Abstract
The lifetime risk for developing endometrial cancer, the fourth most common malignancy in women, is approximately 3%. Endometrial cancer is a hormone-driven cancer, with approximately 80% of endometrial cancers arising attributable to either an excess of estrogen or a lack of progesterone. In the normal endometrium, the proliferative effects of estrogen are normally countered by progesterone, but the absence of progesterone allows estrogen to induce oncogenesis, an effect that is amplified in situations of excess estrogen. One of the major emerging causes of the estrogen/progesterone imbalance is obesity. Obesity is associated with several hormonal derangements as well as dysregulation of insulin/insulin-like growth factor activity, which collectively contribute to hyperplasia and carcinogenesis in the endometrium. In this article, we provide an in-depth description of how obesity mechanistically promotes this hormone and growth factor imbalance. Given that endometrial cancer is clearly associated with obesity, we put forth the hypothesis that a large portion of these cancers might be prevented by treatment with progesterone.
Introduction to Endometrial Cancer
It is generally accepted that all solid tumors rely on the same basic signaling pathways for survival, and approaches that provide therapeutic benefit in one tumor type should act similarly in a tumor at another site. However, we are far from “curing cancer,” and 5-year survival rates for tumors of different origins are vastly different. For example, in the U.S., the current 5-year survival rate for breast cancer is >90% and for prostate cancer nearly 100% (American Cancer Society, 2011). By contrast, incidence and mortality associated with endometrial cancer are on the rise, and survival is significantly worse now than in the decades of the 1970s (84% survival currently vs. 88% survival in the 1970s) (see discussion below). Endometrial cancer is the fourth most common malignancy in women with an estimated 46,470 new cases in 2011. The lifetime risk for developing endometrial cancer is approximately 3%. These statistics highlight that new methods of prevention and treatment are urgently needed that distinguish endometrial cancer from other types of solid tumors (American Cancer Society, 2011).
The vast majority, approximately 80%, of endometrial cancers are likely due to hormonal imbalances; either due to an excess of estrogen or a lack of progesterone. Estrogen promotes endometrial cancer cell growth via direct and indirect regulation of gene transcription. Estrogen binding to its cognate receptors, estrogen receptors (ER) α and β, promotes recruitment of transcriptional cofactors to estrogen-responsive genes and corresponding transcriptional activation. In addition to transcriptional effects, estrogen also acts in the cytoplasm to regulate signaling. For example, 17-β estradiol activates the phosphatidylinositol-3 kinase (PI3K) and mitogen-activated protein kinase (MAPK) proliferative pathways, which are frequently dysregulated in solid tumors (Marino et al., 2005; 2006).
Whereas progesterone delivers a proliferative signal to the breast, this steroid hormone has a myriad of growth-inhibitory effects in the normal endometrium, including countering the estrogen-driven proliferation and inducing glandular differentiation (Yang et al., 2011b). Indeed, progesterone may be the ultimate endometrial tumor suppressor because, through activation of its receptor (progesterone receptor, PR), progesterone induces differentiation, cell cycle arrest, and apoptosis, reduces inflammation, and counters invasion associated with metastatic disease (Yang et al., 2011b).
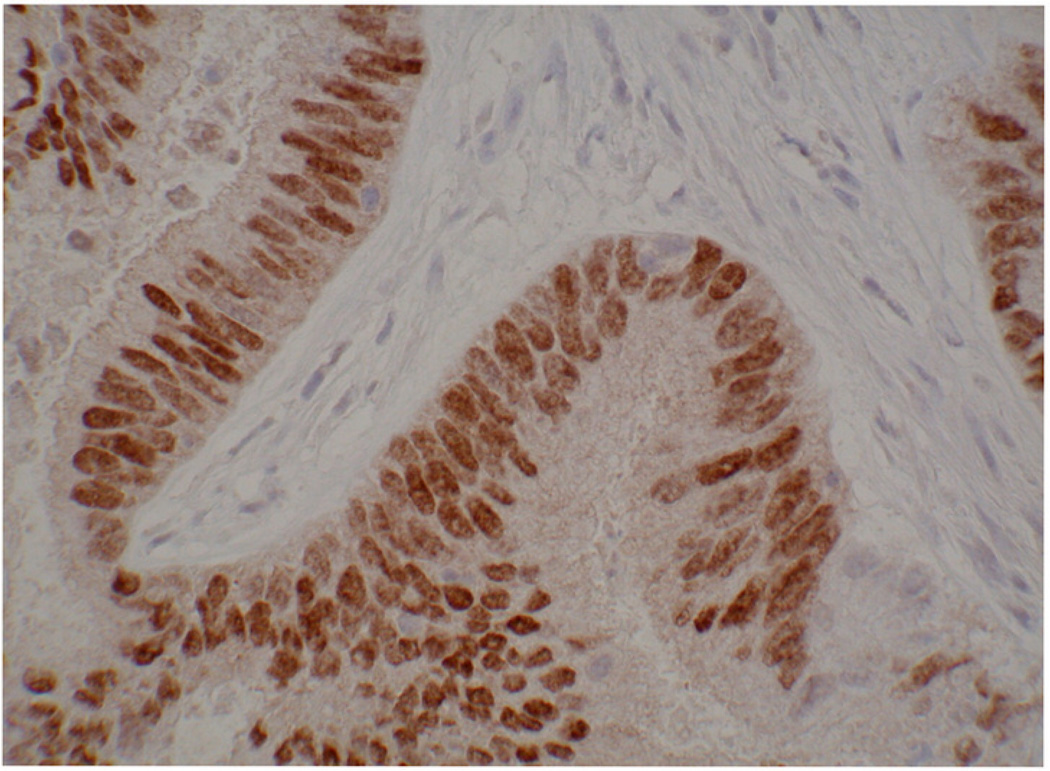
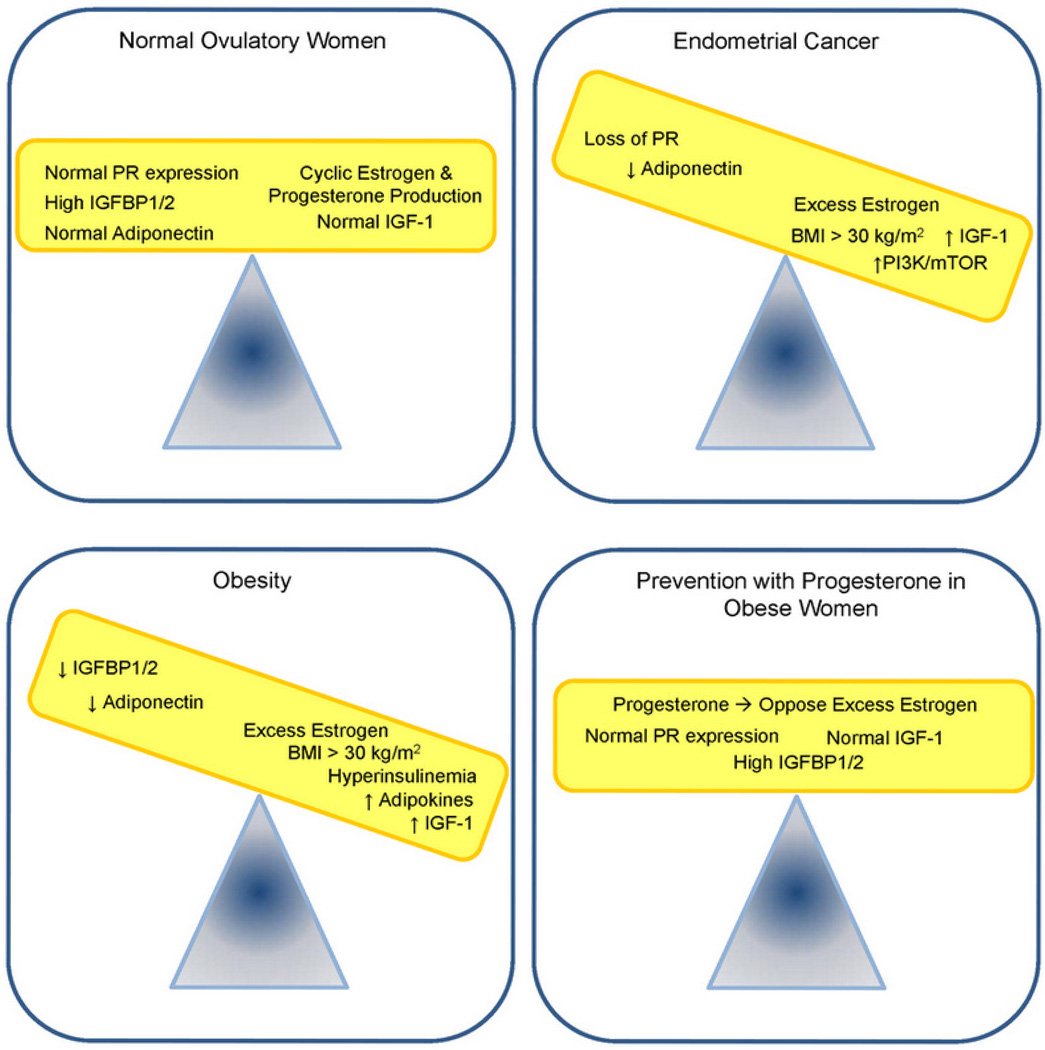
One of the main driving forces behind the estrogen/progesterone hormone imbalance is obesity, which causes several hormonal derangements that potentially contribute to hyperplasia and carcinogenesis in the endometrium (Figure 1). An example of an early grade endometrioid adenocarcinoma with PR expression is provided in Figure 2. In this review, we provide an in-depth description of current knowledge regarding how obesity mechanistically promotes this deleterious hormone imbalance. The fact that so many diagnosed cancers of the endometrium are associated with obesity leads us to hypothesize that a large portion of these cancers might be preventable, and progesterone as a differentiating factor may hold the key.
Figure 1.
Progesterone receptor levels in an early-grade endometrioid adenocarcinoma. Note the well-differentiated nature of the tumor, with obvious glands and stroma.
Figure 2.
Proposed model in which an imbalance in hormones and growth factors induces endometrial cancer. Obesity also promotes many of these same imbalances. We propose that endometrial cancer can be prevented in the obese population by treatment with progesterone, which counters the effects of excess estrogen. Additional preventative measures to decrease BMI may serve to further reduce the incidence of endometrial cancer in the obese population.
Obesity and Steroid Hormone Imbalance
Obesity is a well-established risk factor for multiple types of cancer, cancer-related death, and death associated with co-morbidities (Reeves et al., 2007; Bessonova et al., 2011). With regards to endometrial cancer, a recent meta-analysis found that each 5 kg/m2 increase in body mass index (BMI) significantly increases a woman’s risk of developing endometrial cancer (RR=1.59) (Bessonova et al., 2011). Similarly, the Million Women Study in the United Kingdom revealed that each 10 kg/m2 increase in BMI is associated with an increase in relative risk of 2.59 (Reeves et al., 2007). These large studies clearly establish a link between obesity and endometrial cancer, and, in light of the obesity epidemic, may explain why endometrial cancer incidence and associated deaths are on the rise.
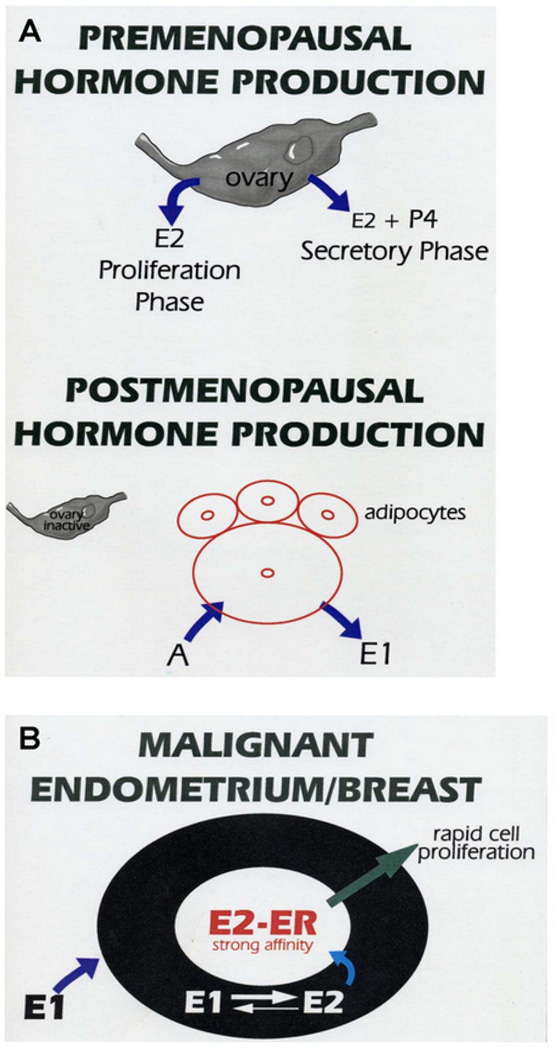
Obesity is thought to cause an increase in endometrial cancer due to abnormal levels of several hormones, most notably increased estrogen levels. Estrogen is a known endometrial growth factor, and, after menopause, the primary source of estrogen is peripheral tissues, including adipose. Adipocytes produce aromatase, which converts androgens to estrone and estradiol. Aromatase levels increase as a function of age and obesity and, as such, levels correspond with an increasing BMI in postmenopausal women (Cauley et al., 1989; Simpson and Mendelson, 1987; Morriset et al., 2006). Accordingly, postmenopausal obese women have >40% increases in both circulating estrone (E1) and estradiol (E2) as compared to postmenopausal women with a BMI in the normal range (Cauley et al., 1989). With increasing adiposity, serum hormone-binding globulin levels are decreased, which serves to amplify the effects of excess estrogen. Serum hormone-binding globulin binds estrogen and testosterone, decreasing their activity. In addition, we have observed (KK Leslie, unpublished observations) that endometrial and breast cancer cell lines contain high levels of 17-β hydroxysteroid dehydrogenase type I, which converts estrone to estradiol, thus serving to increase local estradiol levels (Figure 3). Taken together, these data demonstrate that obesity influences the biologically active levels of these steroid hormones irrespective of de novo hormone synthesis (Schmandt et al., 2011).
Figure 3.
Schematic of estrogen production in postmenopausal women. (A) In premenopausal women, estradiol (E2) is produced primarily by the ovary during the secretory and proliferative phases. Progesterone (P4) is also produced by the ovary during the secretory phase. After menopause, a majority of estradiol is produced by adipocytes, which contain aromatase that converts androgen (A) to estrone (E1). (B) Estrone (E1) is converted to estradiol (E2) by 17-β hydroxysteroid dehydrogenase type I in the endometrium, thereby increasing local E2 levels, activating estrogen receptor (ER) transcriptional activity, and promoting cell proliferation.
In addition to excess estrogen, endometrial hyperplasia and carcinogenesis can also stem from or be exacerbated by a decrease in circulating progesterone levels. In obese women, lack of progesterone due to anovulation, similar to that observed in polycystic ovarian syndrome (PCOS), can contribute to endometrial cancer risk. In addition to ligand-mediated regulation described above, expression and activity of the progesterone receptor are also modulated by a variety of factors, including microRNAs (miRNAs) and epigenetic factors that are frequently dysregulated in cancer. We have extensively described the epigenetic factors controlling loss of PR in endometrial cancer in another recent review article (Yang et al., 2011a). This loss of PR activity translates to the failure of progestin as a therapeutic strategy for many endometrial tumors (Yang et al., 2011a; 2011b), highlighting the need to identify new approaches to maintain PR expression and thereby sensitize tumors to progesterone-based therapy. Given the role of epigenetic modification in progesterone insensitivity, use of agents such as histone deacetylase inhibitors and DNA methyl transferase inhibitors represent the future for endometrial cancer treatment and re-sensitization to progesterone (Yang et al., 2011a).
Estrogen-independent Effects on Obesity and Endometrial Cancer
Hyperinsulinemia
Several other hormonal imbalances that are caused by obesity and lead to higher risks for endometrial carcinogenesis represent novel targets for treatment and prevention strategies (Figure 2). For example, hyperinsulinemia and the insulin-resistant state are associated closely with obesity, with several studies documenting associations between diabetes, obesity, and endometrial cancer (Schoff and Newomb, 1998; Weiderpass et al., 2000; Anderson et al., 2001; Salazar-Martinez et al., 2000; Parazzini et al., 1998). Two independent studies identified a relationship between insulin resistance and endometrial cancer risk, with one study finding that low levels of adiponectin, a surrogate marker for insulin resistance, are also associated with endometrial cancer risk independent of BMI (Soliman et al., 2006; Cust et al., 2007). These studies implicate insulin resistance as an independent risk factor for endometrial cancer.
The specific effects of hyperinsulinemia on the pathogenesis of obesity-associated cancers are not well-defined. Lu and colleagues showed that the proliferative effect of estrogen is enhanced in the endometrium of obese, hyperinsulinemic rats, including a significantly higher expression of proliferative genes cyclin A and c-myc as compared to lean rats (Zhang et al., 2009). Conversely, the obese endometrium has pronounced estrogen-induced suppression of anti-proliferative genes and cell cycle inhibitors (Zhang et al., 2009). This study demonstrates that obesity may shift the balance between pro- and anti-proliferative signals to favor endometrial growth. However, these rodent studies should be interpreted with caution because progesterone has been reported to be pro-proliferative in the rodent endometrium, whereas it is a differentiating signal in the human tissue (Yang et al., 2011b).
Insulin-like growth factor and obesity
Systemic levels of insulin-like growth factor (IGF) have been shown to be dysregulated in obesity (Klotz et al., 2002; Hewitt et al., 2010). While increased estrogen production directly increases IGF-1 synthesis, sustained hyperinsulinemia independent of estrogen results in the decreased synthesis of IGF binding proteins (IGFBP) 1 and 2, which modulate IGF activity by interfering with receptor binding. This downregulation of IGFBP1 and 2 contributes to the increase in IGF activity (Rutanen, 1998; 2000). It is important to recognize that insulin and IGF activate similar signaling pathways. Specifically, upon ligand binding, phosphorylation of the insulin receptor substrate-1 results in activation of PI3K/AKT/mTOR and MAPK pathways, which promote cell survival and proliferation (Pollack, 2008). Thus, the estrogen-independent effects observed with IGF may be replicated by insulin to induce endometrial proliferation.
Adipokines
Adipose tissue is a complex endocrine organ that secretes a variety of both anti- and proinflammatory factors classified as adipokines (Schmandt et al., 2011). Tumor necrosis factor-α (TNF-α) is an adipokine that can disrupt insulin receptor signaling and activate receptors independent of insulin (Nieto-Vazquez et al., 2008). Leptin, IL-6, and resistin are also adipokines that are proinflammatory (Cymbaluk et al., 2008; Ferdeghini et al., 1994; Hlavna et al., 2011). Adiponectin is an anti-inflammatory adipokine that functions to dampen PI3K/AKT/mTOR signaling via activation of the AMP-activated protein kinase (AMPK) pathway (Long et al., 2006). Adiponectin has been shown to be decreased in obesity and may be a marker for endometrial cancer (Salazar-Martinez et al., 2000; Parazzini et al., 1998; Soliman et al., 2006; Cust et al., 2007). This is an area of active research. Increased adiposity (and concomitant inflammation) combined with decreased adiponectin synthesis leads to insulin resistance, which, in turn, causes hyperglycemia and enhanced insulin production (Schmandt et al., 2011). The elevated insulin leads to decreased concentrations of IGFBP and subsequent increased IGF bioavailability. As described above, both insulin and IGF stimulate the AKT/mTOR pathway to induce proliferation in the endometrium. Simultaneous increases in estrogen independently stimulate the endometrium and further increase IGF production. These signaling mechanisms provide one explanation for why obesity and diabetes, independent of BMI, increase the risk for endometrial cancer.
Endometrial Cancer Prevention in the Obesity Epidemic
All of hormonal derangements that are direct effects of obesity are thought to lead to one end result: stimulation of the endometrium. Several prevention strategies for endometrial cancer have been examined, and include screening, hormonal therapy, antidiabetic agents, and weight loss through diet and exercise. In one endometrial cancer prevention study, data were obtained from the SEER database to evaluate four strategies: no intervention, oral contraceptives, annual endometrial biopsy screening starting at age 30, and biannual screening starting at age 30. Using a decision analysis model, none of the strategies had incremental cost-effectiveness ratios of less than $50,000 per year of life saved, and the authors concluded that oral contraceptives and current screening methods are not cost-effective (Kwon and Lu, 2008). However, multivariate analysis of the relationship between obesity-associated factors (i.e., BMI over 30 kg/m2, hyperinsulinemia) and endometrial cancer incidence in these studies is necessary to fully evaluate the effectiveness of screening as a preventative strategy.
Oral contraceptives
Multiple studies have demonstrated that women who use combination oral contraceptive pills (OCP) have as much as a 50% decreased risk of developing endometrial cancer, and that effect can last up to 20 years (Kaufman et al., 1980; Beral et al., 1988; The Cancer and Steroid Hormone Study, 1987). However, no current studies have examined the use of OCP as chemoprevention for endometrial cancer in obese women. It is important to note that some studies have suggested that obese women have a slightly decreased contraceptive efficacy compared to thin women. Future studies that do examine chemoprevention in the obese population should take this into consideration when determining appropriate dosing.
Hormonal therapy
Synthetic progestins are used commonly for the treatment of menorrhagia. These have also been studied in the treatment of endometrial hyperplasia and low-grade cancer. A study by Wheeler et al. (2007) showed a complete remission rate of 67% in women with complex atypical hyperplasia. Moreover, 42% of women with well-differentiated endometrial cancer demonstrated complete remission, although 58% had persistent disease over a 12-month follow-up period.
A case report earlier this year by Brown et al. (2012) described the successful use of a progestin-releasing intrauterine device (IUD) in an 18-year old nulliparous woman diagnosed with grade 2 endometrial adenocarcinoma. Risk factors for the development of cancer in this young woman appeared to be morbid obesity and PCOS, with no evidence for hereditary syndromes associated with endometrial cancer. Because of the patient’s age and desire for eventual pregnancy, she was treated with a progestin-containing IUD. Despite the fact that the tumor was a grade 2, this treatment appeared to completely reverse the tumor, including the shrinkage of a lymph node. This case represents one of the first attempts to treat a grade 2 cancer with uterine preservation. This case is important for two additional reasons: 1) it provides one example that women at a young age who are significantly overweight and do not ovulate can develop endometrial cancer, and 2) it shows that, regardless of grade, endometrial tumors have the potential to respond to hormonal therapy.
The levonorgestrel (progestin)-containing IUD is an attractive candidate for endometrial cancer chemoprevention. The use of IUD alone is associated with a lower risk of endometrial cancer (Beining et al., 2008), and the progestin-containing IUD may further protect the endometrium. A few studies have reported its use in the reversal of endometrial hyperplasia and low-grade cancer. A meta-analysis of six studies utilizing the levonorgestrel IUD was performed (Somboonporn et al., 2011). Four of the included studies examined its use for endometrial protection in women using estrogen hormone replacement therapy (HRT, tamoxifen). The other two included studies examined it for endometrial hyperplasia. In sum, the IUD was as effective as oral progestin for endometrial protection in the case of HRT or hyperplasia (Somboonporn et al., 2011). Recent studies attest to the safety of non-oral progestin therapy by clearly demonstrating that this therapy is not associated with thrombosis (Sweetland et al., 2012). As with OCP, no studies have been conducted on use of progestin-containing IUDs in chemoprevention in obese patients.
Antidiabetic agents
With all of the abnormalities that obesity causes in insulin and IGF and their relation to endometrial cancer, anti-diabetic agents seem to be a logical choice for endometrial cancer prevention. Metformin is an oral antihyperglycemic agent that lowers blood glucose by inhibiting gluconeogenesis and increasing peripheral glucose uptake. Epidemiologic studies have shown that diabetic patients who took metformin have a lower risk of pancreatic cancer than their counterparts who did not, and diabetic patients with breast cancer who took metformin showed a greater rate of pathologic complete response to neoadjuvant chemotherapy (Jiralerspong et al., 2009). Preclinical studies suggest that metformin’s therapeutic benefits are mediated through activation of the growth inhibitory AMPK pathway (action similar to adiponectin), thereby counteracting PI3K/AKT/mTOR proliferative signaling (Zhou et al., 2001; Jalving et al., 2010). Metformin has also been shown to inhibit aromatase expression in vitro and increase PR expression in endometrial cancer cell lines (Brown et al., 2010; Xie et al., 2011). Based on the latter preclinical data, the Gynecologic Oncology Group is considering examination of metformin for the treatment of endometrial cancer.
Diet and exercise
Use of diet and exercise to reduce BMI is an obvious non-pharmacologic strategy to reduce the risk of endometrial cancer as well as some other types of cancers. A recent study of 42,672 postmenopausal women who were enrolled in the American Cancer Society Cancer Prevention Study II Nutrition Cohort evaluated the incidence of endometrial cancer in relation to physical activity and BMI (Patel et al., 2008). Moderate physical activity was associated with a 33% lower endometrial cancer risk, and, not surprisingly, the effect was even more pronounced among women who were overweight or obese. The benefits are likely due to modulation of estrogen levels and IGF/insulin signaling. For example, circulating estrogen levels correlate with increasing BMI, and weight loss is associated with a decrease in estrogen synthesis. Weight loss is also known to reverse insulin resistance and decrease IGF-1 levels. Furthermore, a 10% weight loss results in a decrease in a variety of cytokines (i.e., TNF-α, IL-6, IL-8), which in turn reduces serum glucose and cholesterol (Pendyala et al., 2011). Therefore, weight loss reduces the risk of developing cancer by decreasing the proinflammatory environment and abrogating oncogenic signaling pathways (Schmandt et al., 2011).
Conclusion and Future Directions
We put forth the idea that endometrial cancer is a potentially preventable disease in obese women. It is clear that most of these women are progesterone deficient, at least relative to circulating estrogen levels and increased IGF1/insulin signaling. Weight loss will likely reverse the hormonal imbalances and dysregulation of the IGF/insulin pathway. Unfortunately, most patients are unsuccessful in their weight loss endeavors, and, for those that are successful, the beneficial effects of weight loss on hormone levels may take a year or more to be realized. These individuals need help to protect their endometrium while they are in the process of lifestyle changes in order to lose weight. We propose that a simple intervention is the progestin-containing IUD. Widespread use of progestin-containing IUDs for women at risk could, for the first time in over 30 years, substantially reduce the incidence of this potentially preventable disease.
Acknowledgments
Funding for this work was provided by NIH CA99908 to K.K.L., the Department of Obstetrics and Gynecology Academic Enrichment Fund, and the GOG Core Laboratory for Receptors and Targets funded by NIH CA27469.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- American Cancer Society. Cancer Facts and Figures. [Accessed Nov. 21, 2011]; http://www.cancer.org/acs/groups/content/@epidemiology-surveilance/documents/document/acspc-029771.pdf. [Google Scholar]
- Anderson KE, Anderson E, Mink PJ, Hong CP, Kushi LH, Sellers TA, Lazovich D, Folsom AR. Diabetes and endometrial cancer in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev. 2001;10:611–616. [PubMed] [Google Scholar]
- Beining RM, Dennis LK, Smith EM, Dokras A. Meta-analysis of intrauterine device use and risk of endometrial cancer. Ann Epidemiol. 2008;18:492–499. doi: 10.1016/j.annepidem.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Beral V, Hannaford P, Kay C. Oral contraceptive use and malignancies of the genital tract: results from the Royal College of General Practitioners’ Oral Contraception study. Lancet. 1988;2:1331–1335. doi: 10.1016/s0140-6736(88)90869-0. [DOI] [PubMed] [Google Scholar]
- Bessonova L, Marshall SF, Ziogas A, Largent J, Berstein L, Henderson KD, Ma H, West DW, Anton-Culver H. The association of body mass index with mortality in the California Teachers Study. Int J Cancer. 2011;129(10):2492–2501. doi: 10.1002/ijc.25905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Westin SN, Broaddus RR, Schmeler K. Progestin intrauterine device in an adolescent with grade 2 endometrial cancer. Obstet Gynecol. 2012;119(2 Pt 2):423–426. doi: 10.1097/AOG.0b013e318234d97c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KA, Hunger NI, Docanto M, Simpson ER. Metformin inhibits aromatase expression in human breast adipose stromal cells via stimulation of AMP-activated protein kinase. Breast Cancer Res Treat. 2010;123:591–596. doi: 10.1007/s10549-010-0834-y. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Gutai JP, Kuller LH, LeDonne D, Powell JG. The epidemiology of serum sex hormones in postmenopausal women. Am J Epidemiol. 1989;129:1120–1131. doi: 10.1093/oxfordjournals.aje.a115234. [DOI] [PubMed] [Google Scholar]
- Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, Lukanova A, Rinaldi S, Dossus L, Slimani N, Lundin E, Tjønneland A, Olsen A, Overvad K, Clavel-Chapelon F, Mesrine S, Joulin V, Linseisen J, Rohrmann S, Pischon T, Boeing H, et al. Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J Clin Endocrinol Metab. 2007;92:255–263. doi: 10.1210/jc.2006-1371. [DOI] [PubMed] [Google Scholar]
- Cymbaluk A, Chudecka-Glaz A, Rzepka-Gorska I. Leptin levels in serum depending on body mass index in patients with endometrial hyperplasia and cancer. Eur J Obstet Gynecol Reprod Biol. 2008;136:74–77. doi: 10.1016/j.ejogrb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Ferdeghini M, Gadducci A, Prontera C, Bonuccelli A, Annicchiarico C, Fanucchi A, Facchini V, Bianchi R. Serum interleukin-6 levels in uterine malignancies: preliminary data. Anticancer Res. 1994;14:735–737. [PubMed] [Google Scholar]
- Hewitt SC, Li Y, Li L, Korach KS. Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. J Biol Chem. 2010;285:2676–2685. doi: 10.1074/jbc.M109.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlavna M, Kohut L, Lipkova J, Bienertova-Vasku J, Dostalova Z, Chovanec J, Vasku A. Relationship of resistin levels with endometrial cancer risk. Neoplasma. 2011;58:124–128. doi: 10.4149/neo_2011_02_124. [DOI] [PubMed] [Google Scholar]
- Jalving M, Gietema JA, Lefrandt JD, de Jong S, Reyners AK, Gans RO, de Vries EG. Metformin: taking away the candy for cancer? Eur J Cancer. 2010;46:2369–2380. doi: 10.1016/j.ejca.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi GN, Gonzalez-Angulo AM. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DW, Shapiro S, Slone D, Rosenberg L, Miettinen OS, Stolley PD, Knapp RC, Leavitt T, Jr, Watring WG, Rosenshein NB, Lewis JL, Jr, Schottenfeld D, Engle RL., Jr Decreased risk of endometrial cancer among oral-contraceptive users. N Engl J Med. 1980;303:1045–1047. doi: 10.1056/NEJM198010303031807. [DOI] [PubMed] [Google Scholar]
- Klotz DM, Hewitt SC, Ciana P, Raviscioni M, Lindzey JK, Foley J, Maggi A, DiAugustine RP, Korach KS. Requirement of estrogen receptor-alpha in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem. 2002;277:8531–8537. doi: 10.1074/jbc.M109592200. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Lu KH. Cost-effectiveness analysis of endometrial cancer prevention strategies for obese women. Obstet Gynecol. 2008;112(1):56–63. doi: 10.1097/AOG.0b013e31817d53a4. [DOI] [PubMed] [Google Scholar]
- Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M, Ascenzi P, Acconcia F. S-palmitoylation modulates estrogen receptor alpha localization and functions. Steroids. 2006;71:298–303. doi: 10.1016/j.steroids.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2005;7:497–508. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset AS, Blouin K, Tchernof A. Impact of diet and adiposity on circulating levels of sex hormone-binding globulin and androgens. Nutr Rev. 2006;66:506–516. doi: 10.1111/j.1753-4887.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- Nieto-Vazquez I, Fernandez-Veledo S, Kramer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem. 2008;114:183–194. doi: 10.1080/13813450802181047. [DOI] [PubMed] [Google Scholar]
- Parazzini F, La Vecchia C, Negri E, Riboldi GL, Surace M, Benzi G, Maina A, Chiaffarino F. Diabetes and endometrial cancer: an Italian case-control study. Int J Cancer. 1999;81:539–542. doi: 10.1002/(sici)1097-0215(19990517)81:4<539::aid-ijc6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Patel AV, Feigelson HS, Talbot JT, McCullough ML, Rodriguez C, Patel RC, Thun MJ, Calle EE. The role of bodyweight in the relationship between physical activity and endometrial cancer: results from a large cohort of US women. Int J Cancer. 2008;123:1877–1882. doi: 10.1002/ijc.23716. [DOI] [PubMed] [Google Scholar]
- Pendyala S, Neff LM, Suarez-Farinas M, Holt PR. Diet-induced weight loss reduces colorectal inflammation: implications for colorectal carcinogenesis. Am J Clin Nutr. 2011;93:234–242. doi: 10.3945/ajcn.110.002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutanen EM. Insulin-like growth factors in endometrial function. Gynecol Endocrinol. 1998;12:399–406. doi: 10.3109/09513599809012842. [DOI] [PubMed] [Google Scholar]
- Rutanen EM. Insulin-like growth factors and insulin-like growth factor binding proteins in the endometrium: effect of intrauterine levonorgestrel delivery. Hum Reprod. 2000;15(suppl):173–181. doi: 10.1093/humrep/15.suppl_3.173. [DOI] [PubMed] [Google Scholar]
- Salazar-Martinez E, Lazcano-Ponce EC, Lira-Lira GG, Escudero-De Los Rios P, Salmerón-Castro J, Larrea F, Hernández-Avila M. Case-control study of diabetes, obesity, physical activity and risk of endometrial cancer among Mexican women. Cancer Causes Control. 2000;11:707–711. doi: 10.1023/a:1008913619107. [DOI] [PubMed] [Google Scholar]
- Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. AJOG. 2011;205(6):518–525. doi: 10.1016/j.ajog.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoff SM, Newcomb PA. Diabetes, body size, and risk of endometrial cancer. Am J Epidemiol. 1998;148:234–240. doi: 10.1093/oxfordjournals.aje.a009630. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Mendelson CR. Effect of aging and obesity on aromatase activity of human adipose cells. Am J Clin Nutr. 1987;45:290–295. doi: 10.1093/ajcn/45.1.290. [DOI] [PubMed] [Google Scholar]
- Soliman PT, Wu D, Tortolero-Luna G, Schmeler KM, Slomovitz BM, Bray MS, Gershenson DM, Lu KH. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer. 2006;106:2376–2381. doi: 10.1002/cncr.21866. [DOI] [PubMed] [Google Scholar]
- Somboonporn W, Panna S, Temtanakitpaisan T, Kaewrudee S, Soontrapa S. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: systematic review and meta-analysis. Menopause. 2011;18(10):1060–1066. doi: 10.1097/gme.0b013e31821606c5. [DOI] [PubMed] [Google Scholar]
- Sweetland S, Beral V, Balkwill A, Liu B, Benson VS, Canonico M, Green J, Reeves GK. The Million Women Study Collaborators. Venous thromboembolism risk in relation to use of different types of postmenopausal hormone therapy in a large prospective study. J Thromb Haemost. 2012 Sep. doi: 10.1111/j.1538-7836.2012.04919.x. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. Combination oral contraceptive use and the risk of endometrial cancer. JAMA. 1987;257:796–800. [PubMed] [Google Scholar]
- Weiderpass E, Persson I, Adami HO, Magnusson C, Lindgren A, Baron JA. Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden) Cancer Causes Control. 2000;11:185–192. doi: 10.1023/a:1008946825313. [DOI] [PubMed] [Google Scholar]
- Wheeler DT, Bristow RE, Kurman RJ. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am J Surg Pathol. 2007;31:988–998. doi: 10.1097/PAS.0b013e31802d68ce. [DOI] [PubMed] [Google Scholar]
- Xie Y, Wang YL, Yu L, Hu Q, Ji L, Zhang Y, Liao QP. Metformin progesterone receptor phenotype of breast promotes progesterone receptor expression via inhibition of mammalian target of rapamycin (mTOR) in endometrial cancer cells. J Steroid Biochem Mol Biol. 2011;126:113–120. doi: 10.1016/j.jsbmb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Yang S, Thiel KW, De Geest K, Leslie KK. Endometrial cancer: reviving progesterone therapy in the molecular age. Discov Med. 2011a;12(64):205–212. [PubMed] [Google Scholar]
- Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab. 2011b;22(4):145–152. doi: 10.1016/j.tem.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Shen Q, Celestino J, Milam MR, Westin SN, Lacour RA, Meyer LA, Shipley GL, Davies PJ, Deng L, McCampbell AS, Broaddus RR, Lu KH. Enhanced estrogen-induced proliferation in obese rat endometrium. Am J Obstet Gynecol. 2009;200:186.e1–186.e8. doi: 10.1016/j.ajog.2008.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]