Abstract
Background
Adiposis dolorosa (AD) is a syndrome of obese and non-obese individuals whose hallmark is lipomatosis: unencapsulated painful fatty masses in subcutaneous fat. Lipomatosis may contain excess collagen and multi-nucleated giant (MNG) cells. Case reports suggest metabolic defects in AD.
Objectives
(1) To determine whether women with AD have altered relative resting energy expenditure (REE per total body mass) compared with controls; and (2) to quantitate lipomatosis-associated collagen, MNGs and tissue and blood cytokines that may influence REE.
Methods
A total of 10 women with AD were compared with age, body mass index, fat and weight-matched control women. Adipose tissue was obtained from five women with AD and five controls and evaluated for collagen and macrophages/MNGs. Fat mass and fat-free mass were identified by dual X-ray absorptiometry. REE was by determined indirect calorimetry and related to mass. Adipokines and cytokines were evaluated in blood and tissue.
Results
Relative REE (REE per total body mass) was lower in women with AD compared with controls (P=0.007). Only lipomatosis (group) and total body mass were significant predictors of REE in forward stepwise regression (P<0.0001). Adipose interleukin (IL)-6 levels were elevated (P=0.03) and connective tissue was increased fourfold in lipomatosis compared with control tissue (P<0.0001). There was no difference in adipose tissue macrophages between groups; 30% of women with AD had MNG cells. Anti-inflammatory IL-13 levels were elevated (P=0.03), and cytokines important in the recruitment of monocytes, Fraktalkine (P=0.04) and macrophage inflammatory protein-1β (P=0.009), were significantly lower in the blood of women with AD compared with controls.
Conclusions
The lower relative REE in women with AD compared with controls was associated with increased connective (non-metabolic) tissue in the lipomatosis, and inflammation, although underlying metabolic defects may be important as well. Understanding the pathophysiology and metabolism of lipomatosis in AD may contribute to a better understanding of metabolism in non-lipomatosis obesity.
Keywords: adiposis dolorosa, resting energy expenditure, IL-6, Dercum's disease, lipomatosis
Introduction
Dercum's disease or adiposis dolorosa (AD), first described in 1888 by Dr F Xavier Dercum,1 is a syndrome whose hallmark is lipomatosis: painful fatty growths in subcutaneous fat that can involve large areas of the body from the head to dorsal foot.2 The pain and fatigue associated with AD can be disabling3,4 especially when accompanied by signs and symptoms that affect most systems.2 To date, the underlying pathophysiology remains unknown but metabolic, inflammatory, infectious and autoimmune components have been proposed.5 Obesity is common among AD patients,6 with some describing very rapid weight gain in a short period of time. One reason for persistent obesity is that 100% of individuals with AD are unable to lose mass from the lipomatosis with diet and exercise.2
A survey of 110 individuals with AD showed a twofold higher prevalence of diabetes compared with the 2005 US population for ages 45–64 years for both low and high body mass index (BMI) groups,2 suggesting an alteration in metabolism and insulin resistance in AD even in the absence of obesity. A number of case reports have shown metabolic impairments in individuals with AD. Conversion of oral oleic acid-1−14C to 14CO2 was decreased in two women with AD compared with controls suggesting a defect in fatty acid absorption or utilization.7 In vitro, free fatty acid release was not suppressed from the lipomatosis of a single subject with insulin resistance and AD, when exposed to increasing concentrations of insulin or norepinephrine compared with non-lipomatosis fat.8 In another case, excised lipomatosis fat converted glucose to neutral glycerides at a lower rate than normal fat.9 Fagher et al.10 also found lower heat production from lipomatosis in AD and a lower lipoprotein lipase level in lipomatosis that did not reach statistical significance. These data suggest that fat accumulation may be conferred in part by decreased metabolism in the lipomatosis fat. As both fat mass (FM) and muscle mass or fat-free mass (FFM) contribute significantly to resting energy expenditure (REE) in obesity,11,12 these data lead us to propose that REE would be lower in women with AD relative to total body mass (relative REE).
Qualitative reports on the histology of lipomatosis fat suggest that the lipomatosis contains normal fat cells, but increased dense connective tissue13 and increased fibroblasts.14 A possible explanation for the failure of the lipomatosis to decrease with weight loss could therefore be structural, wherein a majority of the lipomatosis is connective tissue. Alternatively, multi-nucleated giant (MNG) cells have been reported in the lipomatosis,7,15 which are formed by macrophages that are activated and proinflammatory.16 These data suggest that inflammation-driven adipogenesis17 could increase lipomatosis in AD, inhibiting fat loss. In this scenario, we would expect elevated inflammatory cytokines and/or adipokines in the lipomatosis. As systemic inflammation including elevations in interleukin (IL)-6, IL-1β and tumor necrosis factor (TNF)-α are associated with increased REE,18,19 and we predict a decreased relative REE in AD, we would also predict a lack of elevation of systemic inflammation. In addition to testing proinflammatory cytokines, such as IL-1β, IL-6, IL-8, TNF-α, we therefore were also interested in cytokines that would attract macrophages to form MNGs in the lipomatosis fat, such as monocyte chemotactic protein (MCP)-1, macrophage inflammatory protein (MIP)-1α and MIP-1β and the chemokine, Fractalkine, with the ability to attract monocytes, T cells and neutrophils20,21 and which is also thought to contribute to the maintenance of neuropathic pain,22,23 which may be important in AD. IL-13 was also of interest as it suppresses proinflammatory cytokines24 and secreted Fractalkine (sFractalkine)25,26 and diet-induced obesity decreases visceral fat-associated IL-13 in mice.27
In the current report, we examined REE by indirect calorimetry in 10 women with AD and 10 age, BMI, fat and weight-matched control women. Secreted and tissue adipokines, as well as blood lipid levels, were examined, as hypertriglyceridemia increases energy expenditure.28 Connective tissue and macrophages were quantitated in the lipomatosis compared with control fat.
Materials and methods
Subjects
Subjects were recruited from advertisement on forums devoted to AD on the internet (http://health.groups.yahoo.com/group/Dercums_Disease/ and http://health.groups.yahoo.com/group/Dercums_Disease/) and through the National Organization for Rare Diseases Inc., website (http://www. rarediseases.org/research/clinicaltrials). Subjects had an earlier diagnosis of AD or were diagnosed (by KLH) after obtaining a history, performing an examination and obtaining a fat biopsy from affected tissue. Control subjects were recruited based on age, BMI and weight from a pool of women interested in research. All subjects were seen at the General Clinical Research Center at the University of California, San Diego. We certify that all the applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. This study was approved by the Institutional Review Board at University of California, San Diego and all subjects consented to their participation.
Fat biopsy histology and immunohistochemistry
Fat biopsies were obtained from affected areas in five subjects and from matching sites in five controls using a 5-mm side-cutting needle. Lidocaine (1%) was infiltrated in a square field manner, and the biopsy was taken from the center of the field. The fat samples were placed in 10% neutral buffered formalin immediately after removal followed by 70% EtOH after 24 h, then placed in paraffin blocks and sectioned onto double-positive slides. Additional biopsy samples were rinsed in a buffer consisting of 150 mmoll−1 NaCl, 5 mmoll−1 KCl, 1.2 mmoll−1 MgSO4, 1.2 mmoll−1 CaC12, 2.5 mmoll−1 NaH2PO4, 10 mmoll−1 HEPES and 2 mmoll−1 pyruvate, pH 7.4, supplemented with 4% bovine serum albumin then immediately frozen. Tissue sections were stained with hematoxylin and eosin (H&E) and Masson's trichrome staining protocol for collagen fibers, and macrophages were labeled with CD68 (ab955; Abcam, Cambridge, MA, USA) by immunohistochemistry at a 1:300 dilution after a 20 min sodium citrate heat pretreatment, then following the manufacturer's recommendations (Mass Histology, Worcester, MA, USA). Photographs were at ×10 using a digital microscope (Model DC5-163National Microscope, San Antonio, TX, USA). Digital photographs were processed for percentage area of collagen on three representative samples and macrophages were counted in 3–4 representative samples using Motic Images Plus 2.0 software (Richmond, British Columbia, Canada) and are expressed as number per high-power field.
Quantitative PCR
The mRNA levels were quantified in fat samples by subjecting cDNA to TaqMan PCR analysis, in triplicate, using the GeneAmp 5700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) as published earlier.29 Predeveloped sequence detection reagents specific for human TNF- α, IL-1β, IL-6, IL-8 and IL-13 including forward and reverse primers, as well as a fluorogenicTaqMan FAM/TAMRA-labeled hybridization probe were supplied as mixtures and were used at 0.8 μl/25 μl PCR. Variables presented are log transformed.
Dual X-ray absorptiometry
To relate REE to the differences in body composition between AD and controls, FM and FFM were measured by dual X-ray absorptiometry scan for whole-body composition on a Hologic Discovery W (Boston, MA) and analyzed using software QDR DICOM for Windows XP.
Indirect calorimetry
After 12 h of fasting, subjects rested in supine position in hospital beds for 15 min. Subsequently, pulmonary ventilation and gas exchange were measured for 20 min by indirect calorimetry (Deltatrac II Metabolic Monitor, Sensormedics, Yorba Linda, CA, USA) using the dilution principle.30 REE and fat and carbohydrate oxidation rates31 were calculated from steady state values (±5%) for oxygen uptake (V̇O2) and carbon dioxide output (V̇O2) collected during the last 15 min of measurement following manufacturer's specifications. The modified Weir equation (V̇O2 (ml min−1) × 3.94 +V̇O2 mL min−1 × 1.11) × 1.44)32 was used to calculate REE (kcal per day). REE was normalized per kg total body mass, FFM and FM.
Cytokine assays
Blood was drawn after subjects fasted 12 h (overnight), and was processed and stored at −80°F until use. Cytokines and adipokines were measured in duplicate on a Luminex MultiAnalysis system (Luminex Corporation, Austin, TX, USA) at the Mouse Metabolic Phenotyping Center (Cincinnati, OH, USA). Adiponectin and plasminogen activator inhibitor-1 (active) were assayed using the human serum adipokine panel A immunoassay kit (Millipore, Billerica, MA, USA). Leptin was assayed using the human serum adipokine panel B immunoassay kit (Millipore) and IL-1β, IL-6, IL-8, IL-10, IL-13, Fractalkine, MCP-1, MIP-1α, MIP-1β and TNF-α were assayed using the human cytokine/chemokine panel (Millipore, Billerica, MA, USA). Kits used for the measurement of total cholesterol (Sigma Infinity Kit, Sigma Chemical Co., St Louis, MO, USA), triglycerides (Randox Laboratories Ltd. Crumlin, Co. Antrim, UK) and phospholipids and non-esterified fatty acids (Wako Chemicals Inc., Richmond, VA, USA) were utilized following manufacturers specifications.
Statistics
Data in Tables are presented as mean±s.e.m. Differences between groups were examined with non-parametric two-sample Wilcoxon (Mann–Whitney) testing because of small group size and then two sample t-tests with testing for equality of variance. There was no difference in the statistical significance between unadjusted comparisons by non-parametric vs parametric two sample tests. P-values in Tables 2 and 3 are for two sample t-tests. For adipose-associated cytokines, Tukey's post hoc analysis was used. Simple correlation was done by Spearman's rank test and/or simple linear regression. Multiple linear regression modeling was used to evaluate predictors of REE. P-values <0.1 are presented and a value <0.05 was considered statistically significant. Data analyses were performed using GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA, USA) and Stata v 6.0 (StataCorp, College Station, TX, USA).
Table 2.
Body composition and resting energy expenditure in AD and controls (mean±s.e.m.)
| AD | Controls | P-value | |
|---|---|---|---|
| Number | 10 | 10 | |
| Age (years) | 47.7±2.2 | 45.4±2.8 | NS |
| Height (cm) | 163.5±3.0 | 168.1±2.0 | NS |
| Weight (kg) | 101.5±7.5 | 93.5±7.6 | NS |
| BMI (kg m−2) | 37.9±2.6 | 33.0±2.4 | NS |
| Total FM (kg) | 45.1±4.7 | 41.7±3.7 | NS |
| Total FFM (kg) | 52.5±2.9 | 50.5±3.1 | NS |
| Total FM (%) | 44.4±2 | 43.7±1.4 | NS |
| Total FFM (%) | 53.1±2 | 53.8±1.4 | NS |
| Trunkal FM (kg) | 22.4±2.1 | 20.8±2.3 | NS |
| Trunkal FFM (kg) | 27.5±1.6 | 26.0±1.7 | NS |
| Trunkal FM (%) | 43.1±2 | 43.9±1 | NS |
| Trunkal FFM (%) | 55.6±2 | 54.8±1 | NS |
| Extremity FM (kg) | 10.9±1.3 | 10.0±0.8 | NS |
| Extremity FFM (kg) | 10.8±0.7 | 10.5±0.7 | NS |
| Extremity FM (%) | 44.9±2 | 46.6±1.3 | NS |
| Extremity FFM (%) | 47.8±2 | 49.7±1.5 | NS |
| RQ | 0.75±0.03 | 0.78±0.05 | NS |
| REE (kcal per day) | 1684.6±95.4 | 1730.9±97.6 | NS |
| Relative REE (kcal per kg · per day) | 17.0±0.4 | 19.1±0.5 | <0.007 |
| Relative V̇O2 (ml per kg per min) | 2.5±0.05 | 2.8±0.04 | <0.008 |
Abbreviations: BMI, body mass index; FM, fat mass; FFM, fat-free mass; NS, not significant; REE, resting energy expenditure; RQ, respiratory quotient; V̇O2, volume of oxygen transported and utilized by the body.
Table 3.
Predictors of REE in women with AD compared with control women; multiple linear regression with dependent variable, REE
| Independent variables | B coefficient | P-value | AdjustedR2 | Overall model P-value | |
|---|---|---|---|---|---|
| Model 1 | 0.880 | <0.0001 | |||
| Age | 2.29 | 0.5 | |||
| Body mass (kg) | 12.12 | <0.001 | |||
| Group | −149.16 | 0.006 | |||
| Model 2 | 0.878 | <0.0001 | |||
| Age | 4.30 | 0.38 | |||
| Body mass (kg) | 5.51 | 0.83 | |||
| FM (kg) | 3.78 | 0.89 | |||
| FFM (kg) | 12.48 | 0.64 | |||
| Group | −145.76 | 0.009 | |||
| Model 3 | 0.875 | <0.0001 | |||
| Age | 0.93 | 0.79 | |||
| Body mass (kg) | 12.06 | <0.001 | |||
| Percent FM | −54.36 | 0.49 | |||
| Percent FFM | −62.80 | 0.46 | |||
| Group | −144.13 | 0.01 | |||
| Forward stepwise regression: retains variables with P ≤0.2. Variables tested included age, total body mass, FM, FFM and group. Retained variables were as follows: | 0.883 | <0.0001 | |||
| Body mass (kg) | 12.12 | <0.001 | |||
| Group | −143.95 | 0.006 | |||
Abbreviations: REE, resting energy expenditure; FFM, fat free mass; FM, fat mass.
Results
Subjects
Characteristics of women with AD are shown in Table 1. The average age for onset of AD was 32.1±2.8 years, defined by palpable subcutaneous masses, adipose or fibro-adipose tissue by biopsy and signs and symptoms associated with AD.2 Average duration of disease was 15.8±3.8 years. The women with AD were matched to women without AD (control group) by age and body composition. There was no significant difference for age or height between women with AD and controls (Table 2). All women were Caucasian. None of the subjects had thyroid disease or documented allergies. Two women with AD and two women in the control group had diabetes.
Table 1.
Description of AD in subjects
| Age onset of AD (years) | First site of fatty growth | Number of growths | Able to decrease size of growths with weight loss | Diabetes |
|---|---|---|---|---|
| 38 | Axilla | 100s | No | No |
| 29 | Forearm | 100s | No | No |
| 30 | Calcaneus | 25 | No | No |
| 38 | Bicep area | 3 large, many small | No | No |
| 27 | Thigh | 100s | No | No |
| 47 | Abdomen | 4 large, many small | No | No |
| 25 | Forearm | 100s | No | Yes |
| 28 | Thigh | 100s | No | Yes |
| 16 | Thigh | 100s | No | No |
| 43 | Chest | 4 large, many small | No | No |
Abbreviation: AD, adiposis dolorosa.
Body composition
There was no significant difference between groups for weight, BMI, or total or regional FM and FFM by dual X-ray absorptiometry (Table 2). There was also no significant difference in total trunk mass between women with AD and controls (50.6±3.5 vs 47.4±3.7 kg, respectively).
Resting energy expenditure
The respiratory quotient (RQ) between groups was not statistically different nor were rates for fat (107.9±16 and 100±10 g min−1) or carbohydrate oxidation (42.4±27.5 and 71.1±21.1 g min−1, respectively) for the AD and control group. There was no significant difference in unadjusted REE between groups; however, there was a significantly lower level of relative REE, that is, REE per body mass (kg), in women with AD compared with controls (Table 2). There was no significant difference between women with AD and controls, when simply dividing REE by FFM alone (32.2±0.7 vs 34.3±1.2, respectively), but there was a trend toward significance for REE when dividing by FM alone (39.0±2.1 vs 48.5±4.0, respectively; P=0.05). In simple correlation analysis, REE was correlated with total body mass, FM and FFM (P<0.001) but not percent FM or percent FFM. To determine the overall contribution of FM and FFM to the significant difference in relative REE between groups, we performed multiple regression analyses. In multiple linear regression models, there was a significant difference in REE in models adjusting for age, body mass (kg), FM and FFM, as well as percent FM and FFM (overall models P<0.0001; group P≤0.01, see Table 3). To determine which of the variables in the models contributed most to the difference in REE, we used a forward step regression model of REE. In this model, the overall model was significant (P<0.0001; adjusted R2=0.88) and significant predictors of REE included total body mass (P<0.0001) and group (P=0.006; AD vs control women).
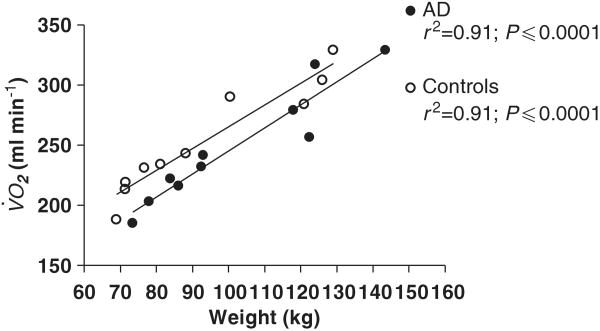
Although controls were matched for BMI and body mass to women with AD, the women with AD had a slightly higher BMI and total body mass than the control group (Table 2). As fat contributes to increased REE11,12 and, in our subjects REE significantly increased with BMI (r2=0.57; P<0.0001) and body mass (r2=0.84; P<0.0001), it was uncertain whether the difference in REE between women with AD and control subjects was driven by the slightly higher body mass in women with AD. Larger individuals consume more oxygen, and oxygen consumption increases with weight, called relative V̇O2.33 Based on weight, it might be predicted that relative V̇O2 would be higher in women with AD. The relative V̇O2 for women with AD, however, was significantly lower than the relative V̇O2 in the control group (Table 2) and the correlation of V̇O2 with weight was highly significant for both the groups (Figure 1). There was no significant difference in slope of the regression lines between women with AD and the controls (1.9±0.2 and 1.8±0.2 ml kg−1, respectively) (Figure 1).
Figure 1.
Oxygen consumption vs body weight in women with AD (open circles) and controls (closed circles). Lines are by simple linear regression.
Histology
Collagen is seen as pink on H&E staining with associated fibroblasts whose nuclei and cell bodies are aligned with tissue planes. There was a significantly higher level of connective tissue in fat biopsy samples after H&E staining from women with AD compared with control women (20.1±5.6 vs 5.1±0.5%; P<0.05). There was also a significantly higher level of connective tissue by trichrome staining (blue) in biopsy samples from women with AD (16.1±2.1%) compared with controls (3.3±0.7%; P<0.0001) (Figure 2).
Figure 2.
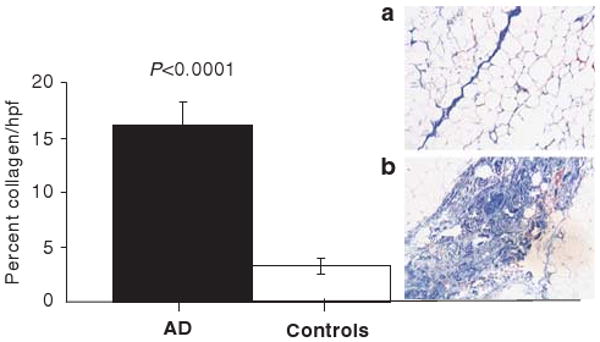
Percent collagen per high power field in women with AD (black bar) and control women (open bar). Inset shows representative trichrome stained fat samples (40 ×) from one control subject (a) and one subject with AD (b). The dark blue-stained septum consisting primarily of collagen that stains blue after trichrome staining in (a) is thin and juxtaposed by fat cells; small blood vessels containing red blood cells can also be seen. In (b), fat cells are found on either side of a blue-stained septum that is much wider and more complex than that seen in (a). P-value refers to between group analyses.
There was no significant difference between the number of macrophages in the lipomatosis from biopsy samples from women with AD (2.9±0.3) compared with adipose biopsy samples from control women (1.8±0.3). We found MNG cells in three lipomatosis fat biopsy samples from women with AD (30%) and none in the control women.
Cytokines
Fat cytokines
There was a significantly higher level of IL-6 in fat from women with AD (1.5±0.1) compared with control women (1.0±0.08; P=0.03). There was no significant difference in the relative expression levels of (GAPDH normalized) MIP-1β (1.2±0.08 vs 1.2±0.2), TNF-α (1.4±0.2 vs 1.5±0.2), IL-13 (0.2±0.1 vs 0.5±0.2), IL-8 (1.0±.3 vs 0.7± 0.2) or IL-1β (1.4±0.2 vs 1.3±0.2) in adipose biopsy samples.
Blood cytokines
There was a significantly lower blood level of MIP-1β in women with AD compared with controls. There was also a trend toward higher levels of IL-13 and lower levels of Fractalkine in women with AD (Table 4). There were no significant differences between the two groups for leptin, adiponectin, plasminogen activator inhibitor-1, IL-1β, IL-6, IL-8, IL-10, MIP-1α, MCP-1 or TNF-α. To confirm the difference in MIP-1β, a larger group of women with AD (n=15) was compared with a control group (n=20). A significantly lower level of MIP-1β in women with AD compared with the control group persisted (83.5±9.3 vs 146.2±16.4 pg ml−1; P=0.009). In addition, Fractalkine was significantly lower in women with AD than controls (21.6±2.9 vs 97.2±35.4 pg ml−1; P=0.04), and IL-13 was significantly higher in women with AD than controls (31.1±4.7 vs 17.9±2.9 pg ml−1; P=0.03). No other significant differences in cytokines were found between the groups. There was no significant difference in BMI between the two larger subject groups (37.3±2.7 vs 34.6±1.3 kg m−2, respectively). There was a significant correlation for BMI and MIP-1b in the control group, which was not found for the AD group. There was no significant correlation between Fractalkine or IL-13 and BMI.
Table 4.
Selected cytokines, insulin and lipids in women with AD and controls (mean±s.e.m.)
| Cytokine or lipid | AD | Controls | P-value |
|---|---|---|---|
| Number | 10 | 10 | NS |
| Adiponectin (pg ml−1) | 29 348±4445 | 36 826±4660 | NS |
| Leptin (pg ml−1) | 40 280±7106 | 37 136±4199 | NS |
| PAI-1 (active) (pg ml−1) | 96.7±20.3 | 75.7±12.5 | NS |
| TNF-α (pg ml−1) | 6.5±1.1 | 7.3±1.3 | NS |
| IL-1β (pg ml−1) | 2.4±0.04 | 2.9±0.3 | NS |
| IL-6 (pg ml−1) | 10.7±3.9 | 13.9±6 | NS |
| IL-8 (pg ml−1) | 6±1.3 | 4.5±1 | NS |
| IL-10 (pg ml−1) | 19.1±10.5 | 24.2±13.6 | NS |
| IL-13 (pg ml−1) | 33.2±5 | 20±5 | 0.09 |
| MCP-1 (pg ml−1) | 341.3±73.2 | 216.2±48.6 | NS |
| MIP-1α (pg ml−1) | 23.1±2.7 | 26.2± 6.3 | NS |
| MIP-1β (pg ml−1) | 66.9±13.6 | 125.6±21.3 | 0.03 |
| Fractalkine (pg ml−1) | 18.7±2.7 | 77.9±29.8 | 0.07 |
| Triglycerides (mg per 100 ml) | 135.2±21.4 | 125.2±13.2 | NS |
| Phospholipids (mg per 100 ml) | 193.2±12.5 | 191.6± 10 | NS |
| NEFA (mEql−1) | 0.8±0.1 | 0.7±0.09 | NS |
| Total cholesterol (mg per 100 ml) | 173.8±14.3 | 195.5±7.8 | NS |
Abbreviations: IL, interleukin; NEFA, non-esterified fatty acids; MIP, macrophage inflammatory protein-1β; MCP, monocyte chemotactic protein-1; PAI, plasminogen activator inhibitor-1; TNF, tumor necrosis factor-α.
Lipids
To evaluate whether the lipomatosis in AD affected lipid levels, we examined fasting lipid levels. There were no differences in lipids between the two groups (Table 4).
Discussion
Adiposis dolorosa is a syndrome characterized by lipomatosis: painful masses of subcutaneous fat. Signs and symptoms from most body systems are altered in AD and contribute to disability in this syndrome. Case reports and other published data support a global decrease in metabolic function in the lipomatosis in individuals with AD.7–10 Our data show a lower relative REE in women with AD compared with matched controls.
Although the standard method for evaluating relative REE has been to divide REE by FFM, in multiple studies of obese women, weight was the best34,35 or an equal36 predictor for REE suggesting that FM may uniquely contribute to REE beyond FFM, at least in obese women.37 We, therefore, compared our groups by REE relative with FM, FFM and total body weight. In this study, we found that women with AD have significantly lower relative REE/total weight than women without AD matched for age, weight, and FM and FFM. Although the location of fat may alter the contribution of FM to REE, that is, women with abdominal obesity can have higher REE than women who are non-abdominally obese,38 there was no difference in trunk or extremity fat or mass between women with AD and controls, suggesting a global type of obesity in both groups. These data, therefore, suggest that for similar locations and amounts of FM and FFM between women with and without AD, the tissue mass in women with AD may be less metabolically active. The lower relative V̇O2 in women with AD compared with controls in our study supports this hypothesis. In multiple regression analyses, there seems to be a difference in REE between women with AD and control women, that is not fully accounted for by total body mass, FM or FFM.
Our data show a fourfold increase in relatively metabolically inactive collagen compared with control adipose, suggesting that lipomatosis fat could be less metabolically active because of the increase in inert collagen tissue. However, the mass of collagen would have been adjusted for by weight in our analyses suggesting an underlying defect in cellular respiration (metabolism) to explain differences in REE between women with AD and controls. More comparisons are needed between areas of lipomatosis within individuals; however, there are no methods currently available to distinguish between normal and lipomatosis fat. The dual X-ray absorptiometry data in this study, although useful for imaging FM and FFM, cannot be used to distinguish between lipomatosis and normal fat. The majority of the lipomatosis fat also does not show up when performing other imaging techniques, including ultrasound, magnetic resonance imaging and computed tomography (data not shown). The lack of a consistent imaging modality for the painful fat also limits our ability to monitor treatment effectively except on a semi-quantitative and qualitative level.
It was surprising to us that IL-6 levels were slightly but significantly higher in the lipomatosis compared with controls as inflammation tends to increase REE.18,19 Whereas the number of macrophages were similar in fat between women with AD and controls, but that MNG cells were found in 30% of the women with AD suggests a need to test a larger volume of lipomatosis for macrophages that could be proinflammatory, producing elevated levels of IL-6 in individuals with AD. Elevated IL-6 levels in cultured cells from adipose tissue inhibited lipoprotein lipase,39 which could explain one of the minor changes in metabolism found in the lipomatosis.10 However, increased IL-6 levels are also associated with a marked loss of fat,40 which is not associated with AD.2 Larger numbers of biopsy samples are needed to evaluate other proinflammatory and anti-inflammatory cytokines to determine further the complexities of metabolism in the lipomatosis.
Lower levels of two chemokines, MIP-1β and Fractalkine were found in the blood of women with AD compared with controls, as well as a higher level of IL-13. The interaction among these immune regulators can possibly shed some light on the underlying pathophysiology of AD. Chemokines are a large family of small-molecular-weight proteins that have primary structure, CXC, CC, C and CX3C, where C,cysteine and X, signify other amino acids. Members of the CC chemokine subfamily, such as MIP-1β (also known as CCL-4) and MIP-1α (also known as CCL-3), whose presentation on the cell surface requires interaction with proteoglycans, are chemotactic for monocytes, subsets of lymphocytes41 and natural killer cells. Fractalkine (CX3CL1) is the only member of the CX3C-chemokine subfamily and has the ability to attract monocytes, T cells, and neutrophils.20,21 Fractalkine is membrane bound and can be shed as a soluble chemotactic form (sFractalkine) by proteolytic cleavage42 subsequently binding to its receptor (CX3CR1) rapidly and firmly.43 The tissue expression of Fractalkine and CX3CR1 are intriguing in relation to the chronic pain associated with AD, because they are constitutively expressed in the central nervous system.20,21 Fractalkine (CX3CL1) is the only member of the CX3C-chemokine subfamily and has the ability to attract monocytes, T cells and neutrophils.20,21
Initially, Fractalkine levels might be predicted to be elevated in association with the chronic pain of AD because of data showing decreased membrane-bound Fractalkine after spinal cord ligation (suggesting a release of sFractalkine),44 and because prolonged release of Fractalkine is thought to contribute to the maintenance of neuropathic pain.22,23 However, CX3CR1 is upregulated after the development of neuropathic pain,45 whereas Fractalkine levels remained unchanged, suggesting a shift from sFractalkine release to receptor-bound Fractalkine. The fact that CX3CR1 receptors are occupied by Fractalkine in neuropathic pain is suggested by blockage of the receptor with a Fractalkine receptor neutralizing antibody leading to attenuation of tolerance, hyperalgesia, allodynia and potentiation of acute morphine analgesia.45 Therefore, CX3CR1 receptors, when occupied with Fractalkine, lead to the promotion of pain and resistance to opioid analgesia, similar to that seen in AD.5
Another explanation for the low levels of the monocyte attractant, Fractalkine, as well as the low levels of another monocyte attractant chemokine, MIP-1β, in women with AD could be the higher levels of IL-13. Interleukin-13 is produced primarily by Th2 lymphocytes, B cells, mast cells, basophils, natural killer T cells and dendritic cells and its receptor is present on a broad range of cell types.46 Along with IL-4, IL-13 is a central mediator in Th2 cytokine pathologies, which are typically associated with allergies, atopy, asthma,47 helminth infections48 and parasite-induced liver and lung fibrosis,49 and is a primary cytokine involved in down-regulation of cytotoxic T-lymphocyte-mediated tumor immunosurveillance.50 IL-13 induces IgE class switching in B cells,51 enhances monocyte/macrophage antigen-presentation ability, suppresses nitric oxide release from macrophages,52 recruits eosinophils,53 increases production of adhesion molecules such as vascular cell adhesion molecule-1,54 and suppresses apoptosis in various cell types.55 IL-13 suppresses LPS-induced production of proinflammatory cytokines24 and relevant to this study, IL-13 is also a potent inhibitor of sFractalkine from human umbilical vein endothelial cells25 and Staphylococcus aureus-induced MIP-1β production.26 The elevated level of IL-13 in women with AD could therefore result in lower levels of Fractalkine and MIP-1β. It is possible that IL-13 participates in the regulation of tissue macrophages in AD. Less metabolically active lipomatosis fat that attracts immune cells differently compared with normal fat could be one reason for the disparate levels of IL-13 between the two groups. Diet-induced obesity decreases natural killer T-cell populations in visceral fat, a major source of IL-13, leading to a decrease in Th2 adipose cytokine production (including IL-13) in wild-type mice.27 As IL-13 is involved in parasitic infection, a parasitic cause for AD may also be considered. Overall, a cause for elevated IL-13 levels in these study subjects will depend on further evaluation of gene expression, immune cell identification and cytokine levels produced by lipomatosis and normal fat.
Conclusions
In addition to greater amounts of connective tissue in lipomatosis, and higher levels of IL-6 and MNG cells compared with control adipose tissue, our data suggest an underlying defect in cellular respiration to explain lower levels of REE in women with AD. Methods are needed to better visualize fatty growths in AD for correlation with metabolic parameters and blood cytokine levels. Understanding the metabolism of lipomatosis may contribute to a better understanding of non-lipomatosis obesity.
Acknowledgments
We thank Patrick Tso for his support, Dana Lee for performing and analyzing the cytokine and lipid data and Tom Storer for his critical read of the indirect calorimetry aspect of this paper. We also extend our sincere gratitude to the women who traveled and took time out from their lives to be a part of this study. This work was supported by GCRC Grant 5M01 RR000827 and NIH NIDDK Grant K23 DK 065038-05.
References
- 1.Dercum FX. A subcutaneous connective-tissue dystrophy of the arms and back, associated with symptoms resembling myxoedema. University Medical Magazine Philadelphia. 1888;1:140–150. [Google Scholar]
- 2.Herbst KL, Asare-Bediako S. Adiposis dolorosa is more than painful fat. Endocrinologist. 2007;17:326–344. [Google Scholar]
- 3.Palmer ED. Dercum's disease: adiposis dolorosa. Am Fam Physician. 1981;24:155–157. [PubMed] [Google Scholar]
- 4.Harris K, Davies K, Dumont S, Stephenson BM. A pain in the groin. Lancet. 1997;350:334. doi: 10.1016/S0140-6736(97)05334-8. [DOI] [PubMed] [Google Scholar]
- 5.Wortham NC, Tomlinson IP. Dercum's disease. Skinmed. 2005;4:157–162. doi: 10.1111/j.1540-9740.2005.03675.x. quiz 163 – 154. [DOI] [PubMed] [Google Scholar]
- 6.Brorson H, Fagher B. Dercum's disease Fatty tissue rheumatism caused by immune defense reaction. Lakartidningen. 1996;93:1430, 1433–1436. [PubMed] [Google Scholar]
- 7.Blomstrand R, Juhlin L, Nordenstam H, Ohlsson R, Werner B, Engstrom J. Adiposis dolorosa associated with defects of lipid metabolism. Acta Derm Venereol. 1971;51:243–250. [PubMed] [Google Scholar]
- 8.Pimenta WP, Paula FJ, Dick-de-Paula I, Piccinato CE, Monteiro CM, Brandao-Neto J, et al. Hormonal and metabolic study of a case of adiposis dolorosa (Dercum's disease) Braz J Med Biol Res. 1992;25:889–893. [PubMed] [Google Scholar]
- 9.Taniguchi A, Okuda H, Mishima Y, Nagata I, Oseko F, Hara M, et al. A case of adiposis dolorosa: lipid metabolism and hormone secretion. Int J Obes. 1986;10:277–281. [PubMed] [Google Scholar]
- 10.Fagher B, Monti M, Nilsson-Ehle P, Akesson B. Fat-cell heat production, adipose tissue fatty acids, lipoprotein lipase activity and plasma lipoproteins in adiposis dolorosa. Clin Sci (Lond) 1991;81:793–798. doi: 10.1042/cs0810793. [DOI] [PubMed] [Google Scholar]
- 11.Weinsier RL, Bracco D, Schutz Y. Predicted effects of small decreases in energy expenditure on weight gain in adult women. Int J Obes Relat Metab Disord. 1993;17:693–700. [PubMed] [Google Scholar]
- 12.Nielsen S, Hensrud DD, Romanski S, Levine JA, Burguera B, Jensen MD. Body composition and resting energy expenditure in humans: role of fat, fat-free mass and extracellular fluid. Int J Obes Relat Metab Disord. 2000;24:1153–1157. doi: 10.1038/sj.ijo.0801317. [DOI] [PubMed] [Google Scholar]
- 13.Steiger WA, Litvin H, Lasche EM, Durant TM. Adiposis dolorsa (Dercum's disease) N Engl J Med. 1952;247:393–396. doi: 10.1056/NEJM195209112471104. [DOI] [PubMed] [Google Scholar]
- 14.Price GE. Adiposis dolorosa. Am J Med. 1905;137:705. [Google Scholar]
- 15.Campen RB, Sang CN, Duncan LM. Case records of the Massachusetts General Hospital Case 25-2006 A 41-year-old woman with painful subcutaneous nodules. N Engl J Med. 2006;355:714–722. doi: 10.1056/NEJMcpc069018. [DOI] [PubMed] [Google Scholar]
- 16.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pond CM. Adipose tissue and the immune system. Prostaglandins Leukot Essent Fatty Acids. 2005;73:17–30. doi: 10.1016/j.plefa.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Kamimura MA, Draibe SA, Dalboni MA, Cendoroglo M, Avesani CM, Manfredi SR, et al. Serum and cellular interleukin-6 in haemodialysis patients: relationship with energy expenditure. Nephrol Dial Transplant. 2007;22:839–844. doi: 10.1093/ndt/gfl705. [DOI] [PubMed] [Google Scholar]
- 19.Roubenoff R, Roubenoff RA, Cannon JG, Kehayias JJ, Zhuang H, Dawson-Hughes B, et al. Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest. 1994;93:2379–2386. doi: 10.1172/JCI117244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 21.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 22.Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O'Connor KA, et al. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- 23.Clark AK, Yip PK, Grist J, Gentry C, Staniland AA, Marchand F, et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci USA. 2007;104:10655–10660. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Waal Malefyt R, Figdor CG, Huijbens R, Mohan-Peterson S, Bennett B, Culpepper J, et al. Effects of IL-13 on phenotype cytokine production, cytotoxic function of human monocytes. Comparison with IL-4 modulation by IFN-gamma or IL-10. J Immunol. 1993;151:6370–6381. [PubMed] [Google Scholar]
- 25.Fraticelli P, Sironi M, Bianchi G, D'Ambrosio D, Albanesi C, Stoppacciaro A, et al. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J Clin Invest. 2001;107:1173–1181. doi: 10.1172/JCI11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Roderiquez G, Oravecz T, Norcross MA. Cytokine regulation of human immunodeficiency virus type 1 entry and replication in human monocytes/macrophages through modulation of CCR5 expression. J Virol. 1998;72:7642–7647. doi: 10.1128/jvi.72.9.7642-7647.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caspar-Bauguil S, Cousin B, Galinier A, Segafredo C, Nibbelink M, Andre M, et al. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. 2005;579:3487–3492. doi: 10.1016/j.febslet.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 28.Alberici LC, Oliveira HC, Patricio PR, Kowaltowski AJ, Vercesi AE. Hyperlipidemic mice present enhanced catabolism and higher mitochondrial ATP-sensitive K+ channel activity. Gastroenterology. 2006;131:1228–1234. doi: 10.1053/j.gastro.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Boyle DL, Rosengren S, Bugbee W, Kavanaugh A, Firestein GS. Quantitative biomarker analysis of synovial gene expression by real-time PCR. Arthritis Res Ther. 2003;5:R352–R360. doi: 10.1186/ar1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper BG, McLean JA, Taylor R. An evaluation of the Deltatrac indirect calorimeter by gravimetric injection and alcohol burning. Clin Phys Physiol Meas. 1991;12:333–341. doi: 10.1088/0143-0815/12/4/003. [DOI] [PubMed] [Google Scholar]
- 31.Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16:23–29. [PubMed] [Google Scholar]
- 32.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medicine ACoS. American College of Sports Medicine's Metabolic Calculations Handbook. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2006. [Google Scholar]
- 34.Dore C, Hesp R, Wilkins D, Garrow JS. Prediction of energy requirements of obese patients after massive weight loss. Hum Nutr Clin Nutr. 1982;36C:41–48. [PubMed] [Google Scholar]
- 35.Muffin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 36.Foster GD, Wadden TA, Mullen JL, Stunkard AJ, Wang J, Feurer ID, et al. Resting energy expenditure, body composition, and excess weight in the obese. Metabolism. 1988;37:467–472. doi: 10.1016/0026-0495(88)90048-0. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham JJ. Body composition as a determinant of energy expenditure: a synthetic review and a proposed general prediction equation. Am J Clin Nutr. 1991;54:963–969. doi: 10.1093/ajcn/54.6.963. [DOI] [PubMed] [Google Scholar]
- 38.Weststrate JA, Dekker J, Stoel M, Begheijn L, Deurenberg P, Hautvast JG. Resting energy expenditure in women: impact of obesity and body-fat distribution. Metabolism. 1990;39:11–17. doi: 10.1016/0026-0495(90)90141-x. [DOI] [PubMed] [Google Scholar]
- 39.Trujillo ME, Sullivan S, Harten I, Schneider SH, Greenberg AS, Fried SK. Interleukin-6 regulates human adipose tissue lipid metabolism and leptin production in vitro. J Clin Endocrinol Metab. 2004;89:5577–5582. doi: 10.1210/jc.2004-0603. [DOI] [PubMed] [Google Scholar]
- 40.Franckhauser S, Elias I, Rotter Sopasakis V, Ferre T, Nagaev I, Andersson CX, et al. Overexpression of Il6 leads to hyperinsulinaemia, liver inflammation and reduced body weight in mice. Diabetologia. 2008;24:24. doi: 10.1007/s00125-008-0998-8. [DOI] [PubMed] [Google Scholar]
- 41.Schall TJ, Bacon K, Camp RD, Kaspari JW, Goeddel DV. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–1826. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsou CL, Haskell CA, Charo IF. Tumor necrosis factor-alpha-converting enzyme mediates the inducible cleavage of fractalkine. J Biol Chem. 2001;276:44622–44626. doi: 10.1074/jbc.M107327200. [DOI] [PubMed] [Google Scholar]
- 43.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, et al. Identification and molecular characterization of Fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 44.Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2007;21:642–651. doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terabe M, Park J, Berzofsky J. Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Cancer Immunol Immunother. 2004;53:79–85. doi: 10.1007/s00262-003-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 48.Urban Jf, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, et al. IL-13, IL-4Ralpha, Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 49.Chiaramonte MG, Schopf LR, Neben TY, Cheever AW, Donaldson DD, Wynn TA. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J Immunol. 1999;162:920–930. [PubMed] [Google Scholar]
- 50.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 51.Punnonen J, Aversa G, Cocks BG, McKenzie AN, Menon S, Zurawski G, et al. Interleukin 13 induces interleukin 4-independent IgG4 IgE synthesis CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bogdan C, Thuring H, Dlaska M, Rollinghoff M, Weiss G. Mechanism of suppression of macrophage nitric oxide release by IL-13: influence of the macrophage population (published erratum appears in J Immunol 1999 Mar 1;162(5):3106) J Immunol. 1997;159:4506–4513. [PubMed] [Google Scholar]
- 53.Pope Sm, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, et al. IL-13 induces eosinophil recruitment into the lung by an IL-5- eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001;108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- 54.Bochner BS, Klunk DA, Sterbinsky SA, Coffman RL, Schleimer RP, et al. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol. 1995;154:799–803. [PubMed] [Google Scholar]
- 55.Manna Sk, Aggarwal BB. IL-13 suppresses TNF-induced activation of nuclear factor-kappa B activation protein-1 apoptosis. J Immunol. 1998;161:2863–2872. [PubMed] [Google Scholar]