is well accepted that IL-6 may activate multiple signaling pathways, in particular, the JAK/STAT3 and mitogen-activated protein kinase (MAPK) pathways (29). Activation of the JAK/STAT3 pathway transmits IL-6-mediated signals from cell-surface receptor to the target genes in nucleus and is critical for regulation of prostate cancer cellular growth and differentiation (30). Indeed, previous reports demonstrated that androgen-refractory PCa DU145 and PC3 cells constitutively express IL-6 and activate phosphorylation of STAT3 (7). Moreover, the JAK/STAT3 pathway is involved in the signaling cross-talking with the AR. Activation of STAT3 not only ensures maintenance of the AR signal but also increases AR activation by other signals such as EGF and IL-6 (31). In the present study, we found that IL-6 stimulates cell growth and JAK/STAT3 pathway in PCa cells. IL-6 can also stimulate AR transactivation in LNCaP cells. For constitutive STAT3 phosphorylation, only DU145 cells showed positive, presumably via endogenous expression of IL-6. Thus, our data further support the role for IL-6 and its associated activation of the JAK2/STAT3 pathway in PCa growth. Moreover, interference of IL-6-stimulated JAK2/STAT3 pathway may yield a promising way to repress or prevent prostate cancer progression.
Numerous epidemiologic studies have shown that consumption of cruciferous vegetables is protective against various types of human cancers (32). ITCs which naturally occur in cruciferous vegetables have recently received much attention as possible chemopreventive agents (33-35). PEITC, a well-studied member of ITCs, has shown anti-proliferative effects on PCa and preneoplastic cells (36). In the present study, consistent with previous reports (37), we found that PEITC can significantly inhibit PCa proliferation, cause cell cycle G2-M phase arrest in DU145 cells. Another similar isothiocyanate substance, PITC, which is a structural analogue of PEITC but lacks the -CH2 spacers that link the aromatic ring to the -N=C=S group, has no significant inhibitory effects on DU145 cell growth, suggesting that inhibition of PCa proliferation by ITCs is structure-dependent. We have also detected an activation of STAT3 and JAK2 phosphorylation in DU145 cells after stimulation with a recombinant human IL-6. For the first time we showed that PEITC, but not PITC, effectively represses both constitutive and IL-6-induced phosphorylation of JAK and STAT3 in cultured PCa cells, suggesting that PEITC represses PCa growth via inhibition of JAK/STAT3 activation. This is further supported by our observation that PEITC inhibits IL-6-induced AR-activation in an androgen sensitive prostate cancer cell line (i.e., LNCaP cells). Activation the JAK/STAT3 pathway has been implicated in the activation of AR-dependent gene expression induced by IL-6 in PCa cells (38) independent of androgens. Indeed, we showed that PEITC, but not PITC, down-regulates IL-6-induced AR transcriptional activity on hK2-3ARE and PSA promoter activity in LNCaP cells.
ROS may stimulate cell proliferation and induce genetic instability, and their increase in cancer cells is often viewed as an adverse event. Increased ROS can be exploited to selectively kill cancer cells (20). Several drugs against the cancer perform their function through generation of ROS (39-40). It has been reported recently that ITCs, including PEITC, can increase intracellular ROS production (20-23). However, in this study we found that IL-6/STAT3 pathway is affected by ROS induced by PEITC only at the early stage of its administration. Further, NAC can only block growth inhibitory effect at early PEITC treatment. These results were one of the most significant findings in this report, and consistently suggest that PEITC induced ROS at early time of its application which in turn interfered with STAT3 activation with consequent cell growth inhibition. Evidence also clearly showed that PEITC can increase oxidative stress and mediate the activation of antioxidant responsive element containing genes such as heme oxygenase-1 (41). Conceivably, those thiol containing antioxidants such as glutathione or 2-mercaptoethanol might have similar effects to NAC (42). Alternatively, it has been reported that NAC can interact chemically with PEITC through its free thiol group interacting with the ITC moiety of PEITC to reverse inhibitory effects on the proliferation of prostate cancer cells (43). Therefore, a plausible explanation was proposed by other studies (44) that thiocarbamoylation of glutathione or the cysteinyl thiol group containing proteins may be a major mechanism for activation of certain critical cellular events by PEITC.
In conclusion, our studies provide the evidence that PEITC, but not PITC, inhibits activation of the JAK/STAT3 pathway and consequently, cell growth of PCa cells. Moreover, inhibition of cell growth and JAK/STAT3 activation by PEITC at the early stage is, at least in part, associated with certain redox mediated mechanisms such as the production of ROS. However, the interesting question of what exact mechanism that ROS is involved in JAK/STAT inactivation is will require further investigation in the near future.
Acknowledgments
This work was, in part, supported by NIH grant 88900.
The abbreviations used are
- PEITC
Phenethyl isothiocyanate
- PITC
Phenyl isothiocyanate
- ITCs
isothiocyanates
- JAK
Janus kinase
- STAT3
signal transducers and activators of transcription factor 3
- PCa
Prostate cancer
- ROS
reactive oxygen species
- NAC
N-acetyl-L-cysteine
- IL-6
Interleukin 6
- AR
androgen receptor
Reference
- 1.Zhang SS, Liu MG, Kano A, Zhang C, Fu XY, Barnstable CJ. STAT3 activation in response to growth factors or cytokines participates in retina precursor proliferation. Exp Eye Res. 2005;81(1):103–15. doi: 10.1016/j.exer.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Lou W, Ni Z, Dyer K, Tweardy DJ, Gao AC. Interleukin-6 induces prostate cancer cell growth accompanied by activation of STAT3 signaling pathway. Prostate. 2000;42:239–242. doi: 10.1002/(sici)1097-0045(20000215)42:3<239::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Heinrich P, Behmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling though the gp130/Jak/STAT pathway. Biochem. J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallner L, Dai J, Escara-Wilke J, Zhang J, Yao Z, Lu Y, Trikha M, Nemeth JA, Zaki MH, Keller ET. Inhibition of interleukin-6 with CNTO328, an anti-interleukin-6 monoclonal antibody, inhibits conversion of androgen-dependent prostate cancer to an androgen-independent phenotype in orchiectomized mice. Cancer. Res. 2006;66(6):3087–95. doi: 10.1158/0008-5472.CAN-05-3447. [DOI] [PubMed] [Google Scholar]
- 5.Barton BE, Murphy TF, Shu P, Huang HF, Meyenhofer M, Barton A. Novel single-stranded oligonucleotides that inhibit signal transducer and activator of transcription 3 induce apoptosis in vitro and in vivo in prostate cancer cell lines. Mol. Cancer. Ther. 2004;3(10):1183–91. [PubMed] [Google Scholar]
- 6.Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer. Res. 1997;57:141–146. [PubMed] [Google Scholar]
- 7.Mora LB, Buettner R, Seigne J. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–66. [PubMed] [Google Scholar]
- 8.Tam L, Mcglynn LM, Traynor P, Mukherjee R, Bartlett JM, Edwards J. Expression levels of the JAK/STAT pathway in the transition from hormone-sensitive to hormone-refractory prostate cancer. Br. J. Cancer. 2007;97(3):378–83. doi: 10.1038/sj.bjc.6603871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buettner R, Huang M, Gritsko T, Karras J, Enkemann S, Mesa T, Nam S, Yu H, Jove R. Activated signal transducers and activators of transcription 3 signaling induces CD46 expression and protects human cancer cells from complement-dependent cytotoxicity. Mol Cancer Res. 2007;5(8):823–32. doi: 10.1158/1541-7786.MCR-06-0352. [DOI] [PubMed] [Google Scholar]
- 10.Wand T, Niu G, Kortylewski M. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 11.Wand T, Niu G, Kortylewski M. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. 2001;56(1):5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 12.Wand T, Niu G, Kortylewski M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. 2008 May;47(Suppl 2):73–88. doi: 10.1007/s00394-008-2009-8. [DOI] [PubMed] [Google Scholar]
- 13.Xiao D, Lew KL, Zeng Y, Xiao H, Maryniwski SW, Dhir R, Singh SV. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis. 2006;27(11):2223–34. doi: 10.1093/carcin/bgl087. [DOI] [PubMed] [Google Scholar]
- 14.Wang LG, Liu XM, Chial JW. Repression of androgen receptor in prostate cancer cells by phenethyl isothiocyanate. Carcinogenesis. 2006;27(10):2124–2132. doi: 10.1093/carcin/bgl075. [DOI] [PubMed] [Google Scholar]
- 15.Hu J, Straub J, Xiao D, Singh SV, Yang HS, Sonenberg N, Vatsyayan J. Phenethyl isothiocyanate, a cancer chemopreventive constituent of cruciferous vegetables, inhibits cap-dependent translation by regulating the level and phosphorylation of 4E-BP1. Cancer. Res. 2007;67(8):3569–73. doi: 10.1158/0008-5472.CAN-07-0392. [DOI] [PubMed] [Google Scholar]
- 16.Xiao D, Singh SV. Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer. Res. 2007;67(5):2239–46. doi: 10.1158/0008-5472.CAN-06-3645. [DOI] [PubMed] [Google Scholar]
- 17.Chung TDK, Yu J, Spiotto MT, Bartkowski M, Simons JW. Characterization of the role of IL-6 in the progression of prostate cancer. Prostate. 1999;38:199–207. doi: 10.1002/(sici)1097-0045(19990215)38:3<199::aid-pros4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Horvach CM. The Jak-STAT pathway stimulated by interleukin 6. Sci. Stke. 2004;23:260. doi: 10.1126/stke.2602004tr9. [DOI] [PubMed] [Google Scholar]
- 19.Lee SO, Lou W, Gao AC. Interleukin-6 promotes androgen-independent growth in LNCaP human prostate cancer cells. Clin Cancer Res. 2003;9:370–376. [PubMed] [Google Scholar]
- 20.Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P. Selective killing of oncogenically transformed cells though a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10(3):241–52. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Xiao D, Lew KL, Zeng Y, Xiao H, Marynowski SW, Dhir R, Singh SV. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis. 2006;27(11):2223–34. doi: 10.1093/carcin/bgl087. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Trachootham D, Lu W, Carew J, Giles FJ, Keating MJ, Arlinghaus RB, Huang P. Effective killing of Gleevec-resistant CML cells with T315I mutation by a natural compound PEITC through redox-mediated mechanism. Leukemia. 2008;22(6):1191–9. doi: 10.1038/leu.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu C, Yuan X, Pan Z, Shen G, Kim JH, Yu S, Khor TO, Li W, Ma J, Kong AN. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol Cancer Ther. 2006;5(8):1918–26. doi: 10.1158/1535-7163.MCT-05-0497. [DOI] [PubMed] [Google Scholar]
- 24.Chen CD, Welabie DS, Tran C. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 25.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem. 2006;99:333–44. doi: 10.1002/jcb.20794. [DOI] [PubMed] [Google Scholar]
- 26.Rose JS, Waetzig GH, Scheller J, Grotzinger J, Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets. 2007;11(5):613–24. doi: 10.1517/14728222.11.5.613. [DOI] [PubMed] [Google Scholar]
- 27.Giri D, Ozen M, Ittmann M. Interleukin-6 is an autocrine growth factor in human prostate cancer. Am J Pathol. 2001;159:2159–2165. doi: 10.1016/S0002-9440(10)63067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhir R, Ni ZY, Lou W, DeMiguel F, Grandis JR, Gao AC. Stat3 activation in prostatic carcinomas. Prostate. 2002;51:241–246. doi: 10.1002/pros.10079. [DOI] [PubMed] [Google Scholar]
- 29.Ueda T, Bruchovsky N, Sadar MD. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J Biol Chem. 2002;277:7076–85. doi: 10.1074/jbc.M108255200. [DOI] [PubMed] [Google Scholar]
- 30.Smith PC, Hobisch A, Lin DL, Culig Z, Keller ET. Interleukin-6 and prostate cancer progression. Cytokine. Growth Factor Rev. 2001;12:33–40. doi: 10.1016/s1359-6101(00)00021-6. [DOI] [PubMed] [Google Scholar]
- 31.Aaronson DS, Muller M, Neves SR, Chung WC, Jayaram G, Iyengar R, Ram PT. An androgen-IL6-Stat3 autocrine loop re-routes EGF signal in prostate cancer cells. Mol Cell Endocrinol. 2007;270(1-2):50–6. doi: 10.1016/j.mce.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5(28):733–748. [PubMed] [Google Scholar]
- 33.Mi L, Wang X, Govind S, Hood BL, Veenstra TD, Conrads TP, Saha DT, Goldman R, Chung FL. The role of protein binding in induction of apoptosis by phenethyl isothiocyanate and sulforaphane in human non-small lung cancer cells. Cancer Res. 2007;67(13):6409–16. doi: 10.1158/0008-5472.CAN-07-0340. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson LR, Philpott M. Cancer prevention by dietary bioactive components that target the immune response. Curr Cancer Drug Targets. 2007;7(5):459–64. doi: 10.2174/156800907781386605. [DOI] [PubMed] [Google Scholar]
- 35.Xiao D, Zeng Y, Choi S, Lew KL, Nelson JB, Singh SV. Caspase-dependent apoptosis induction by phenethyl isothiocyanate, a cruciferous vegetable-derived cancer chemopreventive agent, is mediated by Bak and Bax. Clin Cancer Res. 2005;11(7):2670–9. doi: 10.1158/1078-0432.CCR-04-1545. [DOI] [PubMed] [Google Scholar]
- 36.Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24(28):4486–95. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 37.Xiao D, Singh SV. Phenethyl isothiocyanate-induced apoptosis in p53-deficient PC-3 human prostate cancer cell line is mediated by extracellular signal-regulated kinases. Cancer Res. 2002;62(13):3615–9. [PubMed] [Google Scholar]
- 38.Matsuda T, Junicho A, Yamamoto T, Kishi H, Korkmaz K, Saatcioglu F, Fuse H, Muraguchi A. Cross-talk between signal transducer and activator of transcription 3 and androgen receptor signaling in prostate carcinoma cells. Biochem Biophys Res Commun. 2001;283:179–87. doi: 10.1006/bbrc.2001.4758. [DOI] [PubMed] [Google Scholar]
- 39.Kim BM, Chung HW. Hypoxia/reoxygenation induces apoptosis through a ROS-mediated caspase-8/Bid/Bax pathway in human lymphocytes. Biochem. Biophys. Res. Commun. 2007;363(3):745–50. doi: 10.1016/j.bbrc.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 40.Ko CH, Shen SC, Yang LY, Lin CW, Chen YC. Gossypol reduction of tumor growth through ROS-dependent mitochondria pathway in human colorectal carcinoma cells. Int. J. Cancer. 2007;121(8):1670–9. doi: 10.1002/ijc.22910. [DOI] [PubMed] [Google Scholar]
- 41.Ko CH, Shen SC, Yang LY, Lin CW, Chen YC. Molecular mechanisms of c-Jun N-terminal kinase-mediated apoptosis induced by anticarcinogenic isothiocyanates. 1998;273(3):1769–75. doi: 10.1074/jbc.273.3.1769. [DOI] [PubMed] [Google Scholar]
- 42.Kim YA, Xiao D, Xiao H, Powolny AA, Lew KL, Reilly ML, Zeng Y, Wang Z, Singh SV. Mitochondria-mediated apoptosis by diallyl trisulfide in human prostate cancer cells is associated with generation of reactive oxygen species and regulated by Bax/Bak. Mol Cancer Ther. 2007;6(5):1599–609. doi: 10.1158/1535-7163.MCT-06-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YA, Xiao D, Xiao H, Powolny AA, Lew KL, Reilly ML, Zeng Y, Wang Z, Singh SV. Modulation of growth of human prostate cancer cells by the N-acetylcysteine conjugate of phenethyl isothiocyanate. 2000;16(6):1215–9. doi: 10.3892/ijo.16.6.1215. [DOI] [PubMed] [Google Scholar]
- 44.Kim YA, Xiao D, Xiao H, Powolny AA, Lew KL, Reilly ML, Zeng Y, Wang Z, Singh SV. Involvement of glutathione metabolism in the cytotoxicity of the phenethyl isothiocyanate and its cysteine conjugate to human leukaemia cells in vitro. 2001;61(2):165–77. doi: 10.1016/s0006-2952(00)00526-8. [DOI] [PubMed] [Google Scholar]