SUMMARY
Objectives
Although the incidence of oropharyngeal squamous cell carcinoma (OPSCC) attributable to human papillomavirus (HPV) is rapidly increasing, patients’ informational and psychosocial needs related to the sexual transmission of HPV remain unexplored. The goal of this exploratory study was to assess informational and psychosocial needs of HPV+ OPSCC patients and identify psychosocial challenges associated with having an HPV+ cancer.
Methods
Patients (N = 62; 87% male; mean age = 56 years) with HPV+ OPSCC and in cohabitating relationships completed paper–pencil questionnaires assessing their HPV-related knowledge (e.g., cancer etiology), information needs (e.g., communicability), psychosocial concerns (e.g., relational consequences, self-blame) and measures of distress and health behaviors. Medical information was obtained from patients’ electronic medical records.
Results
Sixty-six percent of patients correctly identified their HPV status but only 35% of them recognized HPV as their putative cancer cause. The majority of patients disclosed their HPV status to their partner, 41% discussed transmission of the virus, and only 23% felt informed regarding potential transmission risks and precautions. Thirty-nine percent want their oncologist to discuss more about HPV-related issues and 58% sought this from other sources. Over one-third said they would be interested in more HPV-related information. Patients reported moderate levels of distress (mean = 3.52, SD = 2.54, possible range 0–10) and relatively low levels of self-blame (mean = 2.27, SD = 1.23, possible range 1–4) with distress and self-blame being significantly correlated (r = .38, p = .005).
Conclusion
Significant knowledge gaps exist regarding patients’ understanding of the link between HPV and OPSCC and the implications of infectious etiology. Future research is encouraged to establish best practice guidelines.
Keywords: Human papillomavirus (HPV), Oropharyngeal squamous cell carcinoma (OPSCC), Psychosocial needs, Informational needs, Patient–physician communication
Introduction
The human papillomavirus (HPV) has recently been shown to be an independent cause of oropharyngeal squamous cell carcinoma (OPSCC) [1-4]. Although cancers of the oropharynx were rare in people who did not drink or smoke excessively, rates of HPV-positive (HPV+) cases have skyrocketed, increasing 225% between 1988 and 2004 [5], particularly among middle-aged white men [1,6]. Up to 70% of current OPSCCs are believed to be HPV-associated, and the incidence continues to escalate [1,5]. Although the declining incidence of HPV-negative (HPV−) OPSCCs parallels declines in smoking in the United States [7,8], the rise in HPV+ OPSCCs has been associated with a decrease in age at first intercourse with a simultaneous increase in lifetime oral and vaginal sex partners [9]. Despite incidence increases, the psychosocial impact of the sexual transmission of the HPV has not been well investigated except for a recent study describing qualitative data of 10 men with HPV-related head and neck cancer [10]. This empirical gap is surprising as patients may experience confusion, stigma, and anxiety about the cause of their cancers. More specifically, the connection of cancer to sexual behaviors may lead to concerns regarding promiscuity and fidelity as well as fear of possible communicability of the virus and adverse consequences for patients’ sexual relationships and partners.
Physicians can play an important role in furthering patients’ understanding of the link between HPV and cancer and diminishing stigma, fear, and self-blame. However, there are currently no widely accepted guidelines for counseling HPV+ OPSCC patients. Although recommendations exist for addressing the needs of HPV+ cervical cancer patients, the demographics, risks, and experiences of patients with HPV+ OPSCC are vastly different [11]. Likewise, patients with HPV+ oropharyngeal cancers tend to be younger and have a better long-term prognosis than those with HPV-negative head and neck cancers [12] making resumption of sexual relations after cancer treatment an important quality of life concern. The development of evidence-driven and patient-centered counseling guidelines that address the above issues may be critical.
Consequently, we conducted an exploratory study to assess the informational and psychosocial needs of HPV+ OPSCC patients and to identify any social or relationship challenges associated with having an oropharyngeal cancer that is attributed to HPV.
Methods and materials
Participants and procedure
Patients initiating radiotherapy for a newly diagnosed head and neck cancer at a comprehensive cancer center in the southwestern United States were eligible if the patient had an Eastern Cooperative Oncology Group (ECOG) performance status score ≤ 2 [13]; was able to provide informed consent; could speak, read, and understand English; was age 18 years or older; and had a domestic partner with whom they had lived for at least 1 year. All patients provided informed consent prior to any data collection. The study was approved by its institutional review board.
All consecutive patients (N = 302) who met the eligibility criteria were asked to participate in the study. Patients who participated in the parent study (N = 124) were asked to complete and return written surveys in sealed envelopes within 2 weeks of the patient starting treatment for head and neck cancer. Because HPV most commonly occurs in OPSCC patients, only patients with primaries of the oropharynx were asked questions about HPV.
Of the 124 patients who participated in the parent study, 79 (64%) had OPSCC.
We extracted HPV-related information from these patients’ pathology reports to verify HPV status. Patients were identified as HPV+ if their tumors were positive for high-risk HPV by in situ hybridization (ISH) or p16 by immunohistochemical analysis as a surrogate marker [14,15]. The tumors of 62 patients were identified as HPV+ (78%), 7 as HPV− (9%), and 10 were not classified (13%). The current study reports the data of the 62 positive cases.
Measures
HPV-related measures
Patients were asked whether they had HPV infection (yes or no) and whether it caused their cancer. Response options for this item were on a 4-point Likert-type scale from 1 (not at all) to 4 (completely). Additionally, patients were asked an open-ended question regarding their cancer cause. Furthermore, patients were asked if they feel they need to keep their HPV infection a secret from others (7-point Likert-type scale from 0 [not at all] to 6 [completely]), and if so, why (open-ended). They were also asked whether they disclosed their HPV status to their current sexual partner (yes or no), whether they thought that their HPV infection had increased their partner’s risk of developing cancer (7-point Likert-type scale), and whether they had talked with their partner about the likelihood of HPV transmission (yes or no). Patients were asked to rate the extent to which the knowledge that their cancer was caused by HPV negatively impacted their relationship with their partner (7-point Likert-type scale), and if it had, how (open ended). Finally, using the same Likert-type scale as mentioned above, patients were asked to rate the following: how informed they felt about the need to safeguard their partner against HPV, the extent to which their physician had discussed HPV-related information, and the extent to which they had actively sought out information related to HPV from sources other than their physician. Patients were further asked to describe their informational needs by responding to the open-ended question “What information would you be interested in regarding HPV and cancer?”.
Other measures
Patients were asked to provide demographic information (e.g., marital and occupational status, ethnicity) on the study questionnaire. Medical variables (e.g., stage and disease subsite) were abstracted from patients’ electronic medical records. Patients completed an 8-item measure developed by Gritz et al. [16] assessing present and past nicotine use and the 10-item Alcohol Use Disorders Identification Test (AUDIT) [17] with a cut off score of ≥8 indicating hazardous alcohol consumption [17,18]. Further, patients were asked to indicate their level of distress on the National Comprehensive Cancer Network’s (NCCN) distress thermometer, a visual analogue scale with labels at 0 (no distress), 5 (moderate distress), and 10 (extreme distress) [19]. Scores ≥4 suggest the need for further psychological evaluation. Self-blame was assessed with the Glinder and Compas one-item measure of behavioral blame (i.e., “How much do you blame yourself for the kinds of things you did, that is, for any behaviors that may have led to your cancer?”) [20]. Participants responded using a 4-point Likert-type scale (1 = not at all; 4 = completely).
Data analysis strategy
All data were coded and analyzed using SPSS v19. Descriptive statistics (e.g., means, standard deviations (SDs), correlations, and frequencies) were calculated where appropriate. For simplicity, instead of describing the percentages of patients endorsing each of the answer choices on items with a Likert-type scale, we grouped the responses as follows: not at all for scores of 0 or 1, somewhat for 2 to 4, and completely for 5 and 6. Responses to open-ended questions were tabulated, categorized, and reported in summary fashion.
Results
Sample characteristics
Medical and demographic characteristics as well as alcohol consumption and smoking behaviors of the 62 HPV+ OPSCC patients are presented in Table 1. Of the 62 HPV+ tumors, 61 were p16 positive (54 also HPV ISH positive) and only one was p16 negative but HPV ISH positive.
Table 1.
Patient characteristics of study cohort (N = 62).
| Variable | HPV+ OPSCC Patients (N = 62) |
|---|---|
| Age in years (mean ± SD) | 55.9 ± 6.4 |
| Years in relationship (mean ± SD) | 22.3 ± 12.4 |
| Weeks since diagnosis (mean ± SD) | 6.9 ± 9.9 |
| Sex (%) | |
| Male | 86.5 |
| Ethnicity (%) | |
| Caucasian | 91.9 |
| African–American | 1.6 |
| Hispanic | 6.5 |
| Marital status (%) | |
| Married | 96.2 |
| Cohabitating | 3.8 |
| Employment status (%) | |
| Full-time | 75.5 |
| Retired | 6.1 |
| ECOG performance (%) | |
| 0 | 89.7 |
| 1 | 10.3 |
| 2 | 0 |
| Education (%) | |
| High school degree | 23.7 |
| Some college | 28.3 |
| College degree | 32.2 |
| Advanced degree | 15.3 |
| Cancer stage at diagnosis (%) | |
| I | 3.2 |
| II | 4.8 |
| III | 12.9 |
| IVA | 71.0 |
| IVB | 3.2 |
| Post-operative | 1.6 |
| Disease subsite (%) | |
| Base of tongue | 59.7 |
| Tonsil | 33.9 |
| Smoking history (%) | |
| Current smoker | 0 |
| Former smoker | 59 |
| Never smoker | 11 |
| Current use of other tobacco products (e.g., cigars and snuff) | 39 |
| Alcohol consumption (%) | |
| Yes | 65 |
| Problem drinkersa | 10 |
Note: HPV, human papillomavirus; +, positive; OPSCC, oropharyngeal squamous cell carcinoma; SD, standard deviation; ECOG, Eastern Cooperative Oncology Group.
Approximately 30% of patients indicated marked distress (score ≥ 4); however, distress levels were moderate (mean = 3.38, SD = 2.53, range = 0–9), and patients reported relatively low levels of behavioral self-blame (mean = 2.27, SD = 1.24, range = 1–4). Blame and distress were significantly correlated (r = .38, p = .005) but there were no significant differences regarding distress and self-blame for those who self-declared to be HPV+ compared to those who did not or were unsure of their HPV status. Similarly, none of the demographic variables (e.g., age, education and ethnicity) were significantly associated with any of the HPV-related measures except for gender. Women were significantly more likely to state that they felt informed regarding transmission precautions (t = 3.07, p = .004) and that their physician discussed HPV-related information with them (t = 2.17, p = .04) compared to men.
Informational and psychosocial needs assessment results
Although 62 patients were identified as having an HPV positive tumor on the basis of their medical record, only 66% of these patients self-declared as having an HPV positive tumor; 18% said they did not have an HPV positive tumor, and 16% said they were unsure. Interestingly, there was a discrepancy between patients’ responses to our open-ended and Likert-type questions regarding their cancer cause. When asked, “What do you think caused your cancer?” only 35% of the HPV+ patients said it was HPV. The remainder cited a variety of factors including smoking, drinking alcohol, exposure to carcinogens, bad luck, and stress (Fig. 1).
Figure 1.
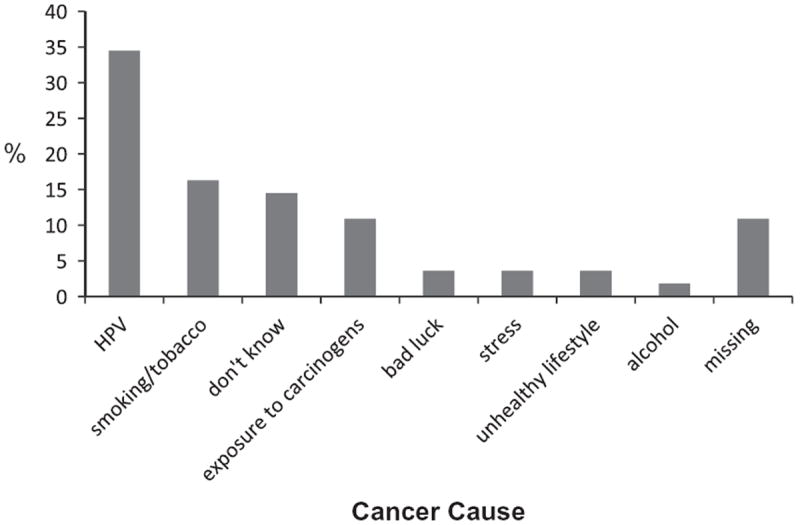
Percentages of HPV+ OPSCC patients who attributed their cancers to HPV and other factors.
Fourteen percent of patients said they fully intended to keep their HPV status secret from others (Fig. 3) (mean = 1.58, SD = 2.02, range = 0–6), and 3% said they did not tell their partner about their HPV status. Reasons for keeping HPV status a secret included mainly embarrassment (25%), stigma (38%), and the belief that their infection is no one else’s business (25%). As Fig. 2 shows, even though the majority of patients discussed their HPV status with their partner, 41% said they had not discussed concerns regarding potential viral transmission. Furthermore, 8% of patients thought their HPV had entirely increased their partner’s risk for developing cancer (mean = 1.92, SD = 1.80, range = 0–6), 42% thought it had somewhat increased their risk, and 29% felt that their HPV did not increase their partner’s risk at all.
Figure 3.
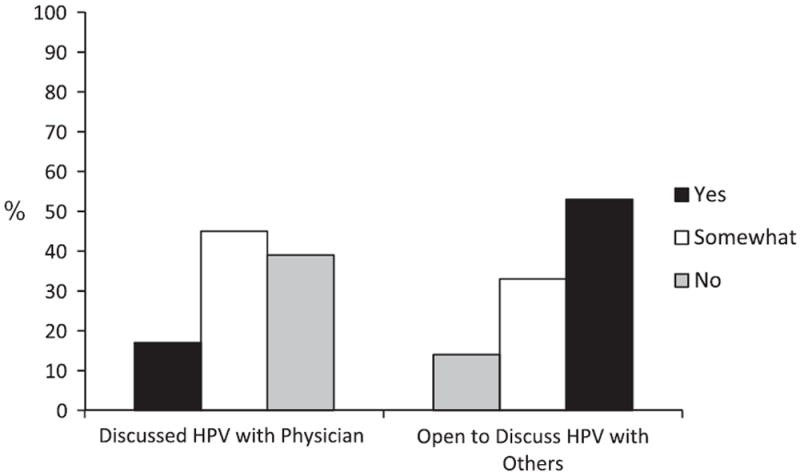
Percentages of HPV+ OPSCC patients discussing their HPV with their physician and others.
Figure 2.
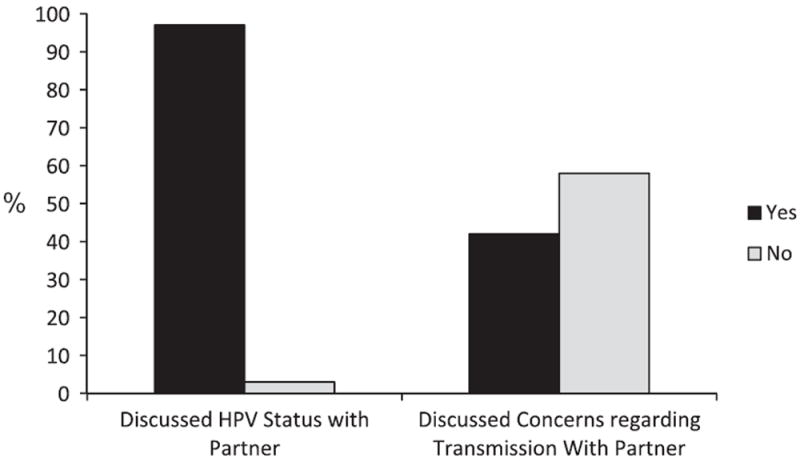
Percentages of HPV+ OPSCC patients discussing their HPV status and concerns regarding transmissions with their partners.
Finally, when asked how having an HPV-related cancer had affected their relationship with their partner, 80% of patients reported that the diagnosis had no negative impact, 14% said it had somewhat of a negative impact, and 6% said it completely negatively impacted their relationship (mean = 0.83, SD = 1.60, range = 0–6). Those who elaborated on their response described reduced trust in the relationship, problems with intimacy and reduced sexual contact because their partner was concerned about HPV transmission, and increased partner worry due to the fact that the patient’s cancer was caused by a sexually transmitted virus.
The majority of patients said they felt uninformed about whether precautions should be taken to safeguard their partner from HPV (mean = 2.63, SD = 2.18, range 0–6); in fact, 34% said they were not at all informed and 43% felt only somewhat informed. Thirty-nine percent patients reported that their oncologist did not discuss issues related to HPV and head and neck cancer with them (Fig. 3), and 45% reported that this information was only somewhat discussed (mean = 2.33, SD = 1.97, range = 0–6). Fifty-eight percent of patients reported seeking this information from sources other than their oncologist (mean = 2.08, SD = 1.68, range = 0–6). When asked about the specific types of information they would be interested in receiving about HPV and cancer, over one-third (37%) said they would be interested in any information; 18% wanted more information about how HPV causes cancer; 15% wanted information about vaccinations for HPV (particularly whether vaccinating their children would prevent them from developing cancer), 10% wanted information about how to prevent transmission to their partner, and 10% were interested to know if there were any treatments available for HPV.
Discussion
This is the first quantitative study to explore the informational needs and concerns of HPV+ OPSCC patients. We found a discrepancy between the number of patients in our study who self-identified as having an HPV-associated cancer, and the number who had HPV+ tumors. In addition, a relatively low percentage (35%) of HPV+ patients attributed their cancer to HPV. The fact that a sizable number of patients (49%) in our sample (similar to previous HPV+ OPSCC’s samples) had a smoking history could have contributed to these discrepancies. Similar to a previous qualitative report [10], our findings suggest that the diagnosis of HPV+ OPSCC may carry significant psychosocial consequences including stigma, self-blame, and relationship problems; and that significant knowledge gaps exist with regard to patients’ understanding of the link between HPV and oropharyngeal cancers as well as the risk for viral transmission and potential adverse consequences for their intimate partners. A significant knowledge gap appears to exist among HPV+ patients regarding the link between HPV and OPSCC as well as HPV and cancer communicability.
In terms of relationship consequences, 20% of our sample reported that knowing that their cancer was caused by HPV had a negative impact on their relationship with their partner. In some cases, the diagnosis led to disclosure of an infidelity, and in others, it resulted in decreased sexual intimacy due to concerns about communicability. These findings underscore the fact that a diagnosis of HPV+ OPSCC may have far-reaching implications for the patient, his/her sexual partner, and their relationship with one another and potentially lead to confusion and unwarranted concerns and anxiety.
Significant knowledge gaps may possibly be related to the fact that 84% of our patients felt that they did not understand these issues to their satisfaction, and 58% reported seeking HPV-related information from sources beyond their healthcare providers. Furthermore, considering that over 1/3 of patients in this study said they would be interested in more information about the link between HPV and cancer, HPV transmission and its prevention, and possible HPV treatment, this study highlights a need to improve patient-provider communication regarding HPV-related information. Although NCCN recommendations caution that HPV information may add anxiety and stress for some patients [15], oncologists can play an important role in educating patients on HPV and its link to OPSCC [21] with goal to reduce unwarranted fears and concerns (e.g., increased cancer risk of sexual partners) because the psychosocial sequelae of misinformation are potentially damaging to both the patient and his/her partner. More research is needed to clarify the role of physicians and other healthcare providers in counseling HPV+ patients and their partners and to specify counseling guidelines.
Chu et al. [21] have recently adapted clinical guidelines from counseling recommendations for cervical cancer patients that offer valuable information for clinicians regarding HPV and OPSCC. Although more research to discern the risk of HPV transmission to spouses and the impact of the diagnosis on the psychosocial well-being of patients and their partners is needed before definitive counseling guidelines can be established, we echo the authors’ sentiment regarding the prudence of providing counseling at the time of diagnosis. Such counseling may serve the dual-purpose of addressing patients’ concerns regarding HPV as well as identifying psychosocial concerns related to the diagnosis and its treatment. If physicians determine that the patient would benefit from psychosocial supportive care, couple-based psychoeducational programs may be particularly beneficial [22]. While head and neck cancer patients report significant decrements in quality of life and their interpersonal relationships over time [23], the challenges for couples coping with HPV+ OPSCCs are likely compounded by concerns about fidelity and sexual intimacy. Consequently, patients and their partners may benefit from programs that provide information about the link between HPV and cancer and reduce fears related to HPV transmission. Baxi and colleagues [10] have developed educational materials for patients which may provide a helpful foundation for such programs to examined in future investigations.
This is the first quantitative study to explore the informational needs and concerns of HPV+ OPSCC patients. Strengths include our assessment of psychosocial implications of the diagnosis as well as patients’ informational needs and the consistency between our sample and the profile of OPSCC patients with regard to age, sex, and tobacco and alcohol history. Limitations include a relatively small sample size and the smaller proportion of female patients. Because these data are part of a larger dyadic study, our sample included only patients in cohabitating relationships and our findings may thus not generalize to single patients who may have additional concerns regarding starting new relationships and how to communicate with new partners about their prior HPV infection and current HPV-associated cancer. Thus, research that includes a more representative sample of the larger population is needed. P16 overexpression is considered the most reliable marker for an HPV-driven cancer and HPV in situ positivity with negative p16 immunohistochemistry may represent false positivity. However in this work, only one patient was HPV in situ positive and p16 negative. In addition, we assessed patients’ perceptions of HPV-related communications and, because there was no documentation regarding physicians or other health care providers addressing HPV during clinic visits, it is impossible to determine if the revealed knowledge deficits are a function of lack of communication or understanding of information that had actually been provided. Finally, in our questionnaire we asked patients about their “infection” with HPV, though we only know of their HPV tumor status and do not know if these patients have an active HPV infection. In fact, it is very likely that patients with HPV positive OPSCC had an oropharyngeal HPV infection many years prior to cancer diagnosis, and only a small portion of HPV DNA remains as incorporated oncogene(s) in the human genome of the cancer cell, which may have resulted in inaccuracies in responses to our questions.
These limitations notwithstanding, our data suggest that given the increasing incidence of HPV+ OPSCCs, it is important to formulate clear and consistent messages aimed at diminishing stigma and self-blame and to develop and evaluate psychoeducational programs that address information gaps and psychosocial needs and concerns of patients and their partners. Although the current state of scientific knowledge diminishes our ability to counsel patients fully about how their cancers evolved, and best practice guidelines may need to be established, physicians, patients, and partners may embark on a partnership in which the role and psychosocial implications of having HPV+ OPSCC are shared and discussed.
Acknowledgments
The authors thank Marshall Posner, M.D., and Erich Sturgis, M.D., for helpful suggestions regarding the development of this manuscript. This parent study of this research was supported by a career development award from the National Cancer Institute (K07CA124668 and 3K07CA124668-3S1) awarded to Dr. Badr and an American Cancer Society post-doctoral Grant (PF-10-0 13-01-CPPB) awarded to Dr. Milbury.
Footnotes
Conflict of interest statement
None declared.
References
- 1.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 2.Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–3. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 3.Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the international agency for research on cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–83. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 4.Sturgis EM, Ang KK. The epidemic of HPV-associated oropharyngeal cancer is here: is it time to change our treatment paradigms? J Natl Compr Cancer Netw. 2011;9:665–73. doi: 10.6004/jnccn.2011.0055. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryerson AB, Peters ES, Coughlin SS, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998–2003. Cancer. 2008;113:2901–9. doi: 10.1002/cncr.23745. [DOI] [PubMed] [Google Scholar]
- 7.(CDC) CfDCaP. Cigarette smoking among adults – United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:1157–61. [PubMed] [Google Scholar]
- 8.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:1429–35. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 9.Bajos N, Bozon M, Beltzer N, et al. Changes in sexual behaviours: from secular trends to public health policies. AIDS. 2010;24:1185–91. doi: 10.1097/QAD.0b013e328336ad52. [DOI] [PubMed] [Google Scholar]
- 10.Baxi SS, Shuman AG, Corner GW, et al. Sharing a diagnosis of HPV-related head and neck cancer: the emotions, the confusion, and what patients want to know. Head Neck. 2012 doi: 10.1002/hed.23182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbyn M, de Sanjose S, Saraiya M, et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J Cancer. 2012;131:1969–82. doi: 10.1002/ijc.27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–6. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 13.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 14.El-Naggar AK, Westra WH. P16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34:459–61. doi: 10.1002/hed.21974. [DOI] [PubMed] [Google Scholar]
- 15.Pfister DG, Ang KK, Brizel DM, et al. Head and neck cancers. J Natl Compr Cancer Netw. 2011;9:596–650. doi: 10.6004/jnccn.2011.0053. [DOI] [PubMed] [Google Scholar]
- 16.Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomar Prevent. 2005;14:2287–93. doi: 10.1158/1055-9965.EPI-05-0224. [DOI] [PubMed] [Google Scholar]
- 17.Saunders J, Aasland O, Babor T, et al. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. Addiction. 1993;88:791–803. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 18.Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Alcohol use disorder identification test. Addiction. 1995;90:1349–56. doi: 10.1046/j.1360-0443.1995.901013496.x. [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network (NCCN) Distress: Treatment guidelines for patients. American Cancer Society; 2005. [Google Scholar]
- 20.Glinder JG, Compas BE. Self-blame attributions in women with newly diagnosed breast cancer: a prospective study of psychological adjustment. Health Psychol. 1999;18:475–81. doi: 10.1037//0278-6133.18.5.475. [DOI] [PubMed] [Google Scholar]
- 21.Chu A, Genden E, Posner M, Sikora A. Patient-centered approach to counseling head and neck cancer patients undergoing HPV testing: a clinician’s guide. Oncologist. 2013;18:180–9. doi: 10.1634/theoncologist.2012-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badr H, Krebs P. A systematic review and meta-analysis of psychosocial interventions for couples coping with cancer. Psycho-Oncology. 2013;22:1688–704. doi: 10.1002/pon.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gritz ER, Carmack CL, de Moor CA. The first year after head and neck cancer: quality of life. J Clin Oncol. 1999;17:352–60. doi: 10.1200/JCO.1999.17.1.352. [DOI] [PubMed] [Google Scholar]


