Abstract
Context
Antipsychotic treatment is the first-line treatment option for schizophrenia. Individual studies suggested they can significantly affect brain structure and account for progressive brain changes observed during the illness.
Objectives
To quantitatively examine the effect of antipsychotics as compared to illness related factors on progressive brain changes in schizophrenia.
Data sources
Electronic databases were searched until April 2012. All magnetic resonance imaging studies reporting progressive brain changes in schizophrenia subjects and antipsychotic exposure were retrieved.
Study selection
30 longitudinal MRI studies with antipsychotic administration in schizophrenia patients met the inclusion criteria.
Data extraction
Brain volumes before and after antipsychotic exposure, duration of illness, severity of psychotic symptoms as well as demographic, clinical, and methodological variables were extracted from each publication, or obtained directly from its authors.
Data synthesis
The overall sample was of 1046 schizophrenia patients and 780 controls for a median duration of follow-up of 72.4 weeks. At baseline, patients showed significant whole brain volume reductions and enlarged lateral ventricle (LV) volumes compared to controls. No baseline volumetric abnormalities were detected in the gray matter volumes (GMV), white matter volumes, cerebrospinal fluid and caudate nucleus. Longitudinally, there were progressive GMV decreases and LV enlargements in patients but not in controls. The GMV decreases were inversely correlated with cumulative exposure to antipsychotic treatments, while no effects were observed for duration of illness or illness severity.
Conclusions
Schizophrenia is characterized by progressive gray matter volume decreases and lateral ventricular volume increases. Some of these neuroanatomical alterations may be associated with antipsychotic treatment.
Keywords: Psychosis, Schizophrenia, Antipsychotic, Neuroimaging, MRI, Structural, Dopamine
1. Introduction
Antipsychotic medication is the mainstay of effective management of schizophrenia. The first-generation ‘conventional’ antipsychotic drugs are predominantly antagonists of dopamine D2 receptors, and are effective against most positive symptoms, but have high rates of extrapyramidal side effects (Miyamoto et al., 2005). The second-generation or ‘atypical’ antipsychotics differ from previous antipsychotic agents in their lower affinity for dopamine- and other neuro-receptors (5-HT2A, adrenergic, acetylcholine, and histamine receptors) (Leucht et al., 2009, Kendall, 2011) with a reduced profile of extrapyramidal side-effects (Miyamoto et al., 2005) but with comparable rates of adverse events such as sedation and weight gain (Leucht et al., 2009). Despite these pharmacodynamic differences, all antipsychotics cross the brain–blood barrier to target receptors distributed in the brain, with a clinical efficacy starting in the first days of treatment and accumulating over time (Agid et al., 2003a). The effect of antipsychotics on brain function starts immediately and can be detected after a single dose using molecular imaging techniques (Handley et al., 2012). There is recent evidence indicating rapid structural remodeling and short-term neural plasticity with acute D2 receptor blockade (Tost et al., 2010). The reversibility of these findings and their clinical meaning, in particular in relation to the long-term outcomes of schizophrenia are unknown (Schaufelberger et al., 2011). However, they provide converging evidence that antipsychotic treatment, even acutely, can significantly impact brain structure and function. This can be particularly relevant to the longitudinal course of the illness and partially account for the observed dynamic brain changes associated with the disorder (Ho et al., 2011). A number of longitudinal structural Magnetic Resonance Imaging (MRI) studies have found progressive brain changes in adults with schizophrenia during the initial years after the onset of the illness (Hulshoff Pol and Kahn, 2008, Kempton et al., 2010). The extent of progressive brain tissue decrease in patients (−0.5% per year) has been estimated as twice that of healthy controls (−0.2% per year) (Hulshoff Pol and Kahn, 2008). These progressive brain changes have been associated with poorer clinical outcomes (Ho et al., 2003; Cahn et al., 2006; van Haren et al., 2008), more negative symptoms, and a decline in neuropsychological performance, although not consistently (van Haren et al., 2003). Currently, it is not clear when these structural brain changes occur and how they develop over time. However, studying individuals at clinical high risk of developing psychosis (Fusar-Poli et al., 2012a) has allowed the investigation of structural brain alterations before the onset of the illness. MRI studies addressing structural alterations in individuals at enhanced risk for psychosis have been recently summarized, confirming that some abnormalities are already present during the prodromal phase (Fusar-Poli et al., 2011) and may be predictive of later transition to psychosis (Smieskova et al., 2010). Despite evidence suggesting early brain changes in psychosis, the specific role played by antipsychotic treatment is strongly debated. Some studies have indicated that higher cumulative dose of antipsychotic medication intake is not associated with brain volume changes, and may even be associated with less prominent volumetric changes (Hulshoff Pol and Kahn, 2008). Conversely, recent investigations in first-episode patients showed increased antipsychotic exposure was associated with brain volume reduction (Ho et al., 2011); and that this association was stronger than illness-related effects. In addition studies from other groups have indicated a differential protective effect of atypical vs. typical antipsychotic on brain volume changes in schizophrenia (Miyamoto et al., 2005).
Besides the potential effect of antipsychotics on brain structure, other factors should be considered when discussing progressive brain changes in schizophrenia. Two commonly investigated clinical variables in the imaging literature are duration of illness (DOI) and severity of illness. A plethora of imaging data are now available at different stages of the illness (Olabi et al., 2011) and a recent voxel-based meta-analysis has directly tested the hypothesis that patients with chronic schizophrenia have more extensive brain abnormalities observed in the same regions as non-psychotic relatives, subjects at high risk for psychosis and first-episode samples (Chan et al., 2009). Subtraction analyses between these groups confirmed that gray matter abnormalities observed in the prodromal phases of schizophrenia become more extensive through first-episode and chronic illness, confirming the significant role played by illness duration on imaging results (Chan et al., 2009). Severity of signs and symptoms is also associated with different brain alterations in schizophrenia. For example MRI studies conducted in antipsychotic-naïve subjects reported negative correlations between positive psychotic symptoms and volumes of temporal areas, and between negative symptoms and volume of fronto-cerebellar areas (Venkatasubramanian, 2010). In particular the association between gray matter reductions in the temporal areas and severity of auditory hallucinations has been confirmed in several MRI studies as well as in extensive voxel-based meta-analyses (Modinos et al., 2012).
Given the above three confounders, the available qualitative reviews addressing progressive brain changes in schizophrenia have yielded inconclusive results (Vita and De Peri, 2007; Navari and Dazzan, 2009; Smieskova et al., 2009; Moncrieff and Leo, 2010). There are no studies investigating the consistency and magnitude of progressive volumetric changes in schizophrenia in a quantitative meta-analysis, controlling at the same time for the above confounders. In the present study we first sought to examine at a meta-analytical level whether schizophrenia is characterized by progressive brain changes as compared to healthy controls. We then aimed to investigate the effect of potential moderators affecting brain structure such as illness duration, illness severity, and antipsychotic treatment.
2. Methods
The details of the research protocol are appended in the supplementary protocol (S1).
2.1. Selection procedures
2.1.1. Search strategies
A systematic search strategy was used to identify relevant studies. Three independent researchers (RS, PFP, SB) conducted a three-step literature search. First, a PubMed and Embase search was performed to identify putative longitudinal MRI studies in schizophrenia. The search was conducted up to end of April 2012, with no time span specified for date of publication. The following search terms were used: “MRI” OR “neuroimaging” AND “schizophrenia” AND “antipsychotic” AND “longitudinal” NOT “review”. In a second step the reference lists of the articles included in the review were manually checked for any studies not identified by the computerized literature search. In the final step, 5 journals with the highest impact factor in the field of psychiatry were additionally searched for potential articles of interests. There was no language restriction, although all the included papers were in English.
2.1.2. Selection criteria
Studies were included if they met the following criteria: (a) were reported in an original paper in a peer-reviewed journal, (b) had involved subjects with DSM-IV, DSM-III-R or ICD-10 schizophrenia (c) had employed volumetric MRI in a longitudinal design (baseline/follow-up study); (d) had evaluated relative contributions of at least one potential moderator (illness duration, antipsychotic treatment, illness severity) of brain volume change. The latter was defined as cumulative exposure of antipsychotics during the inter-scan interval and computed as chlorpromazine equivalents (Ho et al., 2011) (see S1 paragraph 9). When standardized diagnosis of psychotic subjects was not clearly defined, the study was excluded. Voxel Based Morphometry (VBM), Cortical Pattern Matching, Diffusion Tensor Imaging, Tractography or other techniques that do not report absolute brain volumes were not included. When there were two or more studies from the same center we contacted the authors to clarify whether there was overlap in the respective samples (if several articles dealt with the same population, we selected the article with the largest sample). When studies did not report data to compute the chlorpromazine equivalents or other significant data we carefully contacted the respective authors to collect the individual scores and avoid biases in the literature search.
2.1.3. Recorded variables
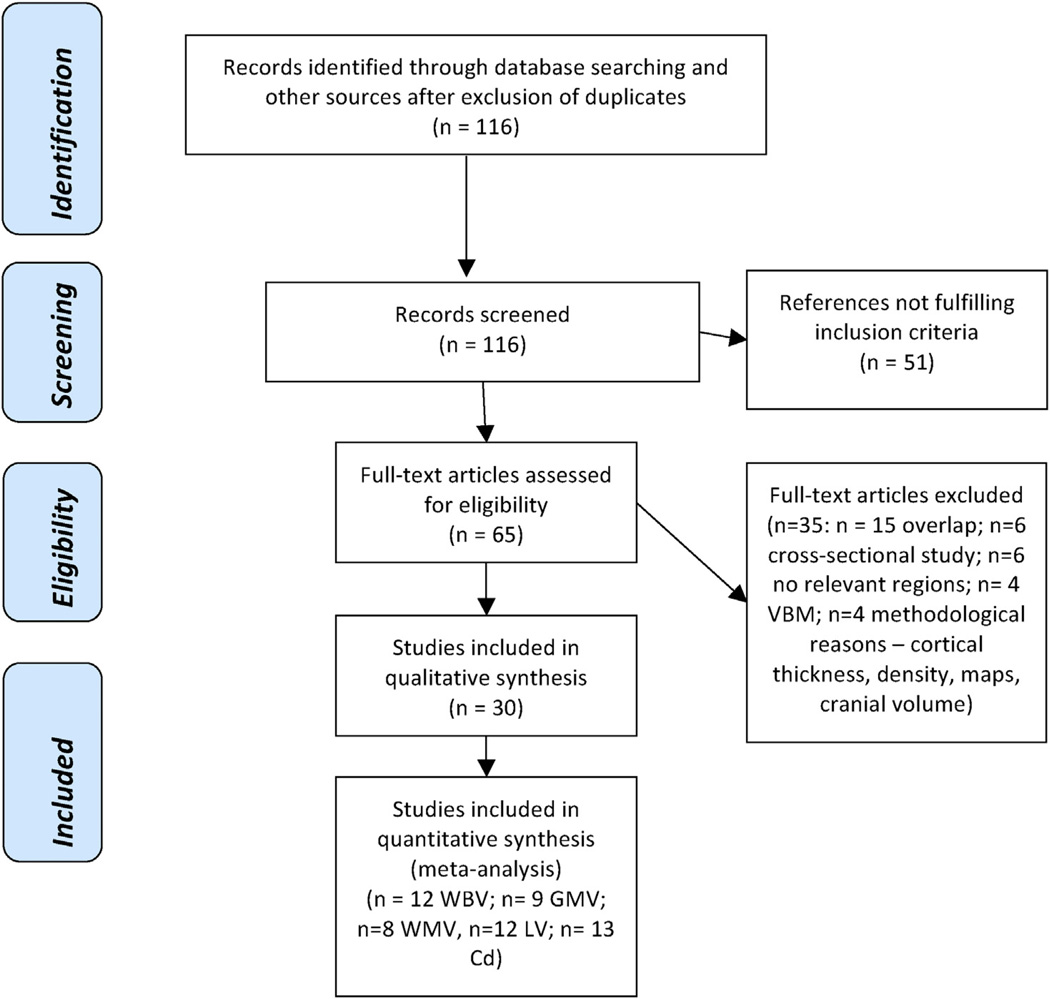
The variables recorded from each article included in the meta-analysis were: sample size, year of publication, gender (proportion of females), mean age of participants, duration of follow-up, duration of illness, type of antipsychotic treatment, daily dose of antipsychotic at the follow-up MRI (chlorpromazine equivalents), previous exposure to antipsychotics, brain volumes (see below), severity of psychotic symptoms (see below). To achieve a high standard of reporting we have adopted ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ (PRISMA) guidelines (Moher et al., 2009) (see Fig. 1).
Fig. 1.
PRISMA Flow Diagram of literature search. All full-text excluded studies together with the reason why they were excluded are listed in the supplementary Table 3. Abbreviations: Cd, caudate nucleus; CSF, cerebrospinal fluid; GMV, gray matter volume; LV, lateral ventricles; VBM, voxel-based morphometry; WBV, whole brain volume; WMV, white matter volume.
2.1.4. Extraction and standardization of psychometric rating scales
There are several scales to measure psychotic symptoms used in studies with schizophrenia patients. Most commonly the Positive and Negative Symptom Scale (PANSS), and the Brief Psychiatric Rating Scale (BPRS), Scale for Assessment of Positive Symptoms (SAPS), and Scale for Assessment of Negative Symptoms (SANS) have been used. There is high correlation among both positive and negative scale totals across these tools (Leucht et al., 2006) with good inter-rater and test-retest reliability (Lyne et al., 2012). We extracted total PANSS, BPRS and SAPS + SANS scores from included studies. Each item of the SAPS and SANS is rated on a six point scale (0–5) and BPRS and PANSS on a seven point scale (1–7). Extracted total scores were transformed to a total score with a baseline of zero, in line with previous studies (Sherwood et al., 2006)(see S1). To investigate the baseline psychotic symptoms in included studies we calculated mean per-item score by dividing the total score by 30 in PANSS, by 18 in BPRS and by 50 in SAPS + SANS. All included studies reported improvement on psychometric score during follow-up and we calculated it as percentage of baseline per-item score (Agid et al., 2003b). The details of clinical variables used in the meta-analysis are described in the supplementary material S1, paragraph 8.
2.1.5. Quality assessment
The quality of the studies was assessed using an item-checklist constructed specifically for the review and similar to the previously published quality assessment (Paulson and Bazemore, 2010). The recorded variables were assessed in terms of precision, directness and consistency of the data. The categories scored in the quality assessment are listed in the table S2 with the range min 0 and max 2 points. The code of the range was developed a priori and modified after the first run of quality assessment (see S1). The disagreements were discussed between the authors and the consensus was put in the table. Quality assessment was conducted in the following categories: (1) study design – random blind, open or case–control, the role of the funding and sample size; (2) demographic and clinical characteristics – clearly reported inclusion and exclusion criteria, substance abuse, included control group, gender, race, IQ, duration of the illness and previous antipsychotic medication; (3) results – reported drop-out rates, psychopathological ratings, statistical thresholds, ROI reliability. The included studies were rated according to the sum of the points and characterized as high quality (above 80% of the maximal sum of points), moderate-high (60–79%), moderate (40–59%), moderate-low (20–39%), and low quality studies (below 19%)(see more details in Tables S2 and S3).
2.2. Statistical analysis
Data were entered into an electronic database and analyzed with a quantitative meta-analytical approach using Comprehensive Meta-Analysis (CMA) Software version 2 (Biostat, Inc., Englewood, NJ, USA). CMA software employs the same computational algorithms used by the Cochrane collaborators to weight studies by the inverse variance method (Borenstein et al., 2005). The primary effect size measure was the difference in brain volumes between patients and controls at baseline and at the end of follow-up (see S1). Meta-analyses were conducted when at least three studies reported the volume of a common brain region. We were thus able to analyze the following regions: gray matter volume (GMV), white matter volume (WMV), cerebrospinal fluid (CSF) and whole brain volume (WBV) as a sum of gray matter plus white matter volume (Courchesne et al., 2000). Most included studies reported volumetric data (WBV and/or GMV and/or WMV) including the volume of cerebellum. For studies where global volumes did not include cerebellar volume, we either present the data received directly from the author (van Haren et al., 2008) or marked them if the authors did not respond (Sporn et al., 2003; Takahashi et al., 2009; Boonstra et al., 2011; Ho et al., 2011) (Table 1) and adjusted our analysis accordingly (sensitivity analysis, see below). We also analyzed lateral ventricle volumes (LV) and the caudate nuclei volumes (Cd), defined as the sum of the left and right nuclei caudate. After completing a meta-analysis of progressive brain changes in patients and controls (at baseline and follow-up) we specifically tested the effect of putative moderator factors: cumulative antipsychotic exposure, duration of illness and illness severity.
Table 1.
Longitudinal magnetic resonance imaging studies of schizophrenia subjects and antipsychotic exposure included in the meta-analysis.
| Author & year | Brain volumetric data used in analysis |
Follow-up duration | Type of antipsychotic |
Previous antipsychotic medication |
Substance abuse |
Schizophrenia patients | Healthy controls | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weeks means |
SD | Y (%) | Y/N | n | Age years | SD | % F | n | Age years | SD | % F | |||
| Arango 2012 (Arango et al., 2012) | WBV, GMV, WMV, CSF | 104.8 | 12.00 | MIX | Y (96) | N | 25 | 15.50 | 2.00 | 28.00 | 94 | 15.30 | 1.50 | 67.00 |
| Boonstra 2011 (Boonstra et al., 2011) | WBV, GMV*, WMV*, LV, NC | 57.27 | 8.55 | ATYP | Y (100) | Y | 8 FE AF | 27.97 | 8.24 | 25.00 | 20 | 27.97 | 5.63 | 33.30 |
| 54.00 | 0.84 | ATYP | Y (100) | Y | 8 FE | 29.56 | 5.72 | 25.00 | ||||||
| Chakos 1994 (Chakos et al., 1994) | LV, NC | 72.00 | . | TYP | Y (27.5) | N | 29 | 25.20 | 6.30 | 41.30 | 10 | 30.50 | 4.90 | 20.00 |
| Crespo-Facorro 2008 (Crespo-Facorro et al., 2008) | WBV, GMV, WMV, LV, NC | 54.60 | 4.60 | TYP | NA | Y | 18 Hal | 29.76 | 7.88 | 38.90 | 38 | Matched | . | 31.60 |
| 55.30 | 4.20 | ATYP | NA | Y | 18 Olan | 28.00 | 5.09 | 27.80 | . | . | . | |||
| 53.70 | 3.50 | ATYP | NA | Y | 16 Risp | 24.99 | 5.96 | 18.70 | . | . | . | |||
| DeLisi 2004 (DeLisi et al., 2004) | LV | 520.00 | . | MIX | Y | NA | 26 | 26.80 | 7.08 | 34.60 | 20 | 25.50 | . | 40.00 |
| Frazier 1996 (Frazier et al., 1996) | LV, NC | 104.00 | . | Clo/MIX | Y | NA | 8 | 15.10 | 2.30 | 13.00 | 8 | 15.40 | 3.10 | 12.50 |
| Garver 2005 (Garver et al., 2005) | WMV, CSF | 4.00 | . | TYP | Y | N | 6 | 32.20 | 14.80 | 33.00 | 7 | 29.00 | 9.00 | 28.60 |
| 4.00 | . | ATYP | Y | N | 13 | 31.60 | 10.20 | 31.00 | . | . | . | . | ||
| Gur 1998 (Gur et al., 1998) | WBV | 119.20 | 48.80 | MIX | N (100) | NA | 20 FE | 29.20 | 8.00 | 42.50 | 17 | 31.90 | 8.90 | 23.50 |
| WBV | 119.20 | 48.80 | MIX | Y (100) | NA | 20 Ch | 29.20 | 8.00 | 42.50 | . | . | . | . | |
| Heitmiller 2004 (Heitmiller et al., 2004) | NC | 120.80 | . | ATYP | N | NA | 14 | 26.30 | 6.80 | 50.00 | 14 | 26.70 | 11.30 | 50.00 |
| Ho 2011 (Ho et al., 2011) | GMV* | 374.40 | 202.80 | MIX | Y (86) | Y | 211 | 26.30 | 7.60 | 28.00 | . | . | . | . |
| Ho 2003 (Ho et al., 2003) | WBV | 171.00 | 83.20 | MIX | Y (55) | NA | 73 | 24.50 | 4.67 | 27.00 | 23 | 26.90 | 1.60 | 34.80 |
| James 2004 (James et al., 2004) | WBV | 125.84 | 82.68 | Cloz/ATYP | Y (70) | NA | 9 M | 17.70 | 1.70 | . | 9 M | 15.70 | 2.00 | . |
| 88.92 | 27.04 | Cloz/ATYP | Y (38) | . | 7 F | 15.30 | 1.50 | . | 7 F | 16.40 | 2.10 | . | ||
| Keshavan 1994 (Keshavan et al., 1994) | NC | 43.57 | TYP | N | NA | 11 | . | . | . | . | . | . | . | |
| Lang 2004 (Lang et al., 2004) | NC | 56.00 | 17.10 | ATYP | Y (100) | N | 10 | 35.30 | 8.80 | 30.00 | 23 | 23.30 | 7.40 | 47.80 |
| 52.10 | 6.90 | ATYP | Y (100) | N | 14 | 23.70 | 3.30 | 28.60 | . | . | . | . | ||
| 42.20 | 12.10 | ATYP | Y (100) | N | 13 | 25.60 | 8.20 | 23.10 | . | . | . | . | ||
| Lieberman 2005 (Lieberman et al., 2005) | WBV, GMV, LV | 52.00 | . | TYP | Y (67.1) | N | 79 | 24.11 | 4.64 | 10.00 | 62 | 25.53 | 4.13 | 35.50 |
| 52.00 | . | ATYP | Y (76.8) | N | 82 | 23.60 | 4.64 | 21.00 | ||||||
| Massana 2005 (Massana et al., 2005) | NC | 9.00 | . | ATYP | N | NA | 11 | 23.00 | 4.00 | 27.00 | . | . | . | . |
| McClure 2008 (McClure et al., 2008) | NC | 12.00 | . | ATYP/Cloz | Y | N | 10 | 36.70 | 7.70 | 10.00 | . | . | . | . |
| Molina 2005 (Molina et al., 2005) | WBV, GMV, WMV | 102.40 | 39.60 | ATYP | N (100) | N | 49 | 25.60 | 4.00 | 31.00 | 11 | 28.40 | 6.20 | 54.50 |
| 114.80 | 47.20 | Clo | Y | N | 29Ch | 31.00 | 5.90 | |||||||
| Nakamura 2007 (Nakamura et al., 2007) | WMV, LV | 72.40 | 46.40 | ATYP | Y | N | 17 FE | 24.70 | 7.00 | 17.70 | 26 | 23.60 | 4.10 | 15.40 |
| Puri 2001 (Puri et al., 2001) | LV | 30.90 | 6.20 | MIX | Y | N | 24 | 28.47 | 8.45 | . | 12 | 27.92 | 6.14 | . |
| Reig 2009 (Reig et al., 2009) | GMV, WMV, CSF | 104.00 | . | Cloz/ATYP | Y | N | 21 | 15.70 | 1.70 | 23.80 | 34 | 15.20 | 1.40 | 38.20 |
| Saijo 2001 (Saijo et al., 2001) | LV | 520.00 | . | NA | NA | N | 18 | 37.50 | 8.90 | 50.00 | 12 | 37.10 | 4.20 | 41.70 |
| Scheepers 2001 (Scheepers et al., 2001) | WBV, NC | 24.00 | . | CLOZ | Y (100) | N | 29 | 35.23 | 10.34 | 30.80 | . | . | . | . |
| Sporn 2003 (Sporn et al., 2003) | WBV*, GMV*, LV | 176.80 | 72.80 | MIX | NA | N | 39 | 15.00 | 2.30 | 38.50 | 43 | 14.80 | 2.20 | 37.20 |
| Takahashi 2009 (Takahashi et al., 2009) | WBV* | 105.04 | 39.52 | MIX | Y | N | 23 FE | 21.60 | 3.50 | 30.40 | 26 | 25.60 | 9.10 | 42.30 |
| 125.32 | 50.44 | MIX | Y | N | 11Ch | 32.70 | 7.60 | 9.00 | . | . | . | . | ||
| Tauscher-Wisniewski 2005 (Tauscher-Wisniewski et al., 2005) | NC | 12.00 | . | ATYP | N (100) | NA | 14 | 22.60 | 3.7 | 21.43 | 37 | 25.80 | 6.20 | 40.50 |
| Tauscher-Wisniewski 2002 (Tauscher-Wisniewski et al., 2002) | NC | 260.00 | . | MIX | Y (47) | NA | 15 | 23.00 | 6.20 | 33.30 | 10 | 29.40 | 8.60 | 30.00 |
| Taylor 2005 (Taylor et al., 2005) | NC | 4.00 | . | MIX | N | N | 11 | 34.70 | 12.40 | . | 11 | 26.80 | 6.60 | . |
| van Haren 2008 (van Haren et al., 2008) | WBV, GMV, WMV*, LV | 260.00 | . | MIX | Y | Y | 96 | 32.22 | 11.10 | 27.00 | 113 | 35.28 | 12.30 | 32.70 |
| Whitworth 2005 (Whitworth et al., 2005) | LV | 132.10 | 41.60 | MIX | NA | NA | 21 FE | 25.00 | 4.80 | 0.00 | 20 | 31.50 | 4.90 | 0.00 |
| 171.10 | 63.40 | MIX | NA | NA | 17Ch | 28.40 | 4.00 | 0.00 | . | . | . | . | ||
Abbreviations: AN, antipsychotic naïve; ATYP, atypical antipsychotic; Clo, clozapine; Ch, chronic; CSF, cerebrospinal fluid; F, female; FE, first episode; GMV, gray matter volume; Hal, haloperidol; LV, lateral ventricle; M, male; MIX, both typical and atypical antipsychotics; n, amount of individuals; N, no; NA, not announced; NC, caudate nucleus; Ola, olanzapine; Ris, risperidone; TYP, typical antipsychotic; WBV, whole brain; WMV, white matter volume; Y, yes;
volumetric data were presented without cerebellar volume.
The effect size was estimated by calculating Hedges’ unbiased g. For the cross-sectional analysis, negative values reflected less gray matter volumes in the patients as compared to healthy controls. For the longitudinal analysis, negative values reflected brain volumes reductions at follow-up as compared to baseline. To limit risk of false positive (type I) errors arising from multiple comparisons we adjusted p < 0.05 by dividing α with the number of meta-analyses conducted. The Q statistic was used to determine between-group differences. To determine whether categorical factors (i.e. substance abuse) modified the progressive brain changes, subgroup analyses were performed (Paulson and Bazemore, 2010). The influence of continuous moderator variables (antipsychotic exposure, duration of illness and illness severity and study duration) was tested using meta-regression analyses. Meta-regressions (fixed effect models) were performed when at least seven independent studies were available for the outcome of interest. The slope of meta-regression (β-coefficient: direct (+) or inverse (−)) of the regression line indicates the strength of a relationship between moderator and outcome. The confounding effect of potential outliers on the meta-regression was controlled with the Cook’s distance test (Cook and Weisberg, 1982).
Heterogeneity among study point estimates was assessed with the Q statistic (Paulson and Bazemore, 2010) with magnitude of heterogeneity being evaluated with the I2 index (Lipsey and Wilson, 2000). For homogeneous data, we calculated the global effect size, using a fixed effect model. In the absence of significant heterogeneity, the use of a fixed effect model is legitimate and may provide greater statistical power than the random effect model (Szoke et al., 2008). For heterogeneous data we employed random effects models which are more conservative than fixed-effect models, and appear to better address heterogeneity between studies and study populations, allowing for greater flexibility in parsing effect size variability (Cooper et al., 2009). Studies with negative results are less likely to be published than studies with statistically significant results. The possibility of small publication biases in the present study was examined by visually inspecting funnel plots and applying the regression intercept of Egger et al. (1997). In this way we assessed whether there was a tendency for selective publication of studies based on the nature and direction of their results. In addition, we used the fail-safe procedure (Orwin, 1983), to generate a number of unpublished studies that would be needed to move estimates to a non significant threshold. In case of publication bias we adopted the ‘trim and fill’ method, which aims both to identify and correct for funnel plot asymmetry arising from publication bias (Duval and Tweedie, 2000). To assess the robustness of the results, we performed sensitivity analyses by sequentially removing each study and rerunning the analysis. We also conducted a separate analysis excluding studies with quality ratings in the lowest third to determine if potential methodological weaknesses influenced meta-analytic estimates. Finally we conducted a sensitivity analysis excluding the studies that had not included cerebellar volumes.
3. Results
3.1. Database
The initial literature search uncovered 116 potential studies. Out of the 65 full-text assessed studies, 35 did not meet inclusion criteria and were excluded. The final database comprised 30 original independent studies published between 1994 and 2012. The overall sample was of 1046 schizophrenia patients and 780 controls for a median duration of follow-up of 72.4 weeks (range 4–520). The details of the literature search are described in the PRISMA flowchart, while the characteristics of the included and excluded studies are detailed in Table 1 and supplementary material S4 respectively.
3.2. Baseline differences in brain volumes
After correcting for multiple comparisons, we found significant baseline volumetric differences in the WBV, with patients showing reduced volumes as compared to controls (p = 0.002, Table 2). Conversely, patients showed enlarged LV volumes as compared to controls (p < 0.001). There were trend-level differences in the reduced GMV, which however did not survive correction for multiple comparisons. Increased cerebrospinal fluid was observed in patients but this occurred in the presence of publication bias (see below). There were no significant baseline differences in WMV or caudate nucleus (Cd) volume.
Table 2.
Meta-analyses of baseline volumetric differences between schizophrenia patients and healthy controls. Negative values of Hedge’s g indicate reduced volume in patients vs. controls.
| Brain region | N | SCZ | C | Hedge’s g | Z score | p | Test for Heterogeneity | FSN | ERT | Trimm and Fill | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | CI95% | Q | I2 | p | Mean | CI95% | ||||||||||
| WBV | 11 | 581 | 429 | −0.252 | −0.414 | −0.091 | −3.063 | 0.002* | 13.561 | 26.26 | 0.194 | 27 | 0.411 | −0.252 | −0.414 | −0.091 |
| GMV | 8 | 399 | 382 | −0.192 | −0.343 | −0.041 | −2.493 | 0.013 | 3.635 | 3.7 | 0.821 | 18 | 0.111 | −0.192 | −0.343 | −0.041 |
| WMV | 6 | 199 | 261 | −0.012 | −0.294 | 0.269 | −0.087 | 0.931 | 8.717 | 42.64 | 0.121 | 0 | 0.393 | −0.012 | −0.294 | 0.269 |
| CSF | 3 | 60 | 98 | 0.451 | 0.088 | 0.813 | 1.434 | 0.045 | 2.173 | 7.90 | 0.337 | 3 | 0.069 | 0.325 | −0.037 | 0.686 |
| LV | 11 | 549 | 347 | 0.309 | 0.144 | 0.467 | 4.046 | <0.001* | 12.084 | 17.25 | 0.279 | 44 | 0.139 | 0.309 | 0.144 | 0.467 |
| Cd | 9 | 192 | 171 | 0.116 | −0.107 | 0.339 | 1.020 | 0.308 | 8.589 | 6.85 | 0.378 | 0 | 0.592 | 0.116 | −0.107 | 0.339 |
WBV, whole brain volume; GMV, grey matter volume; WMV, white matter volume; CSF, cerebrospinal fluid; LV, lateral ventricules; Cd, Caudate nucleus; N = number of studies included in each meta-analysis; SCZ, number of patients with schizophrenia; C, number of controls;
Surviving correction for multiple comparisons (p = 0.008);
FSN, Fail Safe Number; ERT, Egger’s Regression Test.
3.3. Longitudinal difference in brain volumes
When WBV at the end of the follow-up was compared with the baseline values, there was an overall decrease, which however was not statistically significant for both the patient (p = 0.339) and control (p = 0.333) groups (between groups difference p = 0.923, Table 3). Similarly, there were non-significant volume decreases in the Cd for both patients (p = 0.913) and controls (p = 0.160) groups (between groups difference p = 0.318).
Table 3.
Meta-analyses of longitudinal volumetric differences between schizophrenia patients and healthy controls. Negative values of Hedge’s g indicate reduced volume at follow-up vs baseline.
| Brain region | N | Group | Hedge’s g | Z score | p | Between groups effect |
FSN | ERT | Trim and Fill | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | CI95% | Q | p | Mean | CI95% | |||||||||
| WBV | 12 | CTRL | −0.069 | −0.208 | 0.070 | −0.969 | 0.333 | 0.009 | 0.923 | 0 | 0.662 | −0.069 | −0.208 | 0.070 |
| SCZ | −0.060 | −0.183 | 0.063 | −0.956 | 0.339 | −0.060 | −0.183 | 0.063 | ||||||
| GMV | 9 | CTRL | −0.143 | −0.293 | 0.008 | −1.555 | 0.094 | 5.974 | 0.044 | 21 | 0.742 | −0.143 | −0.293 | 0.008 |
| SCZ | −0.249 | −0.399 | −0.093 | −3.154 | 0.002* | −0.249 | −0.399 | −0.093 | ||||||
| WMV | 8 | CTRL | 0.148 | −0.032 | 0.328 | 1.608 | 0.108 | 1.259 | 0.262 | 0 | 0.341 | 0.148 | −0.032 | 0.328 |
| SCZ | 0.001 | −0.184 | 0.184 | 0.002 | 0.998 | 0.001 | −0.184 | 0.184 | ||||||
| CSF | 3 | CTRL | 0.199 | −0.256 | 0.654 | 0.858 | 0.391 | 1.759 | 0.185 | 0 | 0.351 | 0.199 | −0.256 | 0.654 |
| SCZ | 0.007 | −0.339 | 0.352 | 0.037 | 0.970 | 0.007 | −0.339 | 0.352 | ||||||
| LV | 12 | CTRL | 0.129 | −0.025 | 0.283 | 1.637 | 0.102 | 9.566 | 0.029 | 34 | 0.178 | 0.129 | −0.025 | 0.283 |
| SCZ | 0.207 | 0.075 | 0.339 | 3.067 | 0.002* | 0.207 | 0.075 | 0.339 | ||||||
| Cd | 13 | CTRL | −0.149 | −0.357 | 0.059 | −1.405 | 0.160 | 0.996 | 0.318 | 0 | 0.409 | −0.149 | −0.357 | 0.059 |
| SCZ | −0.010 | −0.183 | 0.164 | −0.109 | 0.913 | −0.010 | −0.183 | 0.164 | ||||||
WBV, whole brain volume; GMV, grey matter volume; WMV, white matter volume; CSF, cerebrospinal fluid; LV, lateral ventricules; Cd, Caudate nucleus; N = number of studies included in each meta-analysis; SCZ, number of patients with schizophrenia; C, number of controls;
Surviving correction for multiple comparisons (p = 0.008);
FSN, Fail Safe Number; ERT, Egger’s Regression Test.
Significant longitudinal volumetric changes were observed in the GMV and LV. The first showed significant volumetric decreases in patients (p = 0.002) but not in controls (p = 0.094). The differences in the patient group survived correction for multiple comparisons. There was a significant between group differences in longitudinal GMV changes (Q = 5.974, p = 0.044; Table 3). There were significant LV increases in the patient (p = 0.002) but not in the control group (p = 0.102). The differences in the patient group survived correction for multiple comparisons and a significant between-group difference was detected (Q = 9.566, p = 0.029, Table 3).
WMV and CSF showed non-significant increases at follow-up as compared to baseline across both patients (WMV p = 0.998, CSF p = 0.970) and control (WMV p = 0.108, CSF p = 0.391) groups (WMV between groups differences p = 0.262, CSF between groups differences p = 0.185).
3.4. Effects of moderators
The details of antipsychotic treatment, symptoms severity, and duration of illness are given in supplementary Tables S5 and S6). Meta regression analyses for the selected moderators (cumulative exposure to antipsychotic medication during follow-up time, psychotic symptoms change over follow-up and duration of illness) were tested for both GMV and LV changes in the patient group. Longitudinal GMV decreases in patients were associated with higher cumulative exposure to antipsychotic over time (n of studies = 8 (Sporn et al., 2003; Lieberman et al., 2005; Molina et al., 2005; Crespo-Facorro et al., 2008; van Haren et al., 2008; Boonstra et al., 2011; Ho et al., 2011; Arango et al., 2012), sample = 629 schizophrenia subjects, β = −0.013, CI 95% from −0.033 to −0.001, Q = 8.598, p = 0.048, Fig. 2a) but not with psychotic symptoms change (n of studies = 7 (Sporn et al., 2003; Lieberman et al., 2005; Molina et al., 2005; Crespo-Facorro et al., 2008; van Haren et al., 2008; Reig et al., 2009; Arango et al., 2012), sample = 418 schizophrenia subjects, p > 0.05, Fig. 2b) or DOI (n of studies = 9 (Sporn et al., 2003; Lieberman et al., 2005; Molina et al., 2005; Crespo-Facorro et al., 2008; van Haren et al., 2008; Reig et al., 2009; Boonstra et al., 2011; Ho et al., 2011; Arango et al., 2012), sample = 645 schizophrenia subjects, p > 0.05, Fig. 2c). Longitudinal LV changes in patients were not associated with cumulative exposure to antipsychotics (n of studies = 10 (Chakos et al., 1994; Frazier et al., 1996; Puri et al., 2001; Saijo et al., 2001; Ho et al., 2003; Sporn et al., 2003; Lieberman et al., 2005; Nakamura et al., 2007; van Haren et al., 2008; Boonstra et al., 2011), sample = 533 schizophrenia subjects, p < 0.05) or DOI (n of studies = 11 (Frazier et al., 1996; Puri et al., 2001; Saijo et al., 2001; Ho et al., 2003; Sporn et al., 2003; DeLisi et al., 2004; Lieberman et al., 2005; Whitworth et al., 2005; Nakamura et al., 2007; van Haren et al., 2008; Boonstra et al., 2011), sample = 542 schizophrenia patients, p > 0.05, Fig. 3). Because of missing data (n of studies <7) it was not possible to conduct a meta-regression between LV changes and psychotic symptoms changes in patients. There was no significant confounding effect for study duration on the longitudinal GMV changes (β = −0.0006, CI 95% from −0.001 to 0.001, Z = −1.772, p = 0.096). Although studies with a longer duration tended to detect larger LV enlargements as compared to studies with a short follow-up, this effect was non significant (β = 0.001, CI 95% from −0.001 to 0.002, Z = 1.413, p = 0.158).
Fig. 2.
Meta-regression analysis: (a) progressive GMV changes and cumulative exposure to antipsychotics (β = −0.013, CI 95% from −0.033 to −0.001, Q = 8.598, p = 0.048); (b) progressive GMV changes and duration of illness (DOI, β = 0.001, CI 95% from −0.001 to 0.001, p = 0.653); (c) progressive GMV changes and psychotic symptoms change over follow-up time (β = 0.002, CI 95% from −0.011 to 0.016, p = 0.732). The size of the circle reflects the sample size of the study. Negative vaules on the y axis indicate brain volume reductions at follow-up as compared to baseline. Cumulative exposure to antipsychotics unit was defined in Chlorpromazine Equivalent per day (CPZ-EQ/d) multiplied by the duration of the medication treatment in days (for details see Supplementary material study protocol para. 9). Change in psychotic symptom unit: percentage of baseline per item score (positive values indicate improvement of symptoms at follow-up as compared to baseline; for details see supplementary materials section 8).
Fig. 3.
Meta-regression analysis showing no significant (p > 0.05) correlations between: progressive LV volume changes and duration of illness (DOI) within the schizophrenia patients. The size of the circle reflects the sample size of the study. Positive values on the y axis reflect brain volume increases at follow-up as compared to baseline.
3.5. Publication bias, heterogeneity, sensitivity analysis
As also shown in Table 2, the fail-safe number in the baseline analysis surpassed the number of actual studies by a factor 2.25 (GMV) up to a factor 4 (LV). Conversely, both the fail-safe number and the Egger’s regression test indicated publication bias for CSF. Significance of Hedge’s g did not survive after adjustment by the Trim and Fill method. Fail-safe numbers were generally larger in the longitudinal analysis. Egger’s regression test did not indicate publication bias here and significance of effect sizes did not change after adjustment by the Trim and Fill method. Heterogeneity was low and non-significant in the cross-sectional analysis. The presence of statistically significant heterogeneity of low magnitude in the longitudinal analysis accounted for the exploratory investigations of potential moderator factors. Quality analysis showed that most of the included studies were of high or moderate quality (13.3% high and 73.3% moderate scores). Removing studies with quality ratings in the lowest third did not affect the point estimates by more than 8.5%. The results of the sensitivity analysis excluding studies that did not include cerebellar volumes, did not affect the overall baseline meta-analytical estimates more than 7% (GMV: 7%, WMV 4%, WBV 6%).
4. Discussion
The present meta-analysis investigated longitudinal gray matter changes in schizophrenia addressing the impact of illness duration, severity of psychotic symptoms and antipsychotic treatment. Thirty longitudinal MRI studies were included with final database of 1046 schizophrenia patients and 780 controls. At the baseline cross-sectional analysis, the patients showed significant WBV reductions and enlarged LV volumes as compared to controls but no abnormalities in GMV, WMV, CSF and Cd. Longitudinally, there were progressive GMV decreases and LV enlargements in the schizophrenia group while no significant changes were observed in control group. The higher the cumulative exposure to antipsychotic treatment the greater the GMV decreases in the patient group over follow-up time, while no significant effects were observed for illness duration or severity of symptoms.
Our systematic literature search uncovered a large database of 30 longitudinal MRI studies with antipsychotic administration. The large sample size combined with the absence of significant publication biases (except for CSF), low heterogeneity between studies, strict quality control and careful sensitivity analysis yielded a robust meta-analytical approach. We first conducted a cross-sectional analysis to address putative brain changes prior the initiation of the longitudinal MRI studies. Patients compared to controls had reduced WBV and enlarged LV with trend-level reductions in GMV, which did not survive multiple comparisons. A recent meta-analysis has addressed the cross-sectional brain volume changes in medicated schizophrenia patients; small to medium meta-analytical differences were confirmed in WBV (effect size = −0.17), GMV (−0.43) and LV (0.45) (Haijma et al., 2012). In line with these findings, our cross-sectional analysis detected similar small to medium magnitude in the observed effect sizes: WBV (−0.25), GMV (−0.19), LV (0.31). However, these findings are based on cross-sectional designs and thus it is not possible to establish whether these alterations are secondary to previous antipsychotic treatments, illness duration or illness severity or a mixture of these or other confounding factors. Furthermore, as moderate atrophy of gray matter structures has been observed during normal aging (Long et al., 2012) it is not clear whether similar dynamic alterations occur in healthy individuals.
To address these caveats we have conducted a meta-analytical comparison of WMV, GMV, CSF, WBV, LV, and Cd volumes from longitudinal studies in patients with schizophrenia vs. controls. We found progressive GMV reductions and LV enlargements in schizophrenia patients but not in healthy controls. Previous evidence has indicated that the annualized percentage volume changes in schizophrenia were −0.59% for GMV, and +0.36% for LV (Olabi et al., 2011). The overall effect sizes for our longitudinal LV increases over follow-up time were small to medium: 0.21 in patients and 0.13 in controls. LV increases (approximately 130% the size of normal controls (Wright et al., 2000)) are the earliest (identified by CT imaging in 1976) (Johnstone et al., 1976) and the most consistent volumetric abnormalities reported in schizophrenia (Kempton et al., 2010), in up to 66% of available studies (Sayo et al., 2012). We found no modulating effect of antipsychotic treatment or illness duration on progressive LV increases. Previous works suggested that LV enlargement is globally interrelated with GMV diminution (Horga et al., 2011). In line with this hypothesis we also uncovered longitudinal GMV reductions. Overall the effect size for the longitudinal GMV decreases over follow-up time was again small to medium: −0.25 and in the patient and −0.14 in the control group. Interestingly, the magnitude of progressive GMV decreases was very close to that observed for LV increases, in line with the hypothesis that progressive cortical shrinkage over time might largely explain LV increases. To test that there was a pathological difference in the longitudinal progression of brain changes we compared the magnitude of changes between the patient and control group. The between-groups difference in GMV and LV was statistically significant, indicating that the schizophrenia patients, compared to matched healthy controls, showed pathological progressive GMV decreases and LV increases. However, it is important to note that the lack of significant progressive changes in controls may be a matter of power and variability in included groups. Similarly, our group-level analysis cannot exclude that the observed pathological changes can occur only in a subset of schizophrenia patients (Andreasen et al., 2011).
Given the longitudinal design we then tested the potential effect of the above confounders in the schizophrenia group: antipsychotic cumulative dose during the MRI study, overall duration of illness, and changes of illness severity i.e. psychotic symptoms during the MRI study. The core finding of the present meta-analysis is that longitudinal GMV decreases in schizophrenia patients were associated with higher cumulative exposure to antipsychotic over time, while no effects were observed for duration of illness and severity of symptoms. To our best knowledge this is the first time there is meta-analytical longitudinal evidence for a significant correlation of antipsychotic treatment and progressive GMV changes in a large sample of schizophrenia subjects (n = 629). Our longitudinal result reinforces the previous cross-sectional evidence indicating that GMV loss was more pronounced in patients using a higher dose of antipsychotic medication (atypical but not typical) at time of scanning (Haijma et al., 2012). The merit of our investigation is that rather than using the current dose of antipsychotic medication we employed the cumulative exposure to antipsychotic treatment during follow up, accounting for the exact duration of longitudinal exposure. Our result of structural changes associated with antipsychotic treatment is in line with functional findings indicating that antipsychotics influence neural activity in psychosis, even in the short-term period (Fusar-Poli et al., 2007; Lui et al., 2010). The putative mechanism of action of antipsychotics on GMV is unknown and can only be inferred in vivo from animal studies. Chronic exposure of macaque monkeys to haloperidol or olanzapine was associated with a 10–18% lower glial cell number in the gray matter (Konopaske et al., 2008). A recent investigation tested the hypothesis that chronic treatment with antipsychotic drugs is associated with a decrease in brain cortical volume, whereas treatment with mood stabilizers is associated with an increase in cortical brain volume (Vernon et al., 2012). Chronic haloperidol treatment induced decreases cortical GMV; in contrast, chronic lithium treatment induced increases in GMV. Following drug withdrawal, haloperidol-induced changes in brain volumes normalized (Vernon et al., 2012). However, some studies showed GMV continues to decline in the same patients following discontinuation of antipsychotics (Boonstra et al., 2011). Such a finding reinforces the hypothesis that schizophrenia may be associated with progressive morphologic changes to which antipsychotic drugs may contribute but are not the sole cause (Andreasen et al., 2011). However, the present meta-analysis contradicts explanations that progressive brain losses are simply a correlate of poor clinical outcome, for which antipsychotic medication is only considered any epiphenomenon. In fact, in a recent multimodal voxel-based meta-analysis combining functional and structural MRI studies we showed that anterior cingulate and insula were influenced by exposure to antipsychotics: GMV abnormalities in these regions were significantly more severe in medicated as compared to drug naïve patients (Radua et al., 2012). As GMV alterations were already observed in meta-analyses of antipsychotic-naïve first-episode subjects (Fusar-Poli et al., 2012c), as well as in subjects at high clinical risk for psychosis (Fusar-Poli et al., 2011), antipsychotics may target regions of key pathology in early psychosis, without necessarily causing these alterations (Radua et al., 2012). Consequently, we speculate that progressive brain changes in schizophrenia may be related to a combination of antipsychotic effects as well as illness progression-reflected in concurrent GMV reduction and LV enlargements (Horga et al., 2011). There are limitations to consider in the present study. First, meta-analyses usually carry on the methodological limitations of the individual studies included in their database. Older studies may be characterized by small sample sizes and overall poor quality control (Shepherd et al., 2012). When reporting whole brain volume some studies did and others did not included cerebellar volume in their data. We have controlled for this issue in our analysis. Importantly, cerebellar volume tends to be conserved over disease progression showing no significant differences over time (Andreasen et al., 2011) or are (not significantly) mirroring the pattern of the cerebral GM volume (van Haren et al., 2008). We thus conclude that cerebellar volume, remaining unchanged over time, is not contributing to the longitudinal brain changes we detected. It is also important to note that we could not test the hypothesis that the changes in brain volume are nonlinear (the biggest at the beginning of the illness), as indicated by recent studies (Andreasen et al., 2011). Other methodological issues such as potential role of different scanners used, their upgrades, and software used in calculating brain volume change (de Bresser et al., 2011) could have influenced the results as well. However, to address this potential problem we have conducted a careful quality assessment and used the sum of scores in our analysis, uncovering no significant changes on the principal point estimates. Nonetheless, the pattern of volumetric changes in the brain vary with age: in young adults gray matter declines whilst white matter increases, while in older adults these measures both decline with age. In addition aging has a differential effect on regional brain in males and females (Good et al., 2001). These sources of variability were not integrated in our analysis, but should be considered when interpreting our results. Additionally, there are potential alternative factors that may account for the association between brain volume and medication and which were difficult to control in our meta-analysis. In particular, our approach to quantify moderator variables, (e.g. medication taken during the follow-up period calculated in CPZ equivalents, or difficulty standardizing symptom measures across different scales) were based on assumptions that could differ from the ideal situation where raw individual patient data would be available to analyze. Another limitation underlying volumetric MRI meta-analyses can be that the individual studies usually rely on ROI approaches, which are manually traced. The manual tracing of ROIs, as compared to automated methods such as VBM, can introduce significant heterogeneity in the anatomical definition of brain areas introducing biases in significance of the results reported. In general, ROI analyses can also be affected by publication biases: researchers could perform several exploratory analyses but report only those which yielded significant results (Ioannidis, 2011; Radua and Mataix-Cols, 2012). Additionally, because of limited data available, we were unable to test all our research hypotheses. In particular we were unable to test whether LV changes were longitudinally associated with psychopathological i.e. PANSS changes in schizophrenia patients. Furthermore, we were unable to test the correlation between antipsychotic treatment and DOI and consequently assess the “independent” effects of antipsychotic treatment on brain volume changes. Finally, it is important to note that association is not causation and thus our findings of significant GMV decreases being correlated with cumulative exposure to antipsychotic treatments should be interpreted cautiously. In particular, multifactorial association implicating several alternative causal factors could potentially underlie our core findings. In other words, antipsychotic treatment may not be the only factor associated to longitudinal GMV decreases in schizophrenia. This is supported by progressive brain changes are already present before the onset and may in particular occur during transition of psychosis in antipsychotic-naïve subjects (Pantelis et al., 2003; Borgwardt et al., 2008; Mechelli et al., 2011; Borgwardt et al., 2012). Moreover, as we did not assess effects of conventional vs. atypical antipsychotics we cannot comment on potential differential and modulating effects on progression (Weinberger and McClure, 2002; Lieberman et al., 2005; Hulshoff Pol and Kahn, 2008). Other potential confounders factors (Collin et al., 2012) such as the genetic modulation of progressive brain changes (Andreasen et al., 2012) or the role played by early cognitive deficits (Fusar-Poli et al., 2012b; Koutsouleris et al., 2012) or substance abuse (Rais et al., 2008; Martin-Santos et al., 2010) on gray matter changes should become subject of investigation by future original studies.
5. Conclusions
Schizophrenia is characterized by progressive gray matter volume decreases and lateral ventricular volume increases. Some of these neuroanatomical alterations may be correlated with antipsychotic treatment.
Supplementary Material
Acknowledgments
We wish to thank all the authors for their collaboration in clarifying potential overlaps between samples and providing additional information on the retrieved studies: Crespo-Facoro et al., Lieberman et al., McClure et al., Nakamura et al., Scheepers et al., van Haren et al., and Wood et al.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neubiorev. 2013.06.001.
References
- Agid O, Kapur S, Arenovich T, Zipursky R. Delayed onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch. Gen. Psychiatry. 2003a;60:1228–1235. doi: 10.1001/archpsyc.60.12.1228. [DOI] [PubMed] [Google Scholar]
- Agid O, Kapur S, Arenovich T, Zipursky RB. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch. Gen. Psychiatry. 2003b;60:1228–1235. doi: 10.1001/archpsyc.60.12.1228. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol. Psychiatry. 2011;70:672–679. doi: 10.1016/j.biopsych.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Wilcox MA, Ho BC, Epping E, Ziebell S, Zeien E, Weiss B, Wassink T. Statistical epistasis and progressive brain change in schizophrenia: an approach for examining the relationships between multiple genes. Mol. Psychiatry. 2012;17:1093–1102. doi: 10.1038/mp.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango C, Rapado-Castro M, Reig S, Castro-Fornieles J, González-Pinto A, Otero S, Baeza I, Moreno C, Graell M, Janssen J, Parellada M, Moreno D, Bargalló N, Desco M. Progressive brain changes in children and adolescents with first-episode psychosis. Arch. Gen. Psychiatry. 2012;69:16–26. doi: 10.1001/archgenpsychiatry.2011.150. [DOI] [PubMed] [Google Scholar]
- Boonstra G, van Haren NE, Schnack HG, Cahn W, Burger H, Boersma M, de Kroon B, Grobbee DE, Hulshoff Pol HE, Kahn RS. Brain volume changes after withdrawal of atypical antipsychotics in patients with first-episode schizophrenia. J. Clin. Psychopharmacol. 2011;31:146–153. doi: 10.1097/JCP.0b013e31820e3f58. [DOI] [PubMed] [Google Scholar]
- Borenstein MHL, Higgins J, Rothstein H. Comprehensive Meta-Analysis Version 2. Englewood, NJ, USA: Biostat; 2005. [Google Scholar]
- Borgwardt S, Koutsouleris N, Aston J, Studerus E, Smieskova R, Riecher-Rossler A, Meisenzahl EM. Distinguishing prodromal from first-episode psychosis using neuroanatomical single-subject pattern recognition. Schizophr Bull. 2012 doi: 10.1093/schbul/sbs095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgwardt SJ, McGuire PK, Aston J, Gschwandtner U, Pfluger MO, Stieglitz RDs, Radue EW, Riecher-Rossler A. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr. Res. 2008;106:108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Cahn W, van Haren NE, Hulshoff Pol HE, Schnack HG, Caspers E, Laponder DA, Kahn RS. Brain volume changes in the first year of illness and 5-year outcome of schizophrenia. Br. J. Psychiatry. 2006;189:381–382. doi: 10.1192/bjp.bp.105.015701. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am. J. Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Chan RC, Di X, McAlonan GM, Gong Q. Brain anatomical abnormalities in high risk individuals, first episode and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr. Bull. 2009 doi: 10.1093/schbul/sbp073. (24 July Epub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G, Derks EM, van Haren NE, Schnack HG, Hulshoff Pol HE, Kahn RS, Cahn W. Symptom dimensions are associated with progressive brain volume changes in schizophrenia. Schizophr. Res. 2012;138:171–176. doi: 10.1016/j.schres.2012.03.036. [DOI] [PubMed] [Google Scholar]
- Cook R, Weisberg S. Residuals and Influence in Regression. New York: Chapman & Hall; 1982. [Google Scholar]
- Cooper H, Hedges L, Valentine J. Handbook of Research Synthesis and Meta-Analysis. New York: Russell Sage Foundation; 2009. [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Roiz-Santiáñez R, Pérez-Iglesias R, Pelayo-Terán JM, Rodríguez-Sánchez JM, Tordesillas-Gutiérrez D, Ramírez M, Martínez O, Gutiérrez A, de Lucas EM, Vázquez-Barquero JL. Effect of antipsychotic drugs on brain morphometry. A randomized controlled one-year follow-up study of haloperidol, risperidone and olanzapine. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:1936–1943. doi: 10.1016/j.pnpbp.2008.09.020. [DOI] [PubMed] [Google Scholar]
- de Bresser J, Portegies MP, Leemans A, Biessels GJ, Kappelle LJ, Viergever MA. A comparison of MR based segmentation methods for measuring brain atrophy progression. Neuroimage. 2011;54:760–768. doi: 10.1016/j.neuroimage.2010.09.060. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Maurizio AM, Relja M, Hoff AL. Cerebral ventricular change over the first 10 years after the onset of schizophrenia. Psychiatry Res. 2004;130:57–70. doi: 10.1016/j.pscychresns.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Giedd JN, Kaysen D, Albus K, Hamburger S, Alaghband-Rad J, Lenane MC, McKenna K, Breier A, Rapoport JL. Childhood-onset schizophrenia: brain MRI rescan after 2 years of clozapine maintenance treatment. Am. J. Psychiatry. 1996;153:564–566. doi: 10.1176/ajp.153.4.564. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rössler A, Schultze-Lutter F, Keshavan M, Wood S, Ruhrmann S, Seidman L, Valmaggia L, Cannon T, Velthorst E, De Haan L, Cornblatt B, Birchwood M, McGlashan T, Carpenter W, McGorry P, Klosterkötter J, McGuire P, Yung A. The psychosis high risk state: a comprehensive state of the art review. JAMA Psychiatry. 2012a;70(1):107–120. doi: 10.1001/jamapsychiatry.2013.269. http://dx.doi.org/10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Crescini A, D’Este G, Kempton M, Lawrie S, Guire PM, Sacchetti E. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci. Biobehav. Rev. 2011;35:1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Broome MR, Matthiasson P, Williams SC, Brammer M, McGuire PK. Effects of acute antipsychotic treatment on brain activation in first episode psychosis: an fMRI study. Eur. Neuropsychopharmacol. 2007;17:492–500. doi: 10.1016/j.euroneuro.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Deste G, Smieskova R, Barlati G, Yung AR, Howes O, Stieglitz R, McGuire P, Borgwardt S. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch. Gen. Psychiatry. 2012b;69(6):562–571. doi: 10.1001/archgenpsychiatry.2011.1592. http://dx.doi.org/10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr. Bull. 2012c;38(6):1297–1307. doi: 10.1093/schbul/sbr134. http://dx.doi.org/10.1093/schbul/sbr134, Epub 2011 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver DL, Holcomb JA, Christensen JD. Cerebral cortical gray expansion associated with two second-generation antipsychotics. Biol. Psychiatry. 2005;58:62–66. doi: 10.1016/j.biopsych.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell P, Turetsky BI, Gallacher F, Cannon T, Bilker W, Gur RC. A follow-up magnetic resonance imaging study of schizophrenia. relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch. Gen. Psychiatry. 1998;55:145–152. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr. Bull. 2012 doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley R, Zelaya FO, Reinders AA, Marques TR, Mehta MA, O’Gorman R, Alsop DC, Taylor H, Johnston A, Williams S, McGuire P, Pariante CM, Kapur S, Dazzan P. Acute effects of single-dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum. Brain Mapp. 2012 doi: 10.1002/hbm.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitmiller DR, Nopoulos PC, Andreasen NC. Changes in caudate volume after exposure to atypical neuroleptics in patients with schizophrenia may be sex-dependent. Schizophr. Res. 2004;66:137–142. doi: 10.1016/j.schres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch. Gen. Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horga G, Bernacer J, Dusi N, Entis J, Chu K, Hazlett EA, Haznedar MM, Kemether E, Byne W, Buchsbaum MS. Correlations between ventricular enlargement and gray and white matter volumes of cortex, thalamus, striatum, and internal capsule in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2011;261:467–476. doi: 10.1007/s00406-011-0202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol H, Kahn R. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr. Bull. 2008;34:354–366. doi: 10.1093/schbul/sbm168. (Epub February 2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP. Excess significance bias in the literature on brain volume abnormalities. Arch. Gen. Psychiatry. 2011;68:773–780. doi: 10.1001/archgenpsychiatry.2011.28. [DOI] [PubMed] [Google Scholar]
- James AC, James S, Smith DM, Javaloyes A. Cerebellar, prefrontal cortex, and thalamic volumes over two time points in adolescent-onset schizophrenia. Am. J. Psychiatry. 2004;161:1023–1029. doi: 10.1176/appi.ajp.161.6.1023. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Crow TJ, Frith CD, Husband J, Kreel L. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet. 1976;2:924–926. doi: 10.1016/s0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- Kempton M, Stahl D, Williams SC, DeLisi L. Progressive lateral ventricular enlargement in schizophrenia: a meta-analysis of longitudinal MRI studies. Schizophr. Res. 2010;120:54–62. doi: 10.1016/j.schres.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Kendall T. The rise and fall of the atypical antipsychotics. Br. J. Psychiatry. 2011;199:266–268. doi: 10.1192/bjp.bp.110.083766. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344:1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Dorph-Petersen KA, Sweet RA, Pierri JN, Zhang W, Sampson AR, Lewis DA. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol. Psychiatry. 2008;63:759–765. doi: 10.1016/j.biopsych.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N, Gaser C, Patschurek-Kliche K, Scheuerecker J, Bottlender R, Decker P, Schmitt G, Reiser M, Moller HJ, Meisenzahl EM. Multivariate patterns of brain-cognition associations relating to vulnerability and clinical outcome in the at-risk mental states for psychosis. Hum. Brain Mapp. 2012;33:2104–2124. doi: 10.1002/hbm.21342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang DJ, Kopala LC, Vandorpe RA, Rui Q, Smith GN, Goghari VM, Lapointe JS, Honer WG. Reduced basal ganglia volumes after switching to olanzapine in chronically treated patients with schizophrenia. Am. J. Psychiatry. 2004;161:1829–1836. doi: 10.1176/ajp.161.10.1829. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology. 2006;31:2318–2325. doi: 10.1038/sj.npp.1301147. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kissling W, Davis JM. Second-generation antipsychotics for schizophrenia: can we resolve the conflict? Psychol. Med. 2009;39:1591–1602. doi: 10.1017/S0033291709005455. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch. Gen. Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Lipsey M, Wilson D. Practical Meta-analysis. Thousand Oaks, CA: Sage Publications; 2000. [Google Scholar]
- Long X, Liao W, Jiang C, Liang D, Qiu B, Zhang L. Healthy aging: an automatic analysis of global and regional morphological alterations of human brain. Acad. Radiol. 2012;19:785–793. doi: 10.1016/j.acra.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, Yue Q, Huang X, Chan RC, Collier DA, Meda SA, Pearlson G, Mechelli A, Sweeney JA, Gong Q. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by resting state functional magnetic resonance imaging. Arch. Gen. Psychiatry. 2010;67:783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- Lyne JP, Kinsella A, O’Donoghue B. Can we combine symptom scales for collaborative research projects? J. Psychiatr. Res. 2012;46:233–238. doi: 10.1016/j.jpsychires.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Martin-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, Fusar-Poli P, Borgwardt S, Seal M, Busatto GF, McGuire P. Neuroimaging in cannabis use: a systematic review of the literature. Psychol. Med. 2010;40:383–398. doi: 10.1017/S0033291709990729. [DOI] [PubMed] [Google Scholar]
- Massana G, Salgado-Pineda P, Junqué C, Pérez M, Baeza I, Pons A, Massana J, Navarro V, Blanch J, Morer A, Mercader JM, Bernardo M. Volume changes in gray matter in first-episode neuroleptic-naive schizophrenic patients treated with risperidone. J. Clin. Psychopharmacol. 2005;25:111–117. doi: 10.1097/01.jcp.0000155818.29091.53. [DOI] [PubMed] [Google Scholar]
- McClure RK, Carew K, Greeter S, Maushauer E, Steen G, Weinberger DR. Absence of regional brain volume change in schizophrenia associated with short-term atypical antipsychotic treatment. Schizophr. Res. 2008;98:29–39. doi: 10.1016/j.schres.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Riecher-Rossler A, Meisenzahl EM, Tognin S, Wood SJ, Borgwardt SJ, Koutsouleris N, Yung AR, Stone JM, Phillips LJ, McGorry PD, Valli I, Velakoulis D, Woolley J, Pantelis C, McGuire P. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Arch. Gen. Psychiatry. 2011;68:489–495. doi: 10.1001/archgenpsychiatry.2011.42. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Modinos G, Costafreda SG, van Tol MJ, McGuire PK, Aleman A, Allen P. Neuroanatomy of Auditory Verbal Hallucinations in Schizophrenia: A Quantitative Meta-Analysis of Voxel-Based Morphometry Studies. Cortex. 2012 doi: 10.1016/j.cortex.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina V, Reig S, Sanz J, Palomo T, Benito C, Sánchez J, Sarramea F, Pascau J, Desco M. Increase in gray matter and decrease in white matter volumes in the cortex during treatment with atypical neuroleptics in schizophrenia. Schizophr. Res. 2005;80:61–71. doi: 10.1016/j.schres.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Moncrieff J, Leo J. A systematic review of the effects of antipsychotic drugs on brain volume. Psychol. Med. 2010;40:1409–1422. doi: 10.1017/S0033291709992297. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Salisbury DF, Hirayasu Y, Bouix S, Pohl KM, Yoshida T, Koo MS, Shenton ME, McCarley RW. Neocortical gray matter volume in firstepisode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biol. Psychiatry. 2007;62:773–783. doi: 10.1016/j.biopsych.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol. Med. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol. Psychiatry. 2011;70:88–96. doi: 10.1016/j.biopsych.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Orwin R. A fail-safe N for effect size in meta-analysis. J. Edu. Stat. 1983;8:157–159. [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a crosssectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Paulson JF, Bazemore SD. Prenatal and postpartum depression in fathers and its association with maternal depression: a meta-analysis. JAMA. 2010;303:1961–1969. doi: 10.1001/jama.2010.605. [DOI] [PubMed] [Google Scholar]
- Puri BK, Hutton SB, Saeed N, Oatridge A, Hajnal JV, Duncan L, Chapman MJ, Barnes TR, Bydder GM, Joyce EM. A serial longitudinal quantitative MRI study of cerebral changes in first-episode schizophrenia using image segmentation and subvoxel registration. Psychiatry Res. 2001;106:141–150. doi: 10.1016/s0925-4927(01)00072-5. [DOI] [PubMed] [Google Scholar]
- Radua J, Borgwardt S, Crescini A, Mataix-Cols D, Meyer-Lindenberg A, McGuire P, Fusar-Poli P. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci. Biobehav. Rev. 2012;36:2325–2333. doi: 10.1016/j.neubiorev.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D. Meta-analytic methods for neuroimaging data explained. Biol. Mood Anxiety Disord. 2012;2:6. doi: 10.1186/2045-5380-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rais M, Cahn W, Van Haren N, Schnack H, Caspers E, Hulshoff Pol H, Kahn R. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am. J. Psychiatry. 2008;165:490–496. doi: 10.1176/appi.ajp.2007.07071110. [DOI] [PubMed] [Google Scholar]
- Reig S, Moreno C, Moreno D, Burdalo M, Janssen J, Parellada M, Zabala A, Desco M, Arango C. Progression of brain volume changes in adolescent-onset psychosis. Schizophr. Bull. 2009;35:233–243. doi: 10.1093/schbul/sbm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo T, Abe T, Someya Y, Sassa T, Sudo Y, Suhara T, Shuno T, Asai K, Okubo Y. Ten year progressive ventricular enlargement in schizophrenia: an MRI morphometrical study. Psychiatry Clin. Neurosci. 2001;55:41–47. doi: 10.1046/j.1440-1819.2001.00783.x. [DOI] [PubMed] [Google Scholar]
- Sayo A, Jennings RG, Van Horn JD. Study factors influencing ventricular enlargement in schizophrenia: a 20 year follow-up meta-analysis. Neuroimage. 2012;59:154–167. doi: 10.1016/j.neuroimage.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaufelberger MS, Lappin JM, Duran FL, Rosa PG, Uchida RR, Santos LC, Murray RM, McGuire PK, Scazufca M, Menezes PR, Busatto GF. Lack of progression of brain abnormalities in first-episode psychosis: a longitudinal magnetic resonance imaging study. Psychol. Med. 2011;41:1677–1689. doi: 10.1017/S0033291710002163. [DOI] [PubMed] [Google Scholar]
- Scheepers FE, de Wied CC, Hulshoff Pol HE, van de Flier W, van der Linden JA, Kahn RS. The effect of clozapine on caudate nucleus volume in schizophrenic patients previously treated with typical antipsychotics. Neuropsychopharmacology. 2001;24:47–54. doi: 10.1016/S0893-133X(00)00172-X. [DOI] [PubMed] [Google Scholar]
- Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci. Biobehav. Rev. 2012;36:1342–1356. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Sherwood M, Thornton AE, Honer WG. A meta-analysis of profile and time-course of symptom change in acute schizophrenia treated with atypical antipsychotics. Int. J. Neuropsychopharmacol. 2006;9:357–366. doi: 10.1017/S1461145705005961. [DOI] [PubMed] [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, Radue EW, McGuire PK, Riecher-Rossler A, Borgwardt SJ. The effects of antipsychotics on the brain: what have we learnt from structural imaging of schizophrenia? – A systematic review. Curr. Pharm. Des. 2009;15:2535–2549. doi: 10.2174/138161209788957456. [DOI] [PubMed] [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, Radue EW, McGuire PK, Riecher-Rossler A, Borgwardt SJ. Neuroimaging predictors of transition to psychosis – a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2010;34:1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Sporn AL, Greenstein DK, Gogtay N, Jeffries NO, Lenane M, Gochman P, Clasen LS, Blumenthal J, Giedd JN, Rapoport JL. Progressive brain volume loss during adolescence in childhood-onset schizophrenia. Am. J. Psychiatry. 2003;160:2181–2189. doi: 10.1176/appi.ajp.160.12.2181. [DOI] [PubMed] [Google Scholar]
- Szoke A, Trandafir A, Dupont ME, Meary A, Schurhoff F, Leboyer M. Longitudinal studies of cognition in schizophrenia: meta-analysis. Br. J. Psychiatry. 2008;192:248–257. doi: 10.1192/bjp.bp.106.029009. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Soulsby B, McGorry PD, Tanino R, Suzuki M, Velakoulis D, Pantelis C. Follow-up MRI study of the insular cortex in first-episode psychosis and chronic schizophrenia. Schizophr. Res. 2009;108:49–56. doi: 10.1016/j.schres.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Tauscher-Wisniewski S, Tauscher J, Christensen BK, Mikulis DJ, Zipursky RB. Volumetric MRI measurement of caudate nuclei in antipsychoticnaive patients suffering from a first episode of psychosis. J. Psychiatr. Res. 2005;39:365–370. doi: 10.1016/j.jpsychires.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Tauscher-Wisniewski S, Tauscher J, Logan J, Christensen BK, Mikulis DJ, Zipursky RB. Caudate volume changes in first episode psychosis parallel the effects of normal aging: a 5-year follow-up study. Schizophr. Res. 2002;58:185–188. doi: 10.1016/s0920-9964(01)00406-6. [DOI] [PubMed] [Google Scholar]
- Taylor S, Christensen JD, Holcomb JM, Garver DL. Volume increases in striatum associated with positive symptom reduction in schizophrenia: a preliminary observation. Psychiatry Res. 2005;140:85–89. doi: 10.1016/j.pscychresns.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Tost H, Braus DF, Hakimi S, Ruf M, Vollmert C, Hohn F, Meyer-Lindenberg A. Acute D2 receptor blockade induces rapid, reversible remodeling in human cortical-striatal circuits. Nat. Neurosci. 2010;13:920–922. doi: 10.1038/nn.2572. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Cahn W, Hulshoff Pol HE, Schnack HG, Caspers E, Lemstra A, Sitskoorn MM, Wiersma D, van den Bosch RJ, Dingemans PM, Schene AH, Kahn RS. Brain volumes as predictor of outcome in recent-onset schizophrenia: a multi-center MRI study. Schizophr. Res. 2003;64:41–52. doi: 10.1016/s0920-9964(03)00018-5. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Brans R, Carati I, Rais M, Kahn RS. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol. Psychiatry. 2008;63:106–113. doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G. Neuroanatomical correlates of psychopathology in antipsychotic-naive schizophrenia. Indian. J. Psychiatry. 2010;52:28–36. doi: 10.4103/0019-5545.58892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon AC, Natesan S, Crum WR, Cooper JD, Modo M, Williams SC, Kapur S. Contrasting effects of haloperidol and lithium on rodent brain structure: a magnetic resonance imaging study with postmortem confirmation. Biol. Psychiatry. 2012;71:855–863. doi: 10.1016/j.biopsych.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Vita A, De Peri L. The effects of antipsychotic treatment on cerebral structure and function in schizophrenia. Int. Rev. Psychiatry. 2007;19:429–436. doi: 10.1080/09540260701486332. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, McClure RK. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry: what is happening in the schizophrenic brain? Arch. General Psychiatry. 2002;59:553–558. doi: 10.1001/archpsyc.59.6.553. [DOI] [PubMed] [Google Scholar]
- Whitworth AB, Kemmler G, Honeder M, Kremser C, Felber S, Hausmann A, Walch T, Wanko C, Weiss EM, Stuppaeck CH, Fleischhacker WW. Longitudinal volumetric MRI study in first- and multiple-episode male schizophrenia patients. Psychiatry Res. 2005;140:225–237. doi: 10.1016/j.pscychresns.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am. J. Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.